Efficacy and Safety of Prothrombin Complex Concentrates in Liver Transplantation: Evidence from Observational Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Studies Selection
2.2. Data Extraction and Quality Assessment
2.3. Sensitivity Analyses
2.4. Data Synthesis and Meta-Analysis
3. Results
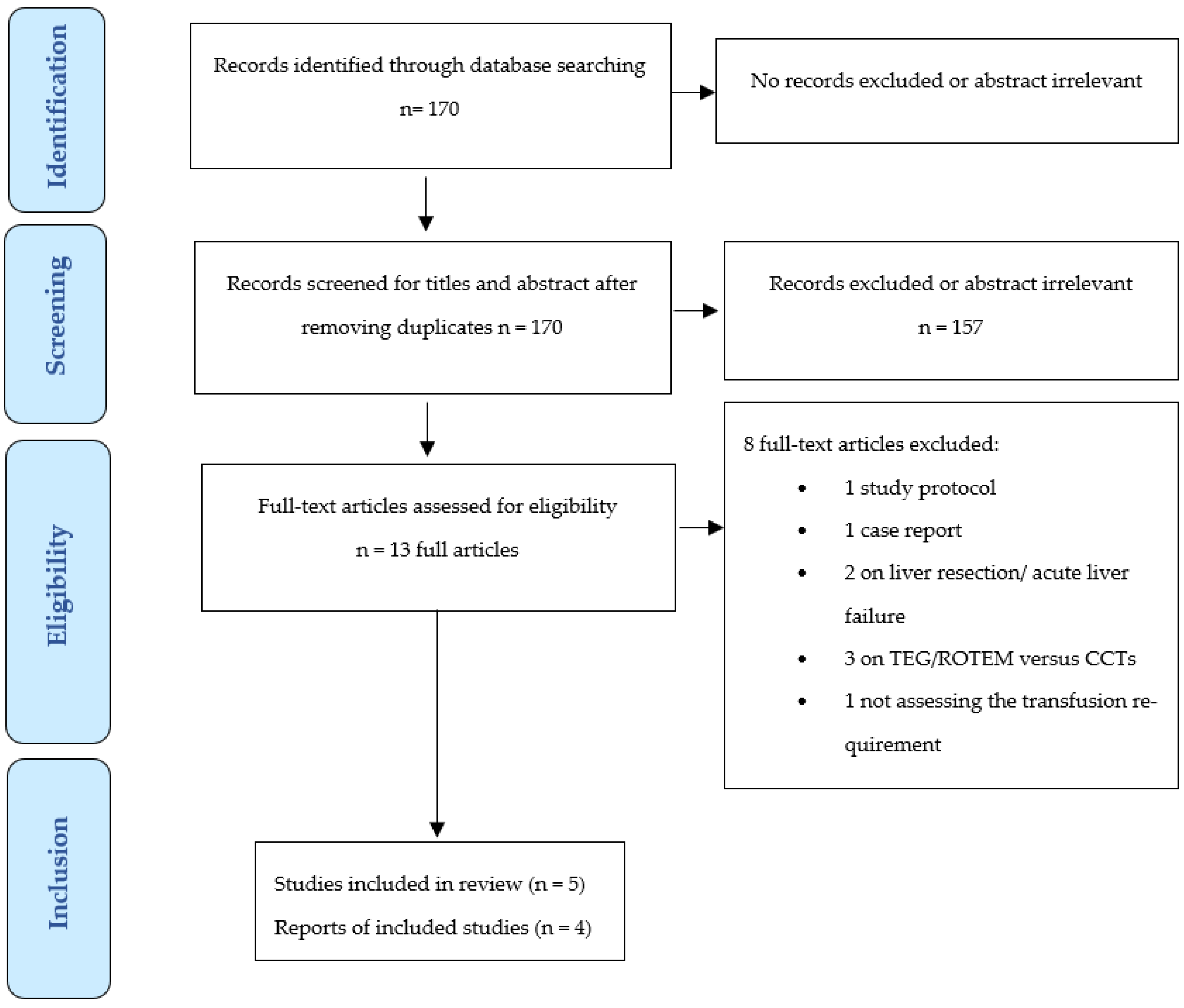
3.1. Studies Selection
3.2. Study Characteristics
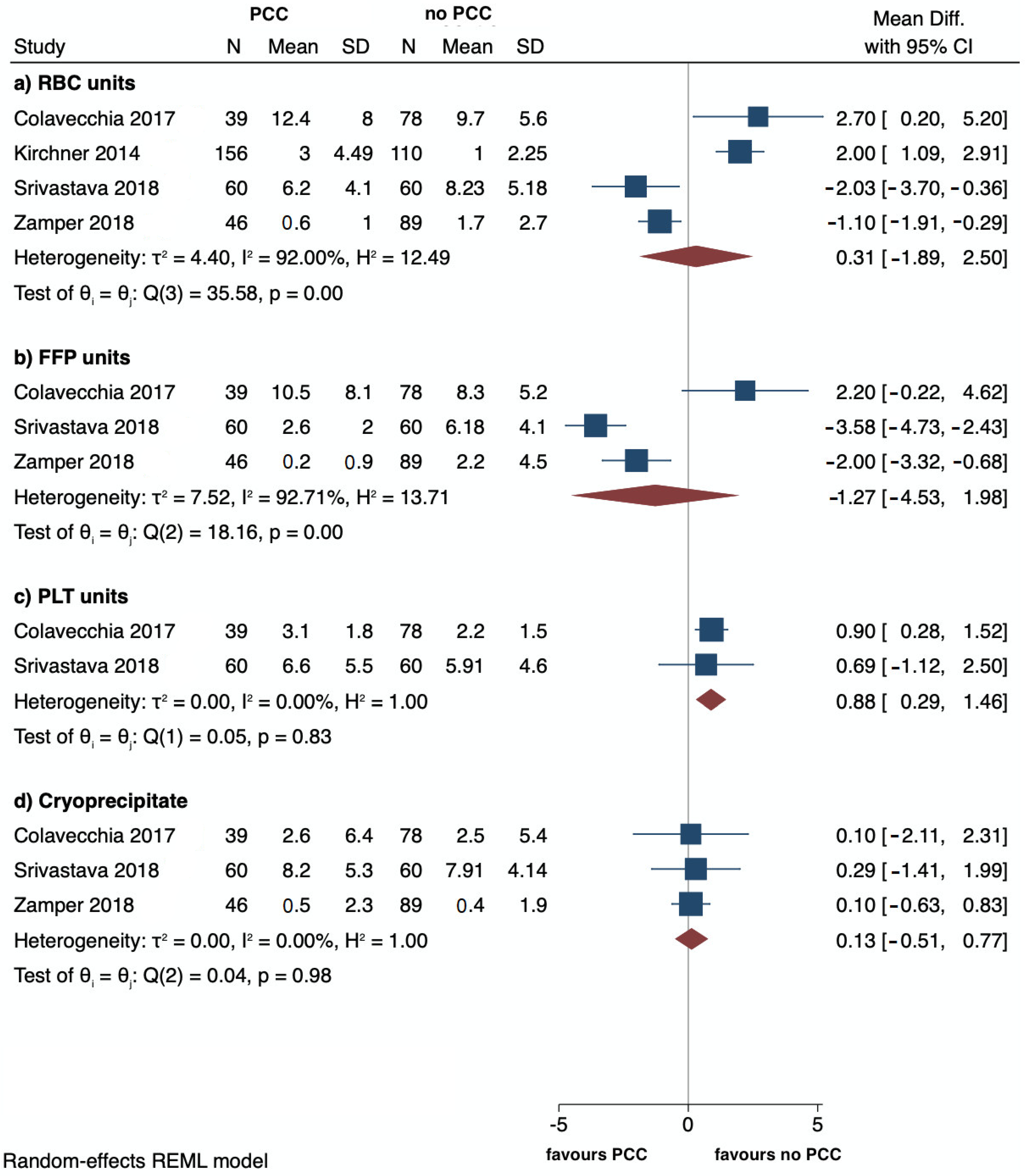
3.3. Primary Outcomes
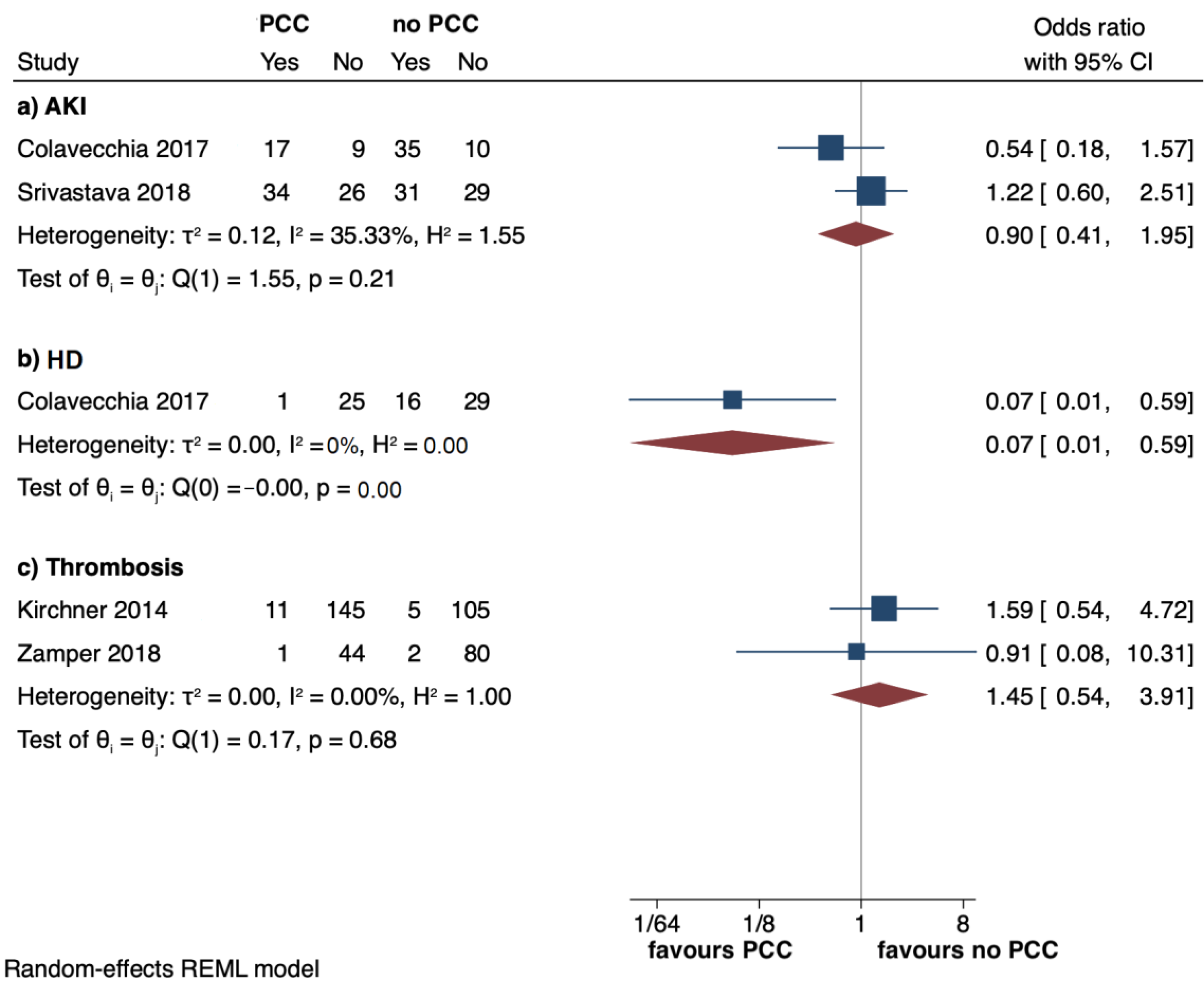
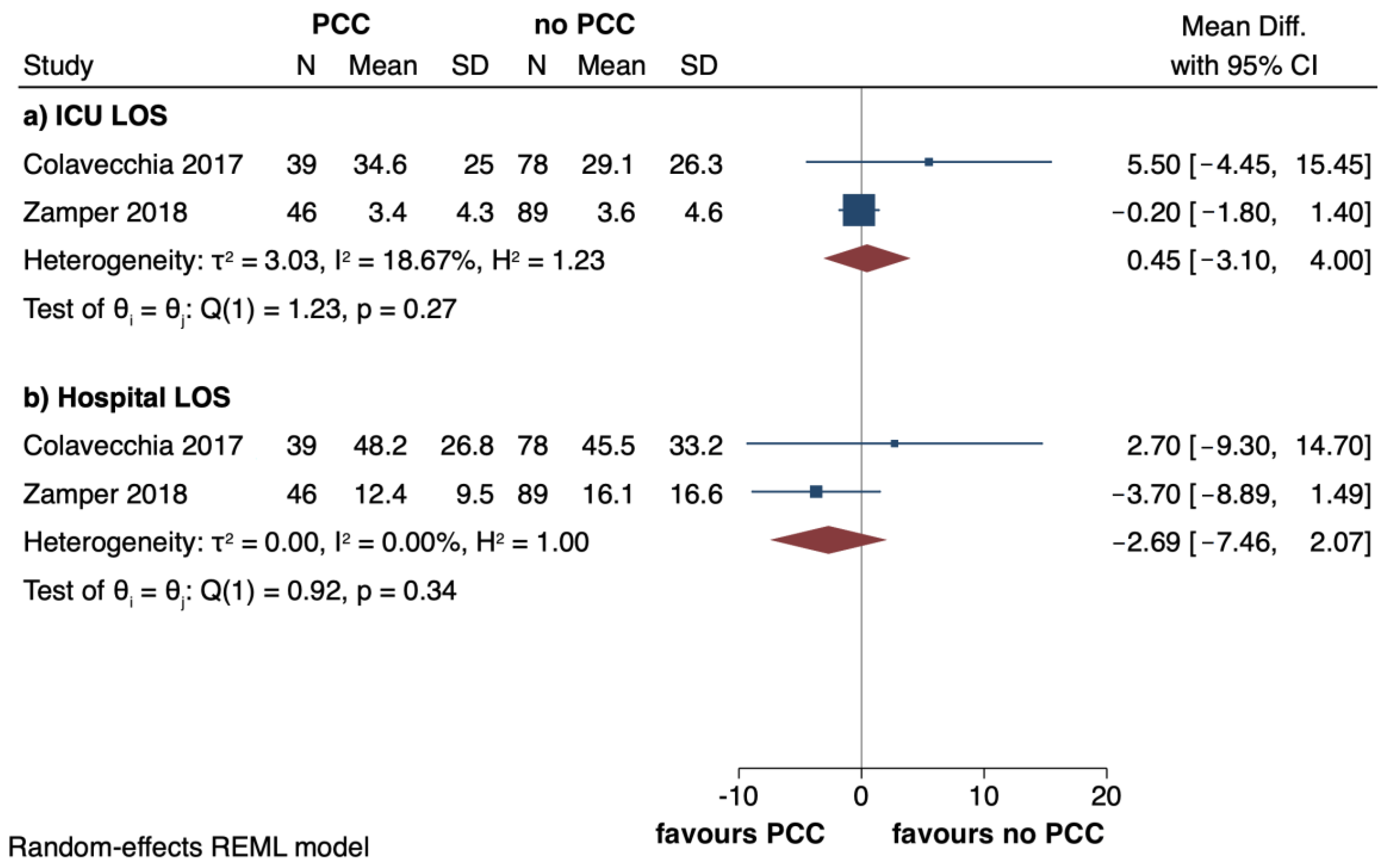
3.4. Secondary Outcomes
3.5. Sensitivity Analyses
3.6. Risk of Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Görlinger, K.; Pérez-Ferrer, A.; Dirkmann, D.; Saner, F.; Maegele, M.; Calatayud, Á.A.P.; Kim, T.Y. The role of evidence-based algorithms for rotational thromboelastometry-guided bleeding management. Korean J. Anesthesiol. 2019, 72, 297–322. [Google Scholar] [CrossRef] [PubMed]
