Cobra Three-Finger Toxins Interact with RNA and DNA: Nucleic Acids as Their Putative Biological Targets
Abstract
1. Introduction
2. Results
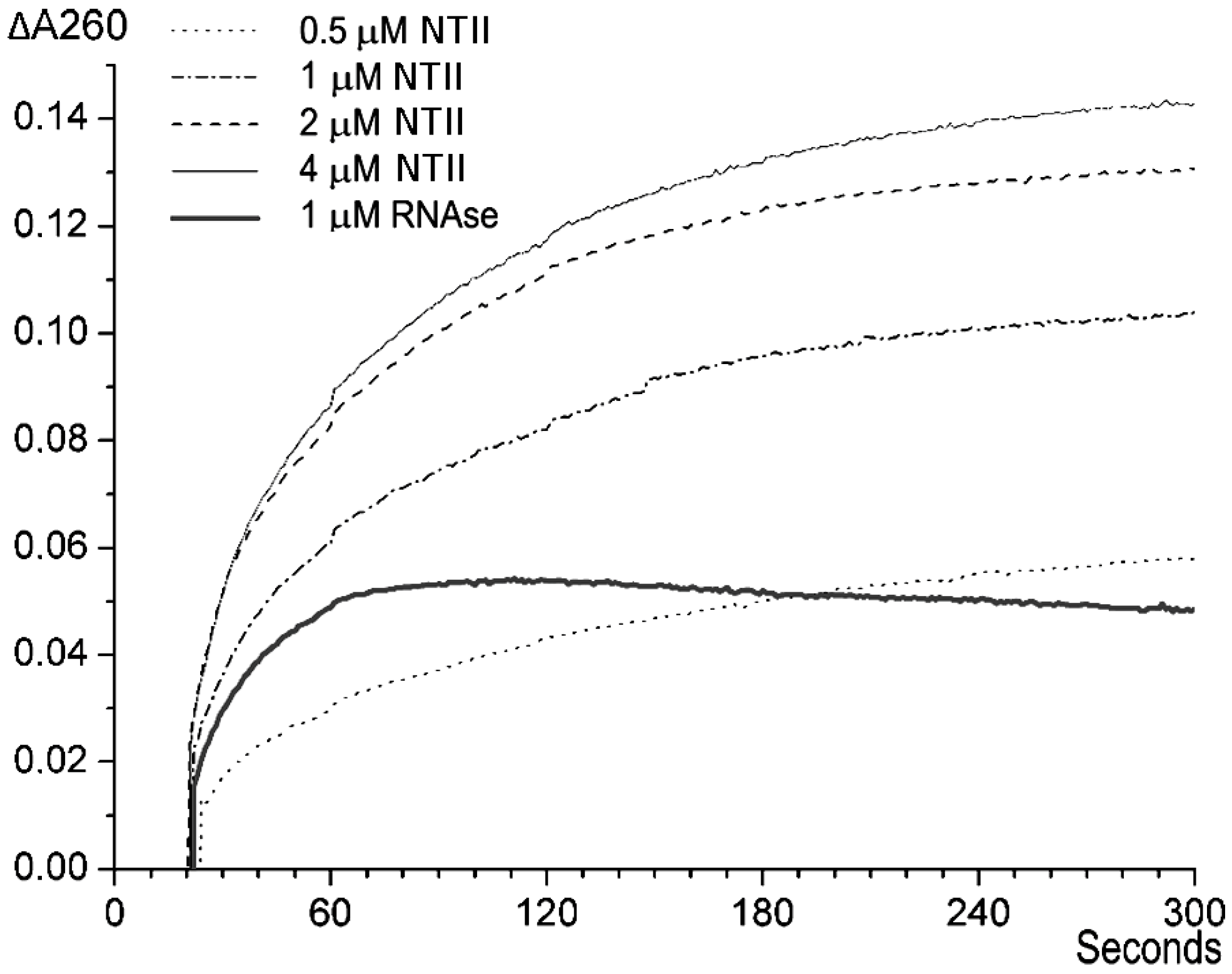
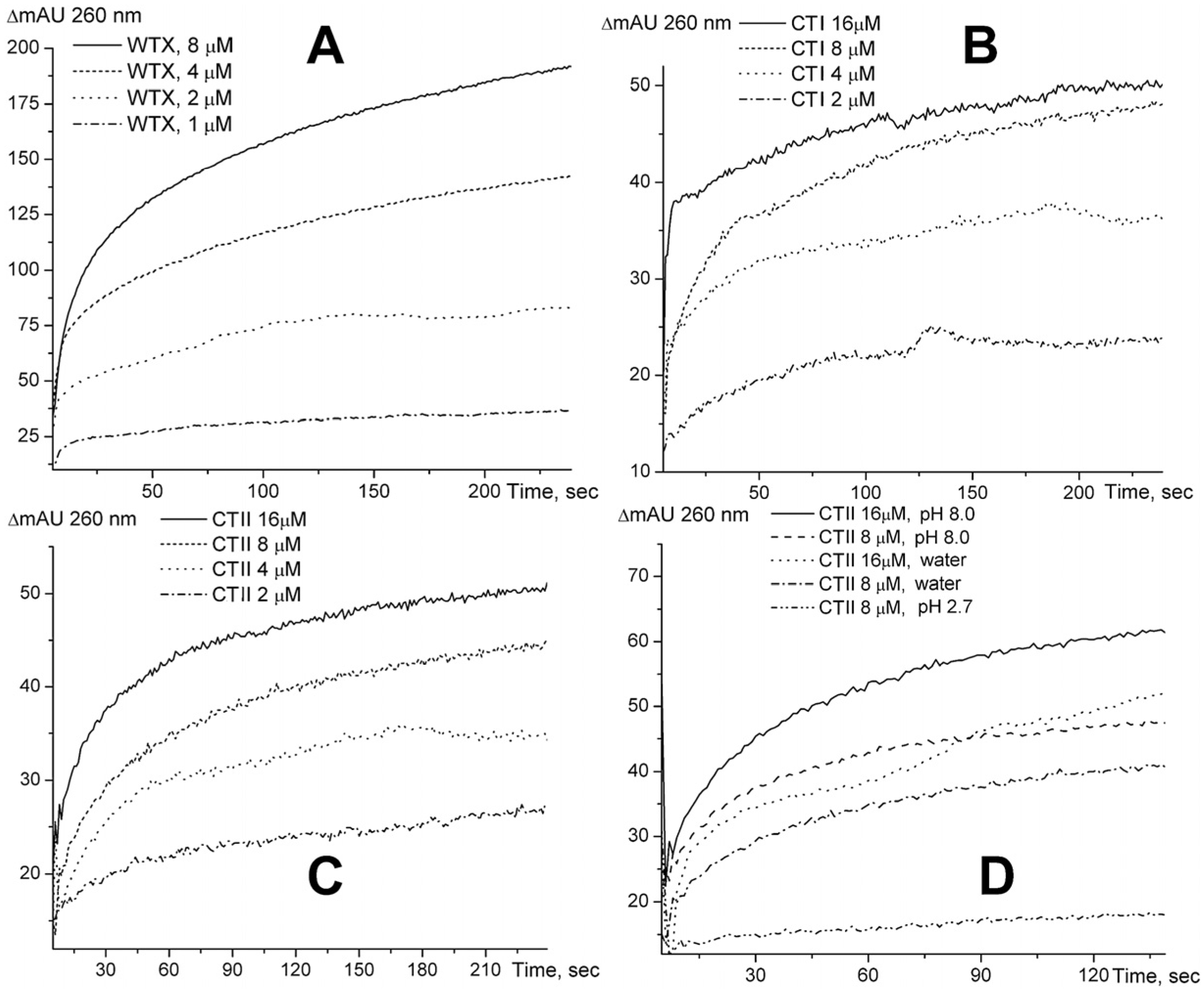
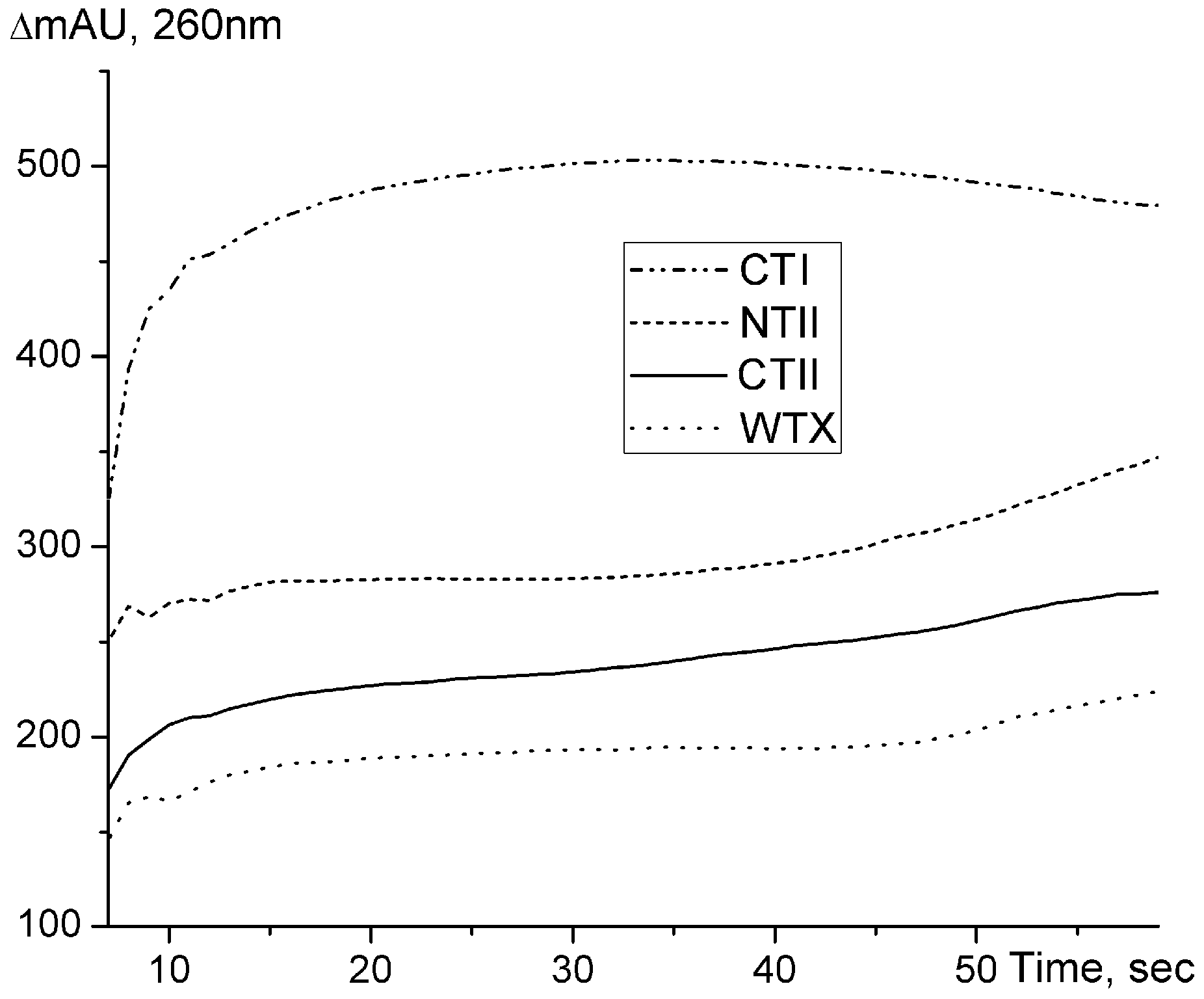
2.1. Spectrophotometry Measurements
2.2. Ion-Paired Reversed-Phase HPLC
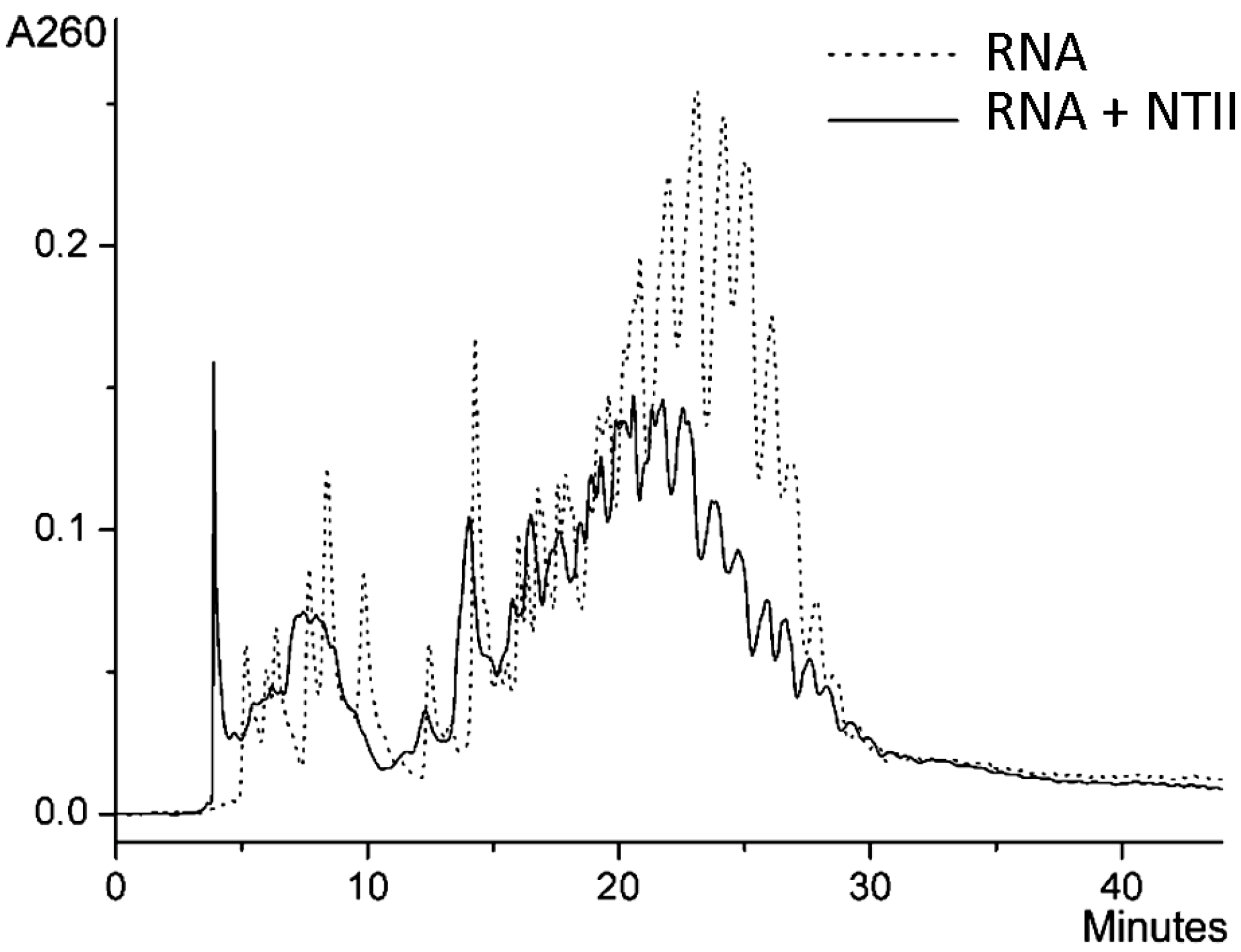
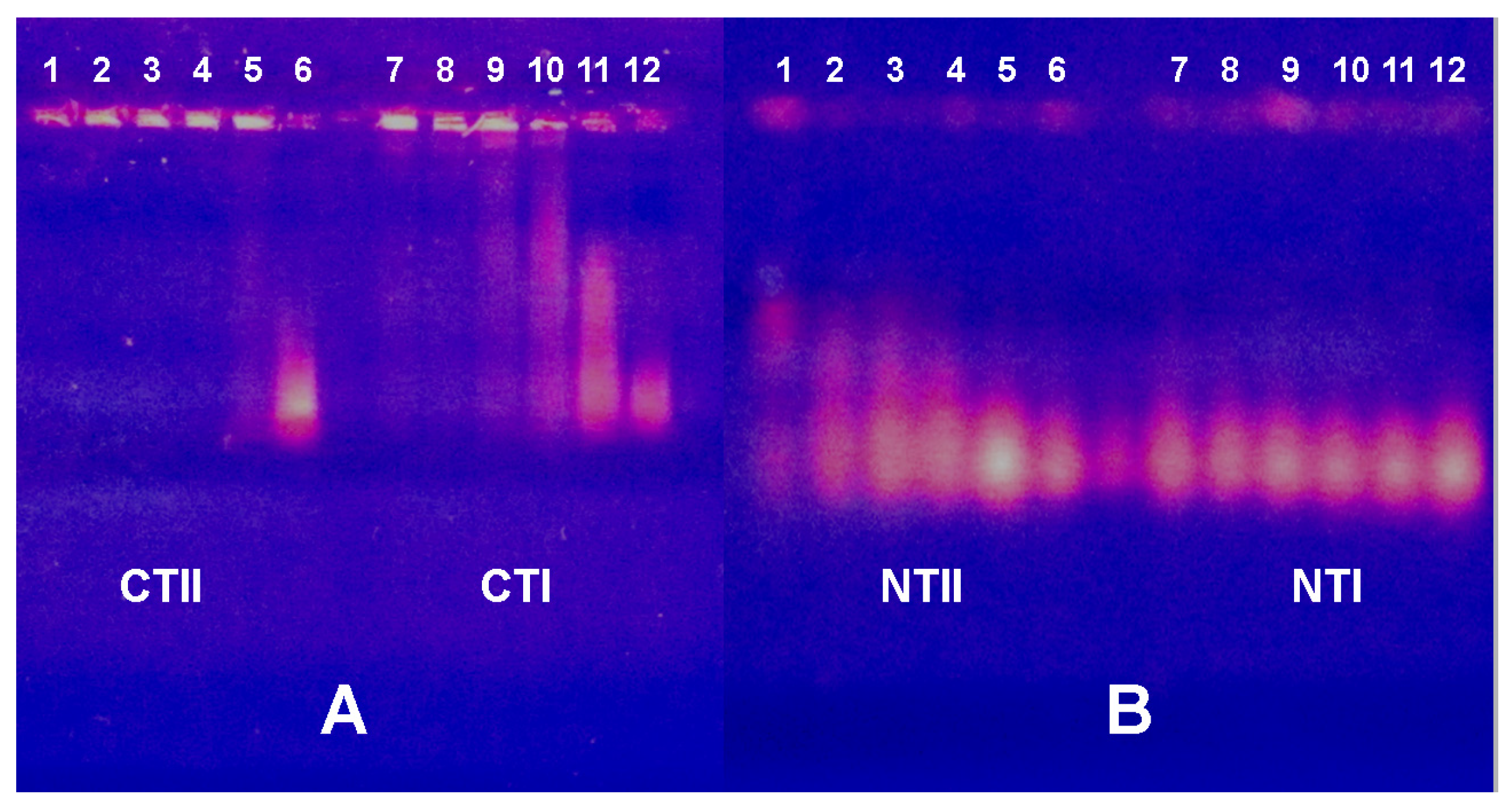
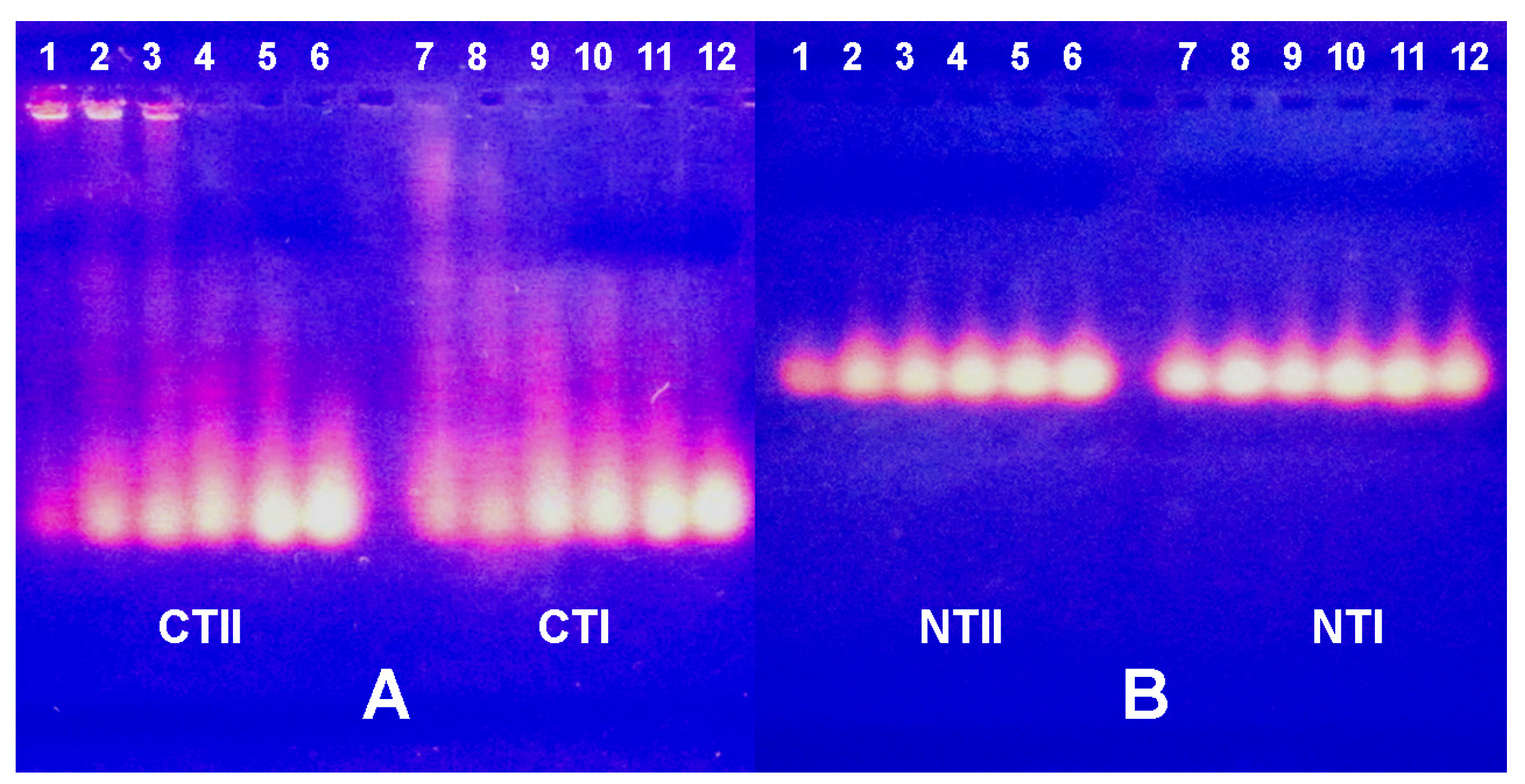
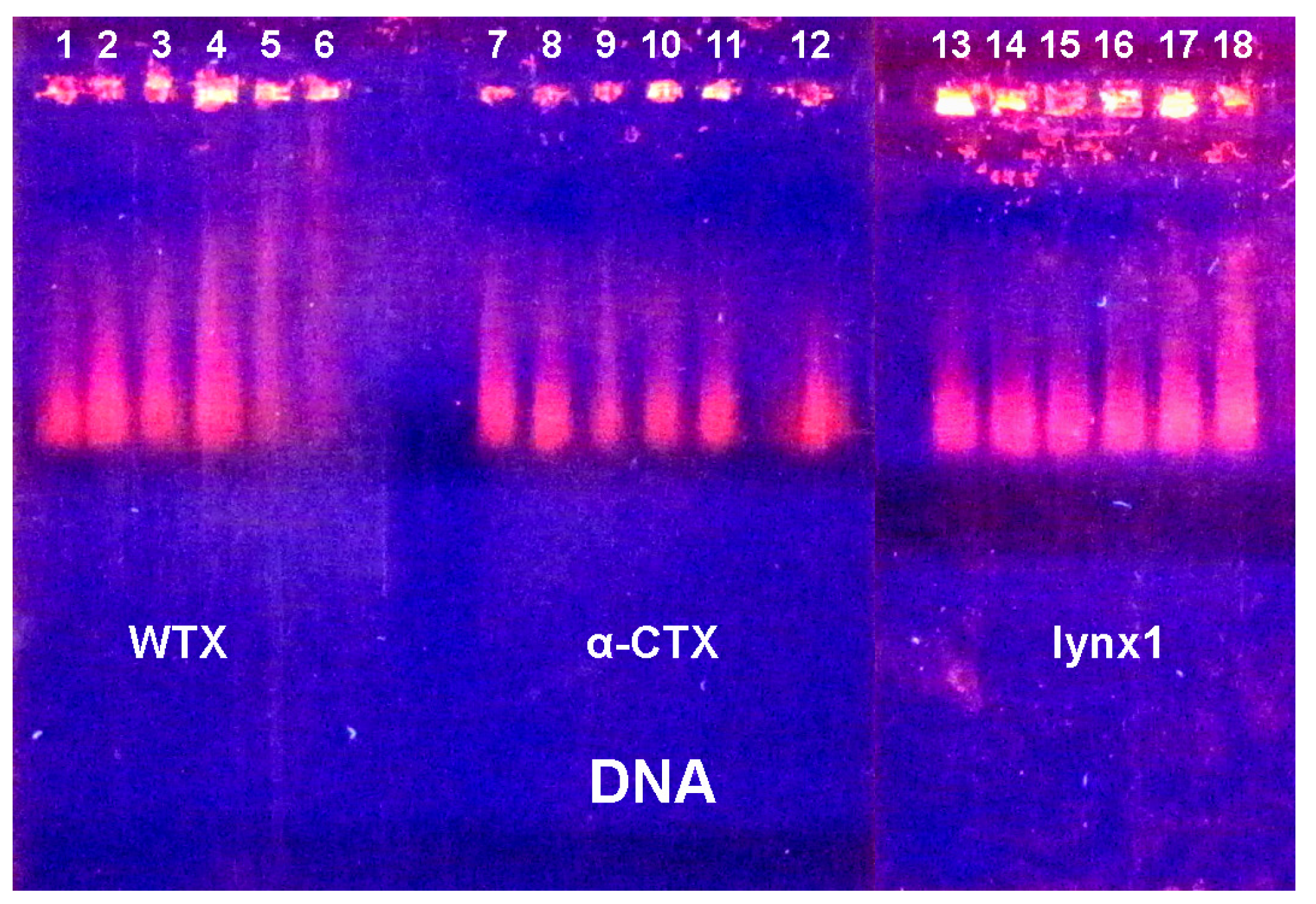
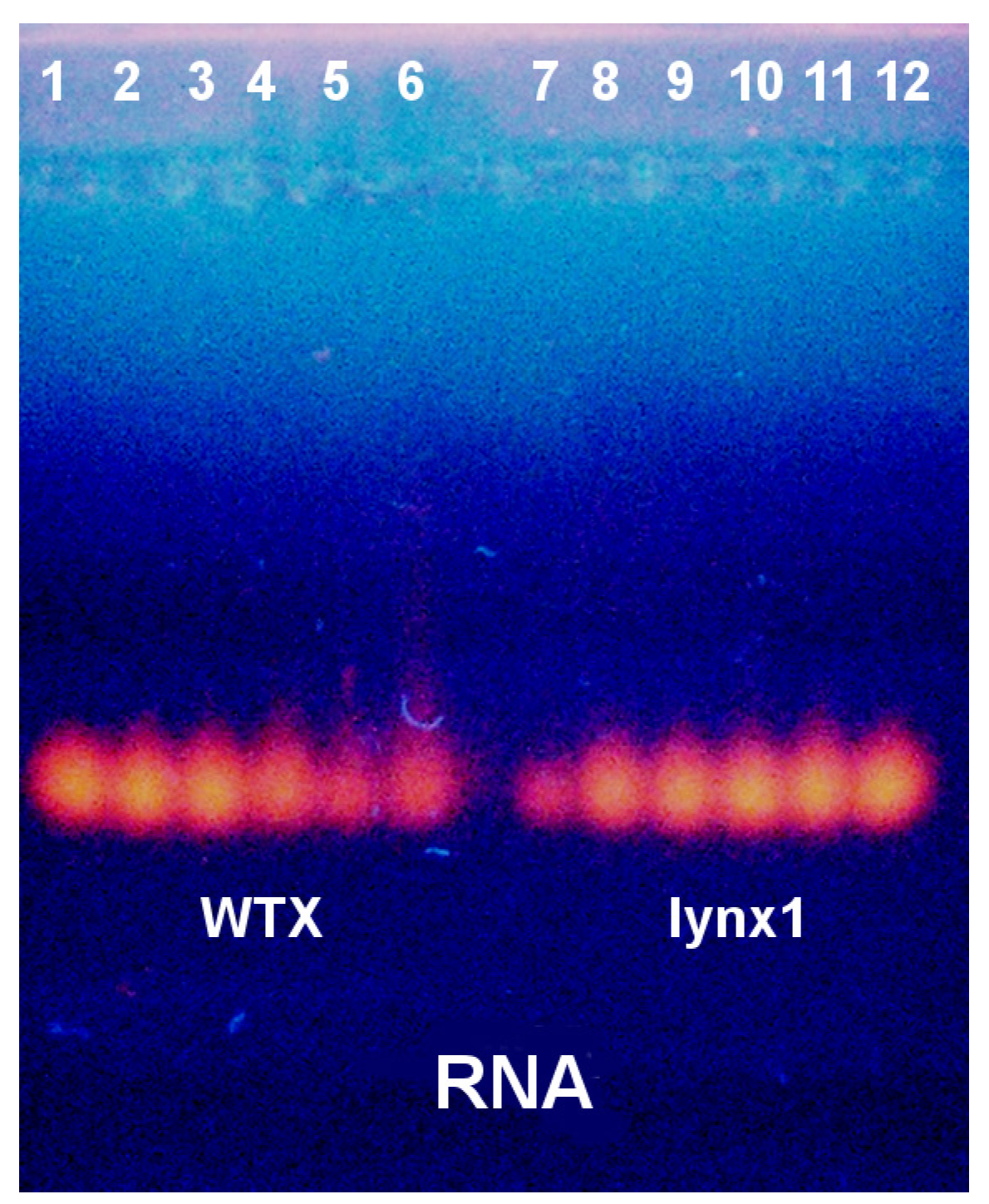
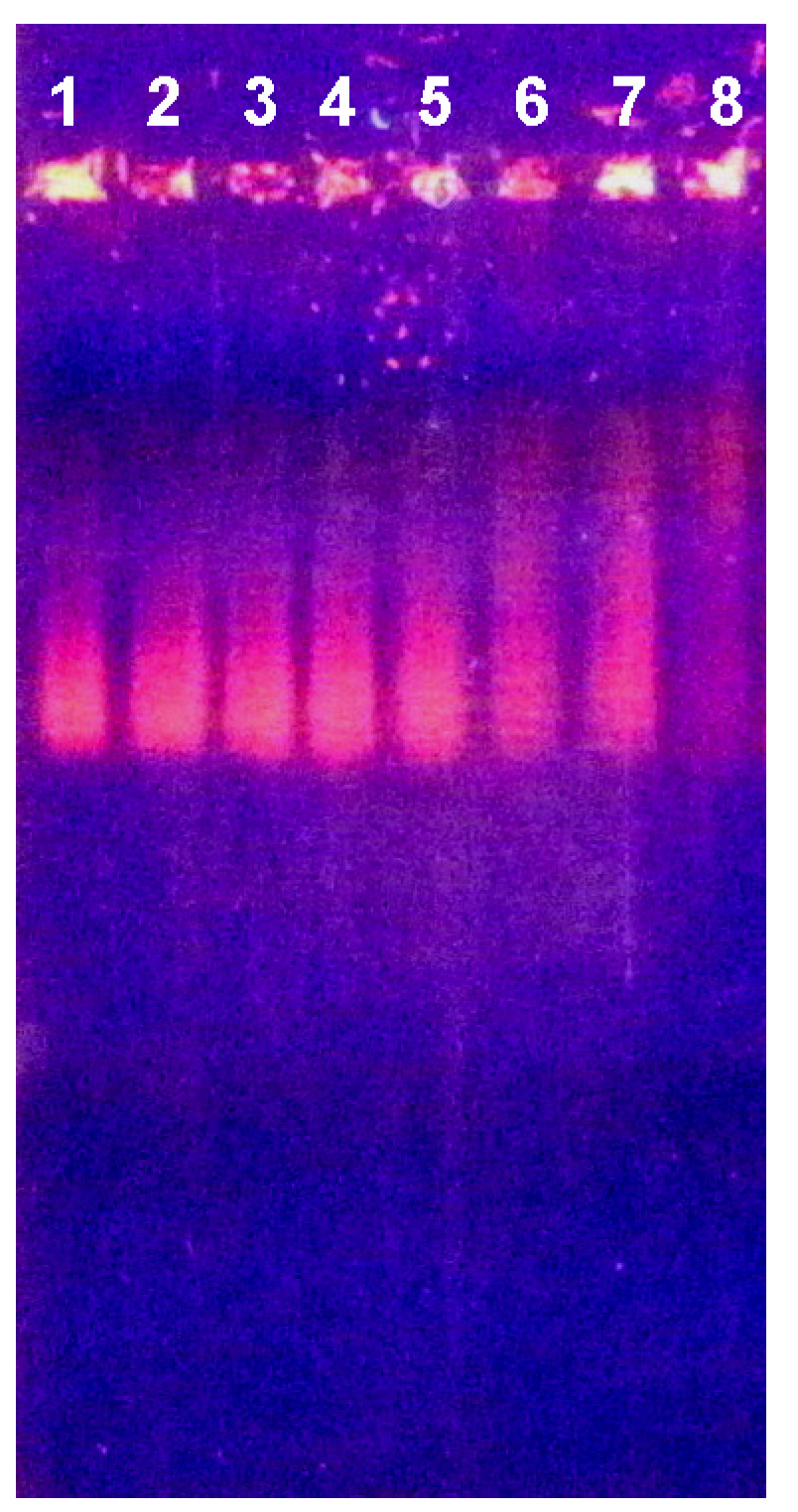
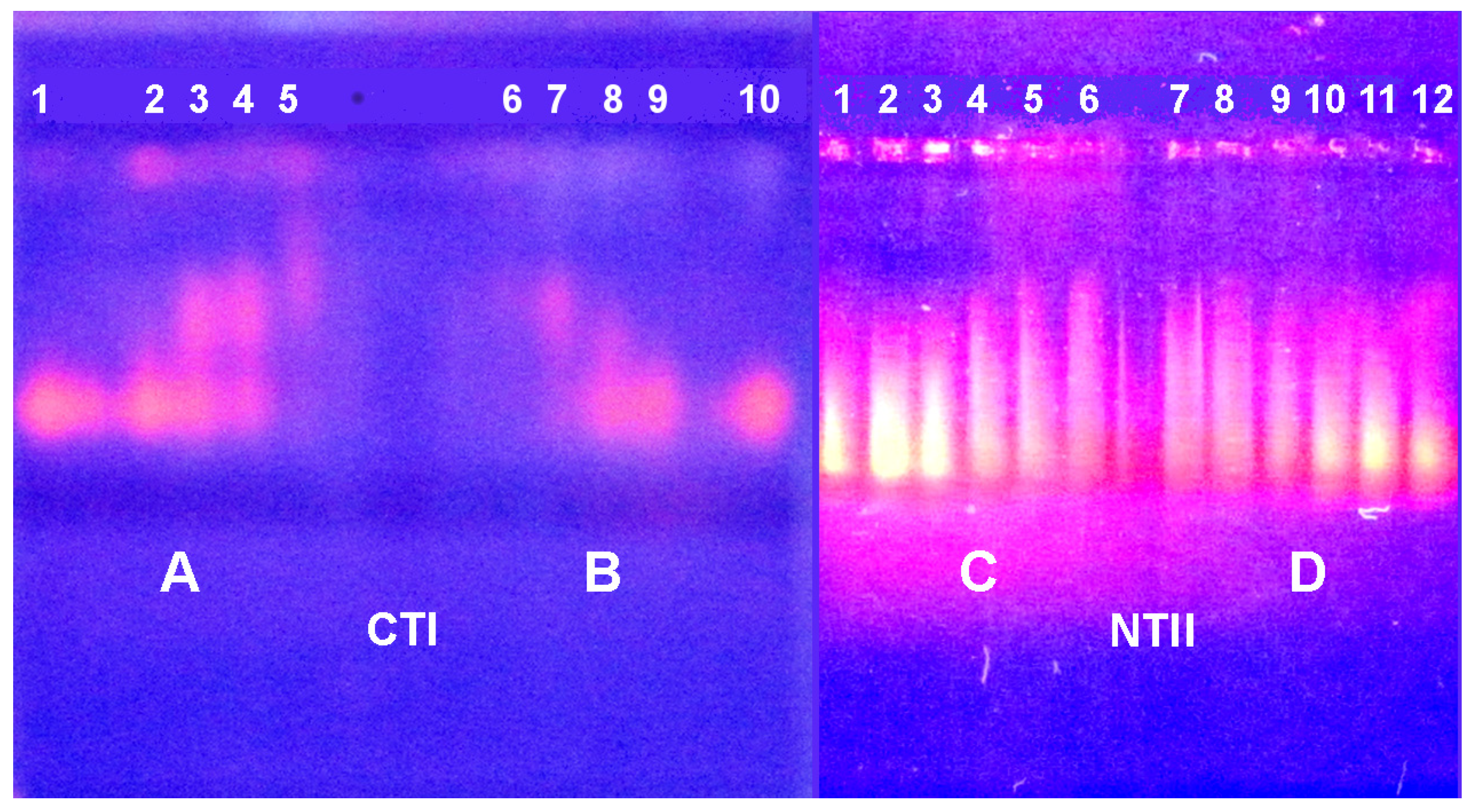
2.3. Electrophoretic Mobility Shift Assay
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Spectrophotometry Measurements
4.3. Ion-Paired Reversed-Phase HPLC
4.4. Electrophoretic Mobility Shift Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| α-CTX | α-Cobratoxin |
| CTI | Cytotoxin I |
| CTII | Cytotoxin II |
| EMSA | Electrophoretic mobility shift assay |
| NTI | Neurotoxin I |
| NTII | Neurotoxin II |
| TFT | Three-finger toxin |
References
- Dutta, S.; Chanda, A.; Kalita, B.; Islam, T.; Patra, A.; Mukherjee, A.K. Proteomic analysis to unravel the complex venom proteome of eastern India Naja naja: Correlation of venom composition with its biochemical and pharmacological properties. J. Proteom. 2017, 156, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Tan, K.Y.; Wong, K.Y.; Tan, N.H.; Chong, H.P. Equatorial Spitting Cobra (Naja sumatrana) from Malaysia (Negeri Sembilan and Penang), Southern Thailand, and Sumatra: Comparative Venom Proteomics, Immunoreactivity and Cross-Neutralization by Antivenom. Toxins 2022, 14, 522. [Google Scholar] [CrossRef]
- McFarlane, L.O.; Pukala, T.L. Proteomic Investigation of Cape Cobra (Naja nivea) Venom Reveals First Evidence of Quaternary Protein Structures. Toxins 2024, 16, 63. [Google Scholar] [CrossRef]
- Utkin, Y.; Sunagar, K.; Jackson, T.N.; Reeks, T.; Fry, B.G. Three-finger toxins (3FTxs). In Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery; Fry, B.G., Ed.; Oxford University Press: New York, NY, USA, 2015; pp. 215–227. [Google Scholar]
- Nirthanan, S. Snake three-finger α-neurotoxins and nicotinic acetylcholine receptors: Molecules, mechanisms and medicine. Biochem. Pharmacol. 2020, 181, 114168. [Google Scholar] [CrossRef] [PubMed]
- Kasaragod, V.B.; Mortensen, M.; Hardwick, S.W.; Wahid, A.A.; Dorovykh, V.; Chirgadze, D.Y.; Smart, T.G.; Miller, P.S. Mechanisms of inhibition and activation of extrasynaptic αβ GABAA receptors. Nature 2022, 602, 529–533. [Google Scholar] [CrossRef]
