Persistent Spinal Pain Syndrome Type 2 (PSPS-T2), a Social Pain? Advocacy for a Social Gradient of Health Approach to Chronic Pain
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection
2.3. Measures
2.4. Statistical Analysis
3. Results
3.1. Participants
3.2. PSC Representation in the PREDIBACK Study and Comparison with the Regional Population
3.3. Social Characteristics of the Patients According to SGH Group
3.4. Association between SGH Group and Medical Assessment Tools
3.5. Association between SGH and Psychological Assessment Tools
3.6. Multivariate Analysis
4. Discussion
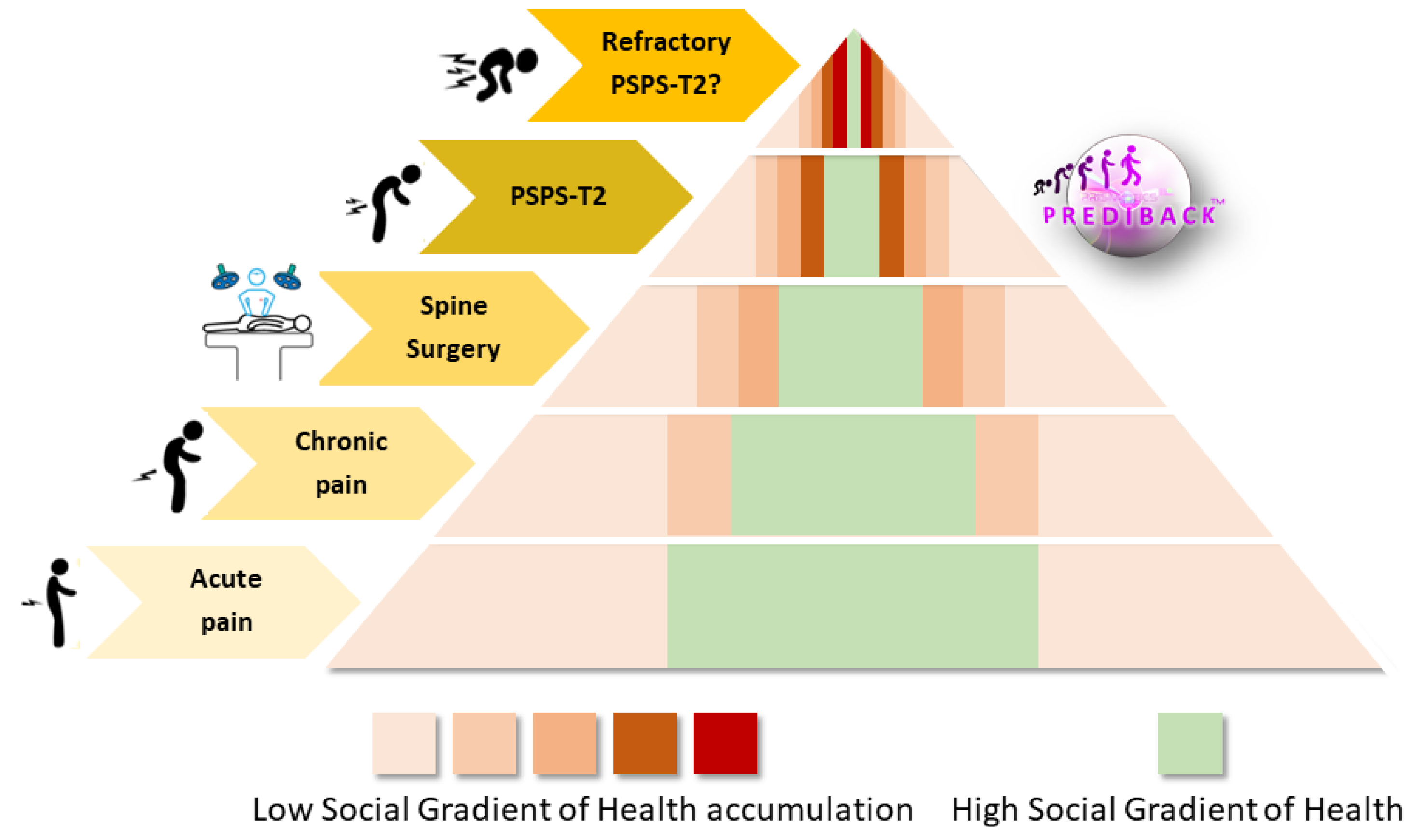
4.1. The Need for an SGH Approach to Stratify the PSPS-T2 Population
4.2. Impact of SGH on PSPS-T2 Prevalence
4.3. Impact of SGH on ODI, FABQ-Work and CSQ-Catastrophizing
4.4. Innovative Model of Multidisciplinary Holistic Therapeutical Care
- Systematic multidisciplinary consultation with the necessary presence of a social worker to optimize the medical care pathway.
