Norfloxacin Oxidative Degradation and Toxicity in Aqueous Media: Reciprocal Effects of Acidity Evolution on Metal Cations and Clay Catalyst Dispersion
Abstract
:1. Introduction
2. Results and Discussion
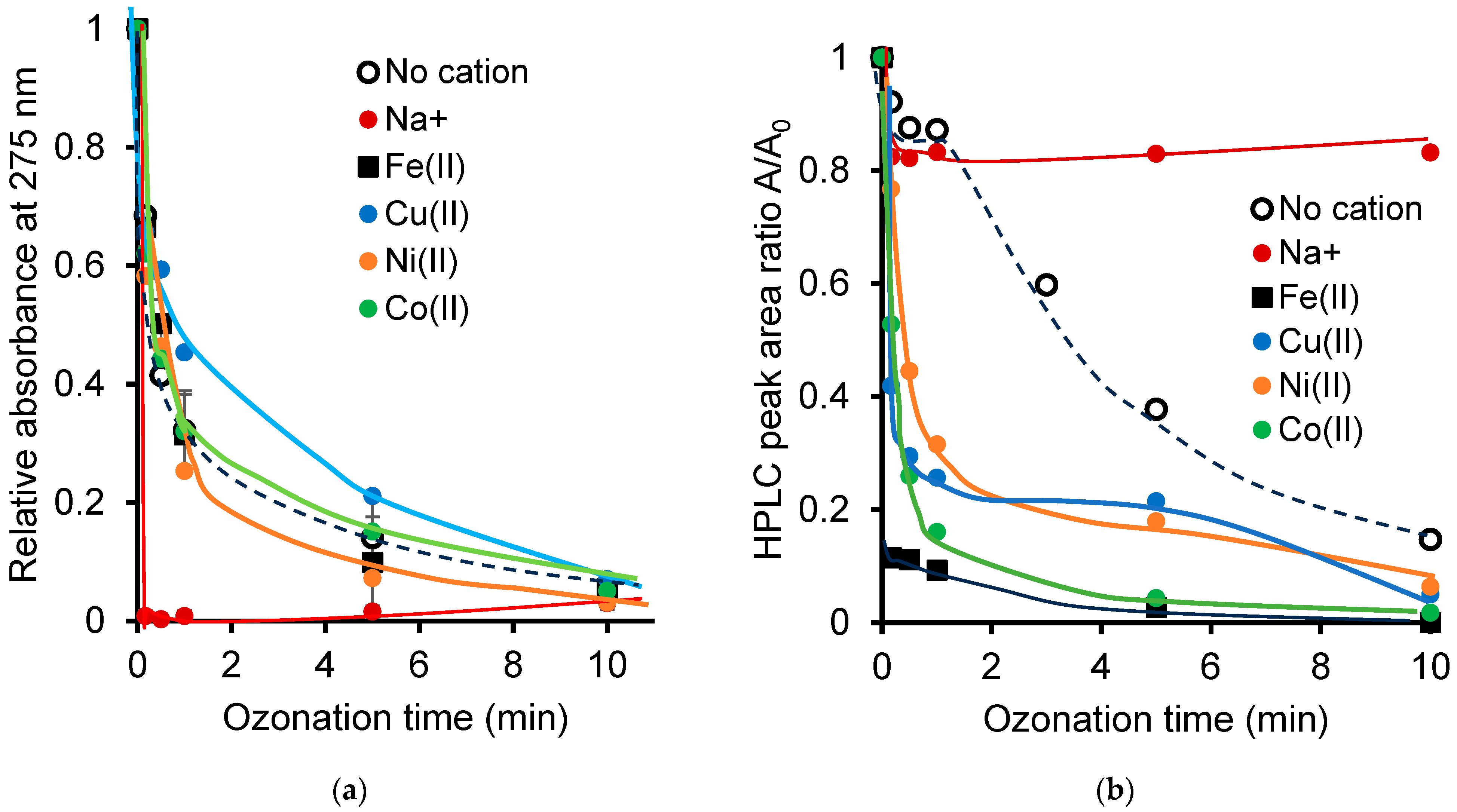
2.1. Effect of Adjusted Initial pH on Non-Catalytic Ozonation
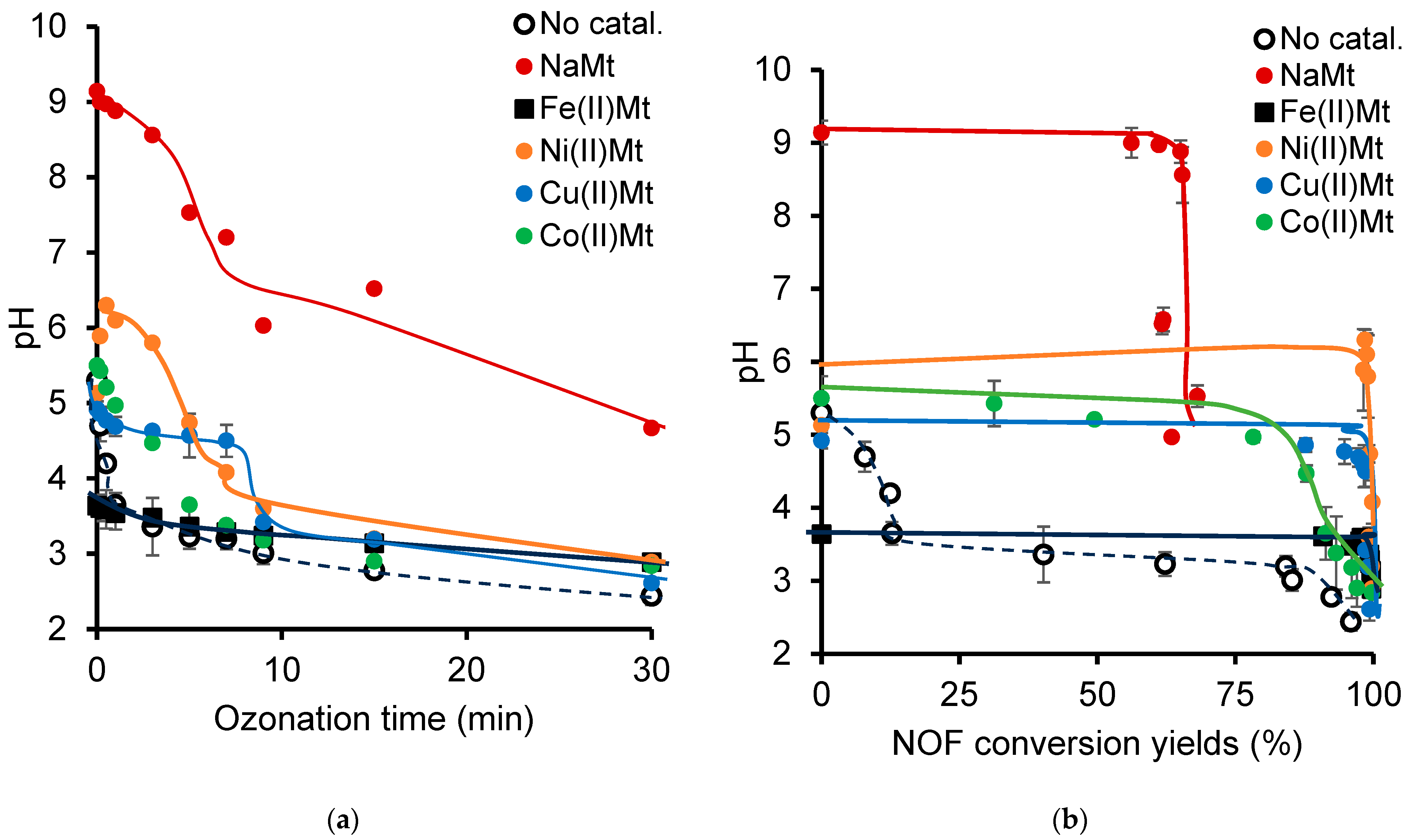
2.2. pH Changes During Cation-Catalyzed Ozonation
| Metal Cation | Dissolved Cation a | Aqueous Medium | Initial Charges of NOF c | pH | ||||
|---|---|---|---|---|---|---|---|---|
| PP a | Lewis Acidity b | |||||||
| R2N | -CO2− | Initial d | Final e | Diff. f | ||||
| None | X | X | Water | X | X | 6.50 | 3.60 | 2.9 |
| NOF(aq) c | +++ | - | 5.28 | 2.41 | 2.87 | |||
| Na+ | 1.0 | 0.16 | Water | X | X | 7.49 | 6.10 | 1.39 |
| NOF(aq) c | ++ | -- | 6.13 | 4.67 | 1.46 | |||
| Fe2+ | 3.3 | 0.34 | Water | X | X | 6.16 | 5.50 | 0.66 |
| NOF(aq) c | + | --- | 6.8 | 3.15 | 3.65 | |||
| Co2+ | 3.6 | 0.40 | Water | X | X | 6.04 | 4.88 | 1.16 |
| NOF(aq) c | ++ | --- | 6.29 | 3.14 | 3.15 | |||
| Ni2+ | 4.2 | 0.50 | Water | X | X | 5.24 | 4.50 | 0.74 |
| NOF(aq) c | +++ | -- | 5.81 | 3.09 | 2.72 | |||
| Cu2+ | 3.8 | 0.45 | Water | X | X | 5.83 | 4.52 | 1.31 |
| NOF(aq) c | ++ | --- | 6.26 | 3.18 | 3.08 | |||
2.3. pH Changes During Clay-Catalyzed Ozonation
2.4. Effect of Cations on Clay Catalyst Dispersion
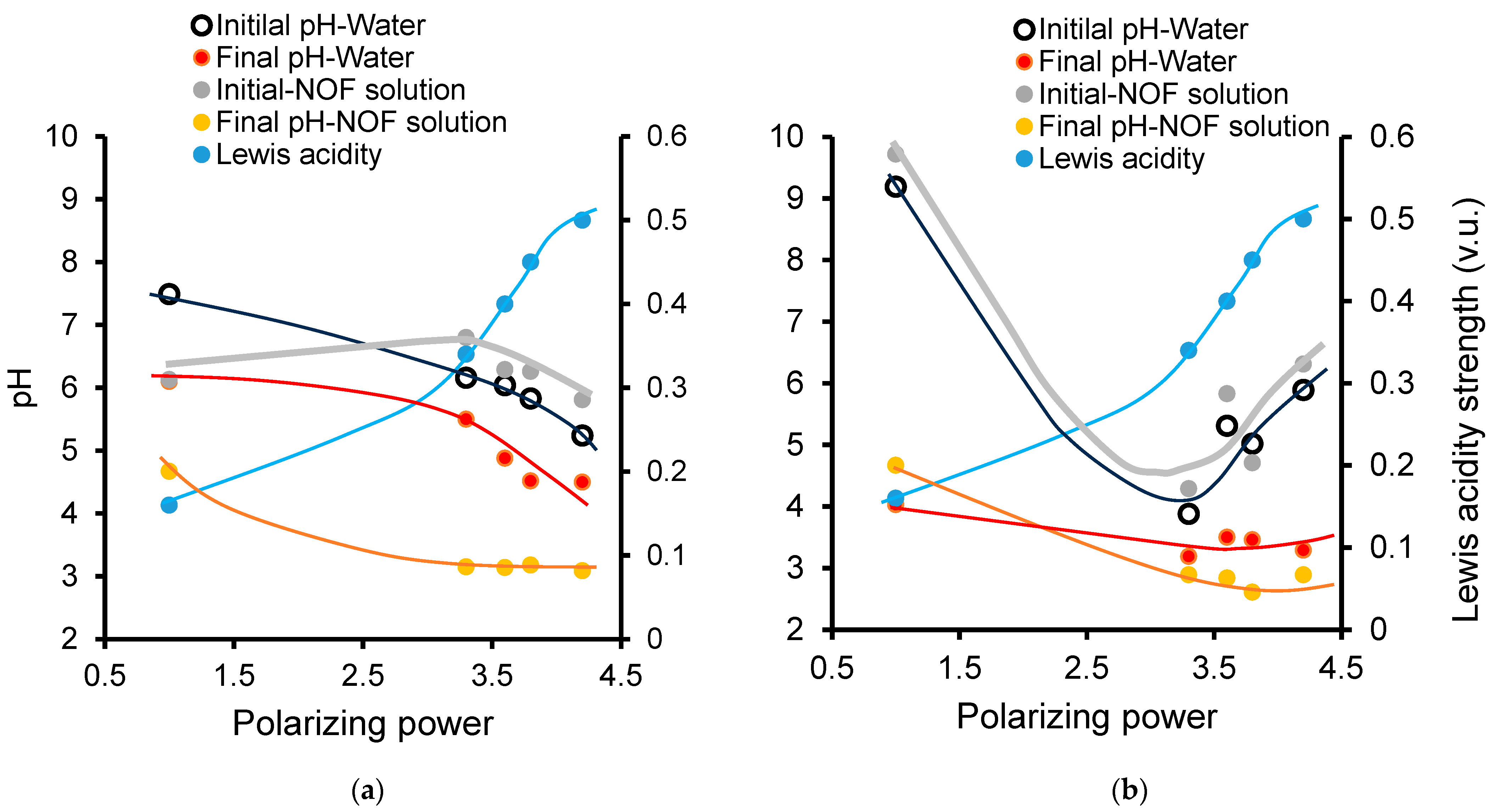
2.5. Correlation-Induced pH-Cation Polarizing Power
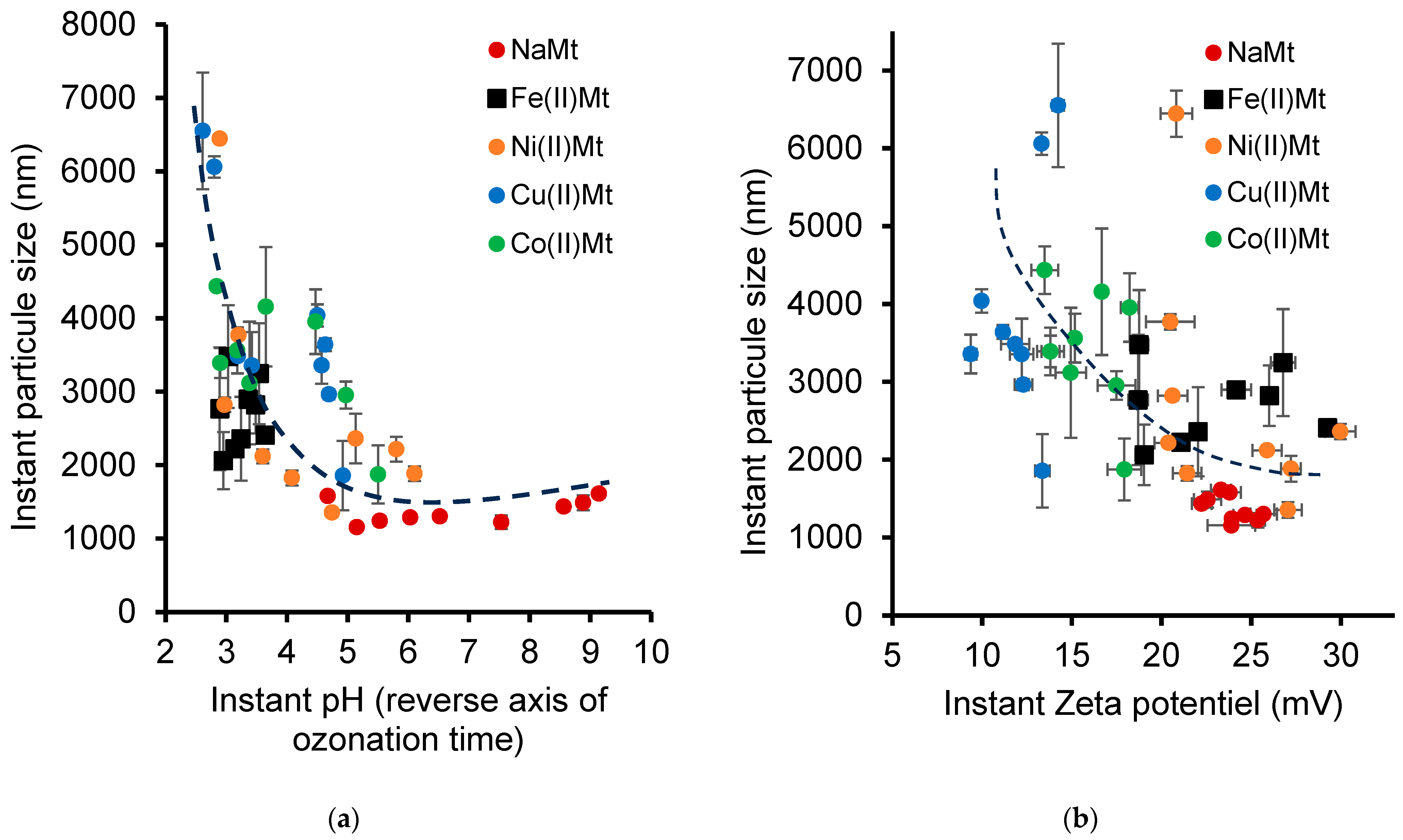
2.6. Evolution of Catalyst Dispersion During Ozonation
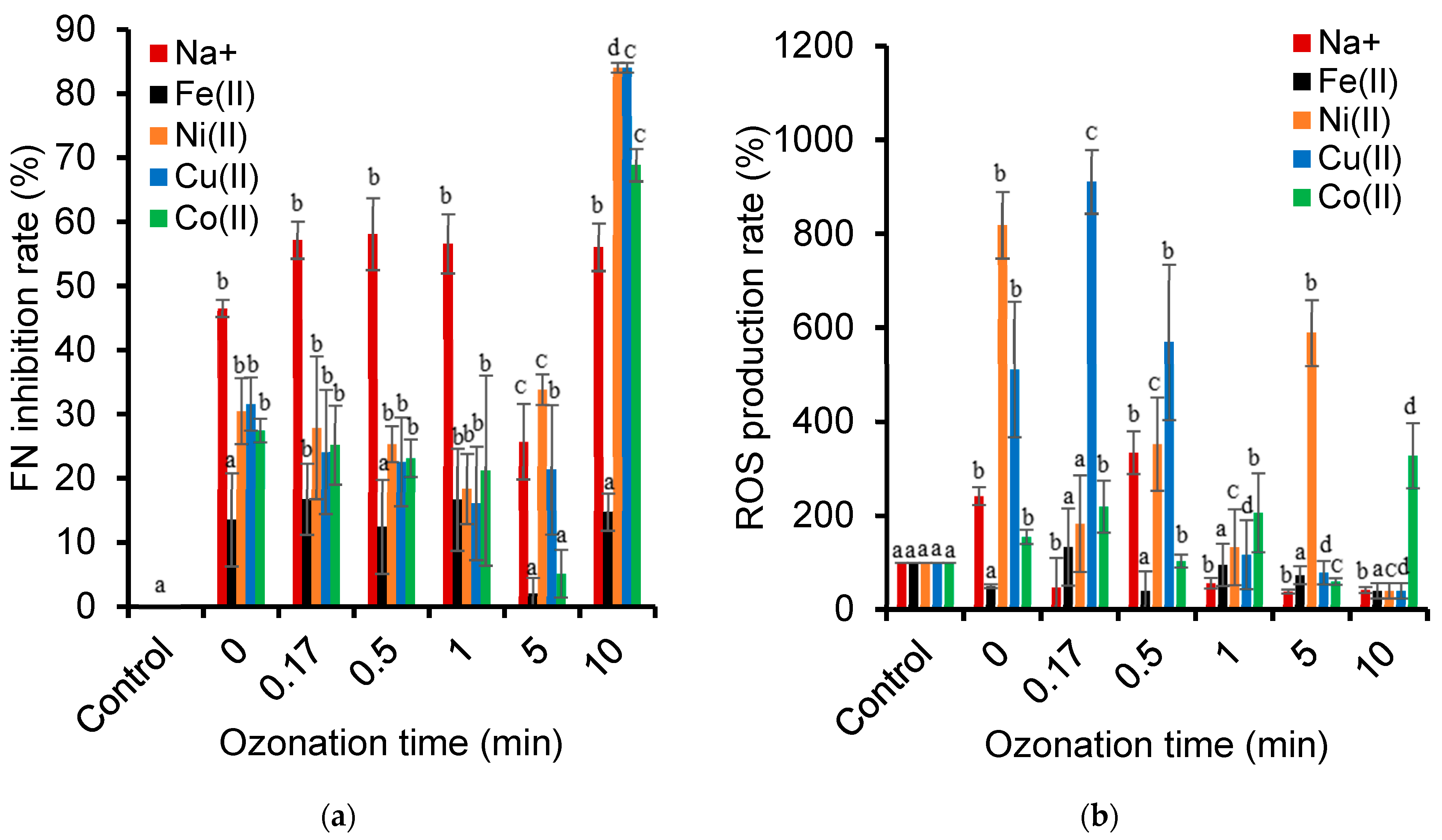
2.7. Electrostatic Interaction Changes During Ozonation
2.8. Chemical Species Interactions During NOF Adsorption
2.9. Clay Dispersion Effect on Toxicity
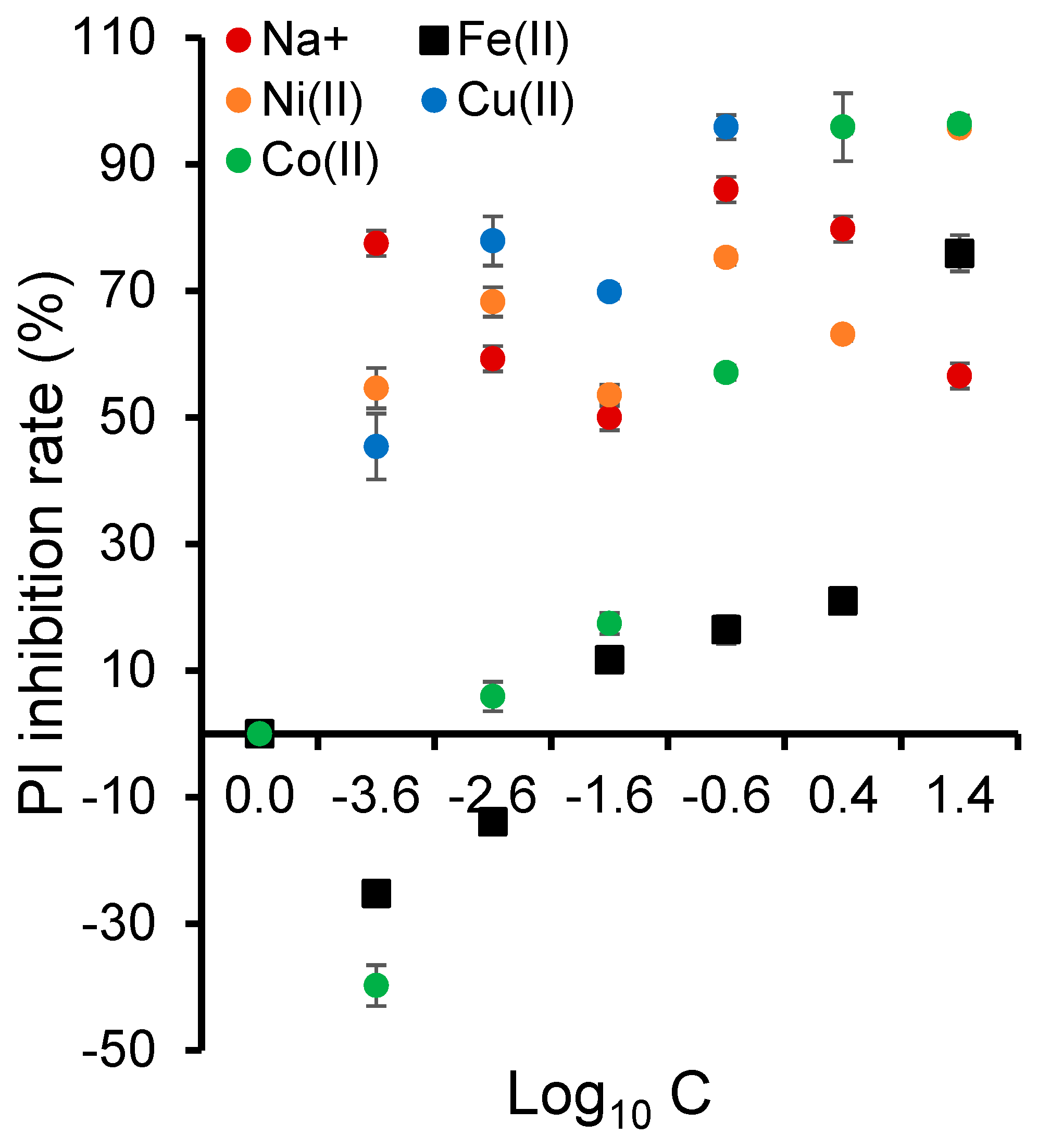
2.10. Cation Intrinsic Toxicity on Frond Number
2.11. Effect on Photosynthetic Efficiency
2.12. Toxicity of Cation-Catalyzed Ozonation
3. Methods and Materials
3.1. Clay Material’s Preparation and Dispersion in Aqueous Suspension
3.2. Adsorption Tests
3.3. Ozonation Tests
3.4. Toxicity Tests on Lemna Minor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, Y.; Gao, Y.; Xu, R.; Li, D.; Waiho, K.; Wang, Y.; Hu, M. Combined toxic effects of nanoplastics and norfloxacin on antioxidant and immune genes in mussels. Mar. Environ. Res. 2024, 193, 106277. [Google Scholar] [CrossRef] [PubMed]
- Salma, A.; Thoröe-Boveleth, S.; Schmidt, T.C.; Tuerk, J. Dependence of transformation product formation on pH during photolytic and photocatalytic degradation of ciprofloxacin. J. Hazard. Mater. 2016, 313, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Thangavelu, K.; Karanikolos, G.N. Bimetallic nanoparticles for antimicrobial applications. Front. Chem. 2020, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Villar, M.; Mateo-Tomás, P.; Sánchez-Barbudo, I.S.; Camarero, P.R.; Taggart, M.A.; Mateo, R. Determinants of the exposure of Eurasian griffon vultures (Gyps fulvus) to fluoroquinolones used in livestock: The role of supplementary feeding stations. Environ. Pollut. 2022, 311, 119923. [Google Scholar] [CrossRef]
- Fang, L.; Chen, C.; Li, S.; Ye, P.; Shi, Y.; Sharma, G.; Sarkar, B.; Shaheen, S.M.; Lee, S.S.; Xiao, R.; et al. A comprehensive and global evaluation of residual antibiotics in agricultural soils: Accumulation, potential ecological risks, and attenuation strategies. Ecotoxicol. Environ. Saf. 2023, 262, 115175. [Google Scholar] [CrossRef]
- Ariyani, M.; Jansen, L.J.; Balzer-Rutgers, P.; Hofstra, N.; van Oel, P.; van de Schans, M.G. Antibiotic residues in the cirata reservoir, Indonesia and their effect on ecology and the selection for antibiotic-resistant bacteria. Environ. Res. 2024, 262, 119992. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, W.; Li, J.; Yuan, M.; Zhang, J.; Xu, F.; Xu, H.; Zheng, X.; Wang, L. Ecotoxicological effects of sulfonamides and fluoroquinolones and their removal by a green alga (Chlorella vulgaris) and a cyanobacterium (Chrysosporum ovalisporum). Environ. Pollut. 2020, 263, 114554. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Tian, L.; Wang, L.; Wei, S.; Zhang, L.; Dong, D.; Guo, Z. Enhanced degradation of enoxacin using ferrihydrite-catalyzed heterogeneous photo-Fenton process. Environ. Res. 2024, 251, 118650. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Li, R.; Yang, H.; Zhou, B.; Wang, X.; Xie, Y. Comparison of chlorination behaviors between norfloxacin and ofloxacin: Reaction kinetics, oxidation products and reaction pathways. Chemosphere 2019, 215, 124–132. [Google Scholar] [CrossRef]