- Zhang, H.L.; Han, R.; Gu, Z.L.; Chen, Z.X.; Chen, B.W.; Reid, P.F.; Raymond, L.N.; Qin, Z.H. A short-chain alpha-neurotoxin from Naja naja atra produces potent cholinergic-dependent analgesia. Neurosci. Bull. 2006, 22, 103–109. [Google Scholar] [PubMed]
- Konshina, A.G.; Boldyrev, I.A.; Utkin, Y.N.; Omel’kov, A.V.; Efremov, R.G. Snake cytotoxins bind to membranes via interactions with phosphatidylserine head groups of lipids. PLoS ONE 2011, 6, e19064. [Google Scholar] [CrossRef]
- Suzuki-Matsubara, M.; Athauda, S.B.; Suzuki, Y.; Matsubara, K.; Moriyama, A. Comparison of the primary structures, cytotoxicities, and affinities to phospholipids of five kinds of cytotoxins from the venom of Indian cobra, Naja naja. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2016, 179, 158–164. [Google Scholar] [CrossRef]
- Patel, H.V.; Vyas, A.A.; Vyas, K.A.; Liu, Y.S.; Chiang, C.M.; Chi, L.M.; Wu, W.G. Heparin and heparan sulfate bind to snake cardiotoxin. Sulfated oligosaccharides as a potential target for cardiotoxin action. J. Biol. Chem. 1997, 272, 1484–1492. [Google Scholar] [CrossRef]
- Kao, P.H.; Lin, S.R.; Chang, L.S. Interaction of Naja naja atra cardiotoxin 3 with H-trisaccharide modulates its hemolytic activity and membrane-damaging activity. Toxicon 2010, 55, 1387–1395. [Google Scholar] [CrossRef]
- Liang, Y.X.; Zhang, Z.Y.; Zhang, R. Antinociceptive Effect of Najanalgesin from Naja naja Atra in a Neuropathic Pain Model via Inhibition of c-Jun NH2-terminal Kinase. Chin. Med. J. 2015, 128, 2340–2345. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, G.; Krishnaswamy, T.; Kumar, S.; Yu, C. Binding of nucleotide triphosphates to cardiotoxin analogue II from the Taiwan cobra venom (Naja naja atra). Elucidation of the structural interactions in the dATP-cardiotoxin analogue ii complex. J. Biol. Chem. 1999, 274, 17869–17875. [Google Scholar] [CrossRef]
- Shi, Y.J.; Chen, Y.J.; Hu, W.P.; Chang, L.S. Detection of Naja atra Cardiotoxin Using Adenosine-Based Molecular Beacon. Toxins 2017, 9, E24. [Google Scholar] [CrossRef] [PubMed]
- Mahalakshmi, Y.V.; Jagannadham, M.V.; Pandit, M.W. Ribonuclease from cobra snake venom: Purification by affinity chromatography and further characterization. IUBMB Life 2000, 49, 309–316. [Google Scholar] [CrossRef]
- Pan, C.T.; Lin, C.C.; Lin, I.J.; Chien, K.Y.; Lin, Y.S.; Chang, H.H.; Wu, W.G. The evolution and structure of snake venom phosphodiesterase (svPDE) highlight its importance in venom actions. eLife 2023, 12, e83966. [Google Scholar] [CrossRef] [PubMed]
