Mechanisms of Viral DNA Replication of Human Papillomavirus: E2 Protein-Dependent Recruitment of E1 DNA Helicase to the Origin of DNA Replication
Abstract
:1. Introduction
2. Results
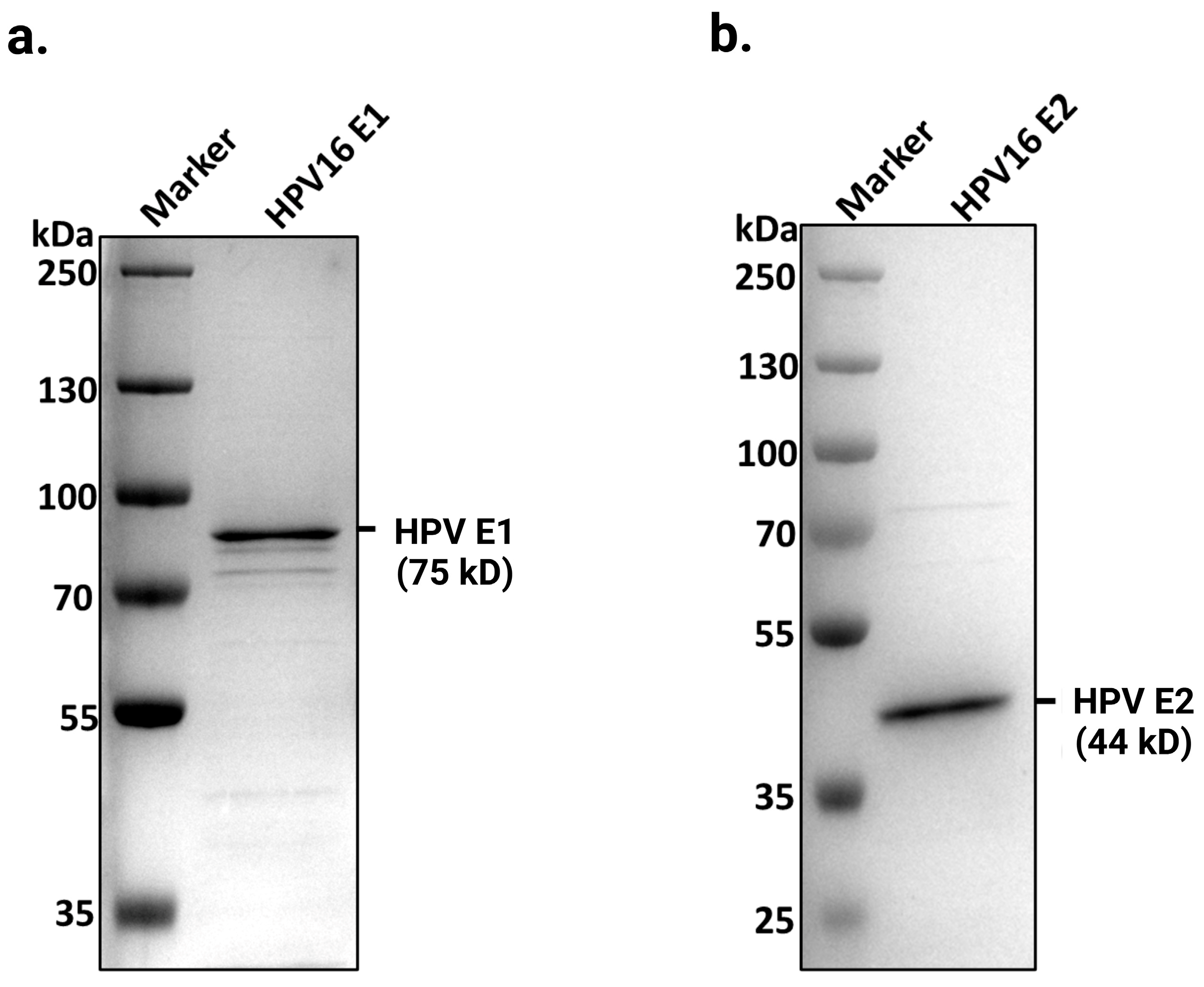
2.1. Purification of Full-Length HPV E1 and E2 Proteins
2.2. Enzymatic Activities of Full-Length HPV E1 Helicase
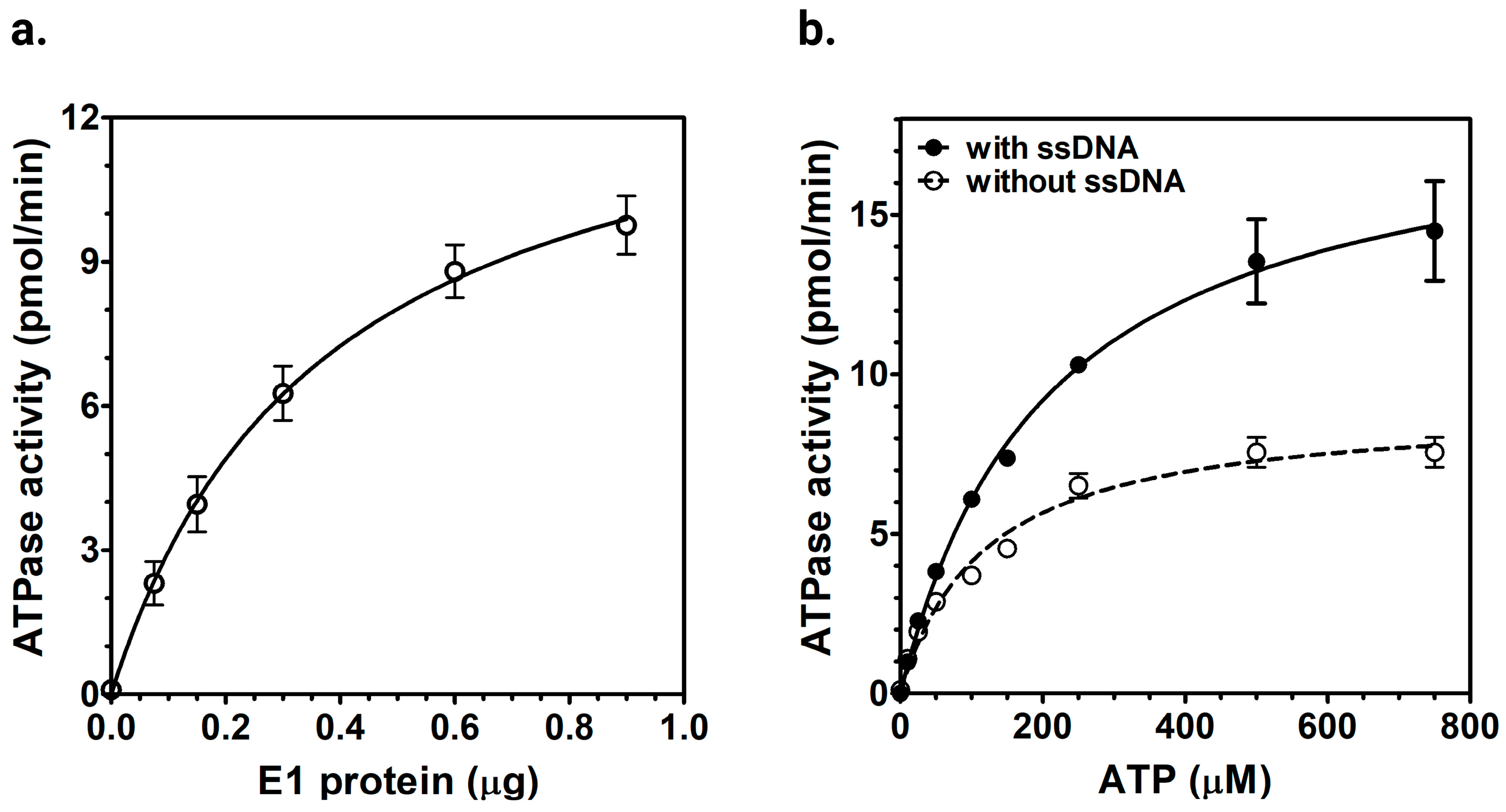
2.2.1. ATPase Activity of Purified E1 Helicase
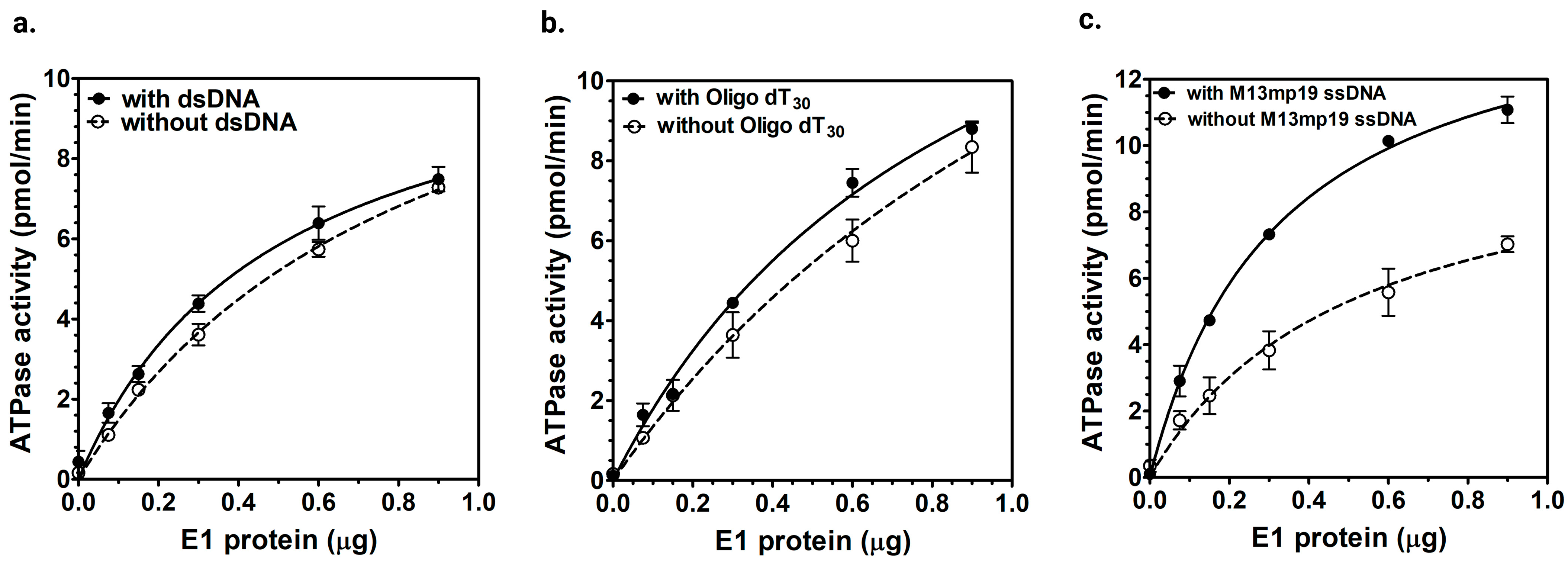
2.2.2. ssDNA-Dependent ATPase Activity of E1 Helicase
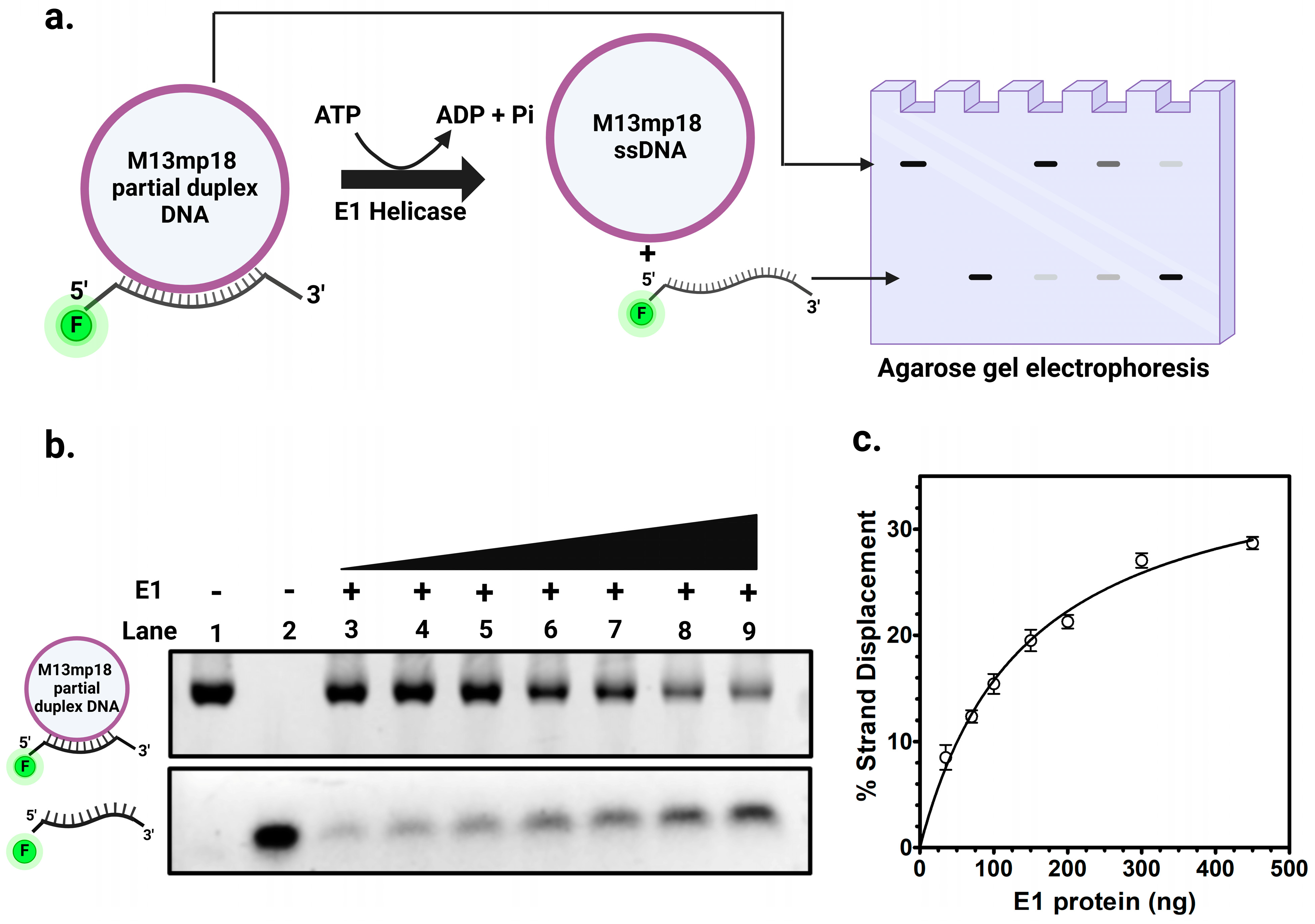
2.2.3. DNA Helicase Activity of E1
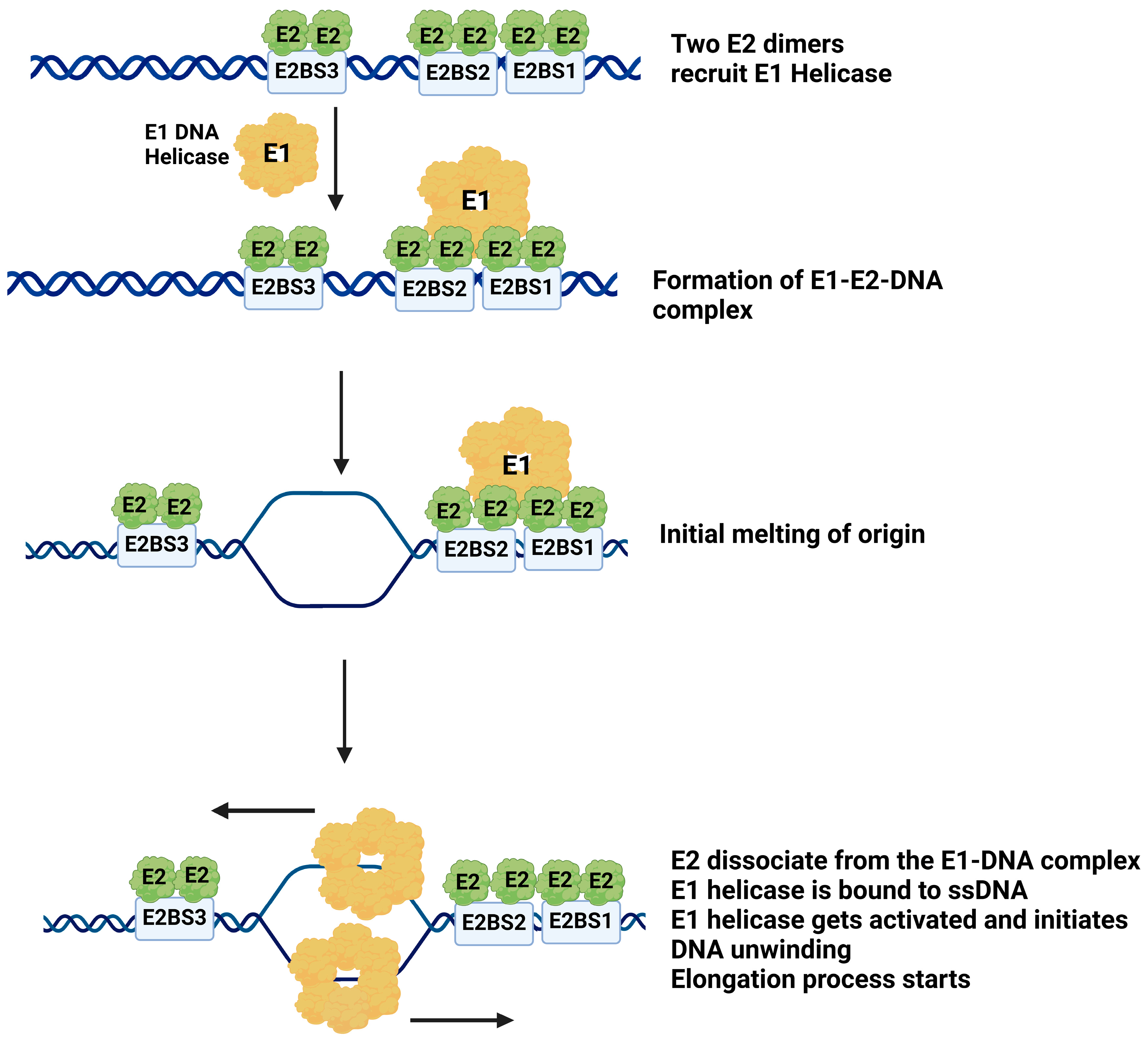
2.3. Mechanism of E1 Helicase Loading to the HPV Origin of DNA Replication
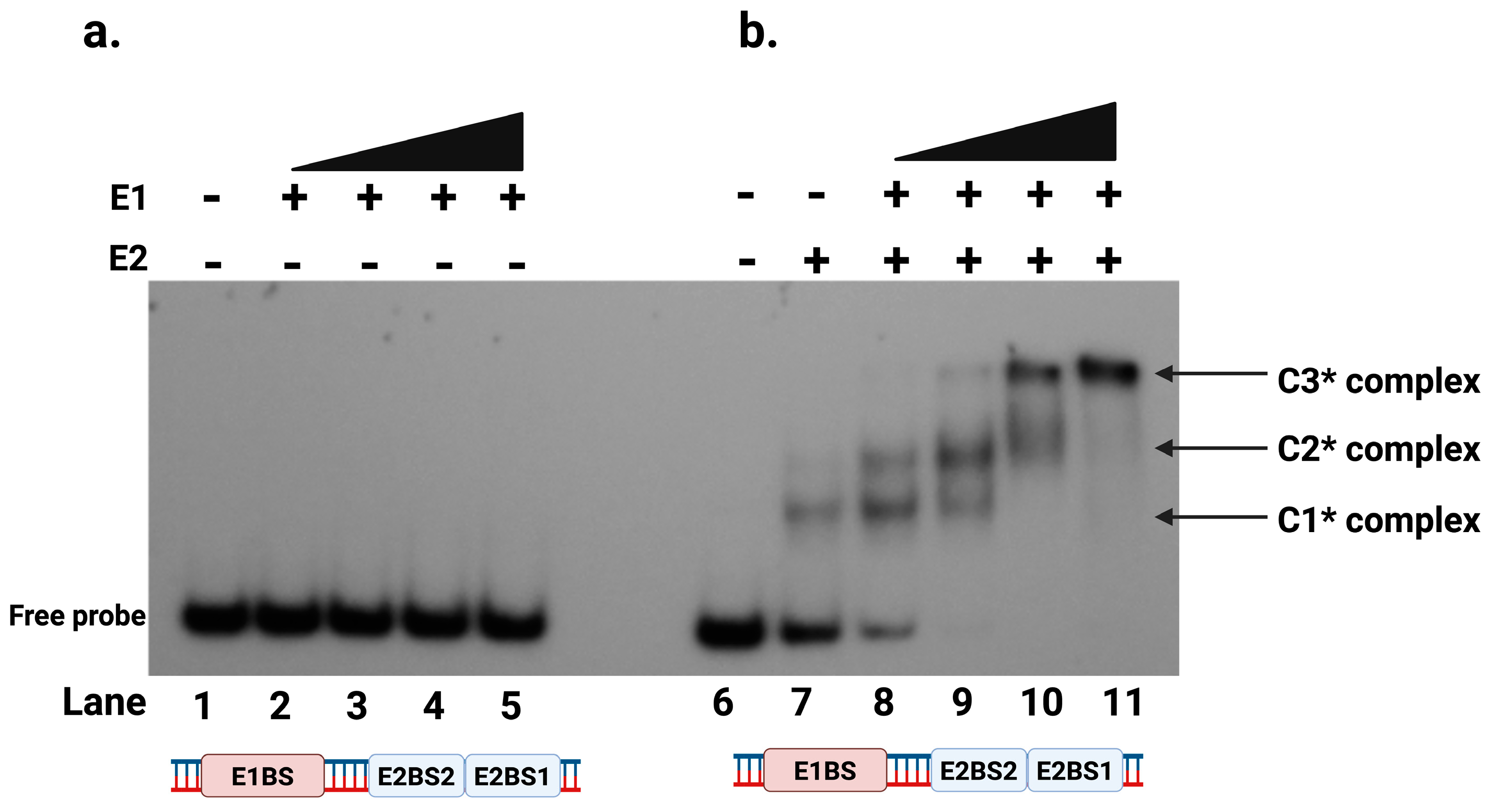
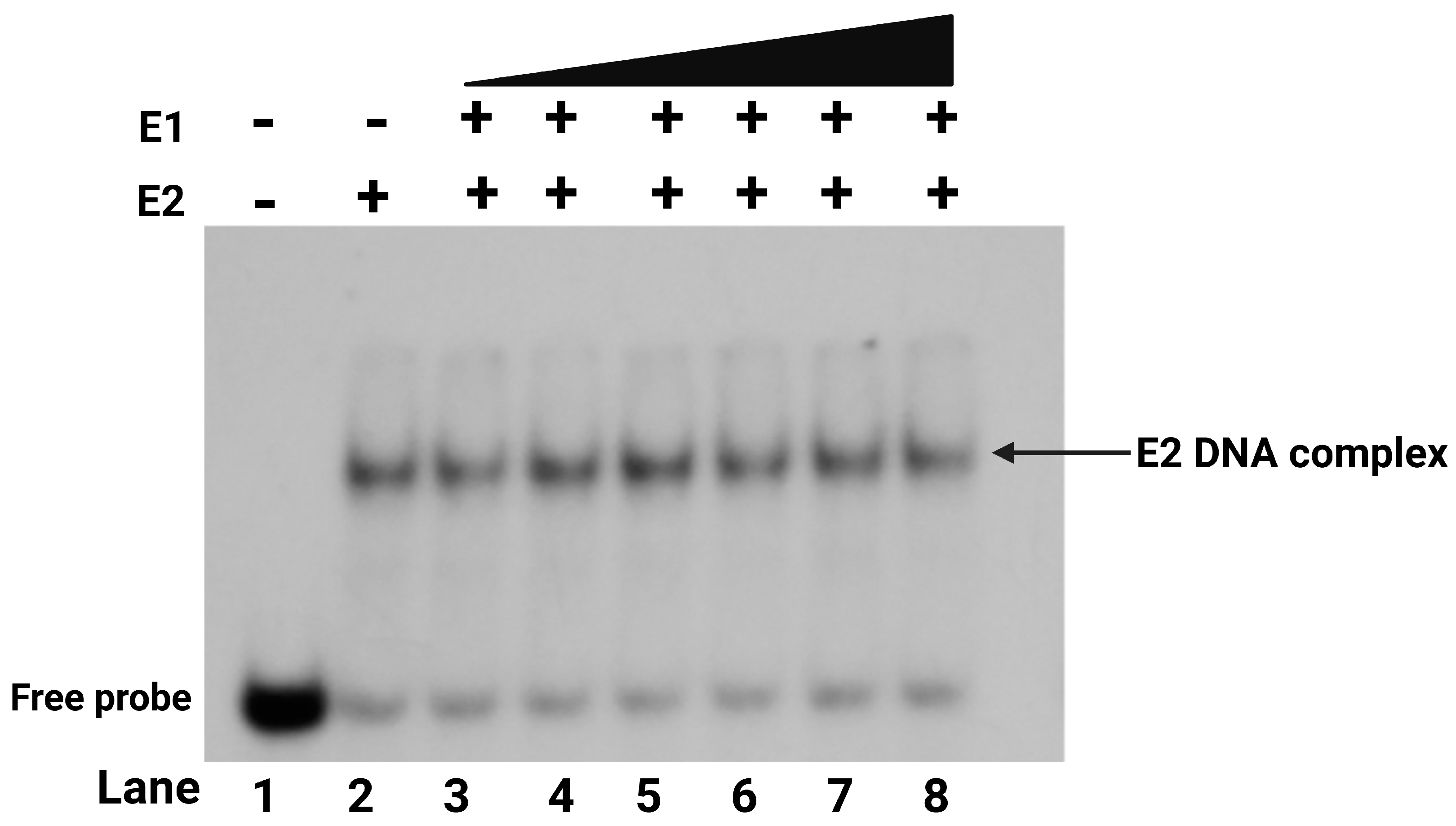
2.4. E2 Protein Is Required for E1 to Bind to the Protein-DNA Complex at the Origin
2.5. E1-E2-DNA Complex Formation Did Not Require a Putative E1 Binding Site
2.6. E1-E2-DNA Complex Formation Requires at Least Two E2 Binding Sites
3. Discussion
3.1. Purification and Functional Characterization of Full-Length HPV16 E1 Helicase
3.2. E1 ATPase Activity Is Stimulated by M13mp19 ssDNA
3.3. E2 Protein Mediates E1 Binding to the Viral Origin
3.4. E1 Binding Site Is Dispensable for E1-E2-DNA Complex Formation
3.5. Two E2 Binding Sites Are Essential for E1-E2-DNA Complex Formation
4. Materials and Methods
4.1. Buffers
4.2. Expression and Purification of Full-Length HPV E1 and E2 Proteins
4.3. ATPase Assay
4.4. Kinetic Analysis of E1 ATPase Activity
4.5. DNA Helicase Assay
4.6. Radiolabeling of Oligonucleotides
4.7. Electrophoretic Mobility Shift Assay (EMSA)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HPV | Human papillomavirus |
| BPV | Bovine papillomavirus |
| EMSA | Electrophoretic mobility shift assay |
| LCR | Long control region |
| BS | Binding site |
| SNV | Single-nucleotide variation |
References
- Mcmurray, H.R.; Nguyen, D.; Westbrook, T.F.; Mcance, D.J. Biology of human papillomaviruses. Int. J. Exp. Pathol. 2001, 82, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Hebner, C.M.; Laimins, L.A. Human papillomaviruses: Basic mechanisms of pathogenesis and oncogenicity. Rev. Med. Virol. 2006, 16, 83–97. [Google Scholar] [CrossRef]
