Effect of Temperature on Heart Rate for Lucilia sericata (syn Phaenicia sericata) and Drosophila melanogaster with Altered Expression of the TrpA1 Receptors
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Fly Lines
2.2. Heart Rates
2.3. Measures of Membrane Potential in Body Wall Muscles
2.4. Survival with Temperature
2.5. Statistical Analysis
3. Results
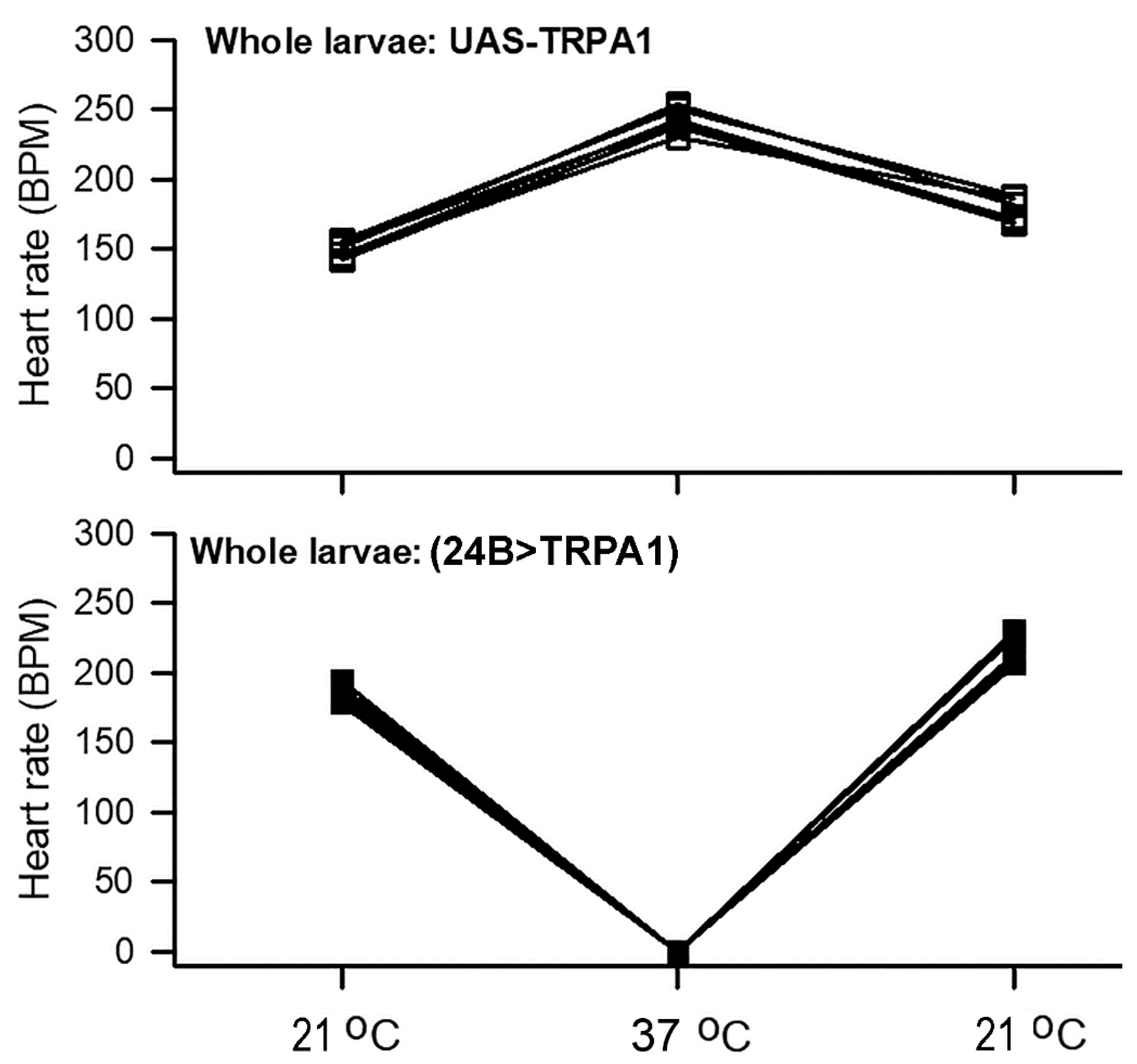
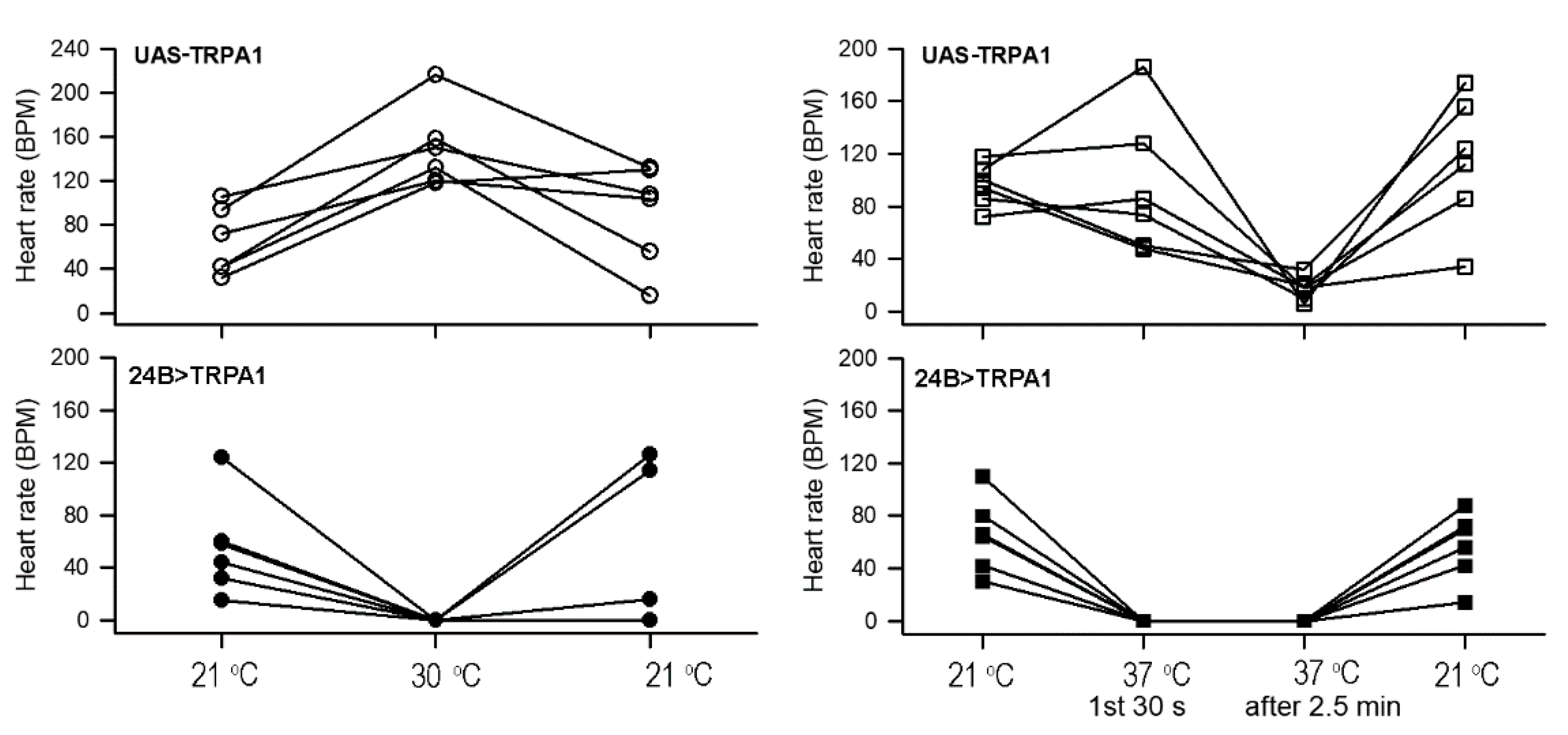
3.1. Heart Rate in Whole Larvae with Increased Temperature
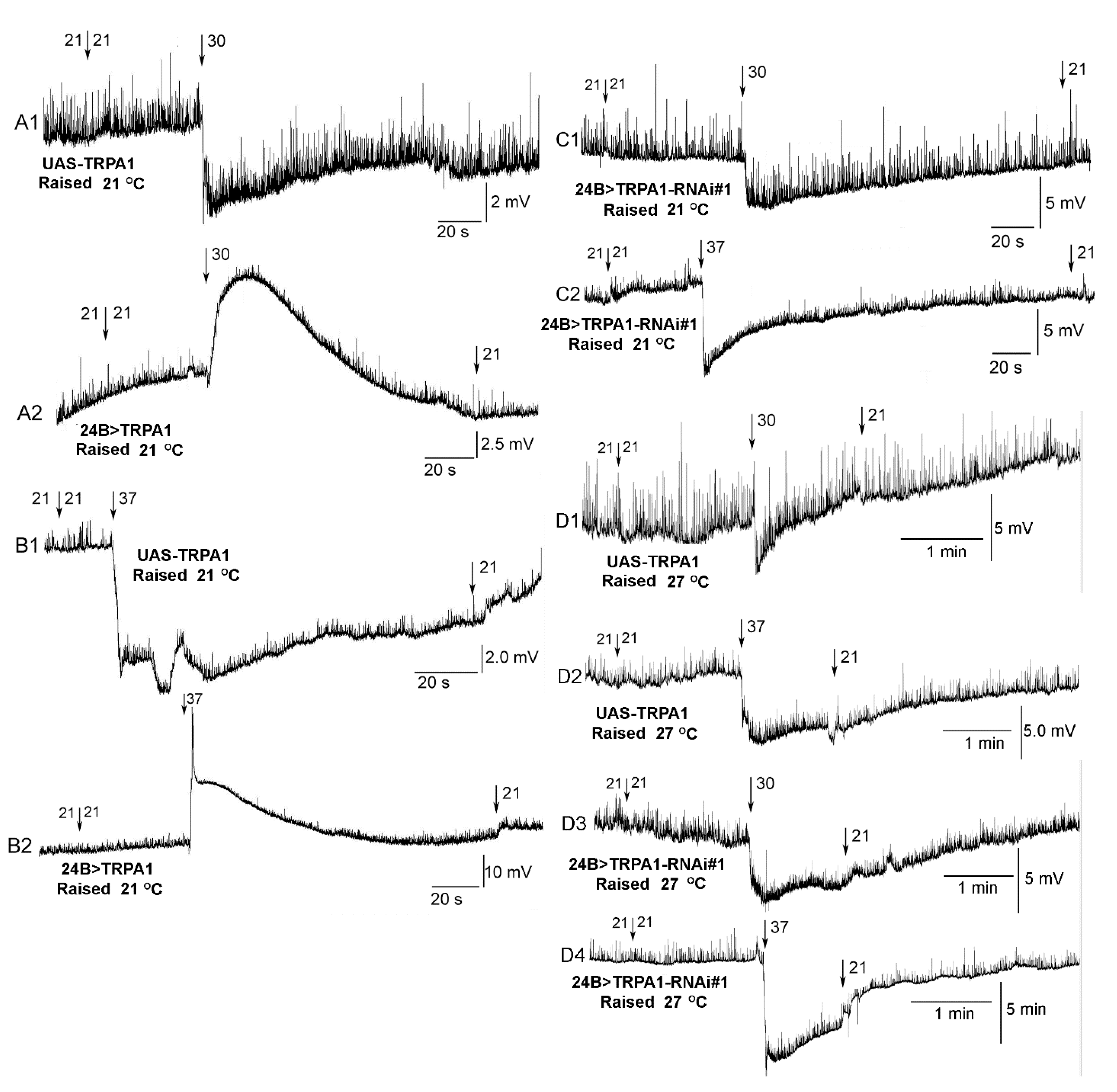
3.2. Heart Rate in Dissected Larvae with Increased Temperature
3.3. Heart-Specific Over-Expression of TrpA1
3.4. RNA Interference with TrpA1
3.5. Effect of Serotonin on Heart Rate with High Temperature
3.6. Resting Membrane Potential of Body Wall Muscles
3.7. Survival with Temperature
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boratyński, J.S.; Szafrańska, P.A. Does Basal Metabolism Set the Limit for Metabolic Downregulation during Torpor? Physiol. Biochem. Zool. 2018, 91, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A.; Sgrò, C.M. Comparative studies of critical physiological limits and vulnerability to environmental extremes in small ectotherms: How much environmental control is needed? Integr. Zool. 2018, 13, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Angilletta, M.J., Jr.; Niewiarowski, P.H.; Navas, C.A. The evolution of thermal physiology in ectotherms. J. Therm. Biol. 2002, 27, 249–268. [Google Scholar] [CrossRef]
- Hochachka, P.; Somero, G. The adaptation of enzymes to temperature. Comp. Biochem. Physiol. 1968, 27, 659–668. [Google Scholar] [CrossRef]
- Parter, M.; Kashtan, N.; Alon, U. Environmental variability and modularity of bacterial metabolic networks. BMC Evol. Biol. 2007, 7, 169. [Google Scholar] [CrossRef]
- Saarinen, K.; Laakso, J.; Lindström, L.; Ketola, T. Adaptation to fluctuations in temperature by nine species of bacteria. Ecol. Evol. 2018, 8, 2901–2910. [Google Scholar] [CrossRef]
- Schulte, P.M. The effects of temperature on aerobic metabolism: Towards a mechanistic understanding of the responses of ectotherms to a changing environment. J. Exp. Biol. 2015, 218, 1856–1866. [Google Scholar] [CrossRef]
