Outcomes of Bone Marrow Compared to Peripheral Blood for Haploidentical Transplantation
Abstract
:1. Introduction
2. Methods
Statistical Methods
3. Results
3.1. Patient Characteristics
3.2. Post-Transplant Outcomes
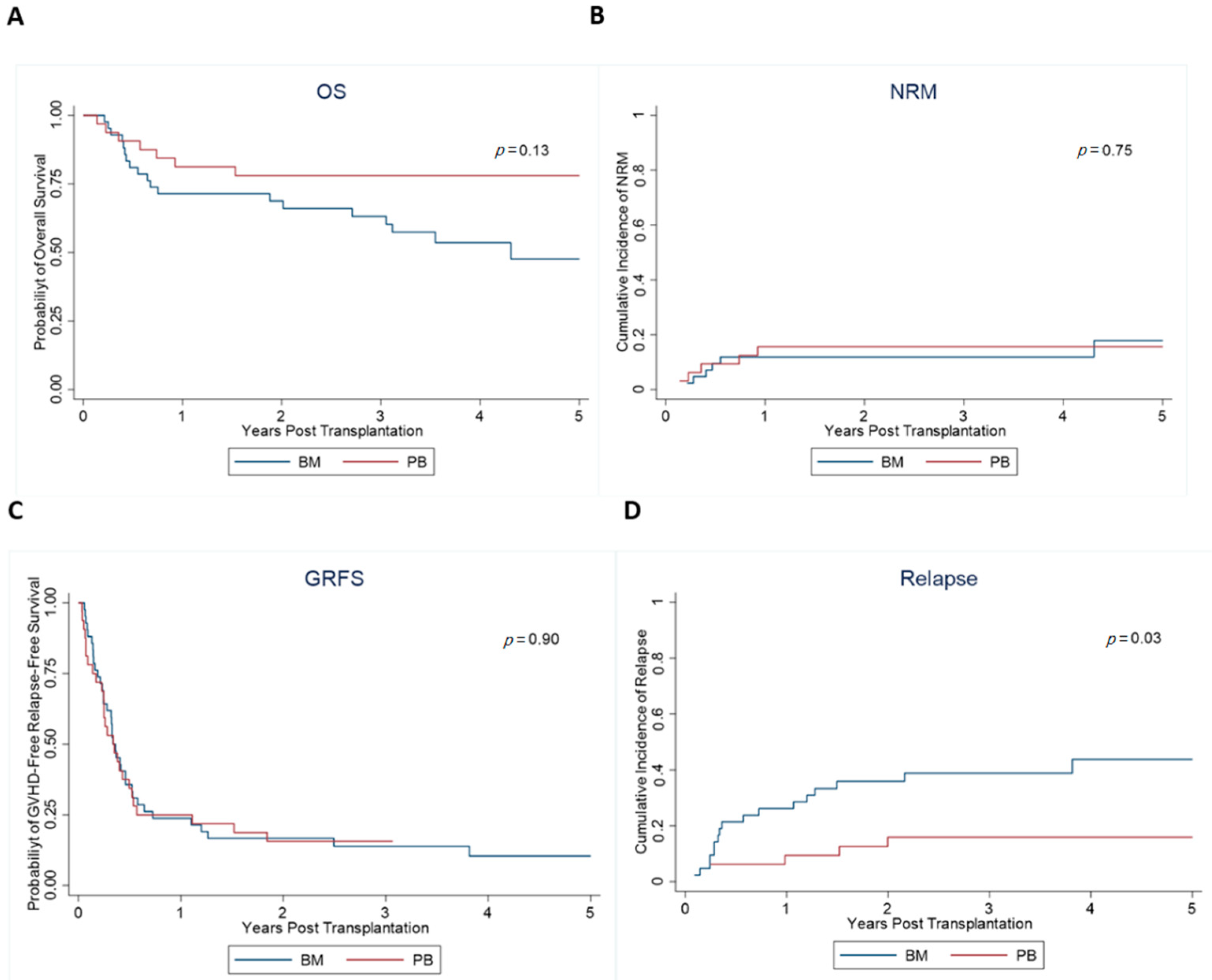
3.3. Survival Outcomes
3.4. Acute and Chronic GVHD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ballen, K.K.; King, R.J.; Chitphakdithai, P.; Bolan, C.D., Jr.; Agura, E.; Hartzman, R.J.; Kernan, N.A. The national marrow donor program 20 years of unrelated donor hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2008, 14, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gragert, L.; Eapen, M.; Williams, E.; Freeman, J.; Spellman, S.; Baitty, R.; Hartzman, R.; Rizzo, J.D.; Horowitz, M.; Confer, D.; et al. HLA Match Likelihoods for Hematopoietic Stem-Cell Grafts in the U.S. Registry. N. Engl. J. Med. 2014, 371, 339–348. [Google Scholar] [CrossRef] [Green Version]
- McCurdy, S.R.; Luznik, L. How we perform haploidentical stem cell transplantation with posttransplant cyclophosphamide. Blood 2019, 134, 1802–1810. [Google Scholar] [CrossRef]
- Aversa, F.; Tabilio, A.; Velardi, A.; Cunningham, I.; Terenzi, A.; Falzetti, F.; Ruggeri, L.; Barbabietola, G.; Aristei, C.; Latini, P.; et al. Treatment of High-Risk Acute Leukemia with T-Cell–Depleted Stem Cells from Related Donors with One Fully Mismatched HLA Haplotype. N. Engl. J. Med. 1998, 339, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Luznik, L.; O’Donnell, P.V.; Symons, H.J.; Chen, A.R.; Leffell, M.S.; Zahurak, M.; Gooley, T.A.; Piantadosi, S.; Kaup, M.; Ambinder, R.F.; et al. HLA-Haploidentical Bone Marrow Transplantation for Hematologic Malignancies Using Nonmyeloablative Conditioning and High-Dose, Posttransplantation Cyclophosphamide. Biol. Blood Marrow Transplant. 2008, 14, 641–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anasetti, C.; Logan, B.R.; Lee, S.J.; Waller, E.K.; Weisdorf, D.J.; Wingard, J.R.; Cutler, C.S.; Westervelt, P.; Woolfrey, A.; Couban, S.; et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N. Engl. J. Med. 2012, 367, 1487–1496. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Logan, B.; Westervelt, P.; Cutler, C.; Woolfrey, A.; Khan, S.P.; Waller, E.K.; Maziarz, R.T.; Wu, J.; Shaw, B.E.; et al. Comparison of Patient-Reported Outcomes in 5-Year Survivors Who Received Bone Marrow vs Peripheral Blood Unrelated Donor Transplantation: Long-term Follow-up of a Randomized Clinical Trial. JAMA Oncol. 2016, 2, 1583–1589. [Google Scholar] [CrossRef]