- Dhananjaya, B.L.; D’Souza, C.J. The pharmacological role of nucleotidases in snake venoms. Cell Biochem. Funct. 2010, 28, 171–177. [Google Scholar] [CrossRef]
- Yap, M.K.K.; Tan, N.H.; Fung, S.Y. Biochemical and toxinological characterization of Naja sumatrana (Equatorial spitting cobra) venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2011, 17, 451–459. [Google Scholar]
- Vasilenko, S.K.; Ryte, V.C. Isolation of highly purified ribonuclease from cobra (Naja oxiana) venom. Biokhimiia. 1975, 40, 578–583. (In Russian) [Google Scholar]
- Nguyen, T.V.; Osipov, A.V. A study of ribonuclease activity in venom of Vietnam cobra. J. Anim. Sci. Technol. 2017, 59, 20. [Google Scholar] [CrossRef]
- Waters, T.R.; Connolly, B.A. Continuous spectrophotometric assay for restriction endonucleases using synthetic oligodeoxynucleotides and based on hyperchromic effect. Anal. Biochem. 1992, 204, 204–209. [Google Scholar] [CrossRef]
- Libonati, M.; Sorrentino, S. Degradation of double-stranded RNA by mammalian pancreatic-type ribonucleases. Methods Enzymol. 2001, 341, 234–248. [Google Scholar] [PubMed]
- Eller, C.H.; Lomax, J.E.; Raines, R.T. Bovine brain ribonuclease is the functional homolog of human ribonuclease 1. J. Biol. Chem 2014, 289, 25996–26006. [Google Scholar] [CrossRef]
- Gotte, G.; Menegazzi, M. Biological Activities of Secretory RNases: Focus on Their Oligomerization to Design Antitumor Drugs. Front. Immunol. 2019, 10, 2626. [Google Scholar] [CrossRef]
- Utkin, Y.N. Last decade update for three-finger toxins: Newly emerging structures and biological activities. World J. Biol. Chem. 2019, 10, 17–27. [Google Scholar] [CrossRef]
- Azarani, A.; Hecker, K.H. RNA analysis by ion-pair reversed-phase high performance liquid chromatography. Nucleic Acids Res. 2001, 29, E7. [Google Scholar] [CrossRef] [PubMed]
- Gjerde, D.T.; Hoang, L.; Hornby, D. RNA Purification and Analysis: Sample Preparation, Extraction, Chromatography; Wiley-VCH Verlag: Weinheim, Germany, 2009; 194p. [Google Scholar]
- Fornstedt, T.; Enmark, M. Separation of therapeutic oligonucleotides using ion-pair reversed-phase chromatography based on fundamental separation science. J. Chromatogr. Open 2023, 3, 100079. [Google Scholar] [CrossRef]
- Laniel, M.A.; Béliveau, A.; Guérin, S.L. Electrophoretic Mobility Shift Assays for the Analysis of DNA-Protein Interactions. In DNA-Protein Interactions: Principles and Protocols, 2nd ed.; Moss, T., Ed.; Humana Press: Totowa, NJ, USA, 2001; Volume 148, pp. 13–30. [Google Scholar]