- Somero, G. Biochemical Adaptation: Mechanism and Process in Physiological Evolution; Oxford University Press: New York, NY, USA, 2002. [Google Scholar]
- Takemoto, K.; Nacher, J.; Akutsu, T. Correlation between structure and temperature in prokaryotic metabolic networks. BMC Bioinform. 2007, 8, 303. [Google Scholar] [CrossRef]
- Sabath, N.; Ferrada, E.; Barve, A.; Wagner, A. Growth Temperature and Genome Size in Bacteria Are Negatively Correlated, Suggesting Genomic Streamlining during Thermal Adaptation. Genome Biol. Evol. 2013, 5, 966–977. [Google Scholar] [CrossRef]
- Wagner, A. Energy Constraints on the Evolution of Gene Expression. Mol. Biol. Evol. 2005, 22, 1365–1374. [Google Scholar] [CrossRef]
- Gracheva, E.O.; Bagriantsev, S.N. Evolutionary adaptation to thermosensation. Curr. Opin. Neurobiol. 2015, 34, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. TRP channels as cellular sensors. Nat. Cell Biol. 2003, 426, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, I.S.; Delling, M.; Clapham, D.E. An introduction to TRP channels. Annu. Rev. Physiol. 2006, 68, 619–647. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, C.E.; Armache, J.-P.; Gao, Y.; Cheng, Y.; Julius, D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nat. Cell Biol. 2015, 520, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Schupp, M.; Zurborg, S.; Heppenstall, P.A. Residues in the pore region of Drosophila transient receptor potential A1 dictate sensitivity to thermal stimuli. J. Physiol. 2013, 591, 185–201. [Google Scholar] [CrossRef]
- Hamada, F.N.; Rosenzweig, M.; Kang, K.; Pulver, S.R.; Ghezzi, A.; Jegla, T.J.; Garrity, P.A. An internal thermal sensor controlling temperature preference in Drosophila. Nat. Cell Biol. 2008, 454, 217–220. [Google Scholar] [CrossRef]
- Cao, D.-S.; Zhong, L.; Hsieh, T.-H.; Abooj, M.; Bishnoi, M.; Hughes, L.; Premkumar, L.S. Expression of Transient Receptor Potential Ankyrin 1 (TRPA1) and Its Role in Insulin Release from Rat Pancreatic Beta Cells. PLoS ONE 2012, 7, e38005. [Google Scholar] [CrossRef]
