Single-Cell Transcriptomics Reveals Stem Cell-Derived Exosomes Attenuate Inflammatory Gene Expression in Pulmonary Oxygen Toxicity
Abstract
:1. Introduction
2. Results
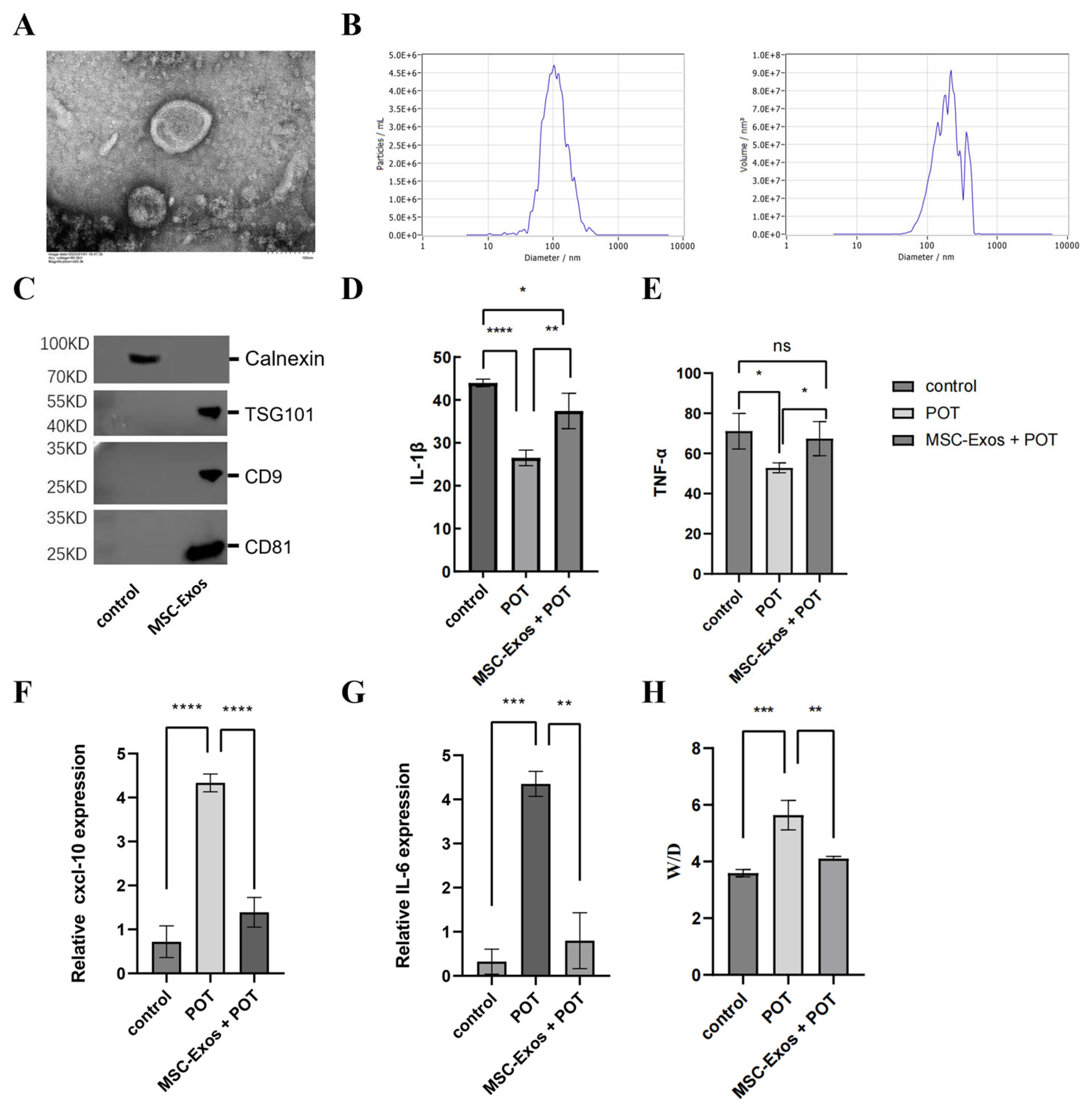
2.1. MSC-Exos Can Alleviate the Inflammatory Response Induced by a Model of Pulmonary Oxygen Toxicity
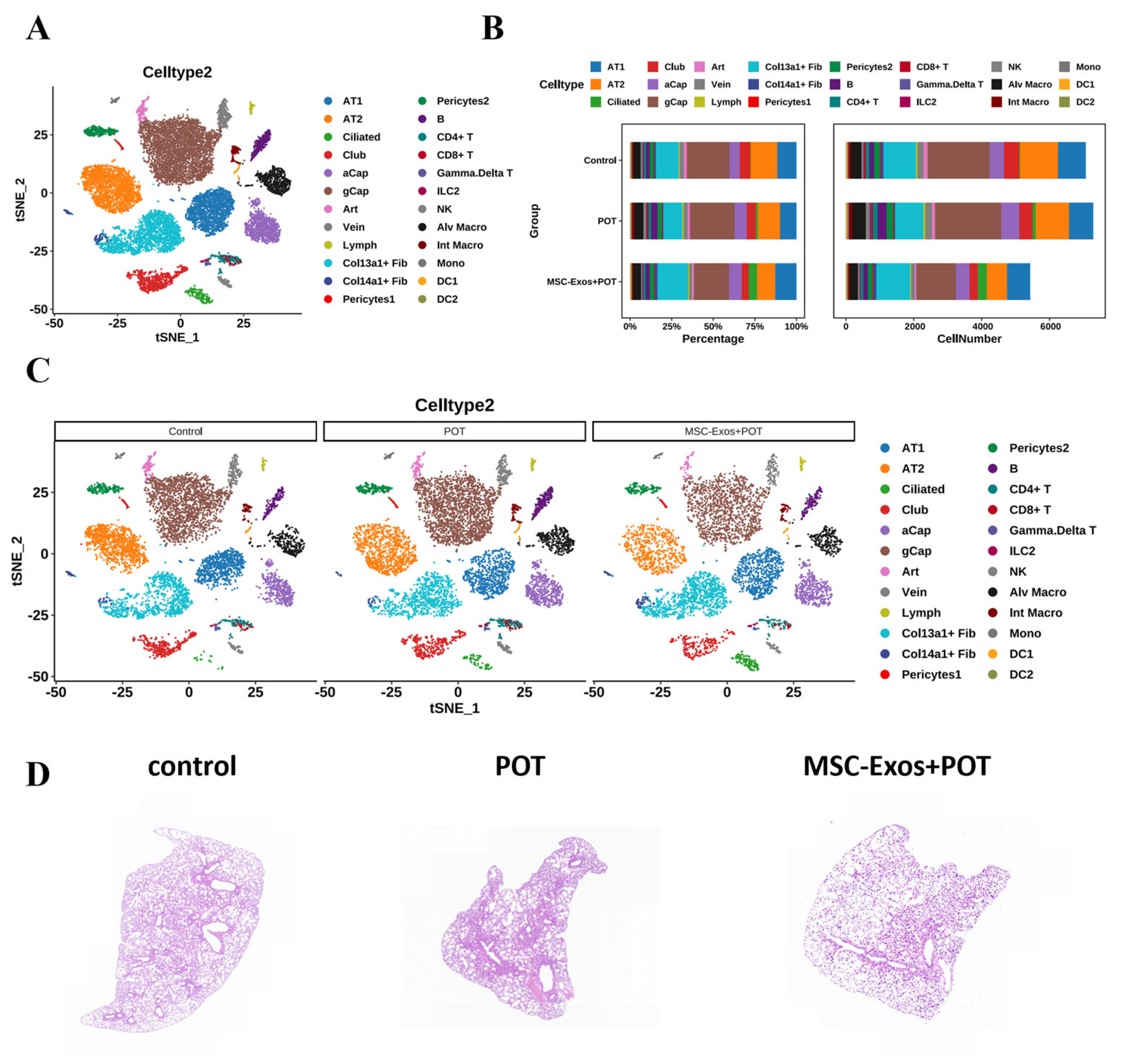
2.2. Interactions Among Cells in POT Fibrotic Niche After MSC-Exos Administration
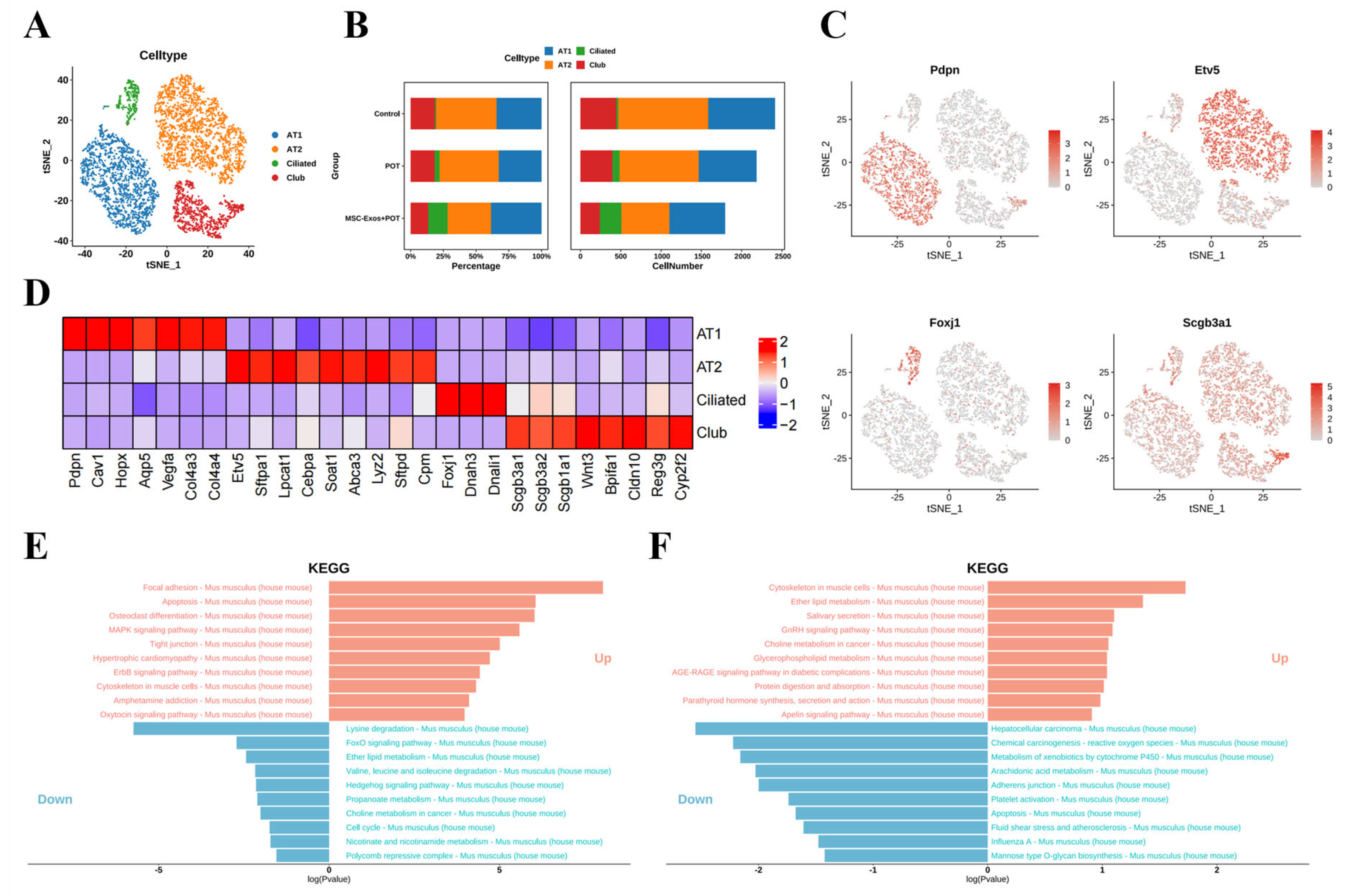
2.3. Exosome Treatment Alters AT1 Populations in Mouse Lungs
2.4. Stem Cell-Derived Exosomes Promote the Expression of Antioxidant Genes in Pulmonary Microvascular Endothelial Cells
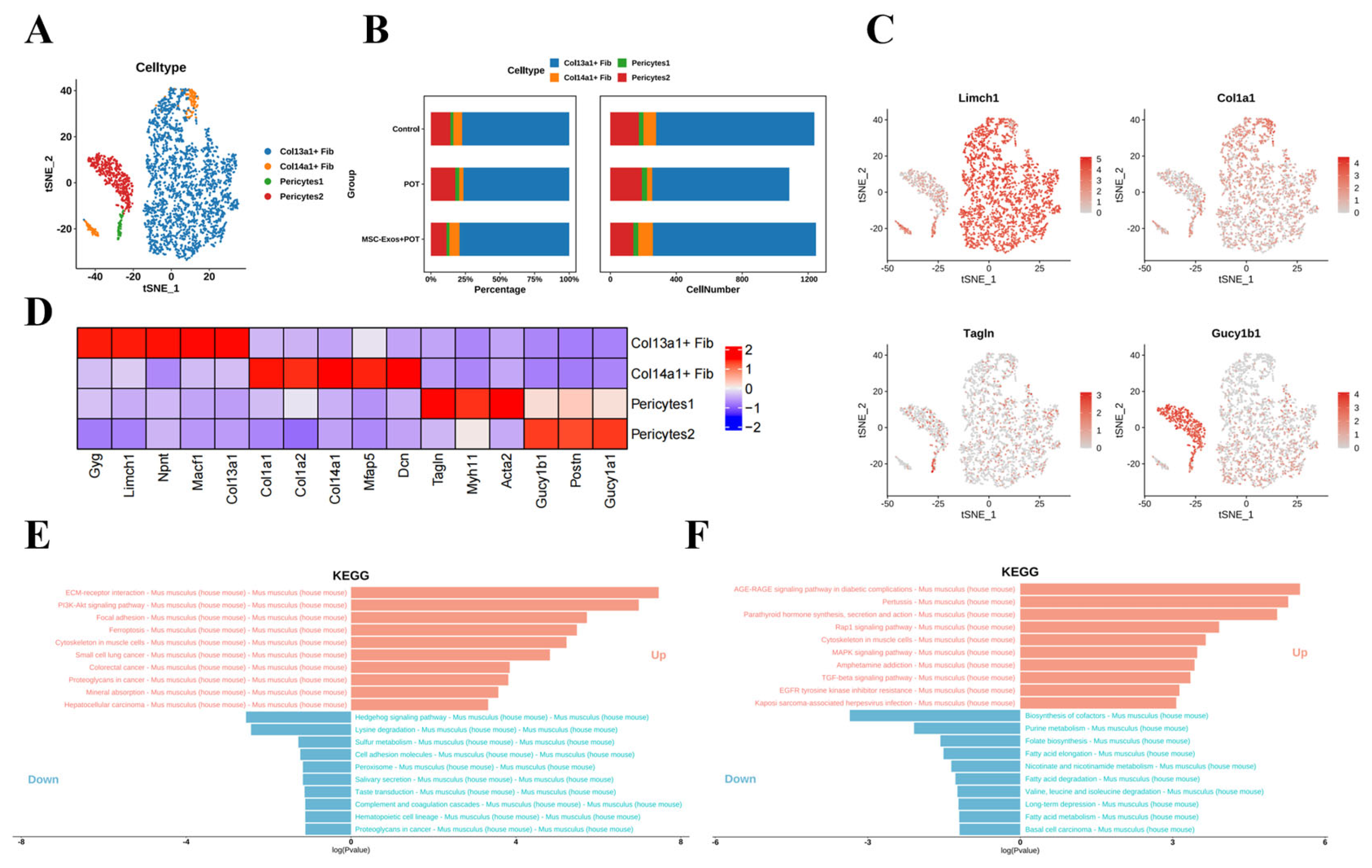
2.5. Stem Cell-Derived Exosome Therapy Induces Dramatic Shifts in Murine Lung Stromal Col13a1 + Fibroblasts and Pericyte Populations
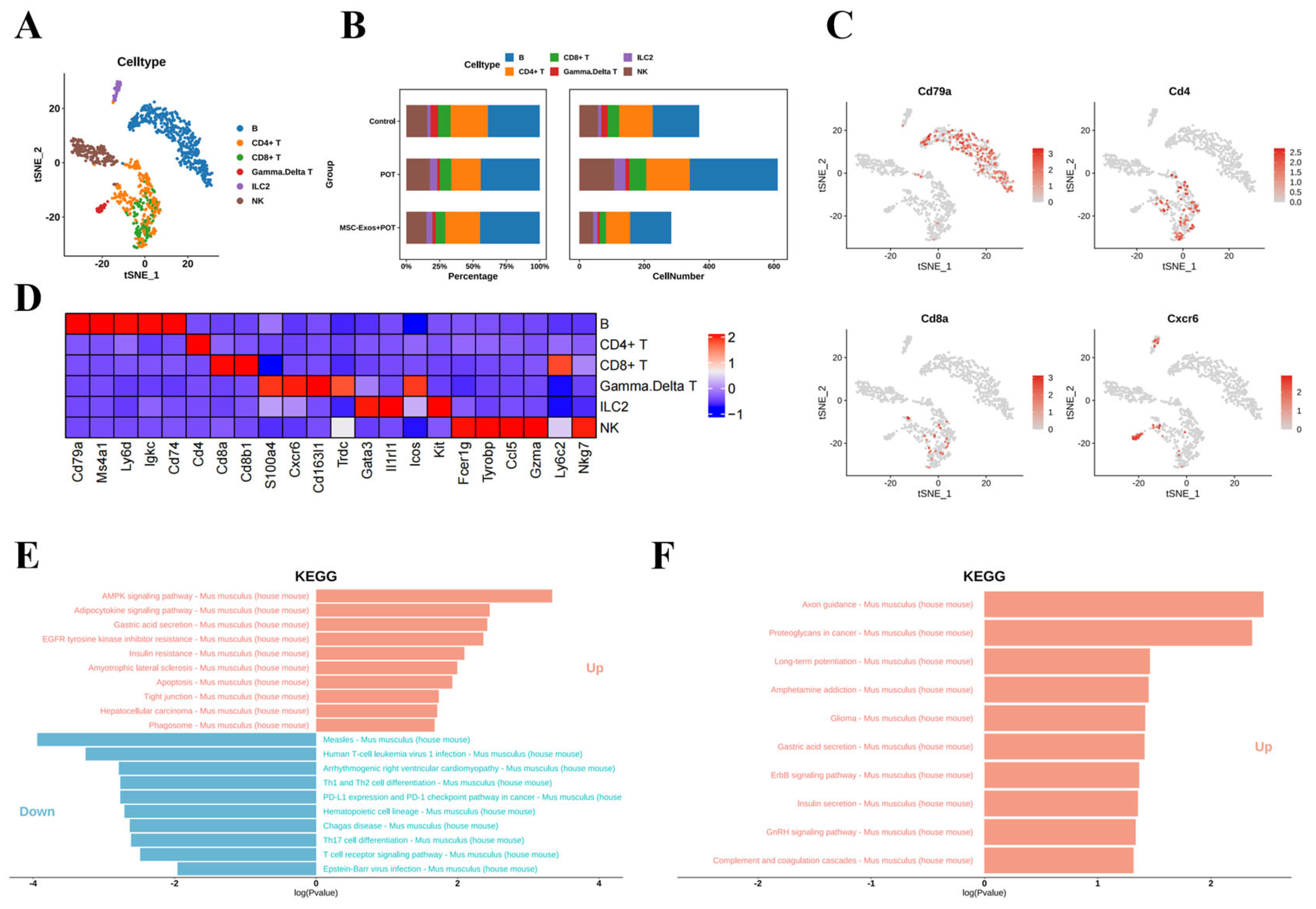
2.6. Stem Cell Exosome Treatment Inhibits Lymphoid NK Cells, B Cells, and CD8+ and CD4+ T Cells in Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Isolation and Characterization of Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes (hUMSC-Exos)
4.3. Characterization of Exosomes
4.4. Histological Analysis
4.5. Quantitative Real-Time Polymerase Chain Reaction (Qrt-PCR) Analysis
4.6. Western Blot Analysis
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Preparation of Single-Cell Suspensions
4.9. Data Quality Control and Preprocessing
4.10. Dimensionality Reduction and Clustering Analysis Based on Single-Cell Sequencing Data
4.11. Identification of Differentially Expressed Genes and Marker Genes
