Scorpion Venom Heat-Resistant Synthetic Peptide Alleviates Neuronal Necroptosis in Alzheimer’s Disease Model by Regulating Lnc Gm6410 Under PM2.5 Exposure
Abstract
1. Introduction
2. Results
2.1. PM2.5 Component Analysis
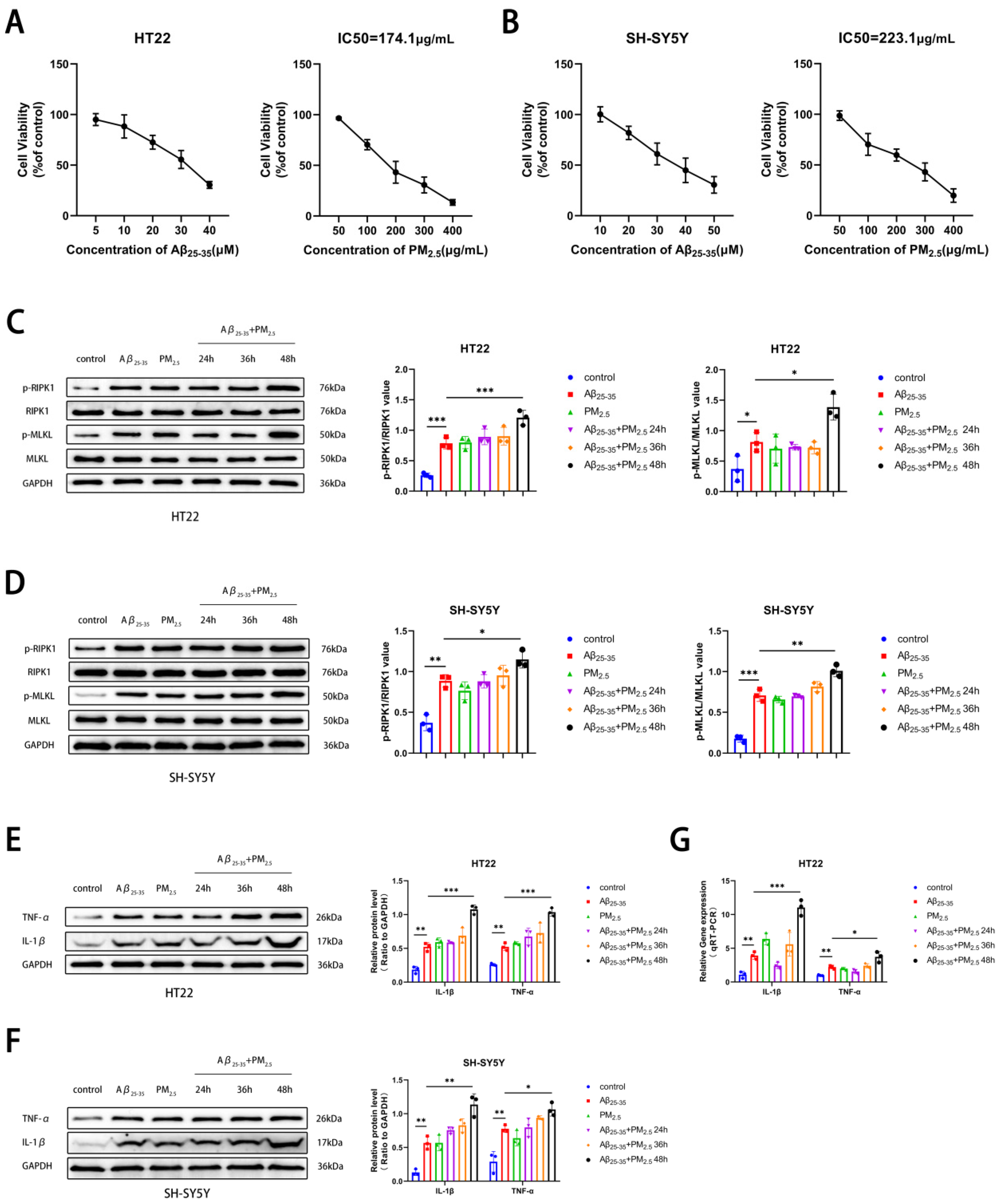
2.2. PM2.5 Exposure Promoted the Necroptosis of AD Neuron Cells
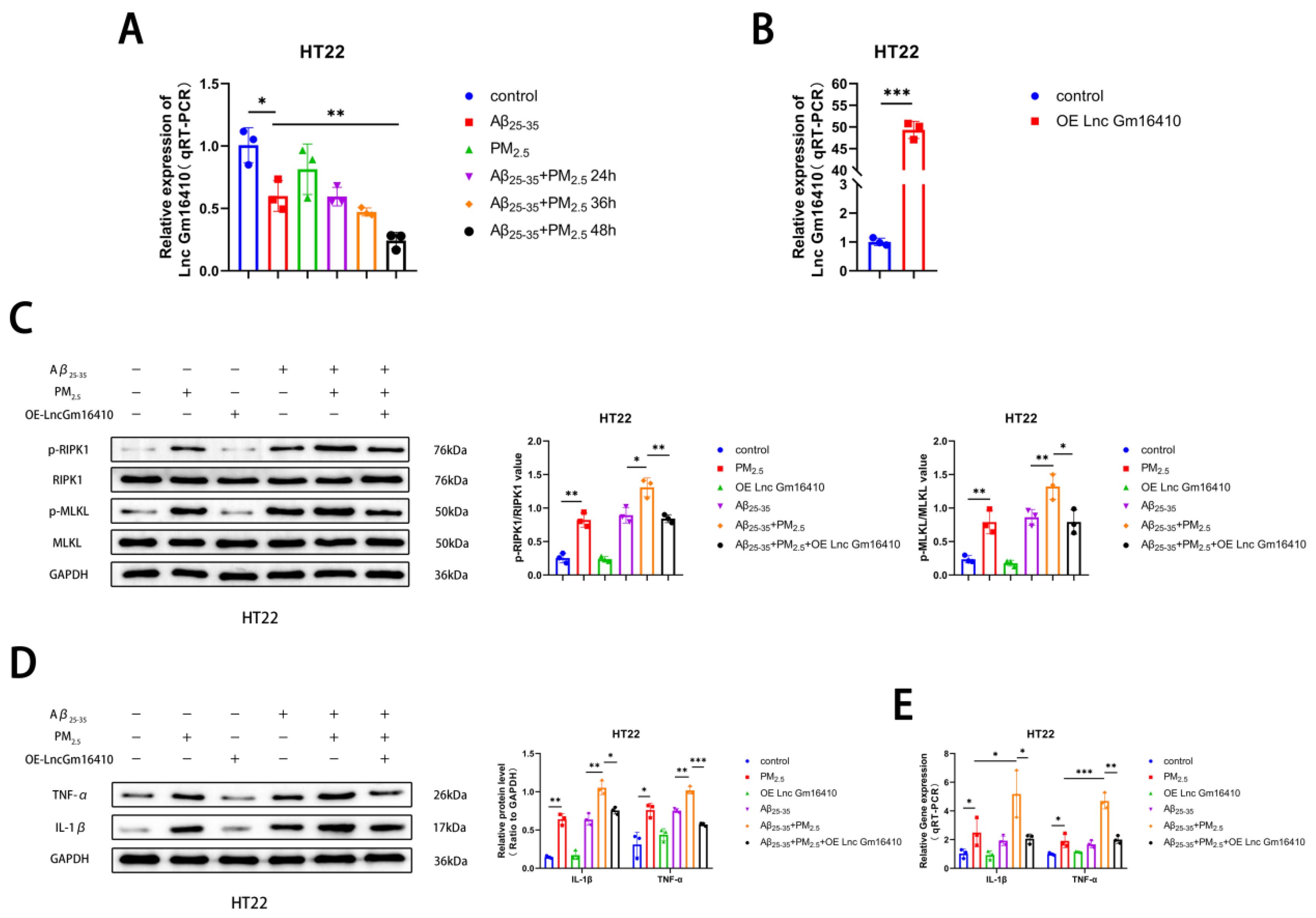
2.3. Lnc Gm16410 Plays a Role in the Regulation of Neuronal Necroptosis in AD Following Exposure to PM2.5
2.4. Lnc Gm16410 Is Involved in the Regulation of Neuronal Necroptosis in AD Under PM2.5 Exposure via p38 MAPK Pathway
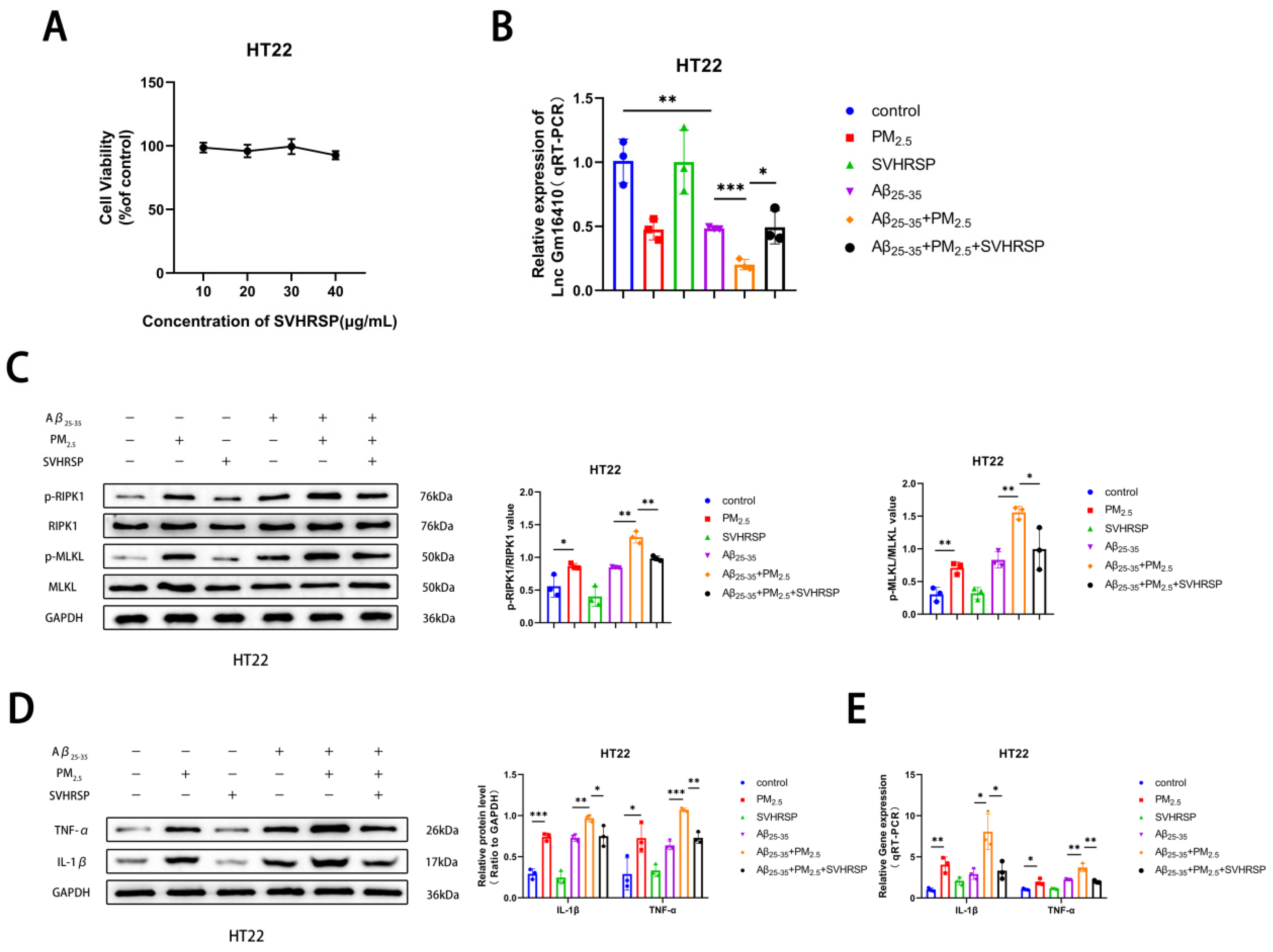
2.5. SVHRSP Alleviates Neuronal Necroptosis in AD Under PM2.5 Exposure by Lnc Gm16410
2.6. SVHRSP Alleviates Cognitive Impairment in AD Mice Exposed to PM2.5
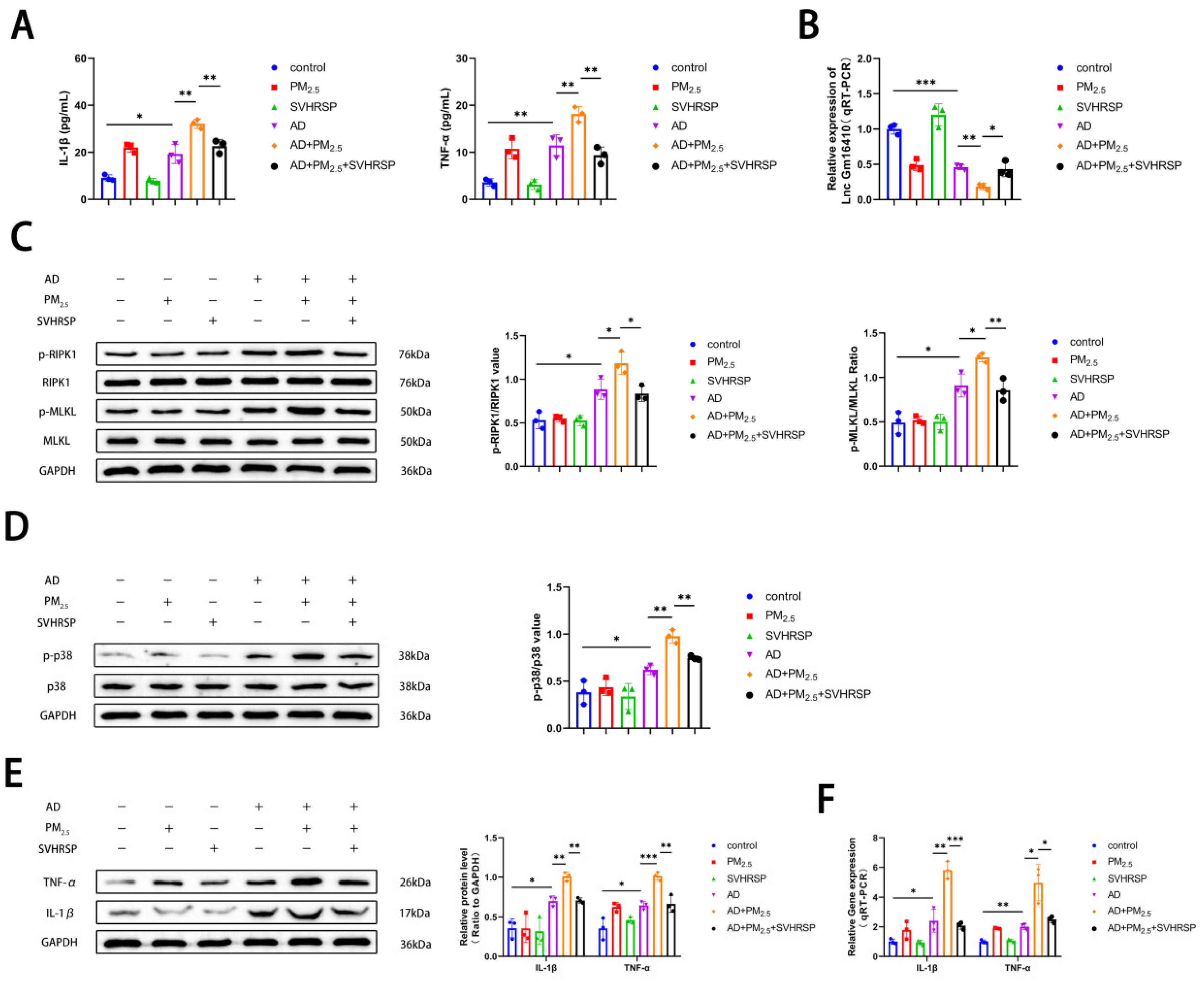
2.7. SVHRSP Regulates the Expression of Lnc Gm16410 and Necroptosis in AD Mice Exposed to PM2.5
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Reagents
5.2. Extraction and Preparation of PM2.5 Samples
5.3. Preparation and Processing of Aβ Protein
5.4. Preparation of SVHRSP
5.5. Animal Experiments and Experiment Design
5.6. Cell Culture
5.7. CCK8
5.8. Western Blot
5.9. RNA Extraction and Real-Time PCR
5.10. Morris Water Maze
5.11. HE Staining
5.12. Nissl Staining
5.13. ELISA
5.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Münzel, T.; Gori, T.; Al-Kindi, S.; Deanfield, J.; Lelieveld, J.; Daiber, A.; Rajagopalan, S. Effects of gaseous and solid constituents of air pollution on endothelial function. Eur. Heart J. 2018, 39, 3543–3550. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S.; Park, B.; Palanivel, R.; Vinayachandran, V.; Deiuliis, J.A.; Gangwar, R.S.; Das, L.; Yin, J.; Choi, Y.; Al-Kindi, S.; et al. Metabolic effects of air pollution exposure and reversibility. J. Clin. Investig. 2020, 130, 6034–6040. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G. Cut particulate air pollution, save lives. Bmj 2021, 375, n2561. [Google Scholar] [CrossRef]