- Görlinger, K.; Fries, D.; Dirkmann, D.; Weber, C.F.; Hanke, A.A.; Schöchl, H. Reduction of Fresh Frozen Plasma Requirements by Perioperative Point-of-Care Coagulation Management with Early Calculated Goal-Directed Therapy. Transfus. Med. Hemother. 2012, 39, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, R.; Kienast, J.; Otto, U.; Egger, K.; Kiehl, M.; Schreiter, D.; Kwasny, H.; Haertel, S.; Barthels, M. Efficacy and safety of a prothrombin complex concentrate with two virus-inactivation steps in patients with severe liver damage. Eur. J. Gastroenterol. Hepatol. 2003, 15, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, C.; Dirkmann, D.; Treckmann, J.W.; Paul, A.; Hartmann, M.; Saner, F.H.; Görlinger, K. Coagulation management with factor concentrates in liver transplantation: A single-center experience. Transfusion 2014, 54, 2760–2768. [Google Scholar] [CrossRef] [PubMed]
- Colavecchia, A.C.; Cohen, D.A.; Harris, J.E.; Thomas, J.M.; Lindberg, S.; Leveque, C.; Salazar, E. Impact of intraoperative factor concentrates on blood product transfusions during orthotopic liver transplantation. Transfusion 2017, 57, 3026–3034. [Google Scholar] [CrossRef]
- Srivastava, P.; Agarwal, A.; Jha, A.; Rodricks, S.; Malik, T.; Makki, K.; Singhal, A.; Vij, V. Utility of prothrombin complex concentrate as first-line treatment modality of coagulopathy in patients undergoing liver transplantation: A propensity score-matched study. Clin. Transpl. 2018, 32, e13435. [Google Scholar] [CrossRef]
- Zamper, R.P.C.; Amorim, T.C.; Queiroz, V.N.F.; Lira, J.D.O.; Costa, L.G.V.; Takaoka, F.; Juffermans, N.P.; Neto, A.S. Association between viscoelastic tests-guided therapy with synthetic factor concentrates and allogenic blood transfusion in liver transplantation: A before-after study. BMC Anesthesiol. 2018, 18, 198. [Google Scholar] [CrossRef]
- Hartmann, M.; Walde, C.; Dirkmann, D.; Saner, F.H. Safety of coagulation factor concentrates guided by ROTEM™-analyses in liver transplantation: Results from 372 procedures. BMC Anesthesiol. 2019, 19, 97. [Google Scholar] [CrossRef]
- Drebes, A.; de Vos, M.; Gill, S.; Fosbury, E.; Mallett, S.; Burroughs, A.; Agarwal, B.; Patch, D.; Chowdary, P. Prothrombin Complex Concentrates for Coagulopathy in Liver Disease: Single-Center, Clinical Experience in 105 Patients. Hepatol. Commun. 2019, 3, 513–524. [Google Scholar] [CrossRef]
- SHOT Annual Report 2021: Summary of Serious Hazards of Transfusion. Available online: https://www.shotuk.org/wp-content/uploads/myimages/SHOT-REPORT-2021-FINAL-bookmarked-V3-November.pdf (accessed on 28 March 2023).
