Role of Masticatory Force in Modulating Jawbone Immunity and Bone Homeostasis: A Review
Abstract
1. Introduction
2. Mechanisms of Bone Homeostasis in the Jawbone
2.1. Bone Cells in Jawbone Homeostasis
2.2. Immune Cells in Jawbone Homeostasis
2.3. Mechanisms of Mechanical Stress in Bone Homeostasis
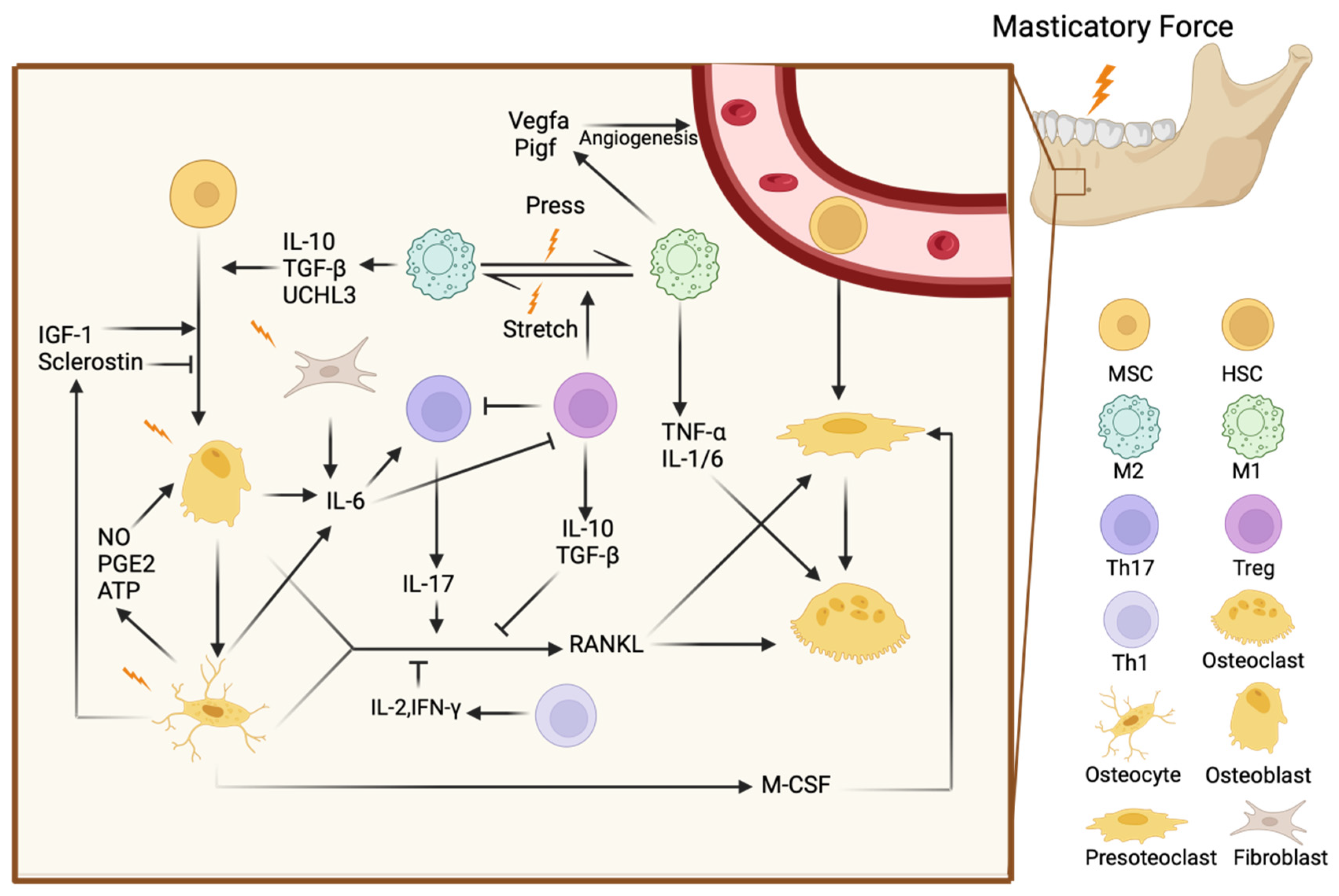
3. The Impact of Masticatory Forces on Immune Cells in the Jawbone
4. The Regulatory Effects of Masticatory Forces on Bone Cells in the Jawbone
4.1. Mechanotransduction and Immune Response in Osteocytes
4.2. Mechanotransduction and Immune Response in Osteoblastes
4.3. Mechanotransduction and Immune Response in Osteoclastes
5. Broader Impacts of Masticatory Forces on Jawbone Immunity and Remodeling
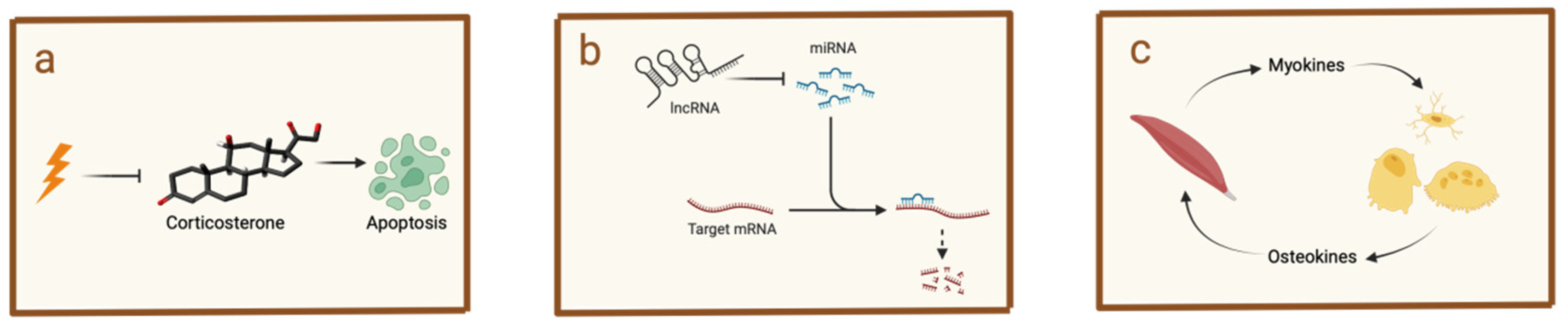
5.1. Hormonal Modulation of Jawbone Immunity by Masticatory Forces
5.2. Influence of Masticatory Forces on Jawbone Immunity Through Non-Coding RNA Regulation
5.3. Muscle–Bone Interaction Mediated by Masticatory Forces
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| RANKL | receptor activator of nuclear factor κ-B ligand |
| RANK | receptor activator of nuclear factor κ-B |
| OPG | osteoprotegerin |
| TNF | tumor necrosis factor |
| IL | interleukin |
| PDLSCs | periodontal ligament stem cells |
| EVs | extracellular vesicles |
| ABs | apoptotic bodies |
| Itgb1 | integrin beta 1 |
| Th1 | T helper 1 cells |
| IFN | interferon |
| Th17 | T helper 17 cells |
| Tregs | regulatory T cells |
| TGF | transforming growth factor |
| TCR | T-cell receptor |
| CCL | C-C chemokine motif ligand |
| M1 | pro-inflammatory macrophages |
| M2 | anti-inflammatory macrophages |
| JPCs | jaw periosteal cells |
| NETs | neutrophil extracellular traps |
| ROS | reactive oxygen species |
| OSM | oncostatin M |
| DCs | dendritic cells |
| ILCs | innate lymphoid cells |
| OTM | orthodontic tooth movement |
| BMSCs | bone marrow mesenchymal stem cells |
| UCHL3 | ubiquitin carboxyl-terminal hydrolase isozyme L3 |
| Vegfa | vascular endothelial growth factor |
| Pigf | placental growth factor |
| AKT | protein kinase B |
| GSK3β | glycogen synthase kinase 3 β |
| NLRP3 | NOD-like receptor family pyrin domain containing 3 |
| PMD | plasma membrane disruptions |
| LCS | lacunar-canalicular system |
| FSS | fluid shear stress |
| DMP | dentin matrix protein |
| Wnt | Wingless/Int-1 |
| YAP | yes-associated protein |
| TAZ | transcriptional co-activator with PDZ-binding motif |
| IGF | insulin-like growth factor |
| JAK | janus kinase |
| STAT3 | signal transducer and activator of transcription 3 |
| ERK | extracellular signal-regulated kinase |
| CXCL | C-X-C motif chemokine ligand |
| M-CSF | macrophage colony-stimulating factor |
| NO | nitric oxide |
| PGE2 | prostaglandins |
| ATP | adenosine triphosphate |
| OSX | osterix |
| ALP | alkaline phosphatase |
| Glut | glucose transporter |
| SIRT | sirtuin |
| Runx | runt-related transcription factor |
| S1P | sphingosine-1-phosphate |
| MMPs | matrix metalloproteinases |
| Rac1 | ras-related C3 botulinum toxin substrate 1 |
| ANXA3 | annexin A3 |
| H2S | hydrogen sulfide |
| MCP | monocyte chemoattractant protein |
| BMMs | bone marrow macrophages |
| CSF1R | colony-stimulating factor 1 receptor |
| MGF | mechano-growth factor |
| miRNAs | microRNAs |
| lncRNAs | long non-coding RNAs |
| BDNF | brain-derived neurotrophic factor |
| FGF | Fibroblast growth factor |
| BAIBA | β-aminoisobutyric acid |
References
- Wang, Y.; Jia, L.; Zheng, Y.; Li, W. Bone remodeling induced by mechanical forces is regulated by miRNAs. Biosci. Rep. 2018, 38, BSR20180448. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Li, Q.; Zhang, D.; Zhang, X.; Qi, X.; Wang, Q.; Chen, Y.; Liu, C.; Li, H.; Zhang, S.; et al. Mapping the immune microenvironment for mandibular alveolar bone homeostasis at single-cell resolution. Bone Res. 2021, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-M.; Quan, Y. Latest research findings on immune microenvironment regulation in jawbone-related diseases. J. Sichuan Univ. (Med. Sci.) 2022, 53, 528–531. [Google Scholar]
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef]
- Zarrer, J.; Taipaleenmäki, H. The osteoblast in regulation of tumor cell dormancy and bone metastasis. J. Bone Oncol. 2024, 45, 100597. [Google Scholar] [CrossRef]
- Del Fattore, A.; Teti, A.; Rucci, N. Bone cells and the mechanisms of bone remodelling. Front. Biosci. Elite 2012, 4, 2302–2321. [Google Scholar] [CrossRef]
- Lara-Castillo, N.; Kim-Weroha, N.A.; Kamel, M.A.; Javaheri, B.; Ellies, D.L.; Krumlauf, R.E.; Thiagarajan, G.; Johnson, M.L. In vivo mechanical loading rapidly activates β-catenin signaling in osteocytes through a prostaglandin mediated mechanism. Bone 2015, 76, 58–66. [Google Scholar] [CrossRef]