4.12. Cell Clustering Analysis
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arieli, R. The pulmonary oxygen toxicity index. Respir. Physiol. Neurobiol. 2023, 315, 104114. [Google Scholar] [CrossRef] [PubMed]
- Baik, A.H.; Haribowo, A.G.; Chen, X.; Queliconi, B.B.; Barrios, A.M.; Garg, A.; Maishan, M.; Campos, A.R.; Matthay, M.A.; Jain, I.H. Oxygen toxicity causes cyclic damage by destabilizing specific Fe-S cluster-containing protein complexes. Mol. Cell. 2023, 83, 942–960.e949. [Google Scholar] [CrossRef] [PubMed]
- De Jong, F.J.; Brinkman, P.; Wingelaar, T.T.; van Ooij, P.A.; van Hulst, R.A. Pulmonary oxygen toxicity breath markers after heliox diving to 81 metres. Diving Hyperb. Med. 2023, 53, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Alva, R.; Mirza, M.; Baiton, A.; Lazuran, L.; Samokysh, L.; Bobinski, A.; Cowan, C.; Jaimon, A.; Obioru, D.; Al Makhoul, T.; et al. Oxygen toxicity: Cellular mechanisms in normobaric hyperoxia. Cell Biol. Toxicol. 2023, 39, 111–143. [Google Scholar] [CrossRef]
- Hadanny, A.; Zubari, T.; Tamir-Adler, L.; Bechor, Y.; Fishlev, G.; Lang, E.; Polak, N.; Bergan, J.; Friedman, M.; Efrati, S. Hyperbaric oxygen therapy effects on pulmonary functions: A prospective cohort study. BMC Pulm. Med. 2019, 19, 148. [Google Scholar] [CrossRef]
- Hossain, T.; Secor, J.T.; Eckmann, D.M. Hyperbaric oxygen rapidly produces intracellular bioenergetics dysfunction in human pulmonary cells. Chem. Biol. Interact. 2024, 404, 111266. [Google Scholar] [CrossRef]
- Mokra, D.; Mikolka, P.; Kosutova, P.; Mokry, J. Corticosteroids in acute lung injury: The dilemma continues. Chem. Biol. Interact. 2019, 20, 4765. [Google Scholar] [CrossRef]