- Massicotte, L.; Lenis, S.; Thibeault, L.; Sassine, M.P.; Seal, R.F.; Roy, A. Effect of low central venous pressure and phlebotomy on blood product transfusion requirements during liver transplantations. Liver Transpl. 2006, 12, 117–123. [Google Scholar] [CrossRef]
- Refaai, M.A.; Goldstein, J.N.; Lee, M.L.; Durn, B.L.; Milling, T.J., Jr.; Sarode, R. Increased risk of volume overload with plasma compared with four-factor prothrombin complex concentrate for urgent vitamin K antagonist reversal. Transfusion 2015, 55, 2722–2729. [Google Scholar] [CrossRef]
- Teofili, L.; Valentini, C.G.; Aceto, P.; Bartolo, M.; Sollazzi, L.; Agnes, S.; Gaspari, R.; Avolio, A.W. High intraoperative blood product requirements in liver transplantation: Risk factors and impact on the outcome. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 64–75. [Google Scholar] [CrossRef]
- Stanworth, S.J.; Brunskill, S.J.; Hyde, C.J.; McClelland, D.B.; Murphy, M.F. Is fresh frozen plasma clinically effective? A systematic review of randomized controlled trials. Br. J. Haematol. 2004, 126, 139–152. [Google Scholar] [CrossRef]
- Gaspari, R.; Teofili, L.; Aceto, P.; Valentini, C.G.; Punzo, G.; Sollazzi, L.; Agnes, S.; Avolio, A.W. Thromboelastography does not reduce transfusion requirements in liver transplantation: A propensity score-matched study. J. Clin. Anesth. 2021, 69, 110154. [Google Scholar] [CrossRef]
- Smith, N.K.; Kim, S.; Hill, B.; Goldberg, A.; DeMaria, S.; Zerillo, J. Transfusion-Related Acute Lung Injury (TRALI) and Transfusion-Associated Circulatory Overload (TACO) in Liver Transplantation: A Case Report and Focused Review. Semin. Cardiothorac. Vasc. Anesth. 2018, 22, 180–190. [Google Scholar] [CrossRef]
- Kuldanek, S.A.; Kelher, M.; Silliman, C.C. Risk factors, management and prevention of transfusion-related acute lung injury: A comprehensive update. Expert. Rev. Hematol. 2019, 12, 773–785. [Google Scholar] [CrossRef]
- Iyer, M.H.; Kumar, J.E.; Kumar, N.; Gorelik, L.; Hussain, N.; Stein, E.; Bhatt, A.M.; Bhandary, S.; Essandoh, M.K.; Flores, A.S. Transfusion-Related Acute Lung Injury During Liver Transplantation: A Scoping Review. J. Cardiothorac. Vasc. Anesth. 2022, 36, 2606–2615. [Google Scholar] [CrossRef]
- Görlinger, K. Gerinnungsmanagement bei Lebertransplantationen [Coagulation management during liver transplantation]. Hamostaseologie 2006, 26 (Suppl. S1), S64–S76. [Google Scholar]
- O’Leary, J.G.; Greenberg, C.S.; Patton, H.M.; Caldwell, S.H. AGA Clinical Practice Update: Coagulation in Cirrhosis. Gastroenterology 2019, 157, 34–43.e1. [Google Scholar] [CrossRef]
- Huang, W.T.; Cang, W.C.; Derry, K.L.; Lane, J.R.; von Drygalski, A. Four-Factor Prothrombin Complex Concentrate for Coagulopathy Reversal in Patients with Liver Disease. Clin. Appl. Thromb. Hemost. 2017, 23, 1028–1035. [Google Scholar] [CrossRef]
- Sadeghi, N.; Kahn, D.; Sayed, D.; Hoppenstadt, D.; Jeske, W.; Harenberg, J.; Dechristopher, P.; Fareed, J. Compositional differences in commercially available prothrombin complex concentrates. Clin. Appl. Thromb. Hemost. 2014, 20, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Bos, S.; van den Boom, B.; Ow, T.W.; Prachalias, A.; Adelmeijer, J.; Phoolchund, A.; Dunsire, F.; Milan, Z.; Roest, M.; Heaton, N.; et al. Efficacy of pro- and anticoagulant strategies in plasma of patients undergoing hepatobiliary surgery. J. Thromb. Haemost. 2020, 18, 2840–2851. [Google Scholar] [CrossRef] [PubMed]