- Ethier, C.R.; Simmons, C.A. Introductory Biomechanics: From Cells to Organisms; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Uda, Y.; Azab, E.; Sun, N.; Shi, C.; Pajevic, P.D. Osteocyte mechanobiology. Curr. Osteoporos. Rep. 2017, 15, 318–325. [Google Scholar] [CrossRef]
- Yu, K.; Sellman, D.P.; Bahraini, A.; Hagan, M.L.; Elsherbini, A.; Vanpelt, K.T.; Marshall, P.L.; Hamrick, M.W.; McNeil, A.; McNeil, P.L.; et al. Mechanical loading disrupts osteocyte plasma membranes which initiates mechanosensation events in bone. J. Orthop. Res. 2018, 36, 653–662. [Google Scholar] [CrossRef]
- Gruber, R. Osteoimmunology: Inflammatory osteolysis and regeneration of the alveolar bone. J. Clin. Periodontol. 2019, 46, 52–69. [Google Scholar] [CrossRef]
- Dutzan, N.; Abusleme, L.; Bridgeman, H.; Greenwell-Wild, T.; Zangerle-Murray, T.; Fife, M.E.; Bouladoux, N.; Linley, H.; Brenchley, L.; Wemyss, K.; et al. On-going mechanical damage from mastication drives homeostatic Th17 cell responses at the oral barrier. Immunity 2017, 46, 133–147. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.A. Control of RANKL gene expression. Bone 2010, 46, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C.; et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Miner. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef]
- Graves, D.T.; Alshabab, A.; Albiero, M.L.; Mattos, M.; Corrêa, J.D.; Chen, S.; Yang, Y. Osteocytes play an important role in experimental periodontitis in healthy and diabetic mice through expression of RANKL. J. Clin. Periodontol. 2018, 45, 285–292. [Google Scholar] [CrossRef]
- Eriksen, E.F. Cellular mechanisms of bone remodeling. Rev. Endocr. Metab. Disord. 2010, 11, 219–227. [Google Scholar] [CrossRef]
- Pacios, S.; Xiao, W.; Mattos, M.; Lim, J.; Tarapore, R.S.; Alsadun, S.; Yu, B.; Wang, C.Y.; Graves, D.T. Osteoblast lineage cells play an essential role in periodontal bone loss through activation of nuclear factor-kappa B. Sci. Rep. 2015, 5, 16694. [Google Scholar] [CrossRef]
- Yang, C.Y.; Jeon, H.H.; Alshabab, A.; Lee, Y.J.; Chung, C.H.; Graves, D.T. RANKL deletion in periodontal ligament and bone lining cells blocks orthodontic tooth movement. Int. J. Oral Sci. 2018, 10, 3. [Google Scholar] [CrossRef]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.C.; Chang, M.S.; Lüthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef]
- Kostenuik, P.J. Osteoprotegerin and RANKL regulate bone resorption, density, geometry and strength. Curr. Opin. Pharmacol. 2005, 5, 618–625. [Google Scholar] [CrossRef]
- Liang, X.; Hou, Y.; Han, L.; Yu, S.; Zhang, Y.; Cao, X.; Yan, J. ELMO1 regulates RANKL-stimulated differentiation and bone resorption of osteoclasts. Front. Cell Dev. Biol. 2021, 9, 702916. [Google Scholar] [CrossRef]
- Zhao, X.; Patil, S.; Xu, F.; Lin, X.; Qian, A. Role of biomolecules in osteoclasts and their therapeutic potential for osteoporosis. Biomolecules 2021, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Elson, A.; Anuj, A.; Barnea-Zohar, M.; Reuven, N. The origins and formation of bone-resorbing osteoclasts. Bone 2022, 164, 116538. [Google Scholar] [CrossRef] [PubMed]
- Jimi, E.; Akiyama, S.; Tsurukai, T.; Okahashi, N.; Kobayashi, K.; Udagawa, N.; Nishihara, T.; Takahashi, N.; Suda, T. Osteoclast differentiation factor acts as a multifunctional regulator in murine osteoclast differentiation and function. J. Immunol. 1999, 163, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Kitaura, H.; Ohori, F.; Noguchi, T.; Marahleh, A.; Ma, J.; Ren, J.; Miura, M.; Fan, Z.; Narita, K.; et al. (D-Ala2) GIP Inhibits Inflammatory Bone Resorption by Suppressing TNF-α and RANKL Expression and Directly Impeding Osteoclast Formation. Int. J. Mol. Sci. 2024, 25, 2555. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Han, G. Advances of mesenchymal stem cells released extracellular vesicles in periodontal bone remodeling. DNA Cell Biol. 2022, 41, 935–950. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Y.; Liu, X.; Fu, J.; Du, J.; Luo, Z.; Xu, J.; Bhawal, U.K.; Liu, Y.; Guo, L.; et al. Mesenchymal stem cell-derived apoptotic bodies alleviate alveolar bone destruction by regulating osteoclast differentiation and function. Int. J. Oral Sci. 2023, 15, 51. [Google Scholar] [CrossRef]
- Muraca, M.; Cappariello, A. The role of extracellular vesicles (EVs) in the epigenetic regulation of bone metabolism and osteoporosis. Int. J. Mol. Sci. 2020, 21, 8682. [Google Scholar] [CrossRef]
- Dai, Z.; Zheng, W.; Li, S. Receptor activator of nuclear factor-κB ligand and tumor necrosis factor-α promotes osteoclast differentiation through the exosomes of inflammatory periodontal ligament stem cells. Hua Xi Kou Qiang Yi Xue Za Zhi = Huaxi Kouqiang Yixue Zazhi = West China J. Stomatol. 2022, 40, 377–385. [Google Scholar]