- Use of SGH as a stratification tool that enhances and orients toward an optimized medical care pathway, notably by providing personalized information and support through patient education programs.
4.5. Study Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inoue, S.; Kamiya, M.; Nishihara, M.; Arai, Y.-C.P.; Ikemoto, T.; Ushida, T. Prevalence, Characteristics, and Burden of Failed Back Surgery Syndrome: The Influence of Various Residual Symptoms on Patient Satisfaction and Quality of Life as Assessed by a Nationwide Internet Survey in Japan. J. Pain Res. 2017, 10, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Schug, S.A.; Lavand’homme, P.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.-D. IASP Taskforce for the Classification of Chronic Pain the IASP Classification of Chronic Pain for ICD-11: Chronic Postsurgical or Posttraumatic Pain. Pain 2019, 160, 45–52. [Google Scholar] [CrossRef]
- Christelis, N.; Simpson, B.; Russo, M.; Stanton-Hicks, M.; Barolat, G.; Thomson, S.; Schug, S.; Baron, R.; Buchser, E.; Carr, D.B.; et al. Persistent Spinal Pain Syndrome: A Proposal for Failed Back Surgery Syndrome and ICD-11. Pain Med. 2021, 22, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Corbin, J.; Strauss, A. Managing Chronic Illness at Home: Three Lines of Work. Qual. Sociol. 1985, 8, 224–247. [Google Scholar] [CrossRef]
- Blond, S.; Mertens, P.; David, R.; Roulaud, M.; Rigoard, P. From “Mechanical” to “Neuropathic” Back Pain Concept in FBSS Patients. A Systematic Review Based on Factors Leading to the Chronification of Pain (Part C). Neurochirurgie 2015, 61 (Suppl. S1), S45–S56. [Google Scholar] [CrossRef] [PubMed]
- Rigoard, P.; Gatzinsky, K.; Deneuville, J.-P.; Duyvendak, W.; Naiditch, N.; Van Buyten, J.-P.; Eldabe, S. Optimizing the Management and Outcomes of Failed Back Surgery Syndrome: A Consensus Statement on Definition and Outlines for Patient Assessment. Pain Res. Manag. 2019, 2019, 3126464. [Google Scholar] [CrossRef]
- Edwards, R.R.; Dworkin, R.H.; Sullivan, M.D.; Turk, D.C.; Wasan, A.D. The Role of Psychosocial Processes in the Development and Maintenance of Chronic Pain. J. Pain 2016, 17, T70–T92. [Google Scholar] [CrossRef] [PubMed]
- Shaw, W.S.; Campbell, P.; Nelson, C.C.; Main, C.J.; Linton, S.J. Effects of Workplace, Family and Cultural Influences on Low Back Pain: What Opportunities Exist to Address Social Factors in General Consultations? Best Pr. Res. Clin. Rheumatol. 2013, 27, 637–648. [Google Scholar] [CrossRef]
- Van Belleghem, V.; Bouhassira, D. Prise en charge des douleurs neuropathiques chroniques sévères: Résultats de l’ “Enquête patients, soins et intervenants de la douleur neuropathique” (Epsidone). Douleurs Évaluation Diagn. Traitement 2009, 10, 283–291. [Google Scholar] [CrossRef]