- Hellstern, P. Production and composition of prothrombin complex concentrates: Correlation between composition and therapeutic efficiency. Thromb. Res. 1999, 95, S7–S12. [Google Scholar] [CrossRef] [PubMed]
- Kalina, U.; Bickhard, H.; Schulte, S. Biochemical comparison of seven commercially available prothrombin complex concentrates. Int. J. Clin. Pract. 2008, 62, 1614–1622. [Google Scholar] [CrossRef]
- Makhoul, T.; Kelly, G.; Kersten, B.; Nadler, M.; Zammit, C.G.; Jones, C.M.C.; Scott, R.; Acquisto, N.M. Incidence of thromboembolic events following administration of four-factor prothrombin complex concentrate (4F-PCC) for oral anticoagulation reversal. Thromb. Res. 2020, 194, 158–164. [Google Scholar] [CrossRef]
- Tischendorf, M.; Fuchs, A.; Zeuzem, S.; Lange, C.M. Use of prothrombin complex concentrates in patients with decompensated liver cirrhosis is associated with thromboembolic events. J. Hepatol. 2019, 70, 800–801. [Google Scholar] [CrossRef]
- Abuelkasem, E.; Hasan, S.; Mazzeffi, M.A.; Planinsic, R.M.; Sakai, T.; Tanaka, K.A. Reduced Requirement for Prothrombin Complex Concentrate for the Restoration of Thrombin Generation in Plasma From Liver Transplant Recipients. Anesth. Analg. 2017, 125, 609–615. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D. The Newcastle Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analysis. 2011. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 30 March 2023).
- Onaca, N.N.; Levy, M.F.; Sanchez, E.Q.; Chinnakotla, S.; Fasola, C.G.; Thomas, M.J.; Weinstein, J.S.; Murray, N.G.; Goldstein, R.M.; Klintmalm, G.B.; et al. A correlation between the pretransplantation MELD score and mortality in the first two years after liver transplantation. Liver. Transpl. 2003, 9, 117–123. [Google Scholar] [CrossRef]
- Lameire, N.H.; Levin, A.; Kellum, J.A.; Cheung, M.; Jadoul, M.; Winkelmayer, W.C.; Stevens, P.E.; Conference Participants. Harmonizing acute and chronic kidney disease definition and classification: Report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2021, 100, 516–526. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Martinez, S.; Garcia, I.; Ruiz, A.; Tàssies, D.; Reverter, J.C.; Colmenero, J.; Beltran, J.; Fondevila, C.; Blasi, A. Is antivitamin K reversal required in patients with cirrhosis undergoing liver transplantation? Transfusion 2021, 61, 3008–3016. [Google Scholar] [CrossRef]
- Arshad, F.; Ickx, B.; van Beem, R.T.; Polak, W.; Grüne, F.; Nevens, F.; Ilmakunnas, M.; Koivusalo, A.M.; Isoniemi, H.; Strengers, P.F.; et al. Prothrombin complex concentrate in the reduction of blood loss during orthotopic liver transplantation: PROTON-trial. BMC Surg. 2013, 13, 22. [Google Scholar] [CrossRef]
- van den Brink, D.P.; Wirtz, M.R.; Neto, A.S.; Schöchl, H.; Viersen, V.; Binnekade, J.; Juffermans, N.P. Effectiveness of prothrombin complex concentrate for the treatment of bleeding: A systematic review and meta-analysis. J. Thromb. Haemost. 2020, 18, 2457–2467. [Google Scholar] [CrossRef]
- Wang, J.; Bao, Y.X.; Bai, M.; Zhang, Y.G.; Xu, W.D.; Qi, X.S. Restrictive vs liberal transfusion for upper gastrointestinal bleeding: A meta-analysis of randomized controlled trials. World J. Gastroenterol. 2013, 28, 6919–6927. [Google Scholar] [CrossRef]
- de Boer, M.T.; Christensen, M.C.; Asmussen, M.; van der Hilst, C.S.; Hendriks, H.G.; Slooff, M.J.; Porte, R.J. The impact of intraoperative transfusion of platelets and red blood cells on survival after liver transplantation. Anesth. Analg. 2008, 106, 32–44. [Google Scholar] [CrossRef]