- Oikawa, T.; Kuroda, Y.; Matsuo, K. Regulation of osteoclasts by membrane-derived lipid mediators. Cell. Mol. Life Sci. 2013, 70, 3341–3353. [Google Scholar] [CrossRef]
- Li, B.; Wang, P.; Jiao, J.; Wei, H.; Xu, W.; Zhou, P. Roles of the RANKL–RANK Axis in Immunity—Implications for Pathogenesis and Treatment of Bone Metastasis. Front. Immunol. 2022, 13, 824117. [Google Scholar] [CrossRef]
- Carbone, F.; Crowe, L.A.; Roth, A.; Burger, F.; Lenglet, S.; Braunersreuther, V.; Brandt, K.J.; Quercioli, A.; Mach, F.; Vallée, J.P.; et al. Treatment with anti-RANKL antibody reduces infarct size and attenuates dysfunction impacting on neutrophil-mediated injury. J. Mol. Cell. Cardiol. 2016, 94, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Kitaura, H.; Kimura, K.; Ishida, M.; Hakami, Z.; Saeed, J.; Sugisawa, H.; Kohara, H.; Yoshimatsu, M.; Takano-Yamamoto, T. The Role of Th1 Cytokines on Mechanical Loading-Induced Osteoclastogenesis and Bone Resorption. In Interface Oral Health Science 2014: Innovative Research on Biosis-Abiosis Intelligent Interface; Springer: Berlin/Heidelberg, Germany, 2015; pp. 269–279. [Google Scholar]
- Amatya, N.; Garg, A.V.; Gaffen, S.L. IL-17 signaling: The yin and the yang. Trends Immunol. 2017, 38, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Seo, S.J.; Kim, J.Y.; Kim, Y.G.; Lee, Y. IL-17 promotes osteoblast differentiation, bone regeneration, and remodeling in mice. Biochem. Biophys. Res. Commun. 2020, 524, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Rossini, M.; Viapiana, O.; Adami, S.; Idolazzi, L.; Fracassi, E.; Gatti, D. Focal bone involvement in inflammatory arthritis: The role of IL17. Rheumatol. Int. 2016, 36, 469–482. [Google Scholar] [CrossRef]
- Tsukasaki, M.; Komatsu, N.; Nagashima, K.; Nitta, T.; Pluemsakunthai, W.; Shukunami, C.; Iwakura, Y.; Nakashima, T.; Okamoto, K.; Takayanagi, H.; et al. Host defense against oral microbiota by bone-damaging T cells. Nat. Commun. 2018, 9, 701. [Google Scholar] [CrossRef]
- Glowacki, A.J.; Yoshizawa, S.; Jhunjhunwala, S.; Vieira, A.E.; Garlet, G.P.; Sfeir, C.; Little, S.R. Prevention of inflammation-mediated bone loss in murine and canine periodontal disease via recruitment of regulatory lymphocytes. Proc. Natl. Acad. Sci. USA 2013, 110, 18525–18530. [Google Scholar] [CrossRef]
- Park, J.Y.; Chung, H.; DiPalma, D.T.; Tai, X.; Park, J.H. Immune quiescence in the oral mucosa is maintained by a uniquely large population of highly activated Foxp3+ regulatory T cells. Mucosal Immunol. 2018, 11, 1092–1102. [Google Scholar] [CrossRef]
- Bettelli, E.; Carrier, Y.; Gao, W.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006, 441, 235–238. [Google Scholar] [CrossRef]
- Ness-Schwickerath, K.J.; Morita, C.T. Regulation and function of IL-17A-and IL-22-producing γδ T cells. Cell. Mol. Life Sci. 2011, 68, 2371–2390. [Google Scholar] [CrossRef]
- Zhu, M.; Belkina, A.C.; DeFuria, J.; Carr, J.D.; Van Dyke, T.E.; Gyurko, R.; Nikolajczyk, B.S. B cells promote obesity-associated periodontitis and oral pathogen-associated inflammation. J. Leukoc. Biol. 2014, 96, 349–357. [Google Scholar] [CrossRef]
- Hu, F.; Liu, H.; Liu, X.; Zhang, X.; Xu, L.; Zhu, H.; Li, Y.; Shi, L.; Ren, L.; Zhang, J.; et al. Pathogenic conversion of regulatory B10 cells into osteoclast-priming cells in rheumatoid arthritis. J. Autoimmun. 2017, 76, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Settem, R.P.; Honma, K.; Chinthamani, S.; Kawai, T.; Sharma, A. B-cell RANKL contributes to pathogen-induced alveolar bone loss in an experimental periodontitis mouse model. Front. Physiol. 2021, 12, 722859. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Meednu, N.; Rosenberg, A.; Rangel-Moreno, J.; Wang, V.; Glanzman, J.; Owen, T.; Zhou, X.; Zhang, H.; Boyce, B.F.; et al. B cells inhibit bone formation in rheumatoid arthritis by suppressing osteoblast differentiation. Nat. Commun. 2018, 9, 5127. [Google Scholar] [CrossRef] [PubMed]
- Sapra, L.; Bhardwaj, A.; Mishra, P.K.; Garg, B.; Verma, B.; Mishra, G.C.; Srivastava, R.K. Regulatory B cells (Bregs) inhibit osteoclastogenesis and play a potential role in ameliorating ovariectomy-induced bone loss. Front. Immunol. 2021, 12, 691081. [Google Scholar] [CrossRef]
- Molofsky, A.B.; Nussbaum, J.C.; Liang, H.E.; Van Dyken, S.J.; Cheng, L.E.; Mohapatra, A.; Chawla, A.; Locksley, R.M. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J. Exp. Med. 2013, 210, 535–549. [Google Scholar] [CrossRef]
- He, F.; Umrath, F.; von Ohle, C.; Reinert, S.; Alexander, D. Analysis of the influence of jaw periosteal cells on macrophages phenotype using an innovative horizontal coculture system. Biomedicines 2021, 9, 1753. [Google Scholar] [CrossRef]