- Shahzad, T.; Chao, C.M.; Hadzic, S.; Behnke, J.; Biebach, L.; Böttcher-Friebertshäuser, E.; Wilhelm, J.; Hilgendorff, A.; Zimmer, K.-P.; Morty, R.E.; et al. TRAIL protects the immature lung from hyperoxic injury. Cell Death Dis. 2022, 13, 614. [Google Scholar] [CrossRef]
- Shykoff, B.E.; Florian, J.P. Pulmonary effects of repeated six-hour normoxic and hyperoxic dives. PLoS ONE 2018, 13, e0202892. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, J.; Tang, R.; Zeng, P.; Li, Z.; You, J.; Li, T.; Zhang, T.; Ma, X.; He, Y.; et al. Edible pueraria lobata-derived exosome-like nanovesicles ameliorate dextran sulfate sodium-induced colitis associated lung inflammation through modulating macrophage polarization. Biomed. Pharmacother. 2024, 170, 116098. [Google Scholar] [CrossRef]
- Dinh, P.C.; Paudel, D.; Brochu, H.; Popowski, K.D.; Gracieux, M.C.; Cores, J.; Huang, K.; Hensley, M.T.; Harrell, E.; Vandergriff, A.C.; et al. Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis. Nat. Commun. 2020, 11, 1064. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Li, J.; Zhang, C. The role of MSCs and CAR-MSCs in cellular immunotherapy. Cell Commun. Signal. 2023, 21, 187. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Cui, T.; Chen, J.; Mao, X.; Zhang, T. Advancements in engineered mesenchymal stem cell exosomes for chronic lung disease treatment. J. Transl. Med. 2023, 21, 895. [Google Scholar] [CrossRef]