- Brentjens, M.H.; Yeung-Yue, K.A.; Lee, P.C.; Tyring, S.K. Human papillomavirus: A review. Dermatol. Clin. 2002, 20, 315–331. [Google Scholar] [CrossRef]
- McLaughlin-Drubin, M.E.; Munger, K. Papillomaviruses. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- zur Hausen, H.; de Villiers, E.M. Human papillomaviruses. Annu. Rev. Microbiol. 1994, 48, 427–447. [Google Scholar] [CrossRef] [PubMed]
- Münger, K. The role of human papillomaviruses in human cancers. Front. Biosci. 2002, 7, d641–d649. [Google Scholar] [CrossRef]
- zur Hausen, H. Papillomaviruses in the causation of human cancers—A brief historical account. Virology 2009, 384, 260–265. [Google Scholar] [CrossRef]
- Naveed, S.; Muhammad, U.; Memoona, R.; Bilal, A. Preventive Strategies against Human Papillomaviruses. In Human Papillomavirus; Rajamanickam, R., Ed.; IntechOpen: London, UK, 2016; Chapter 12. [Google Scholar]
- Bzhalava, D.; Eklund, C.; Dillner, J. International standardization and classification of human papillomavirus types. Virology 2015, 476, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Folliero, V.; Dell’Annunziata, F.; Chianese, A.; Morone, M.V.; Mensitieri, F.; Di Spirito, F.; Mollo, A.; Amato, M.; Galdiero, M.; Dal Piaz, F.; et al. Epigenetic and Genetic Keys to Fight HPV-Related Cancers. Cancers 2023, 15, 5583. [Google Scholar] [CrossRef]
- Graham Sheila, V. The human papillomavirus replication cycle, and its links to cancer progression: A comprehensive review. Clin. Sci. 2017, 131, 2201–2221. [Google Scholar] [CrossRef]
- Kurg, R. The Role of E2 Proteins in Papillomavirus DNA Replication. In DNA Replication-Current Advances; Herve, S., Ed.; IntechOpen: London, UK, 2011; Chapter 28. [Google Scholar]
- Kajitani, N.; Satsuka, A.; Kawate, A.; Sakai, H. Productive Lifecycle of Human Papillomaviruses that Depends Upon Squamous Epithelial Differentiation. Front. Microbiol. 2012, 3, 152. [Google Scholar] [CrossRef]
- Sedman, T.; Sedman, J.; Stenlund, A. Binding of the E1 and E2 proteins to the origin of replication of bovine papillomavirus. J. Virol. 1997, 71, 2887–2896. [Google Scholar] [CrossRef]
- Ustav, M.; Stenlund, A. Transient replication of BPV-1 requires two viral polypeptides encoded by the E1 and E2 open reading frames. Embo J. 1991, 10, 449–457. [Google Scholar] [CrossRef]