- Yoon, U.; Bartoszko, J.; Bezinover, D.; Biancofiore, G.; Forkin, K.T.; Rahman, S.; Spiro, M.; Raptis, D.A.; Kang, Y.; ERAS4OLT.org Working Group. Intraoperative transfusion management, antifibrinolytic therapy, coagulation monitoring and the impact on short-term outcomes after liver transplantation-A systematic review of the literature and expert panel recommendations. Clin. Transplant. 2022, 36, e14637. [Google Scholar] [CrossRef]
- Aceto, P.; Punzo, G.; Di Franco, V.; Teofili, L.; Gaspari, R.; Avolio, A.W.; Del Tedesco, F.; Posa, D.; Lai, C.; Sollazzi, L. Viscoelastic versus conventional coagulation tests to reduce blood product transfusion in patients undergoing liver transplantation: A systematic review and meta-analysis. Eur. J. Anaesthesiol. 2023, 40, 39–53. [Google Scholar] [CrossRef]
- Pérez-Calatayud, A.A.; Hofmann, A.; Pérez-Ferrer, A.; Escorza-Molina, C.; Torres-Pérez, B.; Zaccarias-Ezzat, J.R.; Sanchez-Cedillo, A.; Manuel Paez-Zayas, V.; Carrillo-Esper, R.; Görlinger, K. Patient Blood Management in Liver Transplant-A Concise Review. Biomedicines 2023, 11, 1093. [Google Scholar] [CrossRef]
- Perilli, V.; Aceto, P.; Sacco, T.; Modesti, C.; Ciocchetti, P.; Vitale, F.; Russo, A.; Fasano, G.; Dottorelli, A.; Sollazzi, L. Anaesthesiological strategies to improve outcome in liver transplantation recipients. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3172–3177. [Google Scholar]
| First Author, Year (Country) | Study Design | Sample, n | Age, Mean (SD) or Median [IQR] | Male, n (%) | MELD, Mean (SD) or Median [IQR] | Type of Donor | PCC Product | PCC (UI) and FibC (g) Dose Mean (SD) | Primary Outcome | Secondary Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Colavecchia, 2017 (USA) [5] | R | PCC 39 No PCC 78 | 55 (11.4) 55.7 (9.44) | 23 (58.9) 45 (57.7) | 34.4 (9.2) 35.4 (8.8) | D | Kcentra | PCC 902 (589) FibC 1.7 (1.1) | Requirement of RBC | Requirement of FFP, PLT, cryoprecipitate, OR take-backs within 72 h; total LOS; ICU LOS; incidence of AKI and HD |
| Kirchner, 2014 (Germany) [4] | R | CFCs 156 No CFCs 110 | 51.2 (10.4) 53 (9.4) | 100 (64) 64 (58) | 23 [16–33] 17 [11.5–25] | D | Beriplex P/N | PCC 4090 (3130) FibC 6.3 (5.6) | Safety events | Transfusion requirement |
| Martinez, 2021 (Spain) [34] | R | PCC 11 No PCC 14 | 59 [57–62] 57 [55–61] | 10 (91%) 11 (79%) | 19 [17–20] 20 [18–24] | D | Beriplex | Median dose of PCC 600 [IQR 500–1100] IU | Bleeding, transfusion requirements | Safety of PCC administration |
| Srivastava, 2018 (India) [6] | R | PCC 60 No PCC 60 | 49.5 (5.7) 51.7 (8.98) | 43 (71.7) 46 (76.4) | 33.9 (11.4) 32.7 (12.5) | L | NS | Dose of PCC 25 IU/kg | Requirement of RBC | PO days of VM, incidence of AKI, PO hemorrhagic complication |
| Zamper, 2018 (Brazil) [7] | BAS | PCC 46 No PCC 89 | 53 (11.8) 52.5 (11.9) | 35 (76.1) 71 (79.8) | 21.9 (9.2) 22 (7.9) | D | NS | PCC 195.6 (645.3) FibC 1.4 (2.4) | Transfusion requirement | Use of CFCs or antifibrinolytic; clinical complications related to the procedure; PO duration of VM; ICU and hospital LOS; in-hospital mortality |
| First Author, Year | RBC Mean (SD) or Median [IQR], n (%) | FFP Mean (SD) or Median [IQR], n (%) | PLT Mean (SD), n (%) | FibC or Cryo Mean (SD), n (%) | Safety Events n (%) | AKI n (%) | HD n (%) | Hospital and ICU LOS Mean (SD), n (%) | Others |
|---|---|---|---|---|---|---|---|---|---|
| Colavecchia, 2017 [5] | PCC group 12.4 (8) No PCC group 9.7 (5.6) | PCC group 10.5 (8.1) No PCC group 8.3 (5.2) | PCC group 3.1 (1.8) No PCC group 2.2 (1.5) | Cryo: PCC group 2.6 (6.4) No PCC group 2.5 (5.4) | NS | PCC group 17/26 (65.4) No PCC group 35/45 (77.8) | PCC group 1/26 (3.8) No PCC group 16/45 (35.6) | Hospital LOS: PCC group 48.2 (26.8) No PCC group 45.5 (33.2) ICU LOS: PCC group 34.6 (25) No PCC group 29.1 (26.3) | Operative take-back: PCC group 18 (46.2%) No PCC group 40 (51.3%) |