- Gagnon, K.T.; Maxwell, E.S. Electrophoretic Mobility Shift Assay for Characterizing RNA–Protein Interaction. In RNA. Methods in Molecular Biology; Nielsen, H., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 275–291. [Google Scholar]
- Cozzolino, F.; Iacobucci, I.; Monaco, V.; Monti, M. Protein-DNA/RNA Interactions: An Overview of Investigation Methods in the -Omics Era. J. Proteome Res. 2021, 20, 3018–3030. [Google Scholar] [CrossRef]
- Katkar, G.D.; Sundaram, M.S.; Naveen Kumar, S.K.; Swethakumar, B.; Sharma, R.D.; Paul, M.; Vishalakshi, G.J.; Devaraja, S.; Girish, K.S.; Kemparaju, K. NETosis and lack of DNase activity are key factors in Echis carinatus venom-induced tissue destruction. Nat. Commun. 2016, 7, 11361. [Google Scholar] [CrossRef] [PubMed]
- Swethakumar, B.; Naveen Kumar, S.K.; Katkar, G.D.; Girish, K.S.; Kemparaju, K. Inhibition of Echis carinatus venom by DNA, a promising therapeutic molecule for snakebite management. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1115–1125. [Google Scholar] [CrossRef]
- Chen, P.C.; Hayashi, M.A.; Oliveira, E.B.; Karpel, R.L. DNA-interactive properties of crotamine, a cell-penetrating polypeptide and a potential drug carrier. PLoS ONE 2012, 7, e48913. [Google Scholar] [CrossRef]
- Karpel, R.L. DNA Binding Proteins and Drug Delivery Vehicles: Tales of Elephants and Snakes. Curr. Protein Pept. Sci. 2015, 16, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, L.H.; Shamaileh, H.A.; Edwards, S.L.; Taran, E.; Veedu, R.N. Rapid one-step selection method for generating nucleic acid aptamers: Development of a DNA aptamer against α-bungarotoxin. PLoS ONE 2012, 7, e41702. [Google Scholar] [CrossRef]
- Chen, Y.J.; Tsai, C.Y.; Hu, W.P.; Chang, L.S. Aptamers against Taiwan banded krait α-bungarotoxin recognize Taiwan cobra cardiotoxins. Toxins 2016, 8, 66. [Google Scholar] [CrossRef]
- El Hakim, A.E.; Gamal-Eldeen, A.M.; Shahein, Y.E.; Mansour, N.M.; Wahby, A.F.; Abouelella, A.M. Purification and characterization of a cytotoxic neurotoxin-like protein from Naja haje haje venom that induces mitochondrial apoptosis pathway. Arch. Toxicol. 2011, 85, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Bandopadhyay, P.; Halder, S.; Sarkar, M.; Kumar Bhunia, S.; Dey, S.; Gomes, A.; Giri, B. Molecular modeling of NK-CT1, from Indian monocellate cobra (Naja kaouthia) and its docking interaction with human DNA topoisomerase II alpha. Bioinformation 2016, 12, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.M. Multiple modes of RNA recognition by zinc finger proteins. Curr. Opin. Struct. Biol. 2005, 15, 367–373. [Google Scholar] [CrossRef]