- Ando, Y.; Tsukasaki, M.; Huynh, N.C.; Zang, S.; Yan, M.; Muro, R.; Nakamura, K.; Komagamine, M.; Komatsu, N.; Okamoto, K.; et al. The neutrophil–osteogenic cell axis promotes bone destruction in periodontitis. Int. J. Oral Sci. 2024, 16, 18. [Google Scholar] [CrossRef]
- Bruce, A.G.; Hoggatt, I.H.; Rose, T.M. Oncostatin M is a differentiation factor for myeloid leukemia cells. J. Immunol. 1992, 149, 1271–1275. [Google Scholar] [CrossRef]
- Arizon, M.; Nudel, I.; Segev, H.; Mizraji, G.; Elnekave, M.; Furmanov, K.; Eli-Berchoer, L.; Clausen, B.E.; Shapira, L.; Wilensky, A.; et al. Langerhans cells down-regulate inflammation-driven alveolar bone loss. Proc. Natl. Acad. Sci. USA 2012, 109, 7043–7048. [Google Scholar] [CrossRef]
- Ono, T.; Hayashi, M.; Sasaki, F.; Nakashima, T. RANKL biology: Bone metabolism, the immune system, and beyond. Inflamm. Regen. 2020, 40, 2. [Google Scholar] [CrossRef]
- Elsayed, R.; Kurago, Z.; Cutler, C.W.; Arce, R.M.; Gerber, J.; Celis, E.; Sultan, H.; Elashiry, M.; Meghil, M.; Sun, C.; et al. Role of dendritic cell-mediated immune response in oral homeostasis: A new mechanism of osteonecrosis of the jaw. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 2595. [Google Scholar] [CrossRef]
- Dai, J.; Umrath, F.; Reinert, S.; Alexander, D. Jaw periosteal cells seeded in beta-tricalcium phosphate inhibit dendritic cell maturation. Biomolecules 2020, 10, 887. [Google Scholar] [CrossRef] [PubMed]
- Wojno, E.D.T.; Artis, D. Innate lymphoid cells: Balancing immunity, inflammation, and tissue repair in the intestine. Cell Host Microbe 2012, 12, 445–457. [Google Scholar]
- Qin, X.; Hoda, M.N.; Susin, C.; Wheeler, J.N.; Marshall, B.; Perry, L.; Saad, N.; Yin, L.; Elsayed, R.; Elsalanty, M.; et al. Increased innate lymphoid cells in periodontal tissue of the murine model of periodontitis: The role of AMP-activated protein kinase and relevance for the human condition. Front. Immunol. 2017, 8, 922. [Google Scholar] [CrossRef] [PubMed]
- Bernink, J.H.; Ohne, Y.; Teunissen, M.B.; Wang, J.; Wu, J.; Krabbendam, L.; Guntermann, C.; Volckmann, R.; Koster, J.; van Tol, S.; et al. c-Kit-positive ILC2s exhibit an ILC3-like signature that may contribute to IL-17-mediated pathologies. Nat. Immunol. 2019, 20, 992–1003. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, J.; Liu, P.; Wang, Q.; Liu, L.; Zhao, H. The RANK/RANKL/OPG system and tumor bone metastasis: Potential mechanisms and therapeutic strategies. Front. Endocrinol. 2022, 13, 1063815. [Google Scholar] [CrossRef]
- Nile, M.; Folwaczny, M.; Wichelhaus, A.; Baumert, U.; Janjic Rankovic, M. Fluid flow shear stress and tissue remodeling—An orthodontic perspective: Evidence synthesis and differential gene expression network analysis. Front. Bioeng. Biotechnol. 2023, 11, 1256825. [Google Scholar] [CrossRef]
- Qin, L.; He, T.; Chen, S.; Yang, D.; Yi, W.; Cao, H.; Xiao, G. Roles of mechanosensitive channel Piezo1/2 proteins in skeleton and other tissues. Bone Res. 2021, 9, 44. [Google Scholar] [CrossRef]
- Zong, B.; Yu, F.; Zhang, X.; Pang, Y.; Zhao, W.; Sun, P.; Li, L. Mechanosensitive Piezo1 channel in physiology and pathophysiology of the central nervous system. Ageing Res. Rev. 2023, 90, 102026. [Google Scholar] [CrossRef]
- Gould, N.R.; Torre, O.M.; Leser, J.M.; Stains, J.P. The cytoskeleton and connected elements in bone cell mechano-transduction. Bone 2021, 149, 115971. [Google Scholar] [CrossRef]
- Wang, T.; Liu, X.; Li, J.; Yue, Y.; Li, J.; Wang, M.; Wei, N.; Hao, L. Mechanisms of mechanical force in periodontal homeostasis: A review. Front. Immunol. 2024, 15, 1438726. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Praneetpong, N.; Ono, W.; Ono, N. Mechanisms of osteoclastogenesis in orthodontic tooth movement and orthodontically induced tooth root resorption. J. Bone Metab. 2023, 30, 297. [Google Scholar] [CrossRef] [PubMed]
- Chaushu, S.; Klein, Y.; Mandelboim, O.; Barenholz, Y.; Fleissig, O. Immune changes induced by orthodontic forces: A critical review. J. Dent. Res. 2022, 101, 11–20. [Google Scholar] [CrossRef]
- Lassus, J.; Salo, J.; Jiranek, W.A.; Santavirta, S.; Nevalainen, J.; Matucci-Cerinic, M.; Horák, P.; Konttinen, Y. Macrophage activation results in bone resorption. Clin. Orthop. Relat. Res. (1976–2007) 1998, 352, 7–15. [Google Scholar] [CrossRef]
- Pöllinger, B.; Junt, T.; Metzler, B.; Walker, U.A.; Tyndall, A.; Allard, C.; Bay, S.; Keller, R.; Raulf, F.; Di Padova, F.; et al. Th17 cells, not IL-17+ γδ T cells, drive arthritic bone destruction in mice and humans. J. Immunol. 2011, 186, 2602–2612. [Google Scholar] [CrossRef]
- Blanchard, F.; Duplomb, L.; Baud’huin, M.; Brounais, B. The dual role of IL-6-type cytokines on bone remodeling and bone tumors. Cytokine Growth Factor Rev. 2009, 20, 19–28. [Google Scholar] [CrossRef]
