Management of ERCP-Related Perforations: A Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
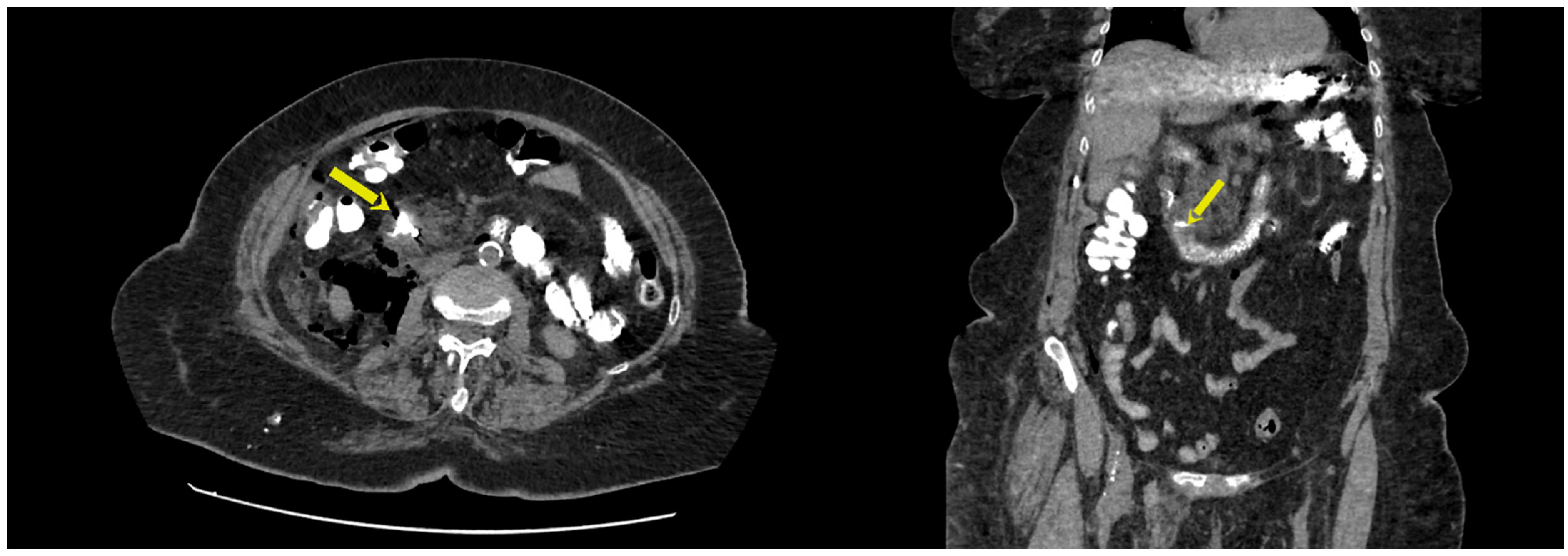
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, J.; Sreevastava, D.; Dwivedi, D.; Gadgi, S.; Sud, S.; Dudeja, P. A Comparison of Stress Response between Insertion of Gastro-Laryngeal Tube and Endotracheal Intubation in Patients Undergoing Upper Gastrointestinal Endoscopic Procedures for Endoscopic Retrograde Cholangiopancreatography. Anesth. Essays Res. 2019, 13, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Tso, D.K.; Almeida, R.R.; Prabhakar, A.M.; Singh, A.K.; Raja, A.S.; Flores, E.J. Accuracy and timeliness of an abbreviated emergency department MRCP protocol for choledocholithiasis. Emerg. Radiol. 2019, 26, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Kodali, S.; Mönkemüller, K.; Kim, H.; Ramesh, J.; Trevino, J.; Varadarajulu, S.; Wilcox, C.M. ERCP-related perforations in the new millennium: A large tertiary referral center 10-year experience. United Eur. Gastroenterol. J. 2015, 3, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Silviera, M.L.; Seamon, M.J.; Porshinsky, B.; Prosciak, M.P.; Doraiswamy, V.A.; Wang, C.F.; Lorenzo, M.; Truitt, M.; Biboa, J.; Jarvis, A.M.; et al. Complications related toendoscopic retrograde cholangiopancreatography: A comprehensiveclinical review. J. Gastrointestin. Liver Dis. 2009, 18, 73–82. [Google Scholar]
- Desirée Armas Ojeda, M.; Marrero, V.O.; Castellano, C.R.; Marrero, J.C.; Mathías Gutierrez, M.d.P.; Ceballos Santos, D.; Marchena Gómez, J. Duodenal Perforations After Endoscopic Retrograde cholangiopancreatography: Spanish Association of Surgeons. Cirugía Española Engl. Ed. 2015, 93, 403–410. [Google Scholar] [CrossRef]
- Freeman, M.L. Complication s of endoscopic retrograde cholangiopancreatography. Gastrointest. Endosc. Clin. N. Am. 2012, 14, 148–155. [Google Scholar] [CrossRef]
- Dubecz, A.; Ottmann, J.; Schweigert, M.; Stadlhuber, R.; Feith, M.; Wiessner, V.; Muschweck, H.; Stein, H. Management of ERCP-related small bowel perforations: The pivotal role of physical investigation. Can. J. Surg. 2012, 55, 99–104. [Google Scholar] [CrossRef]
- Polydorou, A.; Vezakis, A.; Fragulidis, G.; Katsarelias, D.; Vagianos, C.; Polymeneas, G. A tailored approach to themanagement of perforations following endoscopic retrograde cholangiopancreatographyand sphincterotomy. J. Gastrointest. Surg. 2011, 15, 2211–2217. [Google Scholar] [CrossRef]
- Stapfer, M.; Selby, R.R.; Stain, S.C.; Katkhouda, N.; Parekh, D.; Jabbour, N.; Garry, D. Management of duodenal perforation after endoscopic retrograde cholangiopancreatography and sphincterotomy. Ann. Surg. 2000, 232, 191–198. [Google Scholar] [CrossRef]
- Lai, C.H.; Lau, W.Y. Management of endoscopic retrogradecholangiopancreatography-related perforation. Surgeon 2008, 6, 45–48. [Google Scholar] [CrossRef]