- Braveman, P.; Gottlieb, L. The Social Determinants of Health: It’s Time to Consider the Causes of the Causes. Public Health Rep. 2014, 129, 19–31. [Google Scholar] [CrossRef]
- Dalgard, O.S. Social Inequalities in Mental Health in Norway: Possible Explanatory Factors. Int. J. Equity Health 2008, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Donkin, A.J.M. Social Gradient. In The Wiley Blackwell Encyclopedia of Health, Illness, Behavior, and Society; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 2172–2178. ISBN 978-1-118-41086-8. [Google Scholar]
- Wilson, T.C. The Paradox of Social Class and Sports Involvement: The Roles of Cultural and Economic Capital. Int. Rev. Sociol. Sport 2002, 37, 5–16. [Google Scholar] [CrossRef]
- Gidlow, C.; Johnston, L.H.; Crone, D.; Ellis, N.; James, D. A Systematic Review of the Relationship between Socio-Economic Position and Physical Activity. Health Educ. J. 2016, 65, 338–367. [Google Scholar] [CrossRef]
- Sørensen, K.; Pelikan, J.M.; Röthlin, F.; Ganahl, K.; Slonska, Z.; Doyle, G.; Fullam, J.; Kondilis, B.; Agrafiotis, D.; Uiters, E.; et al. Health Literacy in Europe: Comparative Results of the European Health Literacy Survey (HLS-EU). Eur. J. Public Health 2015, 25, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Marmot, M. The Health Gap: The Challenge of an Unequal World: The Argument. Int. J. Epidemiol. 2017, 46, 1312–1318. [Google Scholar] [CrossRef]
- Upton, J. Psychosocial Factors. In Encyclopedia of Behavioral Medicine; Gellman, M.D., Turner, J.R., Eds.; Springer: New York, NY, USA, 2013; pp. 1580–1581. ISBN 9781441910059. [Google Scholar]
- Boonstra, A.M.; Schiphorst Preuper, H.R.; Balk, G.A.; Stewart, R.E. Cut-off Points for Mild, Moderate, and Severe Pain on the Visual Analogue Scale for Pain in Patients with Chronic Musculoskeletal Pain. Pain 2014, 155, 2545–2550. [Google Scholar] [CrossRef] [PubMed]
- Desrosières, A.; Thévenot, L. Les Catégories Socioprofessionelles, 5th ed.; Collection; Repères La Découverte: Paris, France, 1988; ISBN 9782707138569. [Google Scholar]
- INSEE. Professions et Catégories Socioprofessionnelles PCS 2003. Available online: https://www.insee.fr/fr/information/2400059 (accessed on 21 June 2018).
- Oleksiyenko, O.; Życzyńska-Ciołek, D. Structural Determinants of Workforce Participation after Retirement in Poland. J. Popul. Ageing 2018, 11, 83–103. [Google Scholar] [CrossRef] [PubMed]
- Fliesser, M.; De Witt Huberts, J.; Wippert, P.-M. The Choice That Matters: The Relative Influence of Socioeconomic Status Indicators on Chronic Back Pain- a Longitudinal Study. BMC Health Serv. Res. 2017, 17, 800. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Chauvin, P.; Jougla, E.; Laporte, A.; Lombrail, P.; Menvielle, G. Indicateurs de Suivi de l’évolution Des Inégalités Sociales de Santé Dans Les Systèmes d’information En Santé; Haut Conseil de la Santé Publique: Paris, France, 2013; p. 53. [Google Scholar]