- Majedi, F.S.; Hasani-Sadrabadi, M.M.; Thauland, T.J.; Li, S.; Bouchard, L.S.; Butte, M.J. T-cell activation is modulated by the 3D mechanical microenvironment. Biomaterials 2020, 252, 120058. [Google Scholar] [CrossRef]
- Xie, D.; Fu, D.; Fu, S.; Chen, B.; He, W.; Wilson, D.A.; Peng, F. Mechanical activation of immune T cells via a water driven nanomotor. Adv. Healthc. Mater. 2022, 11, 2200042. [Google Scholar] [CrossRef]
- Chansaenroj, J.; Suwittayarak, R.; Egusa, H.; Samaranayake, L.P.; Osathanon, T. Mechanical force modulates inflammation and immunomodulation in periodontal ligament cells. Med. Rev. 2024, 4, 544–548. [Google Scholar] [CrossRef]
- Xu, H.; Guan, J.; Jin, Z.; Yin, C.; Wu, S.; Sun, W.; Zhang, H.; Yan, B. Mechanical force modulates macrophage proliferation via Piezo1-AKT-cyclin D1 axis. FASEB J. 2022, 36, e22423. [Google Scholar] [CrossRef]
- Liang, W.; Ding, P.; Qian, J.; Li, G.; Lu, E.; Zhao, Z. Polarized M2 macrophages induced by mechanical stretching modulate bone regeneration of the craniofacial suture for midfacial hypoplasia treatment. Cell Tissue Res. 2021, 386, 585–603. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Song, Y.; Zhang, Y.; Zhao, W.; Wang, C.; Lin, H.; Al-ani, M.K.; Liu, W.; Xue, R.; Yang, L. Mechanical stretch induces osteogenesis through the alternative activation of macrophages. J. Cell. Physiol. 2021, 236, 6376–6390. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Lu, Y.; Zhong, W.; Wang, T.; Li, Y.; Ruan, X.; Chen, H.; Sun, L.; Guan, Z.; Li, G.; et al. Piezo1-mediated M2 macrophage mechanotransduction enhances bone formation through secretion and activation of transforming growth factor-β1. Cell Prolif. 2023, 56, e13440. [Google Scholar] [CrossRef] [PubMed]
- Pu, P.; Wu, S.; Zhang, K.; Xu, H.; Guan, J.; Jin, Z.; Sun, W.; Zhang, H.; Yan, B. Mechanical force induces macrophage-derived exosomal UCHL3 promoting bone marrow mesenchymal stem cell osteogenesis by targeting SMAD1. J. Nanobiotechnology 2023, 21, 88. [Google Scholar] [CrossRef]
- Han, Y.; Yang, Q.; Huang, Y.; Gao, P.; Jia, L.; Zheng, Y.; Li, W. Compressive force regulates orthodontic tooth movement via activating the NLRP3 inflammasome. FASEB J. 2022, 36, e22627. [Google Scholar] [CrossRef]
- Kulkarni, R.N.; Bakker, A.D.; Everts, V.; Klein-Nulend, J. Mechanical loading prevents the stimulating effect of IL-1β on osteocyte-modulated osteoclastogenesis. Biochem. Biophys. Res. Commun. 2012, 420, 11–16. [Google Scholar] [CrossRef]
- Cabahug-Zuckerman, P.; Stout, R.F., Jr.; Majeska, R.J.; Thi, M.M.; Spray, D.C.; Weinbaum, S.; Schaffler, M.B. Potential role for a specialized β3 integrin-based structure on osteocyte processes in bone mechanosensation. J. Orthop. Res. 2018, 36, 642–652. [Google Scholar] [CrossRef]
- Weinbaum, S.; Cowin, S.C.; Zeng, Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J. Biomech. 1994, 27, 339–360. [Google Scholar] [CrossRef]
- Klein-Nulend, J.; Bakker, A.D.; Bacabac, R.G.; Vatsa, A.; Weinbaum, S. Mechanosensation and transduction in osteocytes. Bone 2013, 54, 182–190. [Google Scholar] [CrossRef]
- Smit, T.H.; Huyghe, J.M.; Cowin, S.C. Estimation of the poroelastic parameters of cortical bone. J. Biomech. 2002, 35, 829–835. [Google Scholar] [CrossRef]
- Wang, L.; You, X.; Zhang, L.; Zhang, C.; Zou, W. Mechanical regulation of bone remodeling. Bone Res. 2022, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Moraes, C.; Sun, Y.; Simmons, C.A. (Micro) managing the mechanical microenvironment. Integr. Biol. 2011, 3, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Takise, S.; Fuchimoto, T.; Kawata, H. Effects of masticatory movement on cranial bone mass and micromorphology of osteocytes and osteoblasts in developing rats. Asia Pac. J. Clin. Nutr. 2009, 18, 96–104. [Google Scholar] [PubMed]
- Wang, L.; Dong, J.; Xian, C.J. Computational investigation on the biomechanical responses of the osteocytes to the compressive stimulus: A poroelastic model. BioMed Res. Int. 2018, 2018, 4071356. [Google Scholar] [CrossRef]
- Bonewald, L.F.; Johnson, M.L. Osteocytes, mechanosensing and Wnt signaling. Bone 2008, 42, 606–615. [Google Scholar] [CrossRef]
- Jackson, E.; Lara-Castillo, N.; Akhter, M.P.; Dallas, M.; Scott, J.M.; Ganesh, T.; Johnson, M.L. Osteocyte Wnt/β-catenin pathway activation upon mechanical loading is altered in ovariectomized mice. Bone Rep. 2021, 15, 101129. [Google Scholar] [CrossRef]
- Zarka, M.; Etienne, F.; Bourmaud, M.; Szondi, D.; Schwartz, J.M.; Kampmann, K.; Helary, C.; Rannou, F.; Haÿ, E.; Cohen-Solal, M. Mechanical loading activates the YAP/TAZ pathway and chemokine expression in the MLO-Y4 osteocyte-like cell line. Lab. Investig. 2021, 101, 1597–1604. [Google Scholar] [CrossRef]