- Cheng, T.; Mao, M.; Liu, Y.; Xie, L.; Shi, F.; Liu, H.; Li, X. The potential therapeutic effect of human umbilical cord mesenchymal stem cell-derived exosomes in bronchopulmonary dysplasia. Life Sci. 2024, 357, 123047. [Google Scholar] [CrossRef]
- Maremanda, K.P.; Sundar, I.K.; Rahman, I. Protective role of mesenchymal stem cells and mesenchymal stem cell-derived exosomes in cigarette smoke-induced mitochondrial dysfunction in mice. Toxicol. Appl. Pharmacol. 2019, 385, 114788. [Google Scholar] [CrossRef]
- Montoro, D.T.; Haber, A.L.; Biton, M.; Vinarsky, V.; Lin, B.; Birket, S.E.; Yuan, F.; Chen, S.; Leung, H.M.; Villoria, J.; et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 2018, 560, 319–324. [Google Scholar] [CrossRef]
- Choi, Y.; Rekers, L.; Dong, Y.; Holzfurtner, L.; Goetz, M.J.; Shahzad, T.; Zimmer, K.-P.; Behnke, J.; Behnke, J.; Bellusci, S.; et al. Oxygen Toxicity to the Immature Lung-Part I: Pathomechanistic Understanding and Preclinical Perspectives. Int. J. Mol. Sci. 2021, 22, 11006. [Google Scholar] [CrossRef]
- Willis, G.R.; Fernandez-Gonzalez, A.; Anastas, J.; Vitali, S.H.; Liu, X.; Ericsson, M.; Kwong, A.; Mitsialis, S.A.; Kourembanas, S. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am. J. Respir. Crit. Care Med. 2018, 197, 104–116. [Google Scholar] [CrossRef]
- Kumar, S.; Mickael, C.; Kumar, R.; Prasad, R.R.; Campbell, N.V.; Zhang, H.; Li, M.; McKeon, B.A.; Allen, T.E.; Graham, B.B.; et al. Single cell transcriptomic analyses reveal diverse and dynamic changes of distinct populations of lung interstitial macrophages in hypoxia-induced pulmonary hypertension. Front. Immunol. 2024, 15, 1372959. [Google Scholar] [CrossRef]