- Morgan, K.A.; Fontenot, B.B.; Ruddy, J.M.; Mickey, S.; Adams, D.B. Endoscopic retrograde cholangiopancreatographygut perforations: When to wait! When to operate! Am. Surg. 2009, 75, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Fatima, J.; Baron, T.H.; Topazian, M.D.; Houghton, S.G.; Iqbal, C.W.; Ott, B.J.; Farley, D.R.; Farnell, M.B.; Sarr, M.G. Pancreaticobiliary andduodenal perforations after periampullary endoscopic procedures: Diagnosis and management. Arch. Surg. 2007, 142, 448–454; discussion 454–455. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, D.; Li, Z. Endoscopic Closure for EUS and ERCP Related Duodenal Perforation by Endoclips. Gastroenterol. Res. Pract. 2016, 2016, 1051597. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Matsumoto, K.; Miyamoto, K.; Matsumi, A.; Morimoto, K.; Terasawa, H.; Yamazaki, T.; Horiguchi, S.; Tsutsumi, K.; Kato, H. Endoscopic treatment for duodenal perforation due to biliary stent dislocation: A case report and brief review of the literature. Medicine 2022, 101, e31868. [Google Scholar] [CrossRef]
- Vezakis, A.; Fragulidis, G.; Polydorou, A. Endoscopic retrograde cholangiopancreatography-related perforations: Diagnosis and management. World J. Gastrointest. Endosc. 2015, 7, 1135–1141. [Google Scholar] [CrossRef]
- Cirocchi, R.; Kelly, M.D.; Griffiths, E.A.; Tabola, R.; Sartelli, M.; Carlini, L.; Ghersi, S.; Di Saverio, S. A systematic review of the management and outcome of ERCP related duodenal perforations using a standardized classification system. Surgery 2017, 15, 379–387. [Google Scholar] [CrossRef]
- Borazan, E.; Konduk, B.T. Comparison of early and delayed diagnosis of mortality in ERCP perforations: A high-volume patient experience. Turk. J. Trauma Emerg. Surg. 2020, 26, 746–753. [Google Scholar] [CrossRef]
- Loperfido, S.; Angelini, G.; Benedetti, G.; Chilovi, F.; Costan, F.; De Berardinis, F.; De Bernardin, M.; Ederle, A.; Fina, P.; Fratton, A. Major early complications from diagnostic and therapeutic ERCP: A prospective multicenter study. Gastrointest. Endosc. 1998, 48, 1–10. [Google Scholar] [CrossRef]
- Enns, R.; Eloubeidi, M.A.; Mergener, K.; Jowell, P.S.; Branch, M.S.; Pappas, T.M.; Baillie, J. ERCP-related perforations: Risk factors and management. Endoscopy 2002, 34, 293–298. [Google Scholar] [CrossRef]
- Paspatis, G.A.; Arvanitakis, M.; Dumonceau, J.-M.; Barthet, M.; Saunders, B.; Turino, S.Y.; Dhillon, A.; Fragaki, M.; Gonzalez, J.-M.; Repici, A.; et al. Diagnosis and management of iatrogenic endoscopic perforations: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement—Update 2020. Endoscopy 2020, 52, 792–810. [Google Scholar] [CrossRef]
- Bell, R.C.; Van Stiegmann, G.; Goff, J.; Reveille, M.; Norton, L.; Pearlman, N.W. Decision for surgical management of perforation following endoscopic sphincterotomy. Proc. Soc. Am. Gastrointest. Endosc. Surg. 1991, 57, 237–240. [Google Scholar]