- Inoue, M.; Ono, T.; Kameo, Y.; Sasaki, F.; Ono, T.; Adachi, T.; Nakashima, T. Forceful mastication activates osteocytes and builds a stout jawbone. Sci. Rep. 2019, 9, 4404. [Google Scholar] [CrossRef]
- Hichijo, N.; Kawai, N.; Mori, H.; Sano, R.; Ohnuki, Y.; Okumura, S.; Langenbach, G.E.; Tanaka, E. Effects of the masticatory demand on the rat mandibular development. J. Oral Rehabil. 2014, 41, 581–587. [Google Scholar] [CrossRef]
- Hao, Z.; Ma, Y.; Wu, J.; Li, X.; Chen, H.; Shen, J.; Wang, H. Osteocytes regulate osteoblast differentiation and osteoclast activity through Interleukin-6 under mechanical loading. RSC Adv. 2017, 7, 50200–50209. [Google Scholar] [CrossRef]
- Bonetto, A.; Aydogdu, T.; Kunzevitzky, N.; Guttridge, D.C.; Khuri, S.; Koniaris, L.G.; Zimmers, T.A. STAT3 activation in skeletal muscle links muscle wasting and the acute phase response in cancer cachexia. PLoS ONE 2011, 6, e22538. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, A.; Kiely, P.A.; Hoey, D.A. Mechanically stimulated osteocytes promote the proliferation and migration of breast cancer cells via a potential CXCL1/2 mechanism. Biochem. Biophys. Res. Commun. 2021, 534, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, L.; Ge, L.; Pathak, J.L. Osteocyte-mediated translation of mechanical stimuli to cellular signaling and its role in bone and non-bone-related clinical complications. Curr. Osteoporos. Rep. 2020, 18, 67–80. [Google Scholar] [CrossRef]
- Sindhavajiva, P.R.; Sastravaha, P.; Arksornnukit, M.; Pavasant, P. Intermittent compressive force induces human mandibular-derived osteoblast differentiation via WNT/β-catenin signaling. J. Cell. Biochem. 2018, 119, 3474–3485. [Google Scholar] [CrossRef] [PubMed]
- Lohberger, B.; Kaltenegger, H.; Stuendl, N.; Payer, M.; Rinner, B.; Leithner, A. Effect of cyclic mechanical stimulation on the expression of osteogenesis genes in human intraoral mesenchymal stromal and progenitor cells. BioMed Res. Int. 2014, 2014, 189516. [Google Scholar] [CrossRef]
- Liu, P.; Tu, J.; Wang, W.; Li, Z.; Li, Y.; Yu, X.; Zhang, Z. Effects of mechanical stress stimulation on function and expression mechanism of osteoblasts. Front. Bioeng. Biotechnol. 2022, 10, 830722. [Google Scholar] [CrossRef]
- Baumert, U.; Golan, I.; Becker, B.; Hrala, B.P.; Redlich, M.; Roos, H.A.; Palmon, A.; Reichenberg, E.; Müßig, D. Pressure simulation of orthodontic force in osteoblasts: A pilot study. Orthod. Craniofacial Res. 2004, 7, 3–9. [Google Scholar] [CrossRef]
- Barthelemi, S.; Robinet, J.; Garnotel, R.; Antonicelli, F.; Schittly, E.; Hornebeck, W.; Lorimier, S. Mechanical forces-induced human osteoblasts differentiation involves MMP-2/MMP-13/MT1-MMP proteolytic cascade. J. Cell. Biochem. 2012, 113, 760–772. [Google Scholar] [CrossRef]
- Sims, N.A.; Gooi, J.H. Bone remodeling: Multiple cellular interactions required for coupling of bone formation and resorption. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2008; Volume 19. [Google Scholar]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef]
- Yamamoto, K.; Yamamoto, T.; Ichioka, H.; Akamatsu, Y.; Oseko, F.; Mazda, O.; Imanishi, J.; Kanamura, N.; Kita, M. Effects of mechanical stress on cytokine production in mandible-derived osteoblasts. Oral Dis. 2011, 17, 712–719. [Google Scholar] [CrossRef]
- Yamamoto, K.; Yamamoto, T.; Kanamura, N.; Kita, M. Role of mechanical stress in mandible bone metabolism. Inflamm. Regen. 2012, 32, 119–123. [Google Scholar] [CrossRef]
- Vahidi, B. Simulation of mechanical modulation of an osteoblast cell due to fluid flow. In Proceedings of the 2018 25th National and 3rd International Iranian Conference on Biomedical Engineering (ICBME), Qom, Iran, 29–30 November 2018; IEEE: Piscataway, NJ, USA, 2018. [Google Scholar]
- Wang, H.; Li, T.; Wang, X.; Guan, Y.; Jiang, Y.; Chen, S.; Zou, S.; Duan, P. Mechanisms of sphingosine-1-phosphate (S1P) signaling on excessive stress-induced root resorption during orthodontic molar intrusion. Clin. Oral Investig. 2021, 26, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Sun, C.; Argraves, K.M. Periodontal inflammation and alveolar bone loss induced by A ggregatibacter actinomycetemcomitans is attenuated in sphingosine kinase 1-deficient mice. J. Periodontal Res. 2016, 51, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhou, Y.; Zhu, L.; Yang, S.; Huang, R.; Shi, W.; Peng, B.; Xiao, Y. SPHK1-S1PR1-RANKL axis regulates the interactions between macrophages and BMSCs in inflammatory bone loss. J. Bone Miner. Res. 2018, 33, 1090–1104. [Google Scholar] [CrossRef]
- Wang, H.; Li, T.; Jiang, Y.; Chen, S.; Zou, S.; Bonewald, L.F.; Duan, P. Force-Loaded Cementocytes Regulate Osteoclastogenesis via S1P/S1PR1/Rac1 Axis. J. Dent. Res. 2023, 102, 1376–1386. [Google Scholar] [CrossRef]