- Brunstein, C.G.; Fuchs, E.J.; Carter, S.L.; Karanes, C.; Costa, L.J.; Wu, J.; Devine, S.M.; Wingard, J.R.; Aljitawi, O.S.; Cutler, C.S.; et al. Alternative donor transplantation after reduced intensity conditioning: Results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood 2011, 118, 282–288. [Google Scholar] [CrossRef] [Green Version]
- Raj, K.; Pagliuca, A.; Bradstock, K.; Noriega, V.; Potter, V.; Streetly, M.; McLornan, D.; Kazmi, M.; Marsh, J.; Kwan, J.; et al. Peripheral Blood Hematopoietic Stem Cells for Transplantation of Hematological Diseases from Related, Haploidentical Donors after Reduced-Intensity Conditioning. Biol. Blood Marrow Transplant. 2014, 20, 890–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruggeri, A.; Labopin, M.; Bacigalupo, A.; Gülbas, Z.; Koc, Y.; Blaise, D.; Bruno, B.; Irrera, G.; Tischer, J.; Diez-Martin, J.L.; et al. Bone marrow versus mobilized peripheral blood stem cells in haploidentical transplants using posttransplantation cyclophosphamide. Cancer 2018, 124, 1428–1437. [Google Scholar] [CrossRef]
- Bashey, A.; Zhang, M.J.; McCurdy, S.R.; St Martin, A.; Argall, T.; Anasetti, C.; Ciurea, S.O.; Fasan, O.; Gaballa, S.; Hamadani, M.; et al. Mobilized Peripheral Blood Stem Cells Versus Unstimulated Bone Marrow As a Graft Source for T-Cell-Replete Haploidentical Donor Transplantation Using Post-Transplant Cyclophosphamide. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 3002–3009. [Google Scholar] [CrossRef]
- Yu, X.; Liu, L.; Xie, Z.; Dong, C.; Zhao, L.; Zhang, J.; Gu, J.; Zhu, H.H. Bone marrow versus peripheral blood as a graft source for haploidentical donor transplantation in adults using post-transplant cyclophosphamide-A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2019, 133, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E.J.; O’Donnell, P.V.; Eapen, M.; Logan, B.; Antin, J.H.; Dawson, P.; Devine, S.; Horowitz, M.M.; Horwitz, M.E.; Karanes, C.; et al. Double unrelated umbilical cord blood vs HLA-haploidentical bone marrow transplantation: The BMT CTN 1101 trial. Blood 2021, 137, 420–428. [Google Scholar] [CrossRef]
- Cieri, N.; Greco, R.; Crucitti, L.; Morelli, M.; Giglio, F.; Levati, G.; Assanelli, A.; Carrabba, M.G.; Bellio, L.; Milani, R.; et al. Post-transplantation Cyclophosphamide and Sirolimus after Haploidentical Hematopoietic Stem Cell Transplantation Using a Treosulfan-based Myeloablative Conditioning and Peripheral Blood Stem Cells. Biol. Blood Marrow Transplant. 2015, 21, 1506–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, S.R.; Sizemore, C.A.; Sanacore, M.; Zhang, X.; Brown, S.; Holland, H.K.; Morris, L.E.; Bashey, A. Total Body Irradiation–Based Myeloablative Haploidentical Stem Cell Transplantation Is a Safe and Effective Alternative to Unrelated Donor Transplantation in Patients Without Matched Sibling Donors. Biol. Blood Marrow Transplant. 2015, 21, 1299–1307. [Google Scholar] [CrossRef] [Green Version]