- Mutke, X.A.; Swiderski, P.; Drees, F.; Akin, O.; Lutze, H.V.; Schmidt, T.C. Efficiency of ozonation and sulfate radical-AOP for removal of pharmaceuticals, corrosion inhibitors, x-ray contrast media and perfluorinated compounds from reverse osmosis concentrates. Water Res. 2024, 255, 121346. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Y.; Ding, S.; Li, X.; Wang, W.; Dong, N.; Nie, M.; Chen, P. Investigation into the Synergistic Effect of the Zinc Peroxide/Peroxymonosulfate Double-Oxidation System for the Efficient Degradation of Tetracycline. Molecules 2024, 29, 4120. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Chen, X.; Su, L.; Dong, S.; Yi, L.; He, P.; Yang, W.; Lu, Z. The effect of interlayer water of metal-modified montmorillonite for catalytic ozonation. Chemosphere 2023, 312, 137200. [Google Scholar] [CrossRef] [PubMed]
- Benghaffour, A.; Azzouz, A.; Dewez, D. Ecotoxicity of Diazinon and Atrazine Mixtures after Ozonation Catalyzed by Na+ and Fe2+ Exchanged Montmorillonites on Lemna minor. Molecules 2023, 28, 6108. [Google Scholar] [CrossRef]
- Novikau, R.; Lujaniene, G. Adsorption behaviour of pollutants: Heavy metals, radionuclides, organic pollutants, on clays and their minerals (raw, modified and treated): A review. J. Environ. Manag. 2022, 309, 114685. [Google Scholar] [CrossRef]
- Hamilton, A.; Hutcheon, G.; Roberts, M.; Gaskell, E. Formulation and antibacterial profiles of clay–ciprofloxacin composites. Appl. Clay Sci. 2014, 87, 129–135. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, X.; Liu, P.; Zhao, L.; Zou, W. Optimization adsorption of norfloxacin onto polydopamine microspheres from aqueous solution: Kinetic, equilibrium and adsorption mechanism studies. Sci. Total Environ. 2018, 639, 428–437. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S. Clay supported TiO2 nanoparticles for photocatalytic degradation of environmental pollutants: A review. J. Environ. Chem. Eng. 2018, 6, 6088–6107. [Google Scholar] [CrossRef]
- Lin, J.; Jiang, B.; Zhan, Y. Effect of pre-treatment of bentonite with sodium and calcium ions on phosphate adsorption onto zirconium-modified bentonite. J. Environ. Manag. 2018, 217, 183–195. [Google Scholar] [CrossRef]
- Pei, H.; Zhang, S. Molecular dynamics study on the zeta potential and shear plane of montmorillonite in NaCl solutions. Appl. Clay Sci. 2021, 212, 106212. [Google Scholar] [CrossRef]
- El Baktaoui, M.; Hadj-Abdelkader, N.E.H.; Benghaffour, A.; Arus, V.-A.; Bennani-Daouadji, N.; Belkhadem, F.; Roy, R.; Azzouz, A. Clay-Catalyzed Ozonation of Hydrotalcite-Extracted Lactic Acid Potential Application for Preventing Milk Fermentation Inhibition. Molecules 2022, 27, 6502. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, D.; Roy, R.; Azzouz, A. Total removal of oxalic acid via synergistic parameter interaction in montmorillonite catalyzed ozonation. J. Environ. Chem. Eng. 2014, 2, 20–30. [Google Scholar] [CrossRef]
- Shahidi, D.; Roy, R.; Azzouz, A. Advances in catalytic oxidation of organic pollutants–prospects for thorough mineralization by natural clay catalysts. Appl. Catal. B Environ. 2015, 174, 277–292. [Google Scholar] [CrossRef]
- Boudissa, F.; Zekkari, M.; Arus, V.-A.; Ouargli-Saker, R.; Nabil, B.; Roy, R.; Azzouz, A. Clay-catalyzed ozonation of endocrine-disrupting compounds in solvent-free media–to better understand soil catalytic capacity. Dalton Trans. 2020, 49, 16693–16706. [Google Scholar] [CrossRef]