- Goobie, G.C.; Carlsten, C.; Johannson, K.A.; Khalil, N.; Marcoux, V.; Assayag, D.; Manganas, H.; Fisher, J.H.; Kolb, M.R.J.; Lindell, K.O.; et al. Association of Particulate Matter Exposure With Lung Function and Mortality Among Patients With Fibrotic Interstitial Lung Disease. JAMA Intern. Med. 2022, 182, 1248–1259. [Google Scholar] [CrossRef]
- Beentjes, D.; Shears, R.K.; French, N.; Neill, D.R.; Kadioglu, A. Mechanistic Insights into the Impact of Air Pollution on Pneumococcal Pathogenesis and Transmission. Am. J. Respir. Crit. Care Med. 2022, 206, 1070–1080. [Google Scholar] [CrossRef]
- Yue, D.; Zhang, Q.; Zhang, J.; Liu, W.; Chen, L.; Wang, M.; Li, R.; Qin, S.; Song, X.; Ji, Y. Diesel exhaust PM2.5 greatly deteriorates fibrosis process in pre-existing pulmonary fibrosis via ferroptosis. Environ. Int. 2023, 171, 107706. [Google Scholar] [CrossRef] [PubMed]
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R.; et al. European Society of Cardiology: Cardiovascular disease statistics 2021. Eur. Heart J. 2022, 43, 716–799. [Google Scholar] [CrossRef]
- Cory-Slechta, D.A.; Sobolewski, M. Neurotoxic effects of air pollution: An urgent public health concern. Nat. Rev. Neurosci. 2023, 24, 129–130. [Google Scholar] [CrossRef]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Primers. 2021, 7, 33. [Google Scholar] [CrossRef]
- GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef]
- Twarowski, B.; Herbet, M. Inflammatory Processes in Alzheimer’s Disease-Pathomechanism, Diagnosis and Treatment: A Review. Int. J. Mol. Sci. 2023, 24, 6518. [Google Scholar] [CrossRef] [PubMed]
- Martens, Y.A.; Zhao, N.; Liu, C.C.; Kanekiyo, T.; Yang, A.J.; Goate, A.M.; Holtzman, D.M.; Bu, G. ApoE Cascade Hypothesis in the pathogenesis of Alzheimer’s disease and related dementias. Neuron 2022, 110, 1304–1317. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, W.; Ming, C.; Gao, X.; Yuan, H.; Lin, X.; Mao, X.; Wang, C.; Guo, X.; Du, Y.; et al. P-tau217 correlates with neurodegeneration in Alzheimer’s disease, and targeting p-tau217 with immunotherapy ameliorates murine tauopathy. Neuron 2024, 112, 1676–1693.e1612. [Google Scholar] [CrossRef] [PubMed]