- Feng, B.; Feng, X.; Yu, Y.; Xu, H.; Ye, Q.; Hu, R.; Fang, X.; Gao, F.; Wu, J.; Pan, Q.; et al. Mesenchymal stem cells shift the pro-inflammatory phenotype of neutrophils to ameliorate acute lung injury. Stem. Cell Res. Ther. 2023, 14, 197. [Google Scholar] [CrossRef]
- Joshi, N.; Watanabe, S.; Verma, R.; Jablonski, R.P.; Chen, C.I.; Cheresh, P.; Markov, N.S.; Reyfman, P.A.; McQuattie-Pimentel, A.C.; Sichizya, L.; et al. A spatially restricted fibrotic niche in pulmonary fibrosis is sustained by M-CSF/M-CSFR signalling in monocyte-derived alveolar macrophages. Eur. Respir. J. 2020, 55, 1900646. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.S.; Schupp, J.C.; Poli, S.; Ayaub, E.A.; Neumark, N.; Ahangari, F.; Chu, S.G.; Raby, B.A.; DeIuliis, G.; Januszyk, M.; et al. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci. Adv. 2020, 6, eaba1983. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Castaldi, A.; Sunohara, M.; Wang, H.; Ji, Y.; Liu, Y.; Li, F.; Wilkinson, T.A.; Hung, L.; Shen, H.; et al. Integrated Single-Cell RNA-Sequencing Analysis of Aquaporin 5-Expressing Mouse Lung Epithelial Cells Identifies GPRC5A as a Novel Validated Type I Cell Surface Marker. Cells 2020, 9, 2460. [Google Scholar] [CrossRef] [PubMed]
- Kesimer, M.; Ford, A.A.; Ceppe, A.; Radicioni, G.; Cao, R.; Davis, C.W.; Doerschuk, C.M.; Alexis, N.E.; Anderson, W.H.; Henderson, A.G.; et al. Airway Mucin Concentration as a Marker of Chronic Bronchitis. N. Engl. J. Med. 2017, 377, 911–922. [Google Scholar] [CrossRef]