- Boudissa, F.; Mirilà, D.; Arus, V.-A.; Terkmani, T.; Semaan, S.; Proulx, M.; Nistor, I.-D.; Roy, R.; Azzouz, A. Acid-treated clay catalysts for organic dye ozonation–Thorough mineralization through optimum catalyst basicity and hydrophilic character. J. Hazard. Mater. 2019, 364, 356–366. [Google Scholar] [CrossRef]
- Sasmaz, A.; Dogan, I.; Sasmaz, M. Removal of Cr, Ni and Co in the water of chromium mining areas by using Lemna gibba L. and Lemna minor L. Water Environ. J. 2016, 30, 235–242. [Google Scholar] [CrossRef]
- Van Dyck, I.; Vanhoudt, N.; i Batlle, J.V.; Horemans, N.; Van Gompel, A.; Nauts, R.; Wannijn, J.; Wijgaerts, A.; Vassilev, A.; Vangronsveld, J. Uptake of Co, Cs, Mn, Ni and Zn by Lemna minor and their effects on physiological and biochemical functions. Environ. Exp. Bot. 2023, 213, 105440. [Google Scholar] [CrossRef]
- Boudissa, F.; Arus, V.-A.; Foka-Wembe, E.-N.; Zekkari, M.; Ouargli-Saker, R.; Dewez, D.; Roy, R.; Azzouz, A. Role of Silica on Clay-Catalyzed Ozonation for Total Mineralization of Bisphenol-A. Molecules 2023, 28, 3825. [Google Scholar] [CrossRef]
- Zhang, H.; He, Y.; Lai, L.; Yao, G.; Lai, B. Catalytic ozonation of Bisphenol A in aqueous solution by Fe3O4–MnO2 magnetic composites: Performance, transformation pathways and mechanism. Sep. Purif. Technol. 2020, 245, 116449. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Z.; Wei, S. Interaction of heavy metals and polycyclic aromatic hydrocarbons in soil-crop systems: The effects and mechanisms. Environ. Res. 2024, 263, 120035. [Google Scholar] [CrossRef]
- Cao, S.; Zhang, P.; Song, H.; Ge, L.; Niu, J. Photochemical properties and toxicity of fluoroquinolone antibiotics impacted by complexation with metal ions in different pH solutions. J. Environ. Sci. 2025, 150, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Serafin, A.; Stańczak, A. The complexes of metal ions with fluoroquinolones. Russ. J. Coord. Chem. 2009, 35, 81–95. [Google Scholar] [CrossRef]
- Chen, Z.; He, G.; You, T.; Zhang, T.; Liu, B.; Wang, Y. Complex pollution of Fluoroquinolone antibiotics and metal oxides/metal ions in water: A review on occurrence, formation mechanisms, removal and ecotoxicity. J. Environ. Chem. Eng. 2024, 12, 112191. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Guo, J.; Kan, Z.; Jia, Y. Catalytic ozonation mechanisms of Norfloxacin using Cu–CuFe2O4. Environ. Res. 2023, 216, 114521. [Google Scholar]
- Macoustra, G.K.; Jolley, D.F.; Stauber, J.; Koppel, D.J.; Holland, A. Amelioration of copper toxicity to a tropical freshwater microalga: Effect of natural DOM source and season. Environ. Pollut. 2020, 266, 115141. [Google Scholar] [CrossRef]
- Liao, W.; Zhu, Z.; Feng, C.; Yan, Z.; Hong, Y.; Liu, D.; Jin, X. Toxicity mechanisms and bioavailability of copper to fish based on an adverse outcome pathway analysis. J. Environ. Sci. 2023, 127, 495–507. [Google Scholar] [CrossRef]
- Tsai, K.-P. Toxic effects of thallium (Tl+) on prokaryotic alga Microcystis aeruginosa: Short and long-term influences by potassium and humic acid. Chemosphere 2024, 346, 140618. [Google Scholar] [CrossRef]
- Aslanzadeh, M.; Saboora, A.; Moradlou, O. Phytoremediation potential of duckweed (Lemna minor L.) for hexavalent chromium removal in synthetic wastewater: Unveiling physiological response and defense mechanisms against excessive heavy metal uptake. Int. J. Environ. Sci. Technol. 2024, 21, 10155–10174. [Google Scholar] [CrossRef]
- Begović, L.; Mlinarić, S.; Dunić, J.A.; Katanić, Z.; Lončarić, Z.; Lepeduš, H.; Cesar, V. Response of Lemna minor L. to short-term cobalt exposure: The effect on photosynthetic electron transport chain and induction of oxidative damage. Aquat. Toxicol. 2016, 175, 117–126. [Google Scholar] [CrossRef]
- Tarrahi, R.; Khataee, A.; Movafeghi, A.; Rezanejad, F. Toxicity of ZnSe nanoparticles to Lemna minor: Evaluation of biological responses. J. Environ. Manag. 2018, 226, 298–307. [Google Scholar] [CrossRef]