- Kunert-Keil, C.; Bisping, F.; Krüger, J.; Brinkmeier, H. Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genom. 2006, 7, 159. [Google Scholar] [CrossRef]
- Yue, Z.; Xie, J.; Yu, A.S.; Stock, J.; Du, J.; Yue, L. Role of TRP channels in the cardiovascular system. Am. J. Physiol. Circ. Physiol. 2015, 308, H157–H182. [Google Scholar] [CrossRef]
- Falcón, D.; Galeano-Otero, I.; Calderón-Sánchez, E.; Del Toro, R.; Martín-Bórnez, M.; Rosado, J.A.; Hmadcha, A.; Smani, T. TRP Channels: Current Perspectives in the Adverse Cardiac Remodeling. Front. Physiol. 2019, 10, 159. [Google Scholar] [CrossRef]
- Lu, M.Y.; Piplani, P.H.; McAllister, P.S.L.; Hurt, M.C.M.; Gross, E.R. Transient Receptor Potential Ankyrin 1 Activation within the Cardiac Myocyte Limits Ischemia–reperfusion Injury in Rodents. Anesthesiology 2016, 125, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Hof, T.; Chaigne, S.; Récalde, A.; Sallé, L.; Brette, F.; Guinamard, R. Transient receptor potential channels in cardiac health and disease. Nat. Rev. Cardiol. 2019, 16, 344–360. [Google Scholar] [CrossRef] [PubMed]
- Bodkin, J.V.; Thakore, P.; Aubdool, A.A.; Liang, L.; Fernandes, E.S.; Nandi, M.; Spina, D.; Clark, J.E.; Aaronson, P.I.; Shattock, M.J.; et al. Investigating the potential role of TRPA1 in locomotion and cardiovascular control during hypertension. Pharmacol. Res. Perspect. 2014, 2, e00052. [Google Scholar] [CrossRef]
- Andrei, S.R.; Ghosh, M.; Sinharoy, P.; Dey, S.; Bratz, I.N.; Damron, D.S. TrpA1 ion channel stimulation enhances cardiomyocyte contractile function via a CaMKII-dependent pathway. Channels 2017, 11, 587–603. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, R.; Wessels, R.J.; Johnson, E.; Dowse, H. Heart Development and Function. In Comprehensive Molecular Insect Science; Gilbert, L.I., Iatrou, K., Gill, S., Eds.; Elsevier: London, UK, 2004. [Google Scholar]
- Zhu, Y.-C.; Yocum, E.; Sifers, J.; Uradu, H.; Cooper, R.L. Modulatory effects on Drosophila larvae hearts in room temperature, acute and chronic cold stress. J. Comp. Physiol. B 2016, 186, 829–841. [Google Scholar] [CrossRef]