- Gaballa, S.; Palmisiano, N.; Alpdogan, O.; Carabasi, M.; Filicko-O’Hara, J.; Kasner, M.; Kraft, W.K.; Leiby, B.; Martinez-Outschoorn, U.; O’Hara, W.; et al. A Two-Step Haploidentical Versus a Two-Step Matched Related Allogeneic Myeloablative Peripheral Blood Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2016, 22, 141–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singhal, S.; Powles, R.; Kulkarni, S.; Treleaven, J.; Sirohi, B.; Millar, B.; Shepherd, V.; Saso, R.; Rowland, A.; Long, S.; et al. Comparison of marrow and blood cell yields from the same donors in a double-blind, randomized study of allogeneic marrow vs blood stem cell transplantation. Bone Marrow Transplant. 2000, 25, 501–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Donnell, P.V.; Eapen, M.; Horowitz, M.M.; Logan, B.R.; DiGilio, A.; Brunstein, C.; Fuchs, E.J.; Flowers, M.E.D.; Salit, R.; Raj, K.; et al. Comparable outcomes with marrow or peripheral blood as stem cell sources for hematopoietic cell transplantation from haploidentical donors after non-ablative conditioning: A matched-pair analysis. Bone Marrow Transplant. 2016, 51, 1599–1601. [Google Scholar] [CrossRef] [Green Version]
- Castagna, L.; Crocchiolo, R.; Furst, S.; Bramanti, S.; El Cheikh, J.; Sarina, B.; Granata, A.; Mauro, E.; Faucher, C.; Mohty, B.; et al. Bone Marrow Compared with Peripheral Blood Stem Cells for Haploidentical Transplantation with a Nonmyeloablative Conditioning Regimen and Post-transplantation Cyclophosphamide. Biol. Blood Marrow Transplant. 2014, 20, 724–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradstock, K.; Bilmon, I.; Kwan, J.; Blyth, E.; Micklethwaite, K.; Huang, G.; Deren, S.; Byth, K.; Gottlieb, D. Influence of Stem Cell Source on Outcomes of Allogeneic Reduced-Intensity Conditioning Therapy Transplants Using Haploidentical Related Donors. Biol. Blood Marrow Transplant. 2015, 21, 1641–1645. [Google Scholar] [CrossRef] [Green Version]