- Bonser, L.R.; Zlock, L.; Finkbeiner, W.; Erle, D.J. Epithelial tethering of MUC5AC-rich mucus impairs mucociliary transport in asthma. J. Clin. Investig. 2016, 126, 2367–2371. [Google Scholar] [CrossRef]
- Xu, Y.; Mizuno, T.; Sridharan, A.; Du, Y.; Guo, M.; Tang, J.; Wikenheiser-Brokamp, K.A.; Perl, A.-K.T.; Funari, V.A.; Gokey, J.J.; et al. Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight 2016, 1, e90558. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem. Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef]
- Riazifar, M.; Pone, E.J.; Lötvall, J.; Zhao, W. Stem Cell Extracellular Vesicles: Extended Messages of Regeneration. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 125–154. [Google Scholar] [CrossRef]
- Lai, R.C.; Yeo, R.W.; Tan, K.H.; Lim, S.K. Exosomes for drug delivery—A novel application for the mesenchymal stem cell. Biotechnol. Adv. 2013, 31, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Su, C. Design strategies and application progress of therapeutic exosomes. Theranostics 2019, 9, 1015–1028. [Google Scholar] [CrossRef] [PubMed]
- Lou, G.; Chen, Z.; Zheng, M.; Liu, Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp. Mol. Med. 2017, 49, e346. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Tan, Z.; Chen, H.; Gao, Y.; Xia, J.; Huang, T.; Liang, L.; Zhang, J.; Zhang, X.; Shi, X.; et al. Integrative analysis of bulk and single-cell RNA sequencing reveals the gene expression profile and the critical signaling pathways of type II CPAM. Cell Biosci. 2024, 14, 94. [Google Scholar] [CrossRef]