- Naderi Yeganeh, P.; Kwak, S.S.; Jorfi, M.; Koler, K.; Kalatturu, T.; von Maydell, D.; Liu, Z.; Guo, K.; Choi, Y.; Park, J.; et al. Integrative pathway analysis across humans and 3D cellular models identifies the p38 MAPK-MK2 axis as a therapeutic target for Alzheimer’s disease. Neuron 2025, 113, 205–224.e208. [Google Scholar] [CrossRef]
- Migliore, L.; Coppedè, F. Gene-environment interactions in Alzheimer disease: The emerging role of epigenetics. Nat. Rev. Neurol. 2022, 18, 643–660. [Google Scholar] [CrossRef]
- Shi, L.; Wu, X.; Danesh Yazdi, M.; Braun, D.; Abu Awad, Y.; Wei, Y.; Liu, P.; Di, Q.; Wang, Y.; Schwartz, J.; et al. Long-term effects of PM(2·5) on neurological disorders in the American Medicare population: A longitudinal cohort study. Lancet Planet Health 2020, 4, e557–e565. [Google Scholar] [CrossRef]
- Sims, J.R.; Zimmer, J.A.; Evans, C.D.; Lu, M.; Ardayfio, P.; Sparks, J.; Wessels, A.M.; Shcherbinin, S.; Wang, H.; Monkul Nery, E.S.; et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. J. Am. Med. Assoc. 2023, 330, 512–527. [Google Scholar] [CrossRef] [PubMed]
- Congdon, E.E.; Ji, C.; Tetlow, A.M.; Jiang, Y.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease: Current status and future directions. Nat. Rev. Neurol. 2023, 19, 715–736. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Andrews, J.S.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E.; et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimers Dement. 2024, 20, 5143–5169. [Google Scholar] [CrossRef]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef]