- Pereira, S.P.; Jesus, F.; Aguiar, S.; de Oliveira, R.; Fernandes, M.; Ranville, J.; Nogueira, A.J. Phytotoxicity of silver nanoparticles to Lemna minor: Surface coating and exposure period-related effects. Sci. Total Environ. 2018, 618, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Hou, W.; Gao, Y.; Wang, Y.; Lin, L.; Zhang, Z.; Niu, Q.; Ma, R.; Mu, L.; Wang, H. Effects of CuO nanoparticles on Lemna minor. Bot. Stud. 2016, 57, 3. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Zhao, J.; Yu, X.; Lv, K.; Wang, Z.; Xing, B. Interaction of CuO nanoparticles with duckweed (Lemna minor L.): Uptake, distribution and ROS production sites. Environ. Pollut. 2018, 243, 543–552. [Google Scholar] [CrossRef]
- Guo, R.; Lu, D.; Liu, C.; Hu, J.; Wang, P.; Dai, X. Toxic effect of nickel on microalgae Phaeodactylum tricornutum (Bacillariophyceae). Ecotoxicology 2022, 31, 746–760. [Google Scholar] [CrossRef]
- Ebert, I.; Bachmann, J.; Kühnen, U.; Küster, A.; Kussatz, C.; Maletzki, D.; Schlüter, C. Toxicity of the fluoroquinolone antibiotics enrofloxacin and ciprofloxacin to photoautotrophic aquatic organisms. Environ. Toxicol. Chem. 2011, 30, 2786–2792. [Google Scholar] [CrossRef]
- Horvat, T.; Vidaković-Cifrek, Ž.; Oreščanin, V.; Tkalec, M.; Pevalek-Kozlina, B. Toxicity assessment of heavy metal mixtures by Lemna minor L. Sci. Total Environ. 2007, 384, 229–238. [Google Scholar] [CrossRef]
- Guzman-Tordecilla, M.; Pacheco-Bustos, C.; Coronado-Posada, N.; Pedrosa-Gomes, M.; Martinez-Burgos, W.J.; Mejía-Marchena, R.; Zorman-Marques, R. Exploring the ecotoxicological impact of meropenem on Lemna minor: Growth, photosynthetic activity, and oxidative stress. Environ. Res. 2024, 258, 119409. [Google Scholar] [CrossRef]
- de Morais, M.B.; Barbosa-Neto, A.G.; Willadino, L.; Ulisses, C.; Calsa Junior, T. Salt stress induces increase in starch accumulation in duckweed (Lemna aequinoctialis, Lemnaceae): Biochemical and physiological aspects. J. Plant Growth Regul. 2019, 38, 683–700. [Google Scholar] [CrossRef]
- Kumar, K.; Sarkar, P.; Paul, T.; Shukla, S.P.; Kumar, S. Ecotoxicological effects of triclosan on Lemna minor: Bioconcentration, growth inhibition and oxidative stress. Environ. Sci. Pollut. Res. 2024, 31, 56550–56564. [Google Scholar] [CrossRef]
- Elbasan, F.; Arikan-Abdulveli, B.; Ozfidan-Konakci, C.; Yildiztugay, E.; Tarhan, İ.; Çelik, B. Exploring the defense strategies of benzalkonium chloride exposures on the antioxidant system, photosynthesis and ROS accumulation in Lemna minor. Chemosphere 2024, 363, 142924. [Google Scholar] [CrossRef]
- Azzouz, A. Advances in Oxidative Decomposition of Oxalic Acid in Aqueous Media Advances in Chemistry Research; Taylor, J.C., Ed.; Nova-Publishers: New York, NY, USA, 2012; Volume 14. [Google Scholar]
- Azzouz, A.; Mirilà, D.; Nistor, I.D.; Boudissa, F.; Roy, R. Chapter 1: Advances in the Oxidative Degradation of Organic Pollutants: Prospects for Catalyzed Oxidation Processes Targeting Total Mineralization. In Advances in Chemistry Research; Taylor, J.C., Ed.; Nova Science Publishers: New York, NY, USA, 2019; Volume 49, p. 235. [Google Scholar]
- Shahidi, D.; Moheb, A.; Abbas, R.; Larouk, S.; Roy, R.; Azzouz, A. Total mineralization of sulfamethoxazole and aromatic pollutants through Fe2+-montmorillonite catalyzed ozonation. J. Hazard. Mater. 2015, 298, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Kalandarov, P.; Zhunussov, K.; Kassimov, A.; Baibolov, B.; Junussov, N.; Kaliyeva, K. Changes in pH during the ozonation process of surface water. E3S Web Conf. 2024, 563, 01020. [Google Scholar] [CrossRef]
- Azzouz, A.; Kotbi, A.; Niquette, P.; Sajin, T.; Ursu, A.; Rami, A.; Monette, F.; Hausler, R. Ozonation of oxalic acid catalyzed by ion-exchanged montmorillonite in moderately acidic media. React. Kinet. Mech. Catal. 2010, 99, 289–302. [Google Scholar] [CrossRef]
- Larouk, S.; Ouargli, R.; Shahidi, D.; Olhund, L.; Shiao, T.C.; Chergui, N.; Sehili, T.; Roy, R.; Azzouz, A. Catalytic ozonation of Orange-G through highly interactive contributions of hematite and SBA-16–To better understand azo-dye oxidation in nature. Chemosphere 2017, 168, 1648–1657. [Google Scholar] [CrossRef]