- Zhu, Y.-C.; Uradu, H.; Majeed, Z.R.; Cooper, R.L. Optogenetic drive of Drosophila heart rate at different temperatures and Ca2+ concentrations. Physiol. Rep. 2016, 4, e12695. [Google Scholar] [CrossRef]
- Hoffmann, A.A. Physiological climatic limits in Drosophila: Patterns and implications. J. Exp. Biol. 2010, 213, 870–880. [Google Scholar] [CrossRef]
- Choudhary, V.; Choudhary, M.; Pandey, S.; Chauhan, V.D.; Hasnani, J.J. Maggot debridement therapy as primary tool to treat chronic wound of animals. Vet. World 2016, 9, 403–409. [Google Scholar] [CrossRef]
- Firoozfar, F.; Moosa-Kazemi, H.; Baniardalani, M.; Abolhassani, M.; Khoobdel, M.; Rafinejad, J. Mass rearing of Lucilia sericata Meigen (Diptera: Calliphoridae). Asian Pac. J. Trop. Biomed. 2011, 1, 54–56. [Google Scholar] [CrossRef]
- Blice-Baum, A.C.; Zambon, A.C.; Kaushik, G.; Viswanathan, M.C.; Engler, A.J.; Bodmer, R.; Cammarato, A. Modest overexpression of FOXO maintains cardiac proteostasis and ameliorates age-associated functional decline. Aging Cell 2017, 16, 93–103. [Google Scholar] [CrossRef]
- Cooper, A.S.; Rymond, K.E.; Ward, M.A.; Bocook, E.L.; Cooper, R.L. Drosophila melanogaster for Physiological Studies. J. Vis. Exp. 2009, 33, e1596. [Google Scholar] [CrossRef]
- De Castro, C.; Titlow, J.; Majeed, Z.R.; Cooper, R.L. Analysis of various physiological salines for heart rate, CNS function, and synaptic transmission at neuromuscular junctions in Drosophila melanogaster larvae. J. Comp. Physiol. A 2014, 200, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Anyagaligbo, O.; Bernard, J.; Greenhalgh, A.; Cooper, R.L. The effects of bacterial endotoxin (LPS) on cardiac function in a medicinal blow fly (Phaenicia sericata) and a fruit fly (Drosophila melanogaster). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 217, 15–24. [Google Scholar] [CrossRef]
- Malloy, C.; Sifers, J.; Mikos, A.; Samadi, A.; Omar, A.; Hermanns, C.; Cooper, R.L. Using optogenetics to assess neuroendocrine modulation of heart rate in Drosophila melanogaster larvae. J. Comp. Physiol. A 2017, 203, 791–806. [Google Scholar] [CrossRef]