- Ai, Y.; Meng, Y.; Yan, B.; Zhou, Q.; Wang, X. The biochemical pathways of apoptotic, necroptotic, pyroptotic, and ferroptotic cell death. Mol. Cell 2024, 84, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Zhang, J.; Jiang, X.; Yang, Y.; Shan, B.; Zhang, M.; Liu, C.; Yuan, J.; Xu, D. PARP5A and RNF146 phase separation restrains RIPK1-dependent necroptosis. Mol. Cell 2024, 84, 938–954.e938. [Google Scholar] [CrossRef] [PubMed]
- Lyu, P.; Wen, J.; Zhang, W.; Liu, N.; Stolzer, I.; Gießl, A.; Jia, Y.; Mauro, D.; Zhang, F.; Ciccia, F.; et al. Expression of HIF1α in intestinal epithelium restricts arthritis inflammation by inhibiting RIPK3-induced cell death machinery. Ann. Rheum. Dis. 2024, 83, 984–997. [Google Scholar] [CrossRef]
- Hänggi, K.; Li, J.; Gangadharan, A.; Liu, X.; Celias, D.P.; Osunmakinde, O.; Keske, A.; Davis, J.; Ahmad, F.; Giron, A.; et al. Interleukin-1α release during necrotic-like cell death generates myeloid-driven immunosuppression that restricts anti-tumor immunity. Cancer Cell 2024, 42, 2015–2031.e2011. [Google Scholar] [CrossRef]
- Takezaki, A.; Tsukumo, S.I.; Setoguchi, Y.; Ledford, J.G.; Goto, H.; Hosomichi, K.; Uehara, H.; Nishioka, Y.; Ya-sutomo, K. A homozygous SFTPA1 mutation drives necroptosis of type II alveolar epithelial cells in patients with idiopathic pulmonary fibrosis. J. Exp. Med. 2019, 216, 2724–2735. [Google Scholar] [CrossRef]
- Li, N.; Xiong, R.; Li, G.; Wang, B.; Geng, Q. PM2.5 contributed to pulmonary epithelial senescence and ferroptosis by regulating USP3-SIRT3-P53 axis. Free. Radic. Biol. Med. 2023, 205, 291–304. [Google Scholar] [CrossRef]
- Fan, X.; Dong, T.; Yan, K.; Ci, X.; Peng, L. PM2.5 increases susceptibility to acute exacerbation of COPD via NOX4/Nrf2 redox imbalance-mediated mitophagy. Redox Biol. 2023, 59, 102587. [Google Scholar] [CrossRef]
- Fu, X.; Hong, W.; Li, S.; Chen, Z.; Zhou, W.; Dai, J.; Deng, X.; Zhou, H.; Li, B.; Ran, P. Wood smoke particulate matter (WSPM2.5) induces pyroptosis through both Caspase-1/IL-1β/IL-18 and ATP/P2Y-dependent mechanisms in human bronchial epithelial cells. Chemosphere 2022, 307, 135726. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef]