- Eom, K.S.; Cheong, J.S.; Lee, S.J. Structural Analyses of Zinc Finger Domains for Specific Interactions with DNA. J. Microbiol. Biotechnol. 2016, 26, 2019–2029. [Google Scholar] [CrossRef]
- Forwood, J.K.; Jans, D.A. Quantitative analysis of DNA-protein interactions using double-labeled native gel electrophoresis and fluorescence-based imaging. Electrophoresis 2006, 27, 3166–3170. [Google Scholar] [CrossRef]
- Szpotkowski, K.; Wójcik, K.; Kurzyńska-Kokorniak, A. Structural studies of protein-nucleic acid complexes: A brief overview of the selected techniques. Comput. Struct. Biotechnol. J. 2023, 21, 2858–2872. [Google Scholar] [CrossRef]
- Kalita, B.; Utkin, Y.N.; Mukherjee, A.K. Current Insights in the Mechanisms of Cobra Venom Cytotoxins and Their Complexes in Inducing Toxicity: Implications in Antivenom Therapy. Toxins 2022, 14, 839. [Google Scholar] [CrossRef]
- Chong, H.P.; Tan, K.Y.; Tan, C.H. Cytotoxicity of Snake Venoms and Cytotoxins From Two Southeast Asian Cobras (Naja sumatrana, Naja kaouthia): Exploration of Anticancer Potential, Selectivity, and Cell Death Mechanism. Front. Mol. Biosci. 2020, 7, 583587. [Google Scholar] [CrossRef]
- Derakhshani, A.; Silvestris, N.; Hemmat, N.; Asadzadeh, Z.; Abdoli Shadbad, M.; Nourbakhsh, N.S.; Mobasheri, L.; Vahedi, P.; Shahmirzaie, M.; Brunetti, O.; et al. Targeting TGF-β-Mediated SMAD Signaling Pathway via Novel Recombinant Cytotoxin II: A Potent Protein from Naja naja oxiana Venom in Melanoma. Molecules 2020, 25, 5148. [Google Scholar] [CrossRef] [PubMed]
- Hiu, J.J.; Yap, M.K.K. The myth of cobra venom cytotoxin: More than just direct cytolytic actions. Toxicon X 2022, 14, 100123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, F.; Chen, Z.; Shrivastava, I.H.; Gasanoff, E.S.; Dagda, R.K. Naja mossambica mossambica Cobra Cardiotoxin Targets Mitochondria to Disrupt Mitochondrial Membrane Structure and Function. Toxins 2019, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Skok, M. Mitochondrial nicotinic acetylcholine receptors: Mechanisms of functioning and biological significance. Int. J. Biochem. Cell Biol. 2022, 143, 106138. [Google Scholar] [CrossRef]