- Ferreira-Valente, M.A.; Pais-Ribeiro, J.L.; Jensen, M.P. Validity of Four Pain Intensity Rating Scales. Pain 2011, 152, 2399–2404. [Google Scholar] [CrossRef]
- Fairbank, J.C.; Couper, J.; Davies, J.B.; O’Brien, J.P. The Oswestry Low Back Pain Disability Questionnaire. Physiotherapy 1980, 66, 271–273. [Google Scholar]
- Vartiainen, P.; Mäntyselkä, P.; Heiskanen, T.; Hagelberg, N.; Mustola, S.; Forssell, H.; Kautiainen, H.; Kalso, E. Validation of EQ-5D and 15D in the Assessment of Health-Related Quality of Life in Chronic Pain. Pain 2017, 158, 1577–1585. [Google Scholar] [CrossRef]
- Castro, M.M.C.; Quarantini, L.; Batista-Neves, S.; Kraychete, D.C.; Daltro, C.; Miranda-Scippa, A. Validity of the hospital anxiety and depression scale in patients with chronic pain. Rev. Bras. Anestesiol. 2006, 56, 470–477. [Google Scholar]
- Crombez, G.; Vlaeyen, J.W.; Heuts, P.H.; Lysens, R. Pain-Related Fear Is More Disabling than Pain Itself: Evidence on the Role of Pain-Related Fear in Chronic Back Pain Disability. Pain 1999, 80, 329–339. [Google Scholar] [CrossRef]
- Waddell, G.; Newton, M.; Henderson, I.; Somerville, D.; Main, C.J. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the Role of Fear-Avoidance Beliefs in Chronic Low Back Pain and Disability. Pain 1993, 52, 157–168. [Google Scholar] [CrossRef]
- Rosenstiel, A.K.; Keefe, F.J. The Use of Coping Strategies in Chronic Low Back Pain Patients: Relationship to Patient Characteristics and Current Adjustment. Pain 1983, 17, 33–44. [Google Scholar] [CrossRef]
- Swartzman, L.C.; Gwadry, F.G.; Shapiro, A.P.; Teasell, R.W. The Factor Structure of the Coping Strategies Questionnaire. Pain 1994, 57, 311–316. [Google Scholar] [CrossRef]
- Célant, N.; Guillaume, S.; Rochereau, T. L’Enquête santé européenne—Enquête santé et protection sociale (EHIS-ESPS) 2014. Les rapports de l’IRDES 2017, 286. [Google Scholar]
- Ikeda, T.; Sugiyama, K.; Aida, J.; Tsuboya, T.; Watabiki, N.; Kondo, K.; Osaka, K. Socioeconomic Inequalities in Low Back Pain among Older People: The JAGES Cross-Sectional Study. Int. J. Equity Health 2019, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Plouvier, S.; Leclerc, A.; Chastang, J.-F.; Bonenfant, S.; Goldberg, M. Socioeconomic Position and Low-Back Pain—The Role of Biomechanical Strains and Psychosocial Work Factors in the GAZEL Cohort. Scand. J. Work Environ. Health 2009, 35, 429–436. [Google Scholar] [CrossRef]
- Punnett, L. Socioeconomic Differences in Severe Back Morbidity. Occup. Environ. Med. 2006, 63, 369–370. [Google Scholar] [CrossRef]