- Dasari, S.; Cooper, R.L. Direct influence of serotonin on the larval heart of Drosophila melanogaster. J. Comp. Physiol. B 2006, 176, 349–357. [Google Scholar] [CrossRef]
- Fast, I.; Rosenkranz, D. Temperature-dependent small RNA expression in Drosophila melanogaster. RNA Biol. 2018, 15, 308–313. [Google Scholar] [CrossRef]
- Haltenhof, T.; Kotte, A.; De Bortoli, F.; Schiefer, S.; Meinke, S.; Emmerichs, A.-K.; Petermann, K.K.; Timmermann, B.; Imhof, P.; Franz, A.; et al. A Conserved Kinase-Based Body-Temperature Sensor Globally Controls Alternative Splicing and Gene Expression. Mol. Cell 2020, 78, 57–69.e4. [Google Scholar] [CrossRef]
- van Roessel, P.; Brand, A.H. GAL4-mediated Ectopic Gene Expression in Drosophila. In Drosophila Protocols; Sullivan, W., Ashburner, M., Hawley, R.S., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2000. [Google Scholar]
- Sénatore, S.; Reddy, V.R.; Sémériva, M.; Perrin, L.; Lalevée, N. Response to mechanical stress is mediated by the TRPA channel painless in the Drosophila heart. PLoS Genet. 2011, 7. [Google Scholar] [CrossRef]
- Jennings, T.; Ringo, J.; Dowse, H. The relationship of heart function to temperature in Drosophila melanogaster and its heritability. J. Exp. Zool. 2009, 311, 689–696. [Google Scholar] [CrossRef]
- Majeed, Z.R.; Nichols, C.D.; Cooper, R.L. 5-HT stimulation of heart rate in Drosophila does not act through cAMP as revealed by pharmacogenetics. J. Appl. Physiol. 2013, 115, 1656–1665. [Google Scholar] [CrossRef]
- Stanley, C.E.; Mauss, A.S.; Borst, A.; Cooper, R.L. The Effects of Chloride Flux on Drosophila Heart Rate. Methods Protoc. 2019, 2, 73. [Google Scholar] [CrossRef]
- Sanyal, S.; Jennings, T.; Dowse, H.; Ramaswami, M. Conditional mutations in SERCA, the Sarco-endoplasmic reticulum Ca2+-ATPase, alter heart rate and rhythmicity in Drosophila. J. Comp. Physiol. B 2006, 176, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Tracey, W.D., Jr.; Wilson, R.I.; Laurent, G.; Benzer, S. Painless, a Drosophila gene essential for nociception. Cell 2003, 113, 261–273. [Google Scholar] [CrossRef]