- Loo, Y.M.; Melendy, T. Recruitment of replication protein A by the papillomavirus E1 protein and modulation by single-stranded DNA. J. Virol. 2004, 78, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Clower, R.V.; Fisk, J.C.; Melendy, T. Papillomavirus E1 protein binds to and stimulates human topoisomerase I. J. Virol. 2006, 80, 1584–1587. [Google Scholar] [CrossRef] [PubMed]
- Masterson, P.J.; Stanley, M.A.; Lewis, A.P.; Romanos, M.A. A C-terminal helicase domain of the human papillomavirus E1 protein binds E2 and the DNA polymerase alpha-primase p68 subunit. J. Virol. 1998, 72, 7407–7419. [Google Scholar] [CrossRef]
- Chojnacki, M.; Melendy, T. The human papillomavirus DNA helicase E1 binds, stimulates, and confers processivity to cellular DNA polymerase epsilon. Nucleic Acids Res. 2018, 46, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Majerciak, V.; Zheng, Z.M. HPV16 and HPV18 Genome Structure, Expression, and Post-Transcriptional Regulation. Int. J. Mol. Sci. 2022, 23, 4943. [Google Scholar] [CrossRef]
- Evande, R.; Rana, A.; Biswas-Fiss, E.E.; Biswas, S.B. Protein–DNA Interactions Regulate Human Papillomavirus DNA Replication, Transcription, and Oncogenesis. Int. J. Mol. Sci. 2023, 24, 8493. [Google Scholar] [CrossRef]
- Yilmaz, G.; Biswas-Fiss, E.E.; Biswas, S.B. Genetic variations in the DNA replication origins of human papillomavirus family correlate with their oncogenic potential. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 979–990. [Google Scholar] [CrossRef]
- McBride, A.A. The papillomavirus E2 proteins. Virology 2013, 445, 57–79. [Google Scholar] [CrossRef]
- Graham, S.V. Human Papillomavirus E2 Protein: Linking Replication, Transcription, and RNA Processing. J. Virol. 2016, 90, 8384–8388. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, G.; Biswas-Fiss, E.E.; Biswas, S.B. Sequence-Dependent Interaction of the Human Papillomavirus E2 Protein with the DNA Elements on Its DNA Replication Origin. Int. J. Mol. Sci. 2023, 24, 6555. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Mohr, I.; Fouts, E.; Lim, D.A.; Nohaile, M.; Botchan, M. The E1 protein of bovine papilloma virus 1 is an ATP-dependent DNA helicase. Proc. Natl. Acad. Sci. USA 1993, 90, 5086–5090. [Google Scholar] [CrossRef]