- Suman, A.; Bostick, G.P.; Schaafsma, F.G.; Anema, J.R.; Gross, D.P. Associations between Measures of Socio-Economic Status, Beliefs about Back Pain, and Exposure to a Mass Media Campaign to Improve Back Beliefs. BMC Public Health 2017, 17, 504. [Google Scholar] [CrossRef] [PubMed]
- Dionne, C.; Von Korff, M.; Koepsell, T.; Deyo, R.; Barlow, W.; Checkoway, H. Formal Education and Back Pain: A Review. J. Epidemiol. Community Health 2001, 55, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Jonsdottir, S.; Ahmed, H.; Tómasson, K.; Carter, B. Factors Associated with Chronic and Acute Back Pain in Wales, a Cross-Sectional Study. BMC Musculoskelet. Disord. 2019, 20, 215. [Google Scholar] [CrossRef] [PubMed]
- Fouquet, N.; Descatha, A.; Ha, C.; Petit, A.; Roquelaure, Y. An Epidemiological Surveillance Network of Lumbar Disc Surgery to Help Prevention of and Compensation for Low Back Pain. Eur. J. Public Health 2016, 26, 543–548. [Google Scholar] [CrossRef]
- Iderberg, H.; Willers, C.; Borgström, F.; Hedlund, R.; Hägg, O.; Möller, H.; Ornstein, E.; Sandén, B.; Stalberg, H.; Torevall-Larsson, H.; et al. Predicting Clinical Outcome and Length of Sick Leave after Surgery for Lumbar Spinal Stenosis in Sweden: A Multi-Register Evaluation. Eur. Spine J. 2019, 28, 1423–1432. [Google Scholar] [CrossRef]
- Picavet, H.S.J.; Vlaeyen, J.W.S.; Schouten, J.S.A.G. Pain Catastrophizing and Kinesiophobia: Predictors of Chronic Low Back Pain. Am. J. Epidemiol. 2002, 156, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.-N.; Pers, Y.-M.; Mercier, G.; Cambiere, J.-P.; Frasson, N.; Ster, F.; Hérisson, C.; Blotman, F. The Importance of Fear, Beliefs, Catastrophizing and Kinesiophobia in Chronic Low Back Pain Rehabilitation. Ann. Phys. Rehabil. Med. 2010, 53, 3–14. [Google Scholar] [CrossRef]
- Valencia, C.; Robinson, M.E.; George, S.Z. Socioeconomic Status Influences the Relationship between Fear-Avoidance Beliefs Work and Disability. Pain Med. 2011, 12, 328–336. [Google Scholar] [CrossRef][Green Version]
- Goudman, L.; Smet, I.; Mariën, P.; de Jaeger, M.; de Groote, S.; Huysmans, E.; Putman, K.; Van Buyten, J.-P.; Buyl, R.; Moens, M. Is the Self-Reporting of Failed Back Surgery Syndrome Patients Treated with Spinal Cord Stimulation in Line with Objective Measurements? Neuromodulation 2018, 21, 93–100. [Google Scholar] [CrossRef]
- Hoggart, R. The Uses of Literacy: Aspects of Working-Class. Life; Penguin Classics: London, UK, 2009. [Google Scholar]
- Le Breton, D. Anthropologie de la Douleur; Métailié: Paris, France, 1995; ISBN 9782864241911. [Google Scholar]
- Hämmig, O.; Bauer, G.F. The Social Gradient in Work and Health: A Cross-Sectional Study Exploring the Relationship between Working Conditions and Health Inequalities. BMC Public Health 2013, 13, 1170. [Google Scholar] [CrossRef]