- Mirilă, D.-C.; Boudissa, F.; Beltrao-Nuñes, A.-P.; Platon, N.; Didi, M.-A.; Nistor, I.-D.; Roy, R.; Azzouz, A. Organic dye ozonation catalyzed by chemically modified montmorillonite K10–Role of surface basicity and hydrophilic character. Ozone Sci. Eng. 2020, 42, 517–530. [Google Scholar] [CrossRef]
- Brown, I.D. 14—The Bond-Valence Method: An Empirical Approach to Chemical Structure and Bonding. In Industrial Chemistry Library; O’Keeffe, M., Navrotsky, A., Eds.; Elsevier: Amsterdam, The Netherlands, 1981; Volume 2, pp. 1–30. [Google Scholar]
- Hawthorne, F.; Schindler, M. Understanding the weakly bonded constituents in oxysalt minerals. Z. Krist. 2008, 223, 41–68. [Google Scholar] [CrossRef]
- Dmitrieva, V.A.; Tyutereva, E.V.; Voitsekhovskaja, O.V. What can reactive oxygen species (ROS) tell us about the action mechanism of herbicides and other phytotoxins? Free Radic. Biol. Med. 2024, 220, 92–110. [Google Scholar] [CrossRef]
- Benghaffour, A.; Foka-Wembe, E.-N.; Dami, M.; Dewez, D.; Azzouz, A. Insight in natural media remediation through ecotoxicity correlation to clay catalyst selectivity in organic molecule ozonation. Dalton Trans. 2022, 51, 4366–4376. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Rivas, F.J.; Montero-de-Espinosa, R. Iron type catalysts for the ozonation of oxalic acid in water. Water Res. 2005, 39, 3553–3564. [Google Scholar] [CrossRef]
- Cromley, J.T.; O’Connor, J.T. Effect of Ozonation on the Removal of Iron From a Ground Water. J. AWWA 1976, 68, 315–319. [Google Scholar] [CrossRef]
- Hopwood, M.J.; Santana-González, C.; Gallego-Urrea, J.; Sanchez, N.; Achterberg, E.P.; Ardelan, M.V.; Gledhill, M.; González-Dávila, M.; Hoffmann, L.; Leiknes, Ø.; et al. Fe(II) stability in coastal seawater during experiments in Patagonia, Svalbard, and Gran Canaria. Biogeosciences 2020, 17, 1327–1342. [Google Scholar] [CrossRef]
- Sallanko, J.; Esko, L.; Röpelinen, J. Iron Behavior in the Ozonation and Filtration of Groundwater. Ozone Sci. Eng. 2006, 28, 269–273. [Google Scholar] [CrossRef]
- Sulpizi, M.; Gaigeot, M.-P.; Sprik, M. The Silica–Water Interface: How the Silanols Determine the Surface Acidity and Modulate the Water Properties. J. Chem. Theory Comput. 2012, 8, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Zekkari, M.; Ouargli-Saker, R.; Boudissa, F.; Lachachi, A.K.; El Houda Sekkal, K.N.; Tayeb, R.; Boukoussa, B.; Azzouz, A. Silica-catalyzed ozonation of 17α-ethinyl-estradiol in aqueous media-to better understand the role of silica in soils. Chemosphere 2022, 298, 134312. [Google Scholar] [CrossRef]
- Bolt, G.H. Determination of the Charge Density of Silica Sols. J. Phys. Chem. 1957, 61, 1166–1169. [Google Scholar] [CrossRef]
- Sögaard, C.; Funehag, J.; Abbas, Z. Silica sol as grouting material: A physio-chemical analysis. Nano Converg. 2018, 5, 6. [Google Scholar] [CrossRef]
- Pavan, C.; Escolano-Casado, G.; Bellomo, C.; Cananà, S.; Tomatis, M.; Leinardi, R.; Mino, L.; Turci, F. Nearly free silanols drive the interaction of crystalline silica polymorphs with membranes: Implications for mineral toxicity. Front. Chem. 2023, 10, 1092221. [Google Scholar] [CrossRef]
- Foka Wembe, E.N.; Benghafour, A.; Dewez, D.; Azzouz, A. Clay-Catalyzed Ozonation of Organic Pollutants in Water and Toxicity on Lemna minor: Effects of Molecular Structure and Interactions. Molecules 2023, 28, 222. [Google Scholar] [CrossRef]
- Tombácz, E.; Szekeres, M. Surface charge heterogeneity of kaolinite in aqueous suspension in comparison with montmorillonite. Appl. Clay Sci. 2006, 34, 105–124. [Google Scholar] [CrossRef]
- Zhu, Y.; Marchuk, A.; Bennett, J.M. Ionicity of clay–cation bonds in relation to dispersive behavior of Mg and K soil clays as influenced by pH. Clays Clay Miner. 2020, 68, 588–600. [Google Scholar] [CrossRef]
- Rengasamy, P.; Tavakkoli, E.; McDonald, G. Exchangeable cations and clay dispersion: Net dispersive charge, a new concept for dispersive soil. Eur. J. Soil Sci. 2016, 67, 659–665. [Google Scholar] [CrossRef]
- Hamilton, A.; Roberts, M.; Hutcheon, G.; Gaskell, E. Formulation and antibacterial properties of clay mineral-tetracycline and-doxycycline composites. Appl. Clay Sci. 2019, 179, 105148. [Google Scholar] [CrossRef]