- Pachiappan, A.; Thwin, M.M.; Manikandanm, J.; Gopalakrishnakone, P. Glial inflammation and neurodegeneration induced by candoxin, a novel neurotoxin from Bungarus candidus venom: Global gene expression analysis using microarray. Toxicon 2005, 46, 883–899. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, X.; Chen, Z. Screening and functional analysis of glioma-related genes induced by candoxin. Mol. Med. Rep. 2014, 10, 767–772. [Google Scholar] [CrossRef]
- Hiu, J.J.; Yap, M.K.K. The effects of Naja sumatrana venom cytotoxin, sumaCTX on alteration of the secretome in MCF-7 breast cancer cells following membrane permeabilization. Int. J. Biol. Macromol. 2021, 184, 776–786. [Google Scholar] [CrossRef]
- Chen, K.C.; Chiou, Y.L.; Kao, P.H.; Lin, S.R.; Chang, L.S. Taiwan cobra cardiotoxins induce apoptotic death of human neuroblastoma SK-N-SH cells mediated by reactive oxygen species generation and mitochondrial depolarization. Toxicon 2008, 51, 624–634. [Google Scholar] [CrossRef]
- Gasanoff, E.S.; Dagda, R.K. Cobra Venom Cytotoxins as a Tool for Probing Mechanisms of Mitochondrial Energetics and Understanding Mitochondrial Membrane Structure. Toxins 2024, 16, 287. [Google Scholar] [CrossRef]
- Chen, X.; Lv, P.; Liu, J.; Xu, K. Apoptosis of human hepatocellular carcinoma cell (HepG2) induced by cardiotoxin III through S-phase arrest. Exp. Toxicol. Pathol. 2009, 61, 307–315. [Google Scholar] [CrossRef]
- Das, T.; Bhattacharya, S.; Biswas, A.; Gupta, S.D.; Gomes, A.; Gomes, A. Inhibition of leukemic U937 cell growth by induction of apoptosis, cell cycle arrest and suppression of VEGF, MMP-2 and MMP-9 activities by cytotoxin protein NN-32 purified from Indian spectacled cobra (Naja naja) venom. Toxicon 2013, 65, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Bychkov, M.L.; Shulepko, M.A.; Vasileva, V.Y.; Sudarikova, A.V.; Kirpichnikov, M.P.; Lyukmanova, E.N. ASIC1a Inhibitor mambalgin-2 Suppresses the Growth of Leukemia Cells by Cell Cycle Arrest. Acta Naturae 2020, 12, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Song, J. Delivery, structure, and function of bacterial genotoxins. Virulence 2022, 13, 1199–1215. [Google Scholar] [CrossRef] [PubMed]
- Galvani, N.C.; Vilela, T.C.; Domingos, A.C.; Fagundes, M.Í.; Bosa, L.M.; Della Vechia, I.C.; Scussel, R.; Pereira, M.; Steiner, B.T.; Damiani, A.P.; et al. Genotoxicity evaluation induced by Tityus serrulatus scorpion venom in mice. Toxicon 2017, 140, 132–138. [Google Scholar] [CrossRef]