- Cotton, P.B.; Lehman, G.; Vennes, J.; Geenen, J.E.; Russell, R.C.G.; Meyers, W.C.; Liguory, C.; Nickl, N. Endoscopic sphincterotomy complications and their management: An attempt at consensus. Gastrointest. Endosc. 1991, 37, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Preetha, M.; Chung, Y.A.; Chan, W.; Ong, H.; Chow, P.K.H.; Wong, W.; Ooi, L.L.P.J.; Soo, K. Surgical management of endoscopic retrograde cholangiopancreatography-related perforations. ANZ J. Surg. 2003, 73, 1011–1014. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-J.; Jeong, S.; Kim, J.H.; Hwang, J.C.; Yoo, B.M.; Moon, J.H.; Park, S.H.; Kim, H.G.; Lee, D.K.; Jeon, Y.S.; et al. Clinical course and proposed treatment strategy for ERCP-related duodenal perforation: A multicenter analysis. Endoscopy 2013, 45, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, S.; Rosa, F.; Cina, C.; Tortorelli, A.P.; Tringali, A.; Perri, V.; Costamagna, G.; Doglietto, G.B. Management of duodeno-pancreato-biliary perforations after ERCP: Outcomes from an Italian tertiary referral center. Surg. Endosc. 2013, 27, 2005–2012. [Google Scholar] [CrossRef]
- Theopistos, V.; Theocharis, G.; Konstantakis, C.; Kitrou, P.; Kehagias, I.; Triantos, C.; Thomopoulos, K. Non-Operative Management of Type 2 ERCP-Related Retroperitoneal Duodenal Perforations: A 9-Year Experience from a Single Center. Gastroenterol. Res. 2018, 11, 207–212. [Google Scholar] [CrossRef]
- Kumbhari, V.; Sinha, A.; Reddy, A.; Afghani, E.; Cotsalas, D.; Patel, Y.A.; Storm, A.C.; Khashab, M.A.; Kalloo, A.N.; Singh, V.K. Algorithm for the management of ERCP-related perforations. Gastrointest. Endosc. 2016, 83, 934–943. [Google Scholar] [CrossRef]
- Lee, T.H. Primary endoscopic approximation suture under cap-assisted endoscopy of an ERCP-induced duodenal perforation. World J. Gastroenterol. 2010, 16, 2305–2310. [Google Scholar] [CrossRef]
- Aranez, J.L.; Miller, J.; Hughes, M.; DeCross, A.; Kaul, V. A novel, duodenoscope-friendly endoscopic clip for treating massive upper-GI bleeding secondary to a Dieulafoy lesion. VideoGIE 2018, 3, 205–206. [Google Scholar] [CrossRef]
- Samarasena, J.B.; Nakai, Y.; Park, D.H.; Iwashita, T.; Chang, K. Endoscopic closure of an iatrogenic duodenal perforation: A novel technique using endoclips, endoloop, and fibrin glue. Endoscopy 2012, 44 (Suppl. S2), E424–E425. [Google Scholar] [CrossRef]
- Odemis, B.; Oztas, E.; Kuzu, U.B.; Parlak, E.; Disibeyaz, S.; Torun, S.; Kayacetin, E. Can a fully covered self-expandable metallic stent be used temporarily for the management of duodenal retroperitoneal perforation during ercp as a part of conservative therapy? Surg. Laparosc. Endosc. Percutaneous Tech. 2016, 26, e9–e17. [Google Scholar] [CrossRef]

| Type | Definition |
|---|---|
| I | Lateral or medial duodenal wall perforation, endoscope-related |
| II | Periampullary perforations, sphincterotomy-related |
| III | Ductal or duodenal perforations caused by endoscopic instruments |
| IV | Guidewire-related perforation with the presence of retroperitoneal gas on imaging |
| Patient | Age | Gender | Indication for ERCP | Bile Duct Canulated | Sphincterotomy | Stone Extracted | Precut | Perforation Type |
|---|---|---|---|---|---|---|---|---|
| 1 | 80 | F | Bile duct stones | Yes | Yes | No | No | Stapfer 4 |