- Wilson, V.G.; West, M.; Woytek, K.; Rangasamy, D. Papillomavirus E1 proteins: Form, function, and features. Virus Genes 2002, 24, 275–290. [Google Scholar] [CrossRef]
- Castro-Muñoz, L.J.; Manzo-Merino, J.; Muñoz-Bello, J.O.; Olmedo-Nieva, L.; Cedro-Tanda, A.; Alfaro-Ruiz, L.A.; Hidalgo-Miranda, A.; Madrid-Marina, V.; Lizano, M. The Human Papillomavirus (HPV) E1 protein regulates the expression of cellular genes involved in immune response. Sci. Rep. 2019, 9, 13620. [Google Scholar] [CrossRef]
- Baedyananda, F.; Sasivimolrattana, T.; Chaiwongkot, A.; Varadarajan, S.; Bhattarakosol, P. Role of HPV16 E1 in cervical carcinogenesis. Front. Cell. Infect. Microbiol. 2022, 12, 955847. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Evande, R.; Biswas-Fiss, E.; Biswas, S. Abstract 1637 Molecular mechanism of HPV DNA replication initiation, mediated by E1 and E2 proteins. J. Biol. Chem. 2024, 300 (Suppl. S3), 106943. [Google Scholar] [CrossRef]
- Bergvall, M.; Melendy, T.; Archambault, J. The E1 proteins. Virology 2013, 445, 35–56. [Google Scholar] [CrossRef]
- Hughes, F.J.; Romanos, M.A. E1 protein of human papillomavirus is a DNA helicase/ATPase. Nucleic Acids Res. 1993, 21, 5817–5823. [Google Scholar] [CrossRef]
- Clertant, P.; Seif, I. A common function for polyoma virus large-T and papillomavirus E1 proteins? Nature 1984, 311, 276–279. [Google Scholar] [CrossRef]
- Castella, S.; Bingham, G.; Sanders, C.M. Common determinants in DNA melting and helicase-catalysed DNA unwinding by papillomavirus replication protein E1. Nucleic Acids Res. 2006, 34, 3008–3019. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, O.; Earnshaw, D.; Sarginson, G.; Del Vecchio, A.; Tsai, J.; Kallender, H.; Amegadzie, B.; Browne, M. Characterization of the helicase and ATPase activity of human papillomavirus type 6b E1 protein. J. Gen. Virol. 1996, 77, 1805–1809. [Google Scholar] [CrossRef]
- Schuck, S.; Stenlund, A. Surface mutagenesis of the bovine papillomavirus E1 DNA binding domain reveals residues required for multiple functions related to DNA replication. J. Virol. 2006, 80, 7491–7499. [Google Scholar] [CrossRef]