| Kirchner, 2014 [4] | CFC group 3 [0–6] 111 (71.2%) No CFC group 0 [0–3] 58 (52.7%) | CFC group 0 [0–0] 32 (20.5%) No CFC group 0 [0–0] 7 (6.4%) | CFC group 0 [0–1] 64 (41%) No CFC group 0 [0–0] 12 (10.9%) | NS | HAT: CFC group 7 (4.5%) No CFC group 4 (3.6%) PVT and MI: CFC group 1 (0.6%) PE: CFC group 2 (1.28%) No CFC group 1 (0.9%) Stroke: no patient Composite outcome: CFC group 11 (7.1%) No CFC group 5 (4.5%) | NS | NS | NS | NS |
| Martinez, 2021 [34] | PCC group 3.5 [2.2–5.5] 4 (36%) No PCC group 3 [2–7] 7 (50%) | PCC group 500 [500–500] ml 2 (18%) No PCC group 1000 [895–2375] 6 (43%) | PCC group 6 (9%) No PCC group 2 (14%) | FibrC/ cryo: PCC group 6 (54%) No PCC group 8 (57%) | Thrombosis: PCC group 0 No PCC group 1 (7%) | KDIGO 2: PCC group 1 (9) No PCC group 2 (14) | NS | Hospital stay: PCC group 17 (11–31) No PCC group 15 (13–23) | Deaths: PCC group 0 No PCC group 1 (7%) Bleeding: PCC group 2 (18%) No PCC group 1 (7%) Reintervention for bleeding: PCC group 2 (18%) No PCC group 1 (7%) |
| Srivastava, 2018 [6] | PCC group 6.2 (4.1) No PCC group 8.23 (5.18) | PCC group 2.6 (2) No PCC group 6.18 (4.1) | PCC group 6.6 (5.5) No PCC group 5.91 (4.6) | Cryo: PCC group 8.2 (5.3) No PCC group 7.9 (4.1) | Thrombotic events: PCC group 0 No PCC group 0 | PCC group 34 (56.6) No PCC group 31 (51.6) | PCC group 0 No PCC group 0 | LOS > 21 days: PCC group 2 (3.3%) No PCC group 3 (5%) | PO MV > 1 day: PCC group 7 (11.6%) No PCC group 8 (13.3%) Hemorrhagic complications: PCC group 2 (3.3%) No PCC group 7 (11.6%) |
| Zamper, 2018 [7] | PCC group 0.6 (1) No PCC group 1.7 (2.7) | PCC group 0.2 (0.9) No PCC group 2.2 (4.5) | PCC group 0 (0) No PCC group 0.1 (0.6) | Cryo: PCC group 0.5 (2.3) No PCC group 0.4 (1.9) FibC: PCC group 1.4 (2.4) No PCC group 0 (0) | HAT: PCC group 1/45 (2.2) No PCC group 2/82 (2.4) | NS | NS | ICU LOS: PCC group 3.4 (4.3) No PCC group 3.6 (4.6) Hospital LOS: PCC group 12.4 (9.5) No PCC group 16.1 (16.6) | Duration of MV: PCC group 0.5 (1.2) No PCC group 0.9 (1.4) In-hospital mortality: PCC group 1/45 (2.2) No PCC group 5/89 (5.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Punzo, G.; Di Franco, V.; Perilli, V.; Sacco, T.; Sollazzi, L.; Aceto, P. Efficacy and Safety of Prothrombin Complex Concentrates in Liver Transplantation: Evidence from Observational Studies. J. Clin. Med. 2023, 12, 3749. https://doi.org/10.3390/jcm12113749
Punzo G, Di Franco V, Perilli V, Sacco T, Sollazzi L, Aceto P. Efficacy and Safety of Prothrombin Complex Concentrates in Liver Transplantation: Evidence from Observational Studies. Journal of Clinical Medicine. 2023; 12(11):3749. https://doi.org/10.3390/jcm12113749
Chicago/Turabian StylePunzo, Giovanni, Valeria Di Franco, Valter Perilli, Teresa Sacco, Liliana Sollazzi, and Paola Aceto. 2023. "Efficacy and Safety of Prothrombin Complex Concentrates in Liver Transplantation: Evidence from Observational Studies" Journal of Clinical Medicine 12, no. 11: 3749. https://doi.org/10.3390/jcm12113749
APA StylePunzo, G., Di Franco, V., Perilli, V., Sacco, T., Sollazzi, L., & Aceto, P. (2023). Efficacy and Safety of Prothrombin Complex Concentrates in Liver Transplantation: Evidence from Observational Studies. Journal of Clinical Medicine, 12(11), 3749. https://doi.org/10.3390/jcm12113749