- Ferrer, J.; Dimitrova, N. Transcription regulation by long non-coding RNAs: Mechanisms and disease relevance. Nat. Rev. Mol. Cell Biol. 2024, 25, 396–415. [Google Scholar] [CrossRef]
- Herman, A.B.; Tsitsipatis, D.; Gorospe, M. Integrated lncRNA function upon genomic and epigenomic regulation. Mol. Cell 2022, 82, 2252–2266. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Zhang, L.; Min, K.W.; Cho, J.H.; Yeh, C.C.; Moon, H.; Hormaechea-Agulla, D.; Mun, H.; Ko, S.; Lee, J.W.; et al. NEAT1 is essential for metabolic changes that promote breast cancer growth and metastasis. Cell Metab. 2021, 33, 2380–2397.e2389. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Wu, S.; Feng, X.; Wang, B.; Xia, S.; Liang, L.; Zhang, L.; Govindarajalu, G.; Bunk, A.; Kadakia, F.; et al. A nerve injury-specific long noncoding RNA promotes neuropathic pain by increasing Ccl2 expression. J. Clin. Investig. 2022, 132, e153563. [Google Scholar] [CrossRef]
- He, X.; Yang, T.; Lu, Y.W.; Wu, G.; Dai, G.; Ma, Q.; Zhang, M.; Zhou, H.; Long, T.; Yan, Y.; et al. The long noncoding RNA CARDINAL attenuates cardiac hypertrophy by modulating protein translation. J. Clin. Investig. 2024, 134, e169112. [Google Scholar] [CrossRef]
- Xu, J.; Xu, H.; Ma, K.; Wang, Y.; Niu, B.; Zhang, L.; Li, F. lncRNA Gm16410 Mediates PM2.5-Induced Macrophage Activation via PI3K/AKT Pathway. Front. Cell Dev. Biol. 2021, 9, 618045. [Google Scholar] [CrossRef]
- Ma, K.; Li, C.; Xu, J.; Ren, F.; Xu, X.; Liu, C.; Niu, B.; Li, F. LncRNA Gm16410 regulates PM(2.5)-induced lung Endothelial-Mesenchymal Transition via the TGF-β1/Smad3/p-Smad3 pathway. Ecotoxicol. Environ. Saf. 2020, 205, 111327. [Google Scholar] [CrossRef]