- Wang, C.-J.; Li, Z.; Jiang, W.-T.; Jean, J.-S.; Liu, C.-C. Cation exchange interaction between antibiotic ciprofloxacin and montmorillonite. J. Hazard. Mater. 2010, 183, 309–314. [Google Scholar] [CrossRef]
- Cuprys, A.; Pulicharla, R.; Lecka, J.; Brar, S.K.; Drogui, P.; Surampalli, R.Y. Ciprofloxacin-metal complexes–stability and toxicity tests in the presence of humic substances. Chemosphere 2018, 202, 549–559. [Google Scholar] [CrossRef]
- Lage, O.M.; Bondoso, J.; Catita, J.A. Determination of zeta potential in Planctomycetes and its application in heavy metals toxicity assessment. Arch. Microbiol. 2012, 194, 847–855. [Google Scholar] [CrossRef]
- Alfarsi, A.H.; Weird, G.M.; Kumar, A.; Nugegoda, D. Multigenerational Toxicity Effects and Impact of Antibiotics Exposed to Duckweed, Lemna minor. Sci. Total Environ. 2025, 977, 179324. [Google Scholar] [CrossRef]
- Yusefi-Tanha, E.; Fallah, S.; Rostamnejadi, A.; Pokhrel, L.R. Particle size and concentration dependent toxicity of copper oxide nanoparticles (CuONPs) on seed yield and antioxidant defense system in soil grown soybean (Glycine max cv. Kowsar). Sci. Total Environ. 2020, 715, 136994. [Google Scholar] [CrossRef]
- Djidja, R.; Dewez, D.; Azzouz, A. Clay-catalyzed ozonation of Norfloxacin-Effects of metal cation and degradation rate on aqueous media toxicity towards Lemna minor. Chemosphere 2025, 372, 144088. [Google Scholar] [CrossRef]
- Sree, K.S.; Keresztes, Á.; Mueller-Roeber, B.; Brandt, R.; Eberius, M.; Fischer, W.; Appenroth, K.-J. Phytotoxicity of cobalt ions on the duckweed Lemna minor—Morphology, ion uptake, and starch accumulation. Chemosphere 2015, 131, 149–156. [Google Scholar] [CrossRef]
- Razinger, J.; Dermastia, M.; Drinovec, L.; Drobne, D.; Zrimec, A.; Dolenc Koce, J. Antioxidative responses of duckweed (Lemna minor L.) to short-term copper exposure. Environ. Sci. Pollut. Res.-Int. 2007, 14, 194–201. [Google Scholar] [CrossRef]
- Stannard, L.M.; Doherty, A.; Chapman, K.E.; Doak, S.H.; Jenkins, G.J. Multi-endpoint analysis of cadmium chloride-induced genotoxicity shows role for reactive oxygen species and p53 activation in DNA damage induction, cell cycle irregularities, and cell size aberrations. Mutagenesis 2024, 39, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.-X.; Chai, F.; Chen, K.-H.; Huang, Y.-X.; Wei, G.-J.; Yuan, H.; Pang, Y.-F.; Luo, S.-H.; Wang, C.-F.; Chen, W.-C. Reactive Oxygen Species-Mediated Mitophagy and Cell Apoptosis are Involved in the Toxicity of Aluminum Chloride Exposure in GC-2spd. Biol. Trace Elem. Res. 2024, 202, 2616–2629. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-L.; Li, P.; Qu, C.; Lu, R.; Li, Z.-H. Phytotoxicity of environmental norfloxacin concentrations on the aquatic plant Spirodela polyrrhiza: Evaluation of growth parameters, photosynthetic toxicity and biochemical traits. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 258, 109365. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Dos Reis, L.L.; de Abreu, C.B.; Gebara, R.C.; Rocha, G.S.; Longo, E.; Mansano, A.d.S.; Melão, M.d.G.G. Isolated and combined effects of cobalt and nickel on the microalga Raphidocelis subcapitata. Ecotoxicology 2024, 33, 104–118. [Google Scholar] [CrossRef]
- Simmons, J.A. Toxicity of major cations and anions (Na+, K+, Ca2+, Cl−, and SO42−) to a macrophyte and an alga. Environ. Toxicol. Chem. 2012, 31, 1370–1374. [Google Scholar] [CrossRef]
- Rodríguez, A.A.; Vilas, J.M.; Sartore, G.D.; Bezus, R.; Colazo, J.; Maiale, S.J. Field and genetic evidence support the photosynthetic performance index (PIABS) as an indicator of rice grain yield. Plant Physiol. Biochem. 2023, 201, 107897. [Google Scholar]
- Samadani, M.; Perreault, F.; Oukarroum, A.; Dewez, D. Effect of cadmium accumulation on green algae Chlamydomonas reinhardtii and acid-tolerant Chlamydomonas CPCC 121. Chemosphere 2018, 191, 174–182. [Google Scholar] [CrossRef]