- Chen, J.; Ma, S.; Luo, B.; Hao, H.; Li, Y.; Yang, H.; Zhu, F.; Zhang, P.; Niu, R.; Pan, P. Human umbilical cord mesenchymal stromal cell small extracellular vesicle transfer of microRNA-223-3p to lung epithelial cells attenuates inflammation in acute lung injury in mice. J. Nanobiotechnology 2023, 21, 295. [Google Scholar] [CrossRef]
- Wang, J.; Yeung, B.Z.; Cui, M.; Peer, C.J.; Lu, Z.; Figg, W.D.; Wientjes, M.G.; Woo, S.; Au, J.L.-S. Exosome is a mechanism of intercellular drug transfer: Application of quantitative pharmacology. J. Control. Release 2017, 268, 147–158. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, Y.; Chu, J.; Zhang, X.; Xu, C.; Liu, S.; Wan, Z.; Wang, J.; Zhang, L.; Liu, K.; et al. Anti-EGFR ScFv functionalized exosomes delivering LPCAT1 specific siRNAs for inhibition of lung cancer brain metastases. J. Nanobiotechnology 2024, 22, 159. [Google Scholar] [CrossRef]
- Olbrecht, S.; Busschaert, P.; Qian, J.; Vanderstichele, A.; Loverix, L.; Van Gorp, T.; Van Nieuwenhuysen, E.; Han, S.; Van den Broeck, A.; Coosemans, A.; et al. High-grade serous tubo-ovarian cancer refined with single-cell RNA sequencing: Specific cell subtypes influence survival and determine molecular subtype classification. Genome Med. 2021, 13, 111. [Google Scholar] [CrossRef]
- Gu, M.; He, T.; Yuan, Y.; Duan, S.; Li, X.; Shen, C. Single-Cell RNA Sequencing Reveals Multiple Pathways and the Tumor Microenvironment Could Lead to Chemotherapy Resistance in Cervical Cancer. Front. Oncol. 2021, 11, 753386. [Google Scholar] [CrossRef]
- Wang, X.; Feng, J.; Dai, H.; Mo, J.; Luo, B.; Luo, C.; Zhang, W.; Pan, L. MicroRNA-130b-3p delivery by mesenchymal stem cell-derived exosomes confers protection on acute lung injury. Autoimmunity 2022, 55, 597–607. [Google Scholar] [CrossRef]