- Gajski, G.; Domijan, A.M.; Žegura, B.; Štern, A.; Gerić, M.; Novak Jovanović, I.; Vrhovac, I.; Madunić, J.; Breljak, D.; Filipič, M.; et al. Melittin induced cytogenetic damage, oxidative stress and changes in gene expression in human peripheral blood lymphocytes. Toxicon 2016, 110, 56–67. [Google Scholar] [CrossRef]
- de Souza, A.H.; da Rosa, L.G.; Uliano, M.R.; da Silva Prado, L.; Ferraz, A.G.; Conter, L.U.; Grivicich, I.; Dallegrave, E.; Gomez, M.V.; Picada, J.N. Evaluation of DNA damage in spinal cord and mutagenic effect of a Phα1β recombinant toxin with analgesic properties from the Phoneutria nigriventer spider. Basic. Clin. Pharmacol. Toxicol. 2019, 124, 615–620. [Google Scholar] [CrossRef]
- Sjakste, N.; Gajski, G. A Review on Genotoxic and Genoprotective Effects of Biologically Active Compounds of Animal Origin. Toxins 2023, 15, 165. [Google Scholar] [CrossRef]
- Mukherjee, A.K.; Chatterjee, S.; Majumder, S.; Saikia, D.; Thakur, R.; Chatterjee, A. Characterization of a pro-angiogenic, novel peptide from Russell’s viper (Daboia russelii russelii) venom. Toxicon 2014, 77, 26–31. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Riyasdeen, A.; Islam, M. Scorpion Venom Causes Upregulation of p53 and Downregulation of Bcl-xL and BID Protein Expression by Modulating Signaling Proteins Erk1/2 and STAT3, and DNA Damage in Breast and Colorectal Cancer Cell Lines. Integr. Cancer Ther. 2018, 17, 271–281. [Google Scholar] [CrossRef]
- Lewinska, A.; Bocian, A.; Petrilla, V.; Adamczyk-Grochala, J.; Szymura, K.; Hendzel, W.; Kaleniuk, E.; Hus, K.K.; Petrillova, M.; Wnuk, M. Snake venoms promote stress-induced senescence in human fibroblasts. J. Cell Physiol. 2019, 234, 6147–6160. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, E.; Maselli, V.; Falanga, A.; Gesuele, R.; Galdiero, S.; Fulgione, D.; Guida, M. Integrated analysis of the ecotoxicological and genotoxic effects of the antimicrobial peptide melittin on Daphnia magna and Pseudokirchneriella subcapitata. Environ. Pollut. 2015, 203, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.R.T.; Aissa, A.F.; Ribeiro, D.L.; Hernandes, L.C.; Machado, C.S.; Bianchi, M.L.P.; Sampaio, S.V.; Antunes, L.M.G. The toxin BjussuLAAO-II induces oxidative stress and DNA damage, upregulates the inflammatory cytokine genes TNF and IL6, and downregulates the apoptotic-related genes BAX, BCL2 and RELA in human Caco-2 cells. Int. J. Biol. Macromol. 2018, 109, 212–219. [Google Scholar] [CrossRef]
- Grishin, E.V.; Sukhikh, A.P.; Slobodyan, L.N.; Ovchinnikov, Y.A.; Sorokin, V.M. Amino acid sequence of neurotoxin I from Naja naja oxiana venom. FEBS Lett. 1974, 45, 118–121. [Google Scholar] [CrossRef]