- Perreault, F.; Samadani, M.; Dewez, D. Effect of soluble copper released from copper oxide nanoparticles solubilisation on growth and photosynthetic processes of Lemna gibba L. Nanotoxicology 2014, 8, 374–382. [Google Scholar] [CrossRef]
- Jmii, S.; Dewez, D. Toxic responses of palladium accumulation in duckweed (Lemna minor): Determination of biomarkers. Environ. Toxicol. Chem. 2021, 40, 1630–1638. [Google Scholar] [CrossRef]
- Frankart, C.; Eullaffroy, P.; Vernet, G. Photosynthetic responses of Lemna minor exposed to xenobiotics, copper, and their combinations. Ecotoxicol. Environ. Saf. 2002, 53, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.K.; Yadav, V.; Vaculík, M.; Gassmann, W.; Pike, S.; Arif, N.; Singh, V.P.; Deshmukh, R.; Sahi, S.; Tripathi, D.K. Aluminum toxicity and aluminum stress-induced physiological tolerance responses in higher plants. Crit. Rev. Biotechnol. 2021, 41, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Superoxide anion chemistry—Its role at the core of the innate immunity. Int. J. Mol. Sci. 2023, 24, 1841. [Google Scholar] [CrossRef]
- Alp, F.N.; Arikan, B.; Ozfidan-Konakci, C.; Gulenturk, C.; Yildiztugay, E.; Turan, M.; Cavusoglu, H. Hormetic activation of nano-sized rare earth element terbium on growth, PSII photochemistry, antioxidant status and phytohormone regulation in Lemna minor. Plant Physiol. Biochem. 2023, 194, 361–373. [Google Scholar] [CrossRef]
- Anjum, N.A.; Gill, S.S.; Corpas, F.J.; Ortega-Villasante, C.; Hernandez, L.E.; Tuteja, N.; Sofo, A.; Hasanuzzaman, M.; Fujita, M. Recent insights into the double role of hydrogen peroxide in plants. Front. Plant Sci. 2022, 13, 843274. [Google Scholar] [CrossRef]
- Bienert, G.P.; Schjoerring, J.K.; Jahn, T.P. Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta (BBA)-Biomembr. 2006, 1758, 994–1003. [Google Scholar] [CrossRef]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 1596–1604. [Google Scholar] [CrossRef]
- Kehrer, J.P. The Haber–Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Richards, S.L.; Wilkins, K.A.; Swarbreck, S.M.; Anderson, A.A.; Habib, N.; Smith, N.G.; McAinsh, M.; Davies, J.M. The hydroxyl radical in plants: From seed to seed. J. Exp. Bot. 2015, 66, 37–46. [Google Scholar] [CrossRef]
- Gao, M.; Chang, X.; Yang, Y.; Song, Z. Foliar graphene oxide treatment increases photosynthetic capacity and reduces oxidative stress in cadmium-stressed lettuce. Plant Physiol. Biochem. 2020, 154, 287–294. [Google Scholar] [CrossRef]
- Hu, X.; Xu, C.; Tao, H.; Wang, S.; Zhang, M.; Chen, Q.; Zhang, H.; Li, G.; Yan, C. The Effect of Low Temperature and Low Illumination Intensity on the Photosynthetic Characteristics and Antioxidant Enzyme Activity in the Strawberry. Agronomy 2025, 15, 860. [Google Scholar] [CrossRef]
- Tanaka, A.; Ito, H. Chlorophyll degradation and its physiological function. Plant Cell Physiol. 2025, 66, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Riyazuddin, R.; Nisha, N.; Ejaz, B.; Khan, M.I.R.; Kumar, M.; Ramteke, P.W.; Gupta, R. A comprehensive review on the heavy metal toxicity and sequestration in plants. Biomolecules 2021, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Bobu, M.; Yediler, A.; Siminiceanu, I.; Zhang, F.; Schulte-Hostede, S. Comparison of different advanced oxidation processes for the degradation of two fluoroquinolone antibiotics in aqueous solutions. J. Environ. Sci. Health Part A 2013, 48, 251–262. [Google Scholar] [CrossRef]
- Benghaffour, A.; Dewez, D.; Azzouz, A. Correlation of pesticide ecotoxicity with clay mineral dispersion effect on adsorption and ozonation—An approach through impact assessment on Lemna minor. Appl. Clay Sci. 2023, 241, 107001. [Google Scholar] [CrossRef]
- Lunardi, C.N.; Gomes, A.J.; Rocha, F.S.; De Tommaso, J.; Patience, G.S. Experimental methods in chemical engineering: Zeta potential. Can. J. Chem. Eng. 2021, 99, 627–639. [Google Scholar] [CrossRef]
- Chasanah, U.; Yulianto, E.; Zain, A.Z.; Sasmita, E.; Restiwijaya, M.; Kinandana, A.W.; Arianto, F.; Nur, M. Evaluation of titration method on determination of ozone concentration produced by dielectric barrier discharge plasma (DBDP) technology. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2019. [Google Scholar]