- Kook, S.-H.; Jang, Y.-S.; Lee, J.-C. Human periodontal ligament fibroblasts stimulate osteoclastogenesis in response to compression force through TNF-α-mediated activation of CD4+ T cells. J. Cell. Biochem. 2011, 112, 2891–2901. [Google Scholar] [CrossRef]
- Huang, H.; Yang, R.; Zhou, Y.-H. Mechanobiology of periodontal ligament stem cells in orthodontic tooth movement. Stem Cells Int. 2018, 2018, 6531216. [Google Scholar] [CrossRef]
- Wang, Z.; Maruyama, K.; Sakisaka, Y.; Suzuki, S.; Tada, H.; Suto, M.; Saito, M.; Yamada, S.; Nemoto, E. Cyclic stretch force induces periodontal ligament cells to secrete exosomes that suppress IL-1β production through the inhibition of the NF-κB signaling pathway in macrophages. Front. Immunol. 2019, 10, 1310. [Google Scholar] [CrossRef]
- Bauernfeind, F.G.; Horvath, G.; Stutz, A.; Alnemri, E.S.; MacDonald, K.; Speert, D.; Fernandes-Alnemri, T.; Wu, J.; Monks, B.G.; Fitzgerald, K.A.; et al. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009, 183, 787–791. [Google Scholar] [CrossRef]
- Huang, H.M.; Han, C.S.; Cui, S.J.; Zhou, Y.K.; Xin, T.Y.; Zhang, T.; Zhu, S.B.; Zhou, Y.H.; Yang, R.L. Mechanical force-promoted osteoclastic differentiation via periodontal ligament stem cell exosomal protein ANXA3. Stem Cell Rep. 2022, 17, 1842–1858. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, R.; Liu, X.; Zhou, Y.; Qu, C.; Kikuiri, T.; Wang, S.; Zandi, E.; Du, J.; Ambudkar, I.S.; et al. Hydrogen sulfide maintains mesenchymal stem cell function and bone homeostasis via regulation of Ca2+ channel sulfhydration. Cell Stem Cell 2014, 15, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Liu, D.; Liu, Y.; Zhang, C.; Wang, J.; Wang, S. Physiologic levels of endogenous hydrogen sulfide maintain the proliferation and differentiation capacity of periodontal ligament stem cells. J. Periodontol. 2015, 86, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wen, F.; He, D.; Liu, D.; Yang, R.; Wang, X.; Yan, Y.; Liu, Y.; Kou, X.; Zhou, Y. Force-induced H2S by PDLSCs modifies osteoclastic activity during tooth movement. J. Dent. Res. 2017, 96, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kadono, Y.; Takami, M.; Lee, J.; Lee, S.H.; Okada, F.; Kim, J.H.; Kobayashi, T.; Odgren, P.R.; Nakano, H.; et al. Osteoclast differentiation independent of the TRANCE–RANK–TRAF6 axis. J. Exp. Med. 2005, 202, 589–595. [Google Scholar] [CrossRef]
- Kwak, H.B.; Jin, H.M.; Ha, H.; Kang, M.J.; Lee, S.B.; Kim, H.H.; Lee, Z.H. Tumor necrosis factor-α induces differentiation of human peripheral blood mononuclear cells into osteoclasts through the induction of p21 (WAF1/Cip1). Biochem. Biophys. Res. Commun. 2005, 330, 1080–1086. [Google Scholar] [CrossRef]
- Cho, E.S.; Lee, K.S.; Son, Y.O.; Jang, Y.S.; Lee, S.Y.; Kwak, S.Y.; Yang, Y.M.; Park, S.M.; Lee, J.C. Compressive mechanical force augments osteoclastogenesis by bone marrow macrophages through activation of c-Fms-mediated signaling. J. Cell. Biochem. 2010, 111, 1260–1269. [Google Scholar] [CrossRef]
- Ma, Z.; Li, S.; Sun, Y. Effect of enhanced masticatory force on OPG, RANKL and MGF in alveolar bone of ovariectomized rats. J. Appl. Oral Sci. 2020, 28, e20190409. [Google Scholar] [CrossRef]
- Tahara, Y.; Sakurai, K.; Ando, T. Influence of chewing and clenching on salivary cortisol levels as an indicator of stress. J. Prosthodont. 2007, 16, 129–135. [Google Scholar] [CrossRef]
- Abella, V.; Scotece, M.; Conde, J.; Pino, J.; Gonzalez-Gay, M.A.; Gomez-Reino, J.J.; Mera, A.; Lago, F.; Gómez, R.; Gualillo, O. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat. Rev. Rheumatol. 2017, 13, 100–109. [Google Scholar] [CrossRef]
- DeVries, A.C.; Gerber, J.M.; Richardson, H.N.; Moffatt, C.A.; Demas, G.E.; Taymans, S.E.; Nelson, R.J. Stress affects corticosteroid and immunoglobulin concentrations in male house mice (Mus musculus) and prairie voles (Microtus ochrogaster). Comp. Biochem. Physiol. Part A Physiol. 1997, 118, 655–663. [Google Scholar] [CrossRef]
- Yang, S.; Park, Y.; Choi, T. Effects of mastication on antibody production under fasting conditions in mice. Int. J. Med. Sci. 2023, 20, 232. [Google Scholar] [CrossRef] [PubMed]
- Iwawaki, Y.; Mizusawa, N.; Iwata, T.; Higaki, N.; Goto, T.; Watanabe, M.; Tomotake, Y.; Ichikawa, T.; Yoshimoto, K. MiR-494-3p induced by compressive force inhibits cell proliferation in MC3T3-E1 cells. J. Biosci. Bioeng. 2015, 120, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mohammed, A.; Oubaidin, M.; Evans, C.A.; Zhou, X.; Luan, X.; Diekwisch, T.G.; Atsawasuwan, P. Cyclic stretch and compression forces alter microRNA-29 expression of human periodontal ligament cells. Gene 2015, 566, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Quinodoz, S.; Guttman, M. Long noncoding RNAs: An emerging link between gene regulation and nuclear organization. Trends Cell Biol. 2014, 24, 651–663. [Google Scholar] [CrossRef]