| 2 | 75 | F | Bile duct stones | Yes | Yes | Yes | No | Stapfer 2 |
| 3 | 83 | M | Bile duct stones | Yes | Yes | No | Yes | Stapfer 2 |
| 4 | 80 | F | Bile duct stones | Yes | Yes | Yes | Yes | Stapfer 2 |
| 5 | 68 | M | Bile duct stones | Yes | Yes | No | No | Stapfer 2 |
| 6 | 66 | M | Pancreatic cancer | No | No | N/A | Yes | Stapfer 2 |
| 7 | 49 | F | Bile duct stones | Yes | Yes | No | No | Stapfer 4 |
| 8 | 58 | F | Bile duct stones | Yes | Yes | Yes | No | Stapfer 2 |
| CT Features | Initial Lab. Analyses | |||||||
|---|---|---|---|---|---|---|---|---|
| Patient | Perforation Type | Abdominal Pain | Retroperitoneal Air | Pneumoperitoneum | Contrast Leakage | Intraabdominal Liquid | CRP® | WBC Count3 |
| 1 | Stapfer 4 | Yes | Yes | Yes | 27.5 | 3.8 | ||
| 2 | Stapfer 2 | Yes | Yes | Yes | Yes | 5.1 | 4.9 | |
| 3 | Stapfer 2 | Yes | Yes | Yes | Yes | |||
| 4 | Stapfer 2 | Yes | Yes | Yes | Yes | 18.6 | 13.7 | |
| 5 | Stapfer 2 | Yes | Yes | Yes | Yes | Yes | 65 | 11.7 |
| 6 | Stapfer 2 | Yes | Yes | Yes | Yes | |||
| 7 | Stapfer 4 | Yes | Yes | 367 | 19.3 | |||
| 8 | Stapfer 2 | Yes | Yes | Yes | 340 | 11.4 | ||
| ®= mg/L | 3= ×109/L | |||||||
| Treatment | |||||||
|---|---|---|---|---|---|---|---|
| Patient | Perforation Type | Time of Diagnosis | Conservative | Endoscopic | Surgery | Length of Stay | Outcome |
| 1 | Stapfer 4 | later than 24 h | Yes | 27 | Discharged | ||
| 2 | Stapfer 2 | Intraprocedural | Yes | Yes | 15 | Discharged | |
| 3 | Stapfer 2 | Intraprocedural | Yes | Yes | 15 | Discharged | |
| 4 | Stapfer 2 | later than 24 h | Yes | 17 | Discharged | ||
| 5 | Stapfer 2 | within 24 h | Yes | 13 | Discharged | ||
| 6 | Stapfer 2 | within 24 h | Yes | / | Discharged | ||
| 7 | Stapfer 4 | later than 24 h | Yes | 18 | Discharged | ||
| 8 | Stapfer 2 | within 24 h | Yes | 11 | Discharged | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plecic, N.; Malenkovic, A.; Begovic, A.; Pavlovic, A.; Bulajic, M.; Bulajic, M.; Đukic, V.; Milanovic, M.; Savic, P.; Panic, N. Management of ERCP-Related Perforations: A Single-Center Experience. J. Clin. Med. 2025, 14, 1. https://doi.org/10.3390/jcm14010001
Plecic N, Malenkovic A, Begovic A, Pavlovic A, Bulajic M, Bulajic M, Đukic V, Milanovic M, Savic P, Panic N. Management of ERCP-Related Perforations: A Single-Center Experience. Journal of Clinical Medicine. 2025; 14(1):1. https://doi.org/10.3390/jcm14010001
Chicago/Turabian StylePlecic, Nemanja, Ana Malenkovic, Aleksa Begovic, Aleksandra Pavlovic, Milutin Bulajic, Mirko Bulajic, Vladimir Đukic, Miljan Milanovic, Predrag Savic, and Nikola Panic. 2025. "Management of ERCP-Related Perforations: A Single-Center Experience" Journal of Clinical Medicine 14, no. 1: 1. https://doi.org/10.3390/jcm14010001
APA StylePlecic, N., Malenkovic, A., Begovic, A., Pavlovic, A., Bulajic, M., Bulajic, M., Đukic, V., Milanovic, M., Savic, P., & Panic, N. (2025). Management of ERCP-Related Perforations: A Single-Center Experience. Journal of Clinical Medicine, 14(1), 1. https://doi.org/10.3390/jcm14010001