- Liu, J.S.; Kuo, S.R.; Makhov, A.M.; Cyr, D.M.; Griffith, J.D.; Broker, T.R.; Chow, L.T. Human Hsp70 and Hsp40 chaperone proteins facilitate human papillomavirus-11 E1 protein binding to the origin and stimulate cell-free DNA replication. J. Biol. Chem. 1998, 273, 30704–30712. [Google Scholar] [CrossRef] [PubMed]
- Lusky, M.; Hurwitz, J.; Seo, Y.S. The bovine papillomavirus E2 protein modulates the assembly of but is not stably maintained in a replication-competent multimeric E1-replication origin complex. Proc. Natl. Acad. Sci. USA 1994, 91, 8895–8899. [Google Scholar] [CrossRef] [PubMed]
- Liblekas, L.; Piirsoo, A.; Laanemets, A.; Tombak, E.-M.; Laaneväli, A.; Ustav, E.; Ustav, M.; Piirsoo, M. Analysis of the Replication Mechanisms of the Human Papillomavirus Genomes. Front. Microbiol. 2021, 12, 738125. [Google Scholar] [CrossRef]
- Liu, J.S.; Kuo, S.R.; Broker, T.R.; Chow, L.T. The functions of human papillomavirus type 11 E1, E2, and E2C proteins in cell-free DNA replication. J. Biol. Chem. 1995, 270, 27283–27291. [Google Scholar] [CrossRef]
- Seo, Y.S.; Müller, F.; Lusky, M.; Hurwitz, J. Bovine papilloma virus (BPV)-encoded E1 protein contains multiple activities required for BPV DNA replication. Proc. Natl. Acad. Sci. USA 1993, 90, 702–706. [Google Scholar] [CrossRef]
- Thorner, L.K.; Lim, D.A.; Botchan, M.R. DNA-binding domain of bovine papillomavirus type 1 E1 helicase: Structural and functional aspects. J. Virol. 1993, 67, 6000–6014. [Google Scholar] [CrossRef]
- Chen, G.; Stenlund, A. The E1 initiator recognizes multiple overlapping sites in the papillomavirus origin of DNA replication. J. Virol. 2001, 75, 292–302. [Google Scholar] [CrossRef]
- Chen, G.; Stenlund, A. Characterization of the DNA-binding domain of the bovine papillomavirus replication initiator E1. J. Virol. 1998, 72, 2567–2576. [Google Scholar] [CrossRef]
- Sun, Y.N.; Lu, J.Z.; McCance, D.J. Mapping of HPV-11 E1 binding site and determination of other important cis elements for replication of the origin. Virology 1996, 216, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Auster, A.S.; Joshua-Tor, L. The DNA-binding Domain of Human Papillomavirus Type 18 E1: Crystal structure, dimerization, and DNA binding. J. Biol. Chem. 2004, 279, 3733–3742. [Google Scholar] [CrossRef] [PubMed]