- Christensen, A.P.; Corey, D.P. TRP channels in mechanosensation: Direct or indirect activation? Nat. Rev. Neurosci. 2007, 8, 510–521. [Google Scholar] [CrossRef]
- Cooper, R.L.; De Castro, C.; Titlow, J.; Majeed, Z.R.; Malloy, C.; King, K.E. Mechanical and Chemical Factors Required for Maintaining Cardiac Rhythm in Drosophila melanogaster Larva. J. Èntomol. 2019, 16, 62–73. [Google Scholar] [CrossRef]
- Prober, D.A.; Zimmerman, S.; Myers, B.R.; McDermott, B.M.; Kim, S.-H.; Caron, S.; Rihel, J.; Solnica-Krezel, L.; Julius, D.; Hudspeth, A.J.; et al. Zebrafish TRPA1 Channels Are Required for Chemosensation but not for Thermosensation or Mechanosensory Hair Cell Function. J. Neurosci. 2008, 28, 10102–10110. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, Y.A.; Cox, C.D.; Ridone, P.; Rohde, P.R.; Cordero-Morales, J.F.; Vásquez, V.; Laver, D.R.; Martinac, B. Mammalian TRP ion channels are insensitive to membrane stretch. J. Cell Sci. 2019, 132, jcs238360. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-C.; Cooper, R.L. Effect of cold exposure on the physiology of cardiac function and synaptic transmission at the neuromuscular junction in invertebrates: A review. Int. J. Zool. Res. 2018, 14, 49–60. [Google Scholar] [CrossRef][Green Version]
- Fischer, L.; Florey, E. Temperature effects on neuromuscular transmission (Opener muscle of Crayfish, Astacus leptodactylus). J. Exp. Biol. 1981, 94, 251–268. [Google Scholar]
- Harri, M.; Florey, E. The effects of temperature on a neuromuscular system of the crayfish, Astacus leptodactylus. J. Comp. Physiol. A 1977, 117, 47–61. [Google Scholar] [CrossRef]
- Hyde, D.; Pearson, T.; Qari, S.; Bowler, K. Adaptive considerations of temperature dependence of neuromuscular function in two species of summer- and winter-caught Crab (Carcinus maenas and Cancer pagurus). J. Comp. Physiol. B 2015, 185, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; De Castro, L.; Cooper, R.L. Effect of temperature change on synaptic transmission at crayfish neuromuscular junctions. Biol. Open 2018, 7, bio037820. [Google Scholar] [CrossRef]