- Fritz, J.M.; George, S.Z. Identifying Psychosocial Variables in Patients with Acute Work-Related Low Back Pain: The Importance of Fear-Avoidance Beliefs. Phys. Ther. 2002, 82, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Feldman, C.H.; Dong, Y.; Katz, J.N.; Donnell-Fink, L.A.; Losina, E. Association between Socioeconomic Status and Pain, Function and Pain Catastrophizing at Presentation for Total Knee Arthroplasty. Musculoskelet. Disord. 2015, 16, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Siciliani, L.; Verzulli, R. Waiting Times and Socioeconomic Status among Elderly Europeans: Evidence from SHARE. Health Econ. 2009, 18, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Bertolucci, P.; Brucki, S.; Campacci, S.; Juliano, Y. The Mini-Mental State Examination in a General Population: Impact of Educational Status. Arq. Neuropsiquiatr. 1994, 52, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Crum, R.M.; Anthony, J.C.; Bassett, S.S.; Folstein, M.F. Population-Based Norms for the Mini-Mental State Examination by Age and Educational Level. JAMA 1993, 269, 2386–2391. [Google Scholar] [CrossRef] [PubMed]
| Professions and Socio-Professional Categories | PREDIBACK Population | Regional Population | Difference between % | ||
|---|---|---|---|---|---|
| n = 191 | % | n = 1,492,365 | % | ||
| Patients SGH− | 163 | 85.3 | 93,693 | 62.8 | +22.5 |
| Farmers (PSC 1) | 3 | 1.6 | 51,820 | 3.5 | −1.9 |
| Craftsmen, salesmen and managers (PSC 2) | 3 | 1.6 | 136,378 | 9.1 | −7.5 |
| Blue-collar workers (PSC 6) | 64 | 33.5 | 315,381 | 21.1 | +12.4 |
| Lower-grade white-collar workers (PSC 5) | 93 | 48.7 | 433,214 | 29.0 | +19.7 |
| Patients SGH+ | 28 | 14.6 | 555,572 | 37.2 | −22.6 |
| Technicians, associate professionals (PSC 4) | 19 | 9.9 | 348,087 | 23.3 | −13.4 |
| Professionals (PSC 3) | 9 | 4.7 | 207,485 | 13.9 | −9.2 |
| Variables | SGH− | SGH+ | p-Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age (Mean; SD) | 52.1 | 12.6 | 56.7 | 11.6 | 0.080 |
| Gender | |||||
| Women | 84 | 51.5 | 22 | 78.6 | 0.008 |
| Men | 79 | 48.5 | 6 | 21.5 | |
| Educational level | |||||
| <upper secondary education | 123 | 75.9 | 3 | 10.7 | 0.001 |
| ≥upper secondary education | 39 | 24.1 | 25 | 89.3 | |
| Professional situation | |||||
| Active | 29 | 22.1 | 11 | 55.0 | 0.002 |
| Inactive | 102 | 77.9 | 9 | 45.0 | |
| Variables | SGH− | SGH+ | p-Value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| NPRS | 6.0 | 1.5 | 6.1 | 1.4 | 0.889 |
| EQ-5D-5L | 0.267 | 0.256 | 0.239 | 0.254 | 0.586 |
| ODI | 43.9 | 13.9 | 49.2 | 15.0 | 0.071 |
| Variables | SGH− | SGH+ | p-Value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| HAD-D | 8.7 | 4.2 | 8.1 | 3.0 | 0.701 |