- Sui, A.R.; Piao, H.; Xiong, S.T.; Zhang, P.; Guo, S.Y.; Kong, Y.; Gao, C.Q.; Wang, Z.X.; Yang, J.; Ge, B.Y.; et al. Scorpion venom heat-resistant synthesized peptide ameliorates epileptic seizures and imparts neuroprotection in rats mediated by NMDA receptors. Eur. J. Pharmacol. 2024, 978, 176704. [Google Scholar] [CrossRef]
- Xia, Z.; He, D.; Wu, Y.; Kwok, H.F.; Cao, Z. Scorpion venom peptides: Molecular diversity, structural characteristics, and therapeutic use from channelopathies to viral infections and cancers. Pharmacol. Res. 2023, 197, 106978. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Li, N.; Li, D.; Sui, A.; Khan, K.; Ge, B.; Li, S.; Li, S.; Zhao, J. Scorpion venom heat-resistant synthesized peptide ameliorates 6-OHDA-induced neurotoxicity and neuroinflammation: Likely role of Na(v) 1.6 inhibition in microglia. Br. J. Pharmacol. 2021, 178, 3553–3569. [Google Scholar] [CrossRef]
- Zhang, X.; Tu, D.; Li, S.; Li, N.; Li, D.; Gao, Y.; Tian, L.; Liu, J.; Zhang, X.; Hong, J.S.; et al. A novel synthetic peptide SVHRSP attenuates dopaminergic neurodegeneration by inhibiting NADPH oxidase-mediated neuroinflammation in experimental models of Parkinson’s disease. Free Radic. Biol. Med. 2022, 188, 363–374. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Guo, S.; Li, Q.; Kong, Y.; Sui, A.; Ma, J.; Lu, L.; Zhao, J.; Li, S. SVHRSP Alleviates Age-Related Cognitive Deficiency by Reducing Oxidative Stress and Neuroinflammation. Antioxidants 2024, 13, 628. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, H.; Guan, B.; Zhang, Y.; Qin, C.; Li, D.; Zhang, J.; Zhang, B.; Lin, Y.; Li, F. CircCDR1as orchestrates the advancement of asthma triggered by PM(2.5) through the modulation of ferroptosis. Sci. Total Environ. 2024, 950, 175328. [Google Scholar] [CrossRef]
- Thiankhaw, K.; Chattipakorn, N.; Chattipakorn, S.C. PM2.5 exposure in association with AD-related neuropathology and cognitive outcomes. Environ. Pollut. 2022, 292, 118320. [Google Scholar] [CrossRef] [PubMed]