- Dillon, M.E.; Wang, G.; Garrity, P.A.; Huey, R.B. Thermal preference in Drosophila. J. Therm. Biol. 2009, 34, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Gong, J.; Shang, Y.; Wang, F.; Ruppell, K.T.; Ma, Z.; Sheehan, A.E.; Freeman, M.R.; Xiang, Y. Polymodal Nociception in Drosophila Requires Alternative Splicing of TrpA1. Curr. Biol. 2019, 29, 3961–3973.e6. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Shen, W.L.; Montell, C. TRPA1 mediates sensation of the rate of temperature change in Drosophila larvae. Nat. Neurosci. 2017, 20, 34–41. [Google Scholar] [CrossRef]
- Lamaze, A.; Ozturk-Colak, A.; Fischer, R.; Peschel, N.; Koh, K.; Jepson, J.E. Regulation of sleep plasticity by a thermo-sensitive circuit in Drosophila. Sci. Rep. 2017, 7, 40304. [Google Scholar] [CrossRef]
- Sokabe, T.; Chen, H.-C.; Luo, J.; Montell, C. A Switch in Thermal Preference in Drosophila larvae Depends on Multiple Rhodopsins. Cell Rep. 2016, 17, 336–344. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marguerite, N.T.; Bernard, J.; Harrison, D.A.; Harris, D.; Cooper, R.L. Effect of Temperature on Heart Rate for Lucilia sericata (syn Phaenicia sericata) and Drosophila melanogaster with Altered Expression of the TrpA1 Receptors. Insects 2021, 12, 38. https://doi.org/10.3390/insects12010038
Marguerite NT, Bernard J, Harrison DA, Harris D, Cooper RL. Effect of Temperature on Heart Rate for Lucilia sericata (syn Phaenicia sericata) and Drosophila melanogaster with Altered Expression of the TrpA1 Receptors. Insects. 2021; 12(1):38. https://doi.org/10.3390/insects12010038
Chicago/Turabian StyleMarguerite, Nicole T., Jate Bernard, Douglas A. Harrison, David Harris, and Robin L. Cooper. 2021. "Effect of Temperature on Heart Rate for Lucilia sericata (syn Phaenicia sericata) and Drosophila melanogaster with Altered Expression of the TrpA1 Receptors" Insects 12, no. 1: 38. https://doi.org/10.3390/insects12010038
APA StyleMarguerite, N. T., Bernard, J., Harrison, D. A., Harris, D., & Cooper, R. L. (2021). Effect of Temperature on Heart Rate for Lucilia sericata (syn Phaenicia sericata) and Drosophila melanogaster with Altered Expression of the TrpA1 Receptors. Insects, 12(1), 38. https://doi.org/10.3390/insects12010038