- Ciurea, S.O.; Zhang, M.-J.; Bacigalupo, A.A.; Bashey, A.; Appelbaum, F.R.; Aljitawi, O.S.; Armand, P.; Antin, J.H.; Chen, J.; Devine, S.M.; et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood 2015, 126, 1033–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruggeri, A.; Labopin, M.; Bacigalupo, A.; Afanasyev, B.; Cornelissen, J.J.; Elmaagacli, A.; Itälä-Remes, M.; Blaise, D.; Meijer, E.; Koc, Y.; et al. Post-transplant cyclophosphamide for graft-versus-host disease prophylaxis in HLA matched sibling or matched unrelated donor transplant for patients with acute leukemia, on behalf of ALWP-EBMT. J. Hematol. Oncol. 2018, 11, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| All (n = 74) | BM (n = 42) | PB (n = 32) | |||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | p-Value | |
| Age at HCT, median, range | 0.45 | ||||||
| 57.0 | 20–74 | 60.0 | 21–71 | 54.0 | 20–74 | ||
| Donor age, median, range | 0.28 | ||||||
| 31.0 | 19–64 | 34.0 | 19–63 | 28.0 | 20–64 | ||
| Gender, patients | 0.67 | ||||||
| Male | 46 | 62.2 | 27 | 64.3 | 19 | 59.4 | |
| Gender, donor | 0.13 | ||||||
| Male | 55 | 74.3 | 34 | 81.0 | 21 | 65.6 | |
| Recipient-donor gender | 0.10 | ||||||
| M–M | 35 | 47.3 | 24 | 57.1 | 11 | 34.4 | |
| M–F | 11 | 14.9 | 3 | 7.1 | 8 | 25.0 | |
| F–M | 20 | 27.0 | 10 | 23.8 | 10 | 31.3 | |
| F–F | 8 | 10.8 | 5 | 11.9 | 3 | 9.4 | |
| Diagnosis | 0.67 | ||||||
| AA | 1 | 1.4 | 1 | 2.4 | 0 | 0.0 | |
| ALL | 13 | 17.6 | 10 | 23.8 | 3 | 9.4 | |
| AML | 25 | 33.8 | 14 | 33.3 | 11 | 34.4 | |
| CML | 3 | 4.1 | 2 | 4.8 | 1 | 3.1 | |
| CLL | 7 | 9.5 | 3 | 7.1 | 4 | 12.5 | |
| HD | 2 | 2.7 | 1 | 2.4 | 1 | 3.1 | |
| NHL | 11 | 14.9 | 5 | 11.9 | 6 | 18.8 | |
| MDS | 8 | 10.8 | 5 | 11.9 | 3 | 9.4 | |
| MPD | 4 | 5.4 | 1 | 2.4 | 3 | 9.4 | |
| KPS | 0.18 | ||||||
| <90 | 26 | 35.1 | 12 | 28.6 | 14 | 43.8 | |
| ≥90 | 48 | 64.9 | 30 | 71.4 | 18 | 56.3 | |
| Donor type | 0.43 | ||||||
| Matched related | 1 | 1.4 | 0 | 0.0 | 1 | 3.1 | |
| Mismatch related | 73 | 98.6 | 42 | 100.0 | 31 | 96.9 | |
| GVHD prophylaxis | 0.43 | ||||||
| FK combination | 73 | 98.6 | 42 | 100.0 | 31 | 96.9 | |
| Others | 1 | 1.4 | 0 | 0.0 | 1 | 3.1 | |
| Conditioning | 0.54 | ||||||
| MA | 16 | 21.6 | 8 | 19.0 | 8 | 25.0 | |
| RIC | 58 | 78.4 | 34 | 81.0 | 24 | 75.0 | |
| Comorbidity index, median, range | 2.5 | 0–8 | 2 | 0–6 | 3 | 0–8 | |
| 0–1 | 27 | 36.5 | 18 | 42.9 | 9 | 28.1 | 0.48 |
| 2–3 | 21 | 28.4 | 12 | 28.6 | 9 | 28.1 | |
| 4–5 | 23 | 31.1 | 11 | 26.2 | 12 | 37.5 | |
| 5+ | 3 | 4.1 | 1 | 2.4 | 2 | 6.3 | |
| Remission status at transplant | 0.83 | ||||||
| AP | 1 | 1.5 | 1 | 2.6 | 0 | 0.0 | |
| Chronic phase | 1 | 1.5 | 1 | 2.6 | 0 | 0.0 | |
| CR | 47 | 72.3 | 28 | 71.8 | 19 | 73.1 | |
| R/R | 5 | 7.7 | 4 | 10.3 | 1 | 3.8 | |
| PR | 9 | 13.8 | 4 | 10.3 | 5 | 19.2 | |
| NA | 2 | 3.1 | 1 | 2.6 | 1 | 3.8 | |