- Fortier, S.M.; Walker, N.M.; Penke, L.R.; Baas, J.D.; Shen, Q.; Speth, J.M.; Huang, S.K.; Zemans, R.L.; Bennett, A.M.; Peters-Golden, M. MAPK phosphatase 1 inhibition of p38α within lung myofibroblasts is essential for spontaneous fibrosis resolution. J. Clin. Investig. 2024, 134, e172826. [Google Scholar] [CrossRef]
- Folgueira, C.; Herrera-Melle, L.; López, J.A.; Galvan-Alvarez, V.; Martin-Rincon, M.; Cuartero, M.I.; García-Culebras, A.; Dumesic, P.A.; Rodríguez, E.; Leiva-Vega, L.; et al. Remodeling p38 signaling in muscle controls locomotor activity via IL-15. Sci. Adv. 2024, 10, eadn5993. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xu, J.; Duan, K.; Bao, T.; Cheng, Y.; Zhang, H.; Zhang, Y.; Lin, Y.; Li, F. Scorpion venom heat-resistant peptide alleviates mitochondrial dynamics imbalance induced by PM(2.5) exposure by downregulating the PGC-1α/SIRT3 signaling pathway. Toxicol. Res. 2023, 12, 756–764. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Tuffier, S.; Zhang, J.; Bergmann, M.; So, R.; Napolitano, G.M.; Cole-Hunter, T.; Maric, M.; Antic, S.; Brandt, J.; Ketzel, M.; et al. Long-term exposure to air pollution and road traffic noise and incidence of dementia in the Danish Nurse Cohort. Alzheimers Dement. 2024, 20, 4080–4091. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Fang, J.; Tang, S.; Du, H.; Zhao, L.; Wang, Y.; Deng, F.; Liu, Y.; Du, Y.; Cui, L.; et al. PM(2.5) exposure associated with microbiota gut-brain axis: Multi-omics mechanistic implications from the BAPE study. Innovation 2022, 3, 100213. [Google Scholar] [CrossRef]
- Kang, Y.J.; Tan, H.Y.; Lee, C.Y.; Cho, H. An Air Particulate Pollutant Induces Neuroinflammation and Neurodegeneration in Human Brain Models. Adv. Sci. 2021, 8, e2101251. [Google Scholar] [CrossRef]
- Shou, Y.; Huang, Y.; Zhu, X.; Liu, C.; Hu, Y.; Wang, H. A review of the possible associations between ambient PM2.5 exposures and the development of Alzheimer’s disease. Ecotoxicol. Environ. Saf. 2019, 174, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Streit, W.J.; Phan, L.; Bechmann, I. Ferroptosis and pathogenesis of neuritic plaques in Alzheimer’s Disease. Pharmacol. Rev. 2025, 77, 100005. [Google Scholar] [CrossRef] [PubMed]