- Lee, A.J.; Jung, I. Functional annotation of lung cancer-associated genetic variants by cell type—specific epigenome and long-range chromatin interactome. Genom. Inform. 2021, 19, e3. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Li, Y.; Zhao, H.; Yan, C.; Cui, R.; Wen, Y.; Yu, X.; Ding, W.; Zhao, Y.; Fang, Y. Single-Cell Transcriptomics Reveals Stem Cell-Derived Exosomes Attenuate Inflammatory Gene Expression in Pulmonary Oxygen Toxicity. Int. J. Mol. Sci. 2025, 26, 4462. https://doi.org/10.3390/ijms26094462
Shi J, Li Y, Zhao H, Yan C, Cui R, Wen Y, Yu X, Ding W, Zhao Y, Fang Y. Single-Cell Transcriptomics Reveals Stem Cell-Derived Exosomes Attenuate Inflammatory Gene Expression in Pulmonary Oxygen Toxicity. International Journal of Molecular Sciences. 2025; 26(9):4462. https://doi.org/10.3390/ijms26094462
Chicago/Turabian StyleShi, Jing, Yabin Li, Houyu Zhao, Chenyang Yan, Ruxia Cui, Yukun Wen, Xuhua Yu, Wei Ding, Yunpeng Zhao, and Yiqun Fang. 2025. "Single-Cell Transcriptomics Reveals Stem Cell-Derived Exosomes Attenuate Inflammatory Gene Expression in Pulmonary Oxygen Toxicity" International Journal of Molecular Sciences 26, no. 9: 4462. https://doi.org/10.3390/ijms26094462
APA StyleShi, J., Li, Y., Zhao, H., Yan, C., Cui, R., Wen, Y., Yu, X., Ding, W., Zhao, Y., & Fang, Y. (2025). Single-Cell Transcriptomics Reveals Stem Cell-Derived Exosomes Attenuate Inflammatory Gene Expression in Pulmonary Oxygen Toxicity. International Journal of Molecular Sciences, 26(9), 4462. https://doi.org/10.3390/ijms26094462