- Frattini, M.G.; Laimins, L.A. The role of the E1 and E2 proteins in the replication of human papillomavirus type 31b. Virology 1994, 204, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.Y.; Ma, T.; Liu, J.S.; Kuo, S.R.; Jin, G.; Broker, T.R.; Harper, J.W.; Chow, L.T. HeLa cells are phenotypically limiting in cyclin E/CDK2 for efficient human papillomavirus DNA replication. J. Biol. Chem. 2000, 275, 6167–6174. [Google Scholar] [CrossRef]
- Kazlauskas, D.; Krupovic, M.; Venclovas, Č. The logic of DNA replication in double-stranded DNA viruses: Insights from global analysis of viral genomes. Nucleic Acids Res. 2016, 44, 4551–4564. [Google Scholar] [CrossRef]
- Wan, L.; Toland, S.; Robinson-McCarthy, L.R.; Lee, N.; Schaich, M.A.; Hengel, S.R.; Li, X.; Bernstein, K.A.; Van Houten, B.; Chang, Y.; et al. Unlicensed origin DNA melting by MCV and SV40 polyomavirus LT proteins is independent of ATP-dependent helicase activity. Proc. Natl. Acad. Sci. USA 2023, 120, e2308010120. [Google Scholar] [CrossRef]
- Dixon, E.P.; Pahel, G.L.; Rocque, W.J.; Barnes, J.A.; Lobe, D.C.; Hanlon, M.H.; Alexander, K.A.; Chao, S.F.; Lindley, K.; Phelps, W.C. The E1 helicase of human papillomavirus type 11 binds to the origin of replication with low sequence specificity. Virology 2000, 270, 345–357. [Google Scholar] [CrossRef]
- Rocque, W.J.; Porter, D.J.T.; Barnes, J.A.; Dixon, E.P.; Lobe, D.C.; Su, J.-L.; Willard, D.H.; Gaillard, R.; Condreay, J.P.; Clay, W.C.; et al. Replication-Associated Activities of Purified Human Papillomavirus Type 11 E1 Helicase. Protein Expr. Purif. 2000, 18, 148–159. [Google Scholar] [CrossRef]
- Santucci, S.; Bonne-Andréa, C.; Clertant, P. Bovine papillomavirus type 1 E1 ATPase activity does not depend on binding to DNA nor to viral E2 protein. J. Gen. Virol. 1995, 76 Pt 5, 1129–1140. [Google Scholar] [CrossRef]
- Enemark, E.J.; Joshua-Tor, L. Mechanism of DNA translocation in a replicative hexameric helicase. Nature 2006, 442, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Biswas, E.E.; Barnes, M.H.; Moir, D.T.; Biswas, S.B. An essential DnaB helicase of Bacillus anthracis: Identification, characterization, and mechanism of action. J. Bacteriol. 2009, 191, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Stahl, H.; Dröge, P.; Knippers, R. DNA helicase activity of SV40 large tumor antigen. Embo J. 1986, 5, 1939–1944. [Google Scholar] [CrossRef]