- Liao, J.; Yu, X.; Hu, X.; Fan, J.; Wang, J.; Zhang, Z.; Zhao, C.; Zeng, Z.; Shu, Y.; Zhang, R.; et al. lncRNA H19 mediates BMP9-induced osteogenic differentiation of mesenchymal stem cells (MSCs) through Notch signaling. Oncotarget 2017, 8, 53581. [Google Scholar] [CrossRef]
- Buvinic, S.; Balanta-Melo, J.; Kupczik, K.; Vásquez, W.; Beato, C.; Toro-Ibacache, V. Muscle-bone crosstalk in the masticatory system: From biomechanical to molecular interactions. Front. Endocrinol. 2021, 11, 606947. [Google Scholar] [CrossRef]
- Maurel, D.B.; Jähn, K.; Lara-Castillo, N. Muscle–bone crosstalk: Emerging opportunities for novel therapeutic approaches to treat musculoskeletal pathologies. Biomedicines 2017, 5, 62. [Google Scholar] [CrossRef]
- Brotto, M.; Johnson, M.L. Endocrine crosstalk between muscle and bone. Curr. Osteoporos. Rep. 2014, 12, 135–141. [Google Scholar] [CrossRef]
- Brotto, M.; Bonewald, L. Bone and muscle: Interactions beyond mechanical. Bone 2015, 80, 109–114. [Google Scholar] [CrossRef]
- Kitase, Y.; Vallejo, J.A.; Gutheil, W.; Vemula, H.; Jähn, K.; Yi, J.; Zhou, J.; Brotto, M.; Bonewald, L.F. β-aminoisobutyric acid, l-BAIBA, is a muscle-derived osteocyte survival factor. Cell Rep. 2018, 22, 1531–1544. [Google Scholar] [CrossRef]
- Giudice, J.; Taylor, J.M. Muscle as a paracrine and endocrine organ. Curr. Opin. Pharmacol. 2017, 34, 49–55. [Google Scholar] [CrossRef]
- Bonewald, L. Use it or lose it to age: A review of bone and muscle communication. Bone 2019, 120, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Dankbar, B.; Fennen, M.; Brunert, D.; Hayer, S.; Frank, S.; Wehmeyer, C.; Beckmann, D.; Paruzel, P.; Bertrand, J.; Redlich, K.; et al. Myostatin is a direct regulator of osteoclast differentiation and its inhibition reduces inflammatory joint destruction in mice. Nat. Med. 2015, 21, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Peng, Y.; Zhao, W.; Pan, J.; Ksiezak-Reding, H.; Cardozo, C.; Wu, Y.; Pajevic, P.D.; Bonewald, L.F.; Bauman, W.A.; et al. Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: A novel mechanism in muscle-bone communication. J. Biol. Chem. 2017, 292, 11021–11033. [Google Scholar] [CrossRef] [PubMed]
- Goldspink, G. Mechanical signals, IGF-I gene splicing, and muscle adaptation. Physiology 2005, 20, 232–238. [Google Scholar] [CrossRef]
- Tokimasa, C.; Kawata, T.; Fujita, T.; Kaku, M.; Kohno, S.; Tsutsui, K.; Tenjou, K.; Ohtani, J.; Motokawa, M.; Tanne, K. Effects of insulin-like growth factor-I on the expression of osteoclasts and osteoblasts in the nasopremaxillary suture under different masticatory loading conditions in growing mice. Arch. Oral Biol. 2003, 48, 31–38. [Google Scholar] [CrossRef]
- Chowdhury, S.; Schulz, L.; Palmisano, B.; Singh, P.; Berger, J.M.; Yadav, V.K.; Mera, P.; Ellingsgaard, H.; Hidalgo, J.; Brüning, J.; et al. Muscle-derived interleukin 6 increases exercise capacity by signaling in osteoblasts. J. Clin. Investig. 2020, 130, 2888–2902. [Google Scholar] [CrossRef]
- Mo, C.; Romero-Suarez, S.; Bonewald, L.; Johnson, M.; Brotto, M. Prostaglandin E2: From clinical applications to its potential role in bone-muscle crosstalk and myogenic differentiation. Recent Pat. Biotechnol. 2012, 6, 223–229. [Google Scholar] [CrossRef]
- Bonnet, N.; Bourgoin, L.; Biver, E.; Douni, E.; Ferrari, S. RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J. Clin. Investig. 2023, 129, 3214–3223. [Google Scholar] [CrossRef]
- Huang, J.; Romero Suarez, S.; Lara, N.; Mo, C.; Kaja, S.; Brotto, L.; Dallas, S.L.; Johnson, M.L.; Jähn, K.; Bonewald, L.F.; et al. Crosstalk between MLO Y4 osteocytes and C2C12 muscle cells is mediated by the Wnt/β catenin pathway. J. Bone Miner. Res. Plus 2017, 1, 86–100. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Jiao, Y.; Liu, Y.; Guo, L. Role of Masticatory Force in Modulating Jawbone Immunity and Bone Homeostasis: A Review. Int. J. Mol. Sci. 2025, 26, 4478. https://doi.org/10.3390/ijms26104478
Song Y, Jiao Y, Liu Y, Guo L. Role of Masticatory Force in Modulating Jawbone Immunity and Bone Homeostasis: A Review. International Journal of Molecular Sciences. 2025; 26(10):4478. https://doi.org/10.3390/ijms26104478
Chicago/Turabian StyleSong, Yue, Yao Jiao, Yitong Liu, and Lijia Guo. 2025. "Role of Masticatory Force in Modulating Jawbone Immunity and Bone Homeostasis: A Review" International Journal of Molecular Sciences 26, no. 10: 4478. https://doi.org/10.3390/ijms26104478
APA StyleSong, Y., Jiao, Y., Liu, Y., & Guo, L. (2025). Role of Masticatory Force in Modulating Jawbone Immunity and Bone Homeostasis: A Review. International Journal of Molecular Sciences, 26(10), 4478. https://doi.org/10.3390/ijms26104478