- Grishin, E.V.; Sukhikh, A.P.; Lukyanchuk, N.N.; Slobodyan, L.N.; Lipkin, V.M.; Ovchinnikov, Y.A.; Sorokin, V.M. Amino acid sequence of neurotoxin II from Naja naja oxiana venom. FEBS Lett. 1973, 36, 77–78. [Google Scholar] [CrossRef]
- Grishin, E.V.; Sukhikh, A.P.; Adamovich, T.B.; Ovchinnikov, Y.A. Isolation, properties and sequence determination of the two cytotoxins from the venom of the Middle-Asian cobra Naja naja oxiana. Bioorg. Khim. 1976, 2, 1018–1034. (In Russian) [Google Scholar]
- Utkin, Y.N.; Kukhtina, V.V.; Maslennikov, I.V.; Eletsky, A.V.; Starkov, V.G.; Weise, C.; Franke, P.; Hucho, F.; Tsetlin, V.I. First tryptophan-containing weak neurotoxin from cobra venom. Toxicon 2001, 39, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Kukhtina, V.V.; Weise, C.; Osipov, A.V.; Starkov, V.G.; Titov, M.I.; Esipov, S.E.; Ovchinnikova, T.V.; Tsetlin, V.I.; Utkin, Y.N. MALDI-mass spectrometry for identification of new proteins in snake venoms. Russ. J. Bioorganic Chem. 2000, 26, 803–807. (In Russian) [Google Scholar] [CrossRef]
- Shulepko, M.A.; Lyukmanova, E.N.; Kasheverov, I.E.; Dolgikh, D.A.; Tsetlin, V.I.; Kirpichnikov, M.P. Bacterial expression of the water-soluble domain of Lynx1, an endogenous neuromodulator of human nicotinic receptors. Russ. J. Bioorg. Chem. 2011, 37, 543–549. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osipov, A.V.; Starkov, V.G.; Tsetlin, V.I.; Utkin, Y.N. Cobra Three-Finger Toxins Interact with RNA and DNA: Nucleic Acids as Their Putative Biological Targets. Int. J. Mol. Sci. 2025, 26, 4291. https://doi.org/10.3390/ijms26094291
Osipov AV, Starkov VG, Tsetlin VI, Utkin YN. Cobra Three-Finger Toxins Interact with RNA and DNA: Nucleic Acids as Their Putative Biological Targets. International Journal of Molecular Sciences. 2025; 26(9):4291. https://doi.org/10.3390/ijms26094291
Chicago/Turabian StyleOsipov, Alexey V., Vladislav G. Starkov, Victor I. Tsetlin, and Yuri N. Utkin. 2025. "Cobra Three-Finger Toxins Interact with RNA and DNA: Nucleic Acids as Their Putative Biological Targets" International Journal of Molecular Sciences 26, no. 9: 4291. https://doi.org/10.3390/ijms26094291
APA StyleOsipov, A. V., Starkov, V. G., Tsetlin, V. I., & Utkin, Y. N. (2025). Cobra Three-Finger Toxins Interact with RNA and DNA: Nucleic Acids as Their Putative Biological Targets. International Journal of Molecular Sciences, 26(9), 4291. https://doi.org/10.3390/ijms26094291