- Seguin, S.P.; Ireland, A.W.; Gupta, T.; Wright, C.M.; Miyata, Y.; Wipf, P.; Pipas, J.M.; Gestwicki, J.E.; Brodsky, J.L. A screen for modulators of large T antigen’s ATPase activity uncovers novel inhibitors of Simian Virus 40 and BK virus replication. Antivir. Res. 2012, 96, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Ha, T. Need for Speed: Mechanical Regulation of a Replicative Helicase. Cell 2007, 129, 1249–1250. [Google Scholar] [CrossRef]
- Martinez, M.P.; Jones, J.M.; Bruck, I.; Kaplan, D.L. Origin DNA Melting-An Essential Process with Divergent Mechanisms. Genes 2017, 8, 26. [Google Scholar] [CrossRef]
- Jameson, K.H.; Wilkinson, A.J. Control of Initiation of DNA Replication in Bacillus subtilis and Escherichia coli. Genes 2017, 8, 22. [Google Scholar] [CrossRef]
- Burgers, P.M.J.; Kunkel, T.A. Eukaryotic DNA Replication Fork. Annu. Rev. Biochem. 2017, 86, 417–438. [Google Scholar] [CrossRef]
- Chow, L.T.; Broker, T.R. Papillomavirus DNA Replication. Intervirology 2008, 37, 150–158. [Google Scholar] [CrossRef]
- Abbate, E.A.; Berger, J.M.; Botchan, M.R. The X-ray structure of the papillomavirus helicase in complex with its molecular matchmaker E2. Genes Dev. 2004, 18, 1981–1996. [Google Scholar] [CrossRef]
- Bramhill, D.; Kornberg, A. Duplex opening by dnaA protein at novel sequences in initiation of replication at the origin of the E. coli chromosome. Cell 1988, 52, 743–755. [Google Scholar] [CrossRef]
- Hayashi, C.; Miyazaki, E.; Ozaki, S.; Abe, Y.; Katayama, T. DnaB helicase is recruited to the replication initiation complex via binding of DnaA domain I to the lateral surface of the DnaB N-terminal domain. J. Biol. Chem. 2020, 295, 11131–11143. [Google Scholar] [CrossRef] [PubMed]
- Biswas, E.E.; Chen, P.-H.; Biswas, S.B. Modulation of enzymatic activities of Escherichia coli DnaB helicase by single-stranded DNA-binding proteins. Nucleic Acids Res. 2002, 30, 2809–2816. [Google Scholar] [CrossRef]
- Hoskins, J.R.; Wickner, S.; Doyle, S.M. Bacterial Hsp90 ATPase Assays. In Chaperones: Methods and Protocols; Calderwood, S.K., Prince, T.L., Eds.; Springer: New York, NY, USA, 2018; pp. 199–207. [Google Scholar]