- Battino, R.; Rettich, T.R.; Tominaga, T. The solubility of oxygen and ozone in liquids. J. Phys. Chem. Ref. Data 1983, 12, 163–178. [Google Scholar] [CrossRef]
- Egorova, G. Ozone solubility in water. Mosc. Univ. Chem. Bull. 2015, 70, 207–210. [Google Scholar] [CrossRef]
- Roth, J.A.; Sullivan, D.E. Solubility of ozone in water. Ind. Eng. Chem. Fundam. 1981, 20, 137–140. [Google Scholar] [CrossRef]
- Elkhatat, A.M.; Soliman, M.; Ismail, R.; Ahmed, S.; Abounahia, N.; Mubashir, S.; Fouladi, S.; Khraisheh, M. Recent trends of copper detection in water samples. Bull. Natl. Res. Cent. 2021, 45, 218. [Google Scholar] [CrossRef]
- Monroy, M.; Maceda-Veiga, A.; de Sostoa, A. Metal concentration in water, sediment and four fish species from Lake Titicaca reveals a large-scale environmental concern. Sci. Total Environ. 2014, 487, 233–244. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 321–362. [Google Scholar]
- Stirbet, A. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B Biol. 2011, 104, 236–257. [Google Scholar] [CrossRef] [PubMed]

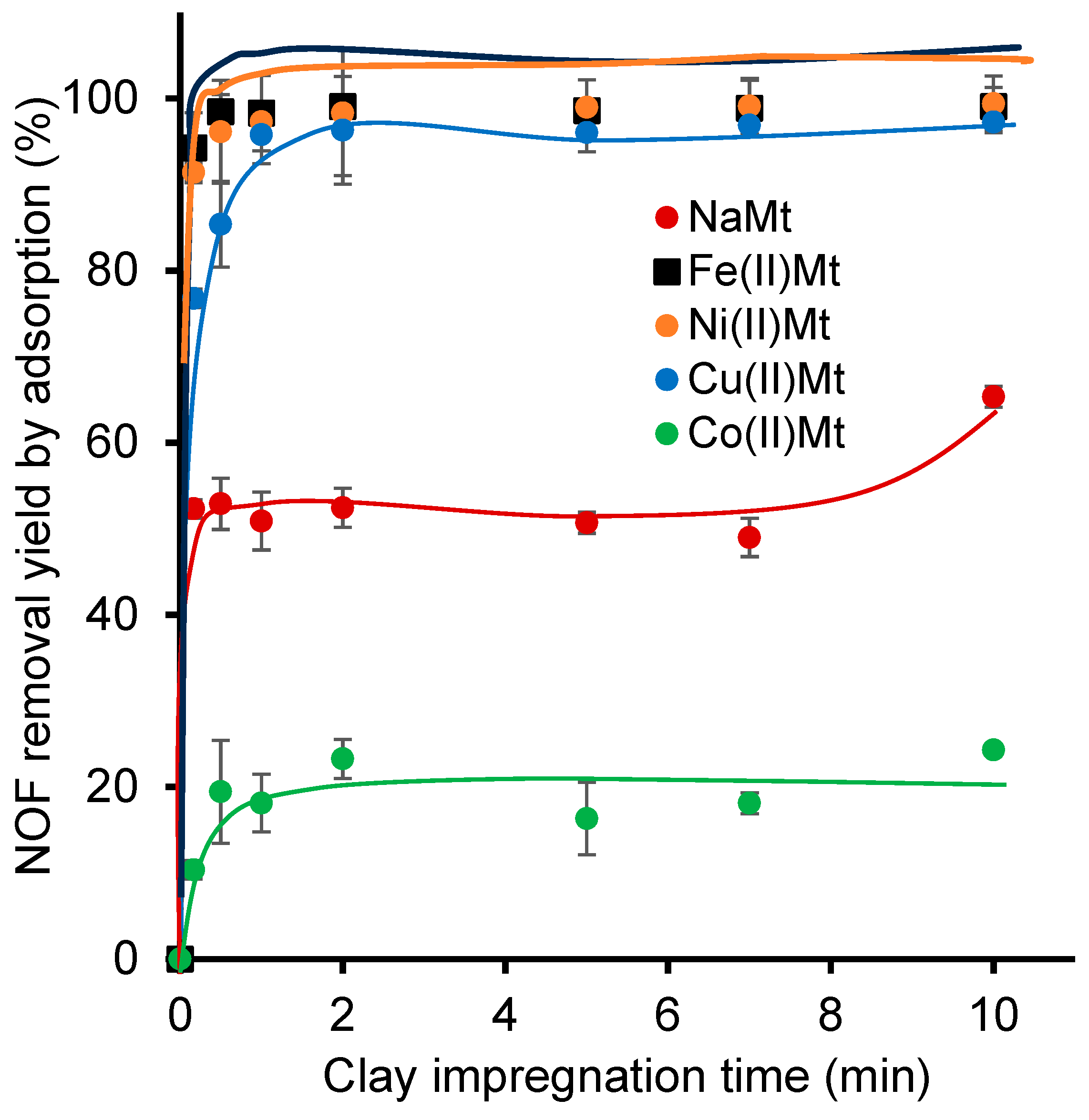
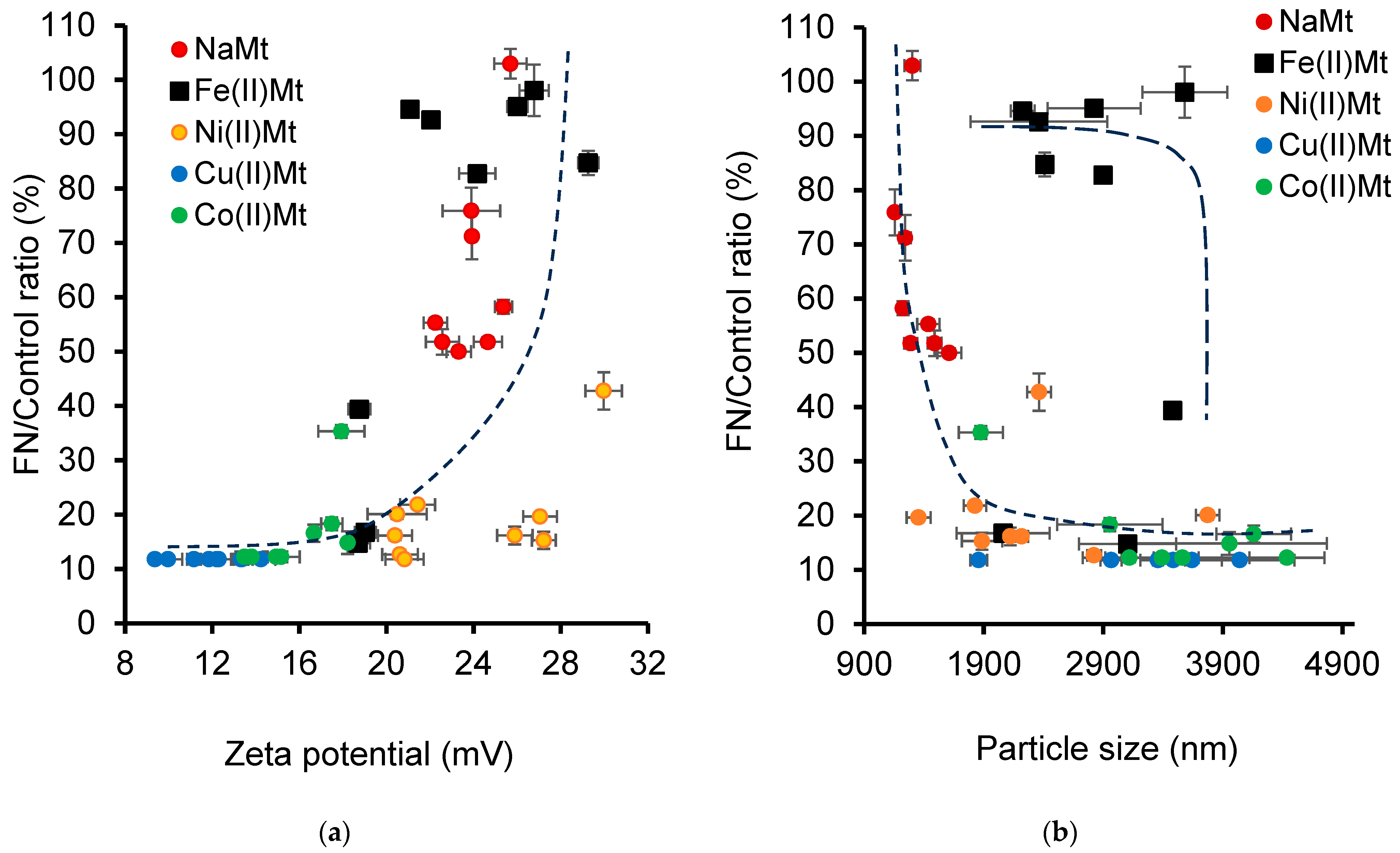

| Clay Catalyst | Aqueous Medium | ZP (mV) a | Standard Deviation | PS b (nm) | Standard Deviation | Initial Charges | pH | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NOF c | Silanols d | |||||||||||
| R2N | -CO2− | Out | In | Initial e | Final f | Diff. g | ||||||
| None | NOF(aq)* | ++ | - | X | X | 5.28 | 2.44 | 2.84 | ||||
| NaMt | Water | −23.45 | 1.663 | 846 | 25.65 | - - - | - - - | 9.19 | 4.03 | 5.16 | ||
| NOF(aq)* | −23.36 | 0.5761 | 1608 | 101.7 | + | - - - | - - - | - - - | 9.72 | 4.67 | 5.05 | |
| Fe(II)Mt | Water | −30.5 | 0.7215 | 2515 | 232.6 | +/- | +/- | 3.88 | 3.19 | 0.69 | ||
| NOF(aq)* | −28.71 | 0.876 | 2409 | 702.4 | +++ | - | +/- | +/- | 4.29 | 2.89 | 1.4 | |
| Co(II)Mt | Water | −19.54 | 0.9465 | 1972 | 397.5 | - - | - | 5.31 | 3.50 | 1.81 | ||
| NOF(aq)* | −17.93 | 1.059 | 1875 | 184 | +++ | - - | - - | - | 5.83 | 2.84 | 2.99 | |
| Ni(II)Mt | Water | −23.54 | 0.3172 | 2147 | 338.5 | - - - | - - | 5.89 | 3.29 | 2.6 | ||
| NOF(aq)* | −29.97 | 0.8394 | 2362 | 98.46 | +++ | - - - | - - | - | 6.31 | 2.89 | 3.42 | |
| Cu(II)Mt | Water | −14.95 | 0.4041 | 5068 | 472.5 | - - | - | 5.02 | 3.46 | 1.56 | ||
| NOF(aq)* | −13.36 | 0.4912 | 1858 | 69.51 | ++ | - - | - - | - | 4.71 | 2.61 | 2.1 | |
| Clay Material | Pseudo-First Order | Pseudo-Second Order | ||
|---|---|---|---|---|
| K1 | R1 2 | K2 | R2 2 | |
| NaMt | 1.165 | 0.486 | 13.944 | 0.966 |
| Fe(II)Mt | 4.048 | 0.205 | 444.532 | 0.999 |
| Co(II)Mt | 2.851 | 0.386 | 32.303 | 0.949 |
| Ni(II)Mt | 3.122 | 0.544 | 123.296 | 0.999 |
| Cu(II)Mt | 2.689 | 0.574 | 58.055 | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djidja, R.; Dewez, D.; Azzouz, A. Norfloxacin Oxidative Degradation and Toxicity in Aqueous Media: Reciprocal Effects of Acidity Evolution on Metal Cations and Clay Catalyst Dispersion. Int. J. Mol. Sci. 2025, 26, 4347. https://doi.org/10.3390/ijms26094347
Djidja R, Dewez D, Azzouz A. Norfloxacin Oxidative Degradation and Toxicity in Aqueous Media: Reciprocal Effects of Acidity Evolution on Metal Cations and Clay Catalyst Dispersion. International Journal of Molecular Sciences. 2025; 26(9):4347. https://doi.org/10.3390/ijms26094347
Chicago/Turabian StyleDjidja, Roumaissa, David Dewez, and Abdelkrim Azzouz. 2025. "Norfloxacin Oxidative Degradation and Toxicity in Aqueous Media: Reciprocal Effects of Acidity Evolution on Metal Cations and Clay Catalyst Dispersion" International Journal of Molecular Sciences 26, no. 9: 4347. https://doi.org/10.3390/ijms26094347
APA StyleDjidja, R., Dewez, D., & Azzouz, A. (2025). Norfloxacin Oxidative Degradation and Toxicity in Aqueous Media: Reciprocal Effects of Acidity Evolution on Metal Cations and Clay Catalyst Dispersion. International Journal of Molecular Sciences, 26(9), 4347. https://doi.org/10.3390/ijms26094347