| cd34 infused, mean, SD | 5.66 | 2.9 | 3.90 | 1.7 | 7.96 | 2.6 | <0.001 |
| cd3 infused, mean, SD | 1.28 | 1.2 | 0.38 | 0.2 | 2.45 | 1.0 | <0.001 |
| Recipient-donor CMV | 0.67 | ||||||
| Pos-Pos | 20 | 27.0 | 9 | 21.4 | 11 | 34.4 | |
| Pos-Neg | 20 | 27.0 | 12 | 28.6 | 8 | 25.0 | |
| Neg-Pos | 13 | 17.6 | 8 | 19.0 | 5 | 15.6 | |
| Neg-Neg | 21 | 28.4 | 13 | 31.0 | 8 | 25.0 | |
| Recipient-donor EBV | 0.81 | ||||||
| Pos-Pos | 65 | 90.3 | 37 | 92.5 | 28 | 87.5 | |
| Pos-Neg | 4 | 5.6 | 2 | 5.0 | 2 | 6.3 | |
| Neg-Pos | 2 | 2.8 | 1 | 2.5 | 1 | 3.1 | |
| Neg-Neg | 1 | 1.4 | 0 | 0.0 | 1 | 3.1 | |
| All (n = 74) | BM (n = 42) | PB (n = 32) | |||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | p-Value | |
| ANC engraftment days, median, range | 17 | 8–31 | 17.5 | 8–31 | 16 | 13–31 | 0.09 |
| Platelet engraftment days, median, range | 27 | 13–159 | 29 | 19–82 | 20 | 13–159 | <0.001 |
| Post-transplant response | 0.18 | ||||||
| CR | 66 | 89.2 | 35 | 83.3 | 31 | 96.9 | |
| Less than CR | 6 | 8.1 | 5 | 11.9 | 1 | 3.1 | |
| Progression | 2 | 2.7 | 2 | 4.8 | 0 | 0.0 | |
| Pulmonary infection | 0.82 | ||||||
| No | 61 | 82.4 | 35 | 83.3 | 26 | 81.3 | |
| Yes | 13 | 17.6 | 7 | 16.7 | 6 | 18.8 | |
| VOD | NA | ||||||
| No | 74 | 100.0 | 42 | 100.0 | 32 | 100.0 | |
| Bacteremia in first D+100 | 0.05 | ||||||
| No | 46 | 70.8 | 23 | 60.5 | 23 | 85.2 | |
| Yes | 19 | 29.2 | 15 | 39.5 | 4 | 14.8 | |
| Viremia in first D+100 | 0.68 | ||||||
| No | 25 | 37.3 | 15 | 39.5 | 10 | 34.5 | |
| Yes | 42 | 62.7 | 23 | 60.5 | 19 | 65.5 | |
| Fungemia in first D+100 | 0.99 | ||||||
| No | 60 | 95.2 | 34 | 94.4 | 26 | 96.3 | |
| Yes | 3 | 4.8 | 2 | 5.6 | 1 | 3.7 | |
| Hemorrhagic cystitis | 0.99 | ||||||
| No | 65 | 87.8 | 37 | 88.1 | 28 | 87.5 | |
| Yes | 9 | 12.2 | 5 | 11.9 | 4 | 12.5 | |
| Graft failure | 1 | 1.2 | 0 | 0.0 | 1 | 3.1 | 0.43 |
| CMV reactivation | |||||||
| No | 38 | 51.35 | 22 | 52.38 | 16 | 50 | |
| Yes | 36 | 48.75 | 20 | 47.62 | 16 | 50 | |
| Overall | BM | PB | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rate | 95% CI | Rate | 95% CI | Rate | 95% CI | |||||
| OS | 0.13 | |||||||||
| Median OS, years | NR | 3.5 | NR | 4.3 | 2.0 | NR | NR | NR | NR | |
| Year 1 | 76% | 64% | 84% | 71% | 55% | 83% | 81% | 63% | 91% | |
| Year 3 | 69% | 57% | 79% | 63% | 46% | 76% | 78% | 59% | 89% | |
| PFS | ||||||||||
| Median PFS, years | 5.8 | 1.4 | NR | 2.2 | 0.6 | NR | NR | 2.0 | NR | |
| Year 1 | 68% | 56% | 77% | 62% | 46% | 75% | 75% | 56% | 87% | |
| Year 3 | 57% | 45% | 68% | 49% | 33% | 63% | 68% | 49% | 82% | |
| GRFS | 0.9 | |||||||||