- Biswas, E.E.; Biswas, S.B. Mechanism of DNA binding by the DnaB helicase of Escherichia coli: Analysis of the roles of domain gamma in DNA binding. Biochemistry 1999, 38, 10929–10939. [Google Scholar] [CrossRef] [PubMed]
- Biswas, E.E.; Biswas, S.B.; Bishop, J.E. The dnaB protein of Escherichia coli: Mechanism of nucleotide binding, hydrolysis, and modulation by dnaC protein. Biochemistry 1986, 25, 7368–7374. [Google Scholar] [CrossRef] [PubMed]
- Maurya, G.K.; Chaudhary, R.; Pandey, N.; Misra, H.S. Molecular insights into replication initiation in a multipartite genome harboring bacterium Deinococcus radiodurans. J. Biol. Chem. 2021, 296, 100451. [Google Scholar] [CrossRef]
- Selby, C.P.; Sancar, A. Human transcription-repair coupling factor CSB/ERCC6 is a DNA-stimulated ATPase but is not a helicase and does not disrupt the ternary transcription complex of stalled RNA polymerase II. J. Biol. Chem. 1997, 272, 1885–1890. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, A.; Yilmaz, G.; Biswas-Fiss, E.E.; Biswas, S. Mechanisms of Viral DNA Replication of Human Papillomavirus: E2 Protein-Dependent Recruitment of E1 DNA Helicase to the Origin of DNA Replication. Int. J. Mol. Sci. 2025, 26, 4333. https://doi.org/10.3390/ijms26094333
Rana A, Yilmaz G, Biswas-Fiss EE, Biswas S. Mechanisms of Viral DNA Replication of Human Papillomavirus: E2 Protein-Dependent Recruitment of E1 DNA Helicase to the Origin of DNA Replication. International Journal of Molecular Sciences. 2025; 26(9):4333. https://doi.org/10.3390/ijms26094333
Chicago/Turabian StyleRana, Anshul, Gulden Yilmaz, Esther E. Biswas-Fiss, and Subhasis Biswas. 2025. "Mechanisms of Viral DNA Replication of Human Papillomavirus: E2 Protein-Dependent Recruitment of E1 DNA Helicase to the Origin of DNA Replication" International Journal of Molecular Sciences 26, no. 9: 4333. https://doi.org/10.3390/ijms26094333
APA StyleRana, A., Yilmaz, G., Biswas-Fiss, E. E., & Biswas, S. (2025). Mechanisms of Viral DNA Replication of Human Papillomavirus: E2 Protein-Dependent Recruitment of E1 DNA Helicase to the Origin of DNA Replication. International Journal of Molecular Sciences, 26(9), 4333. https://doi.org/10.3390/ijms26094333