- Moonen, S.; Koper, M.J.; Van Schoor, E.; Schaeverbeke, J.M.; Vandenberghe, R.; von Arnim, C.A.F.; Tousseyn, T.; De Strooper, B.; Thal, D.R. Pyroptosis in Alzheimer’s disease: Cell type-specific activation in microglia, astrocytes and neurons. Acta. Neuropathol. 2023, 145, 175–195. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Sun, F.; Wang, L.; Gao, M.; Xie, Y.; Sun, Y.; Liu, H.; Yuan, Y.; Yi, W.; Huang, Z.; et al. Virus-induced p38 MAPK activation facilitates viral infection. Theranostics 2020, 10, 12223–12240. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, C.; Li, D.; Zhang, J.; Yin, Z.; Li, F. Scorpion Venom Heat-Resistant Synthetic Peptide Alleviates Neuronal Necroptosis in Alzheimer’s Disease Model by Regulating Lnc Gm6410 Under PM2.5 Exposure. Int. J. Mol. Sci. 2025, 26, 4372. https://doi.org/10.3390/ijms26094372
Qin C, Li D, Zhang J, Yin Z, Li F. Scorpion Venom Heat-Resistant Synthetic Peptide Alleviates Neuronal Necroptosis in Alzheimer’s Disease Model by Regulating Lnc Gm6410 Under PM2.5 Exposure. International Journal of Molecular Sciences. 2025; 26(9):4372. https://doi.org/10.3390/ijms26094372
Chicago/Turabian StyleQin, Chuhao, Dongsheng Li, Jiahui Zhang, Ze Yin, and Fasheng Li. 2025. "Scorpion Venom Heat-Resistant Synthetic Peptide Alleviates Neuronal Necroptosis in Alzheimer’s Disease Model by Regulating Lnc Gm6410 Under PM2.5 Exposure" International Journal of Molecular Sciences 26, no. 9: 4372. https://doi.org/10.3390/ijms26094372
APA StyleQin, C., Li, D., Zhang, J., Yin, Z., & Li, F. (2025). Scorpion Venom Heat-Resistant Synthetic Peptide Alleviates Neuronal Necroptosis in Alzheimer’s Disease Model by Regulating Lnc Gm6410 Under PM2.5 Exposure. International Journal of Molecular Sciences, 26(9), 4372. https://doi.org/10.3390/ijms26094372