| HAS-A | 10.3 | 4.1 | 9.4 | 3.8 | 0.785 |
| CSQ-Distraction | 11.8 | 4.0 | 12.7 | 4.2 | 0.273 |
| CSQ-Reinterpretation | 6.3 | 2.3 | 6.3 | 3.3 | 0.414 |
| CSQ-Catastrophizing | 9.9 | 3.0 | 8.9 | 2.7 | 0.084 |
| CSQ-Ignorance | 9.5 | 3.9 | 9.4 | 3.3 | 0.819 |
| CSQ-Self Encouragement | 10.4 | 2.7 | 10.7 | 2.9 | 0.770 |
| FABQ-W | 18.4 | 11.1 | 13.8 | 11.5 | 0.043 |
| FABQ-PA | 16.4 | 7.0 | 16.2 | 5.0 | 0.436 |
| Representation of the psychologist’s usefulness | 3.7 | 3.6 | 6.0 | 3.6 | 0.002 |
| Psychologist consultation | |||||
| No, never (n; %) | 98 | 65.8 | 10 | 35.7 | 0.003 |
| Yes, at least once (n; %) | 51 | 34.2 | 18 | 64.3 | |
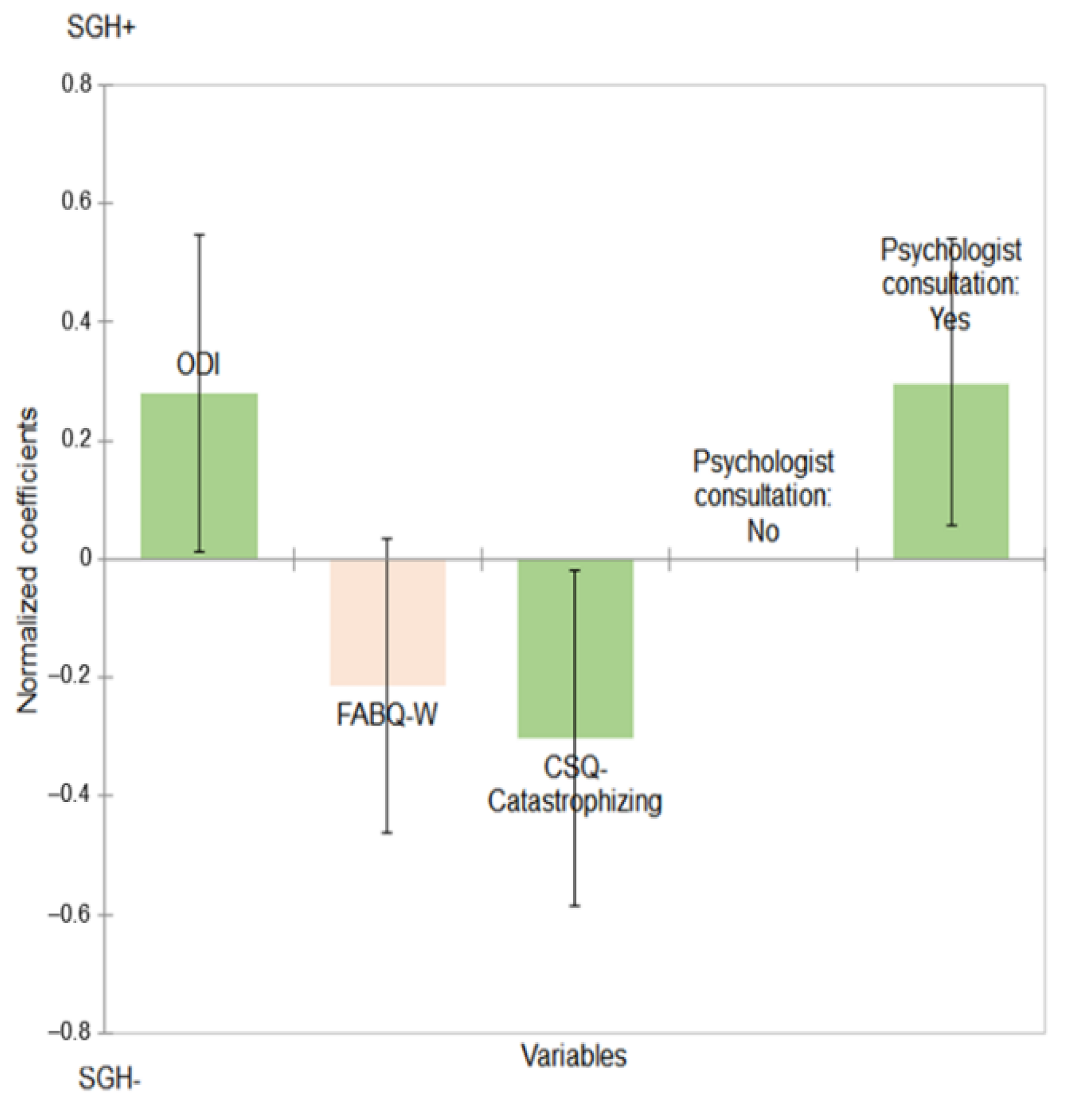
| Variables | Normalized Coefficients * | CI95%. | p-Value |
|---|---|---|---|
| ODI | 0.280 | [0.014; 0.547] | 0.039 |
| CSQ-Catastrophizing | −0.303 | [−0.585; −0.020] | 0.036 |
| FABQ-W | −0.214 | [−0.461; 0.034] | 0.091 |
| No, I have never consulted a psychologist | Reference | Reference | 0.016 |
| Yes, I have consulted a psychologist | 0.299 | [0.055; 0.542] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naiditch, N.; Billot, M.; Moens, M.; Goudman, L.; Cornet, P.; Le Breton, D.; Roulaud, M.; Ounajim, A.; Page, P.; Lorgeoux, B.; et al. Persistent Spinal Pain Syndrome Type 2 (PSPS-T2), a Social Pain? Advocacy for a Social Gradient of Health Approach to Chronic Pain. J. Clin. Med. 2021, 10, 2817. https://doi.org/10.3390/jcm10132817
Naiditch N, Billot M, Moens M, Goudman L, Cornet P, Le Breton D, Roulaud M, Ounajim A, Page P, Lorgeoux B, et al. Persistent Spinal Pain Syndrome Type 2 (PSPS-T2), a Social Pain? Advocacy for a Social Gradient of Health Approach to Chronic Pain. Journal of Clinical Medicine. 2021; 10(13):2817. https://doi.org/10.3390/jcm10132817
Chicago/Turabian StyleNaiditch, Nicolas, Maxime Billot, Maarten Moens, Lisa Goudman, Philippe Cornet, David Le Breton, Manuel Roulaud, Amine Ounajim, Philippe Page, Bertille Lorgeoux, and et al. 2021. "Persistent Spinal Pain Syndrome Type 2 (PSPS-T2), a Social Pain? Advocacy for a Social Gradient of Health Approach to Chronic Pain" Journal of Clinical Medicine 10, no. 13: 2817. https://doi.org/10.3390/jcm10132817
APA StyleNaiditch, N., Billot, M., Moens, M., Goudman, L., Cornet, P., Le Breton, D., Roulaud, M., Ounajim, A., Page, P., Lorgeoux, B., Nivole, K., Pries, P., Swennen, C., Teyssedou, S., Charrier, E., de Montgazon, G. B., Descoins, P. F., Roy-Moreau, B., Grimaud, N., ... Rigoard, P. (2021). Persistent Spinal Pain Syndrome Type 2 (PSPS-T2), a Social Pain? Advocacy for a Social Gradient of Health Approach to Chronic Pain. Journal of Clinical Medicine, 10(13), 2817. https://doi.org/10.3390/jcm10132817