| Median GRFS, years | 0.4 | 0.3 | 0.5 | 0.3 | 0.2 | 0.5 | 0.3 | 0.2 | 0.5 | |
| Year 1 | 24% | 15% | 35% | 23% | 12% | 37% | 25% | 12% | 41% | |
| Year 3 | 14% | 7% | 23% | 14% | 5% | 26% | 16% | 6% | 30% | |
| NRM | 0.76 | |||||||||
| Year 1 | 14% | 7% | 22% | 12% | 4% | 24% | 16% | 6% | 30% | |
| Year 3 | 14% | 7% | 22% | 12% | 4% | 24% | 16% | 6% | 30% | |
| Relapse | 0.03 | |||||||||
| Year 1 | 19% | 11% | 29% | 26% | 14% | 40% | 9% | 2% | 22% | |
| Year 2 | 27% | 18% | 38% | 36% | 22% | 50% | 16% | 6% | 31% | |
| aGVHD,2–4 | ||||||||||
| Day 100 | 59% | 47% | 70% | 57% | 41% | 70% | 63% | 44% | 77% | 0.31 |
| Day 180 | 59% | 47% | 60% | 57% | 41% | 70% | 63% | 44% | 77% | |
| aGVHD, 3–4 | ||||||||||
| Day 100 | 24% | 15% | 35% | 24% | 12% | 37% | 25% | 12% | 41% | 0.79 |
| Day 180 | 24% | 15% | 35% | 24% | 12% | 37% | 25% | 12% | 41% | |
| cGVHD, Extensive/Limited | 0.18 | |||||||||
| Day 365 | 47% | 36% | 58% | 40% | 26% | 55% | 56% | 38% | 71% | |
| cGVHD, Extensive | 0.97 | |||||||||
| Day 365 | 38% | 27% | 49% | 38% | 24% | 52% | 38% | 21% | 54% | |
| HR | 95% CI | p-Value | ||
|---|---|---|---|---|
| Impact on OS PB vs. BM | ||||
| 0.50 | 0.21 | 1.21 | 0.125 | |
| Impact on GRFS | ||||
| PB vs. BM | 1.03 | 0.63 | 1.70 | 0.902 |
| Impact on NRM | ||||
| PB vs. BM | 1.21 | 0.38 | 3.89 | 0.751 |
| Impact on relapse | ||||
| PB vs. BM | 0.33 | 0.13 | 0.88 | 0.027 |
| HR | 95% CI | p | ||
|---|---|---|---|---|
| PB vs. BM | 0.17 | 0.05 | 0.56 | 0.004 |
| Rural | 3.75 | 1.39 | 10.08 | 0.009 |
| Remission status at transplant: CR vs. all others | 1.17 | 0.40 | 3.43 | 0.774 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, N.; Faisal, M.S.; Zhao, Q.; Jiang, J.; Elder, P.; Benson, D.M.; Rosko, A.; Chaudhry, M.; Bumma, N.; Khan, A.; et al. Outcomes of Bone Marrow Compared to Peripheral Blood for Haploidentical Transplantation. J. Clin. Med. 2021, 10, 2843. https://doi.org/10.3390/jcm10132843
Sharma N, Faisal MS, Zhao Q, Jiang J, Elder P, Benson DM, Rosko A, Chaudhry M, Bumma N, Khan A, et al. Outcomes of Bone Marrow Compared to Peripheral Blood for Haploidentical Transplantation. Journal of Clinical Medicine. 2021; 10(13):2843. https://doi.org/10.3390/jcm10132843
Chicago/Turabian StyleSharma, Nidhi, Muhammad Salman Faisal, Qiuhong Zhao, Justin Jiang, Patrick Elder, Don M. Benson, Ashley Rosko, Maria Chaudhry, Naresh Bumma, Abdullah Khan, and et al. 2021. "Outcomes of Bone Marrow Compared to Peripheral Blood for Haploidentical Transplantation" Journal of Clinical Medicine 10, no. 13: 2843. https://doi.org/10.3390/jcm10132843






