Role of T3 in the Regulation of GRP78 on Granulosa Cells in Rat Ovaries
Abstract
:1. Introduction
2. Results
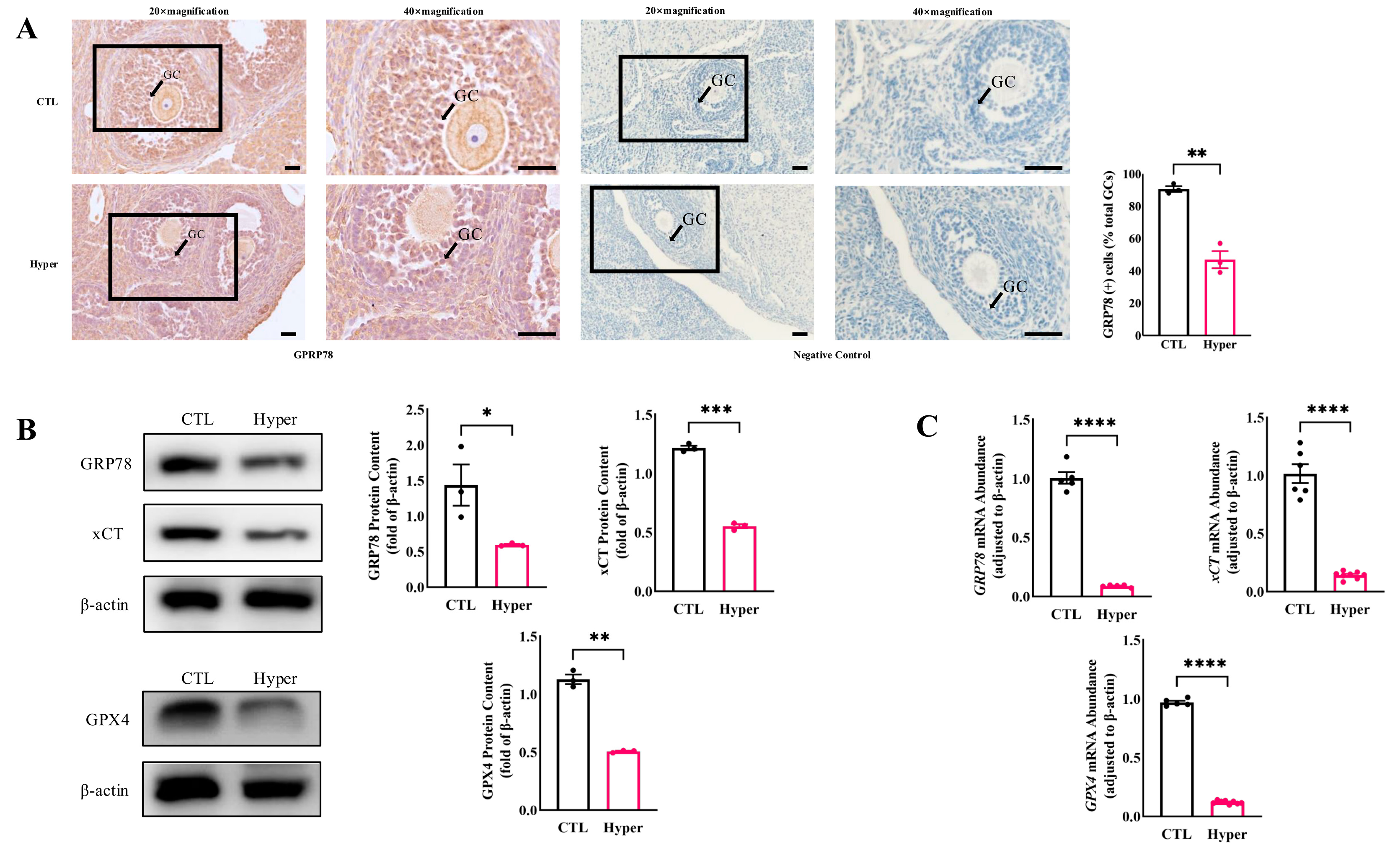
2.1. Decreased Expression of GRP78 and Ferroptosis Markers in Hyperthyroid Rat Ovaries
2.2. GRP78 Overexpression Rescues T3-Induced Ferroptosis in Rat Granulosa Cells
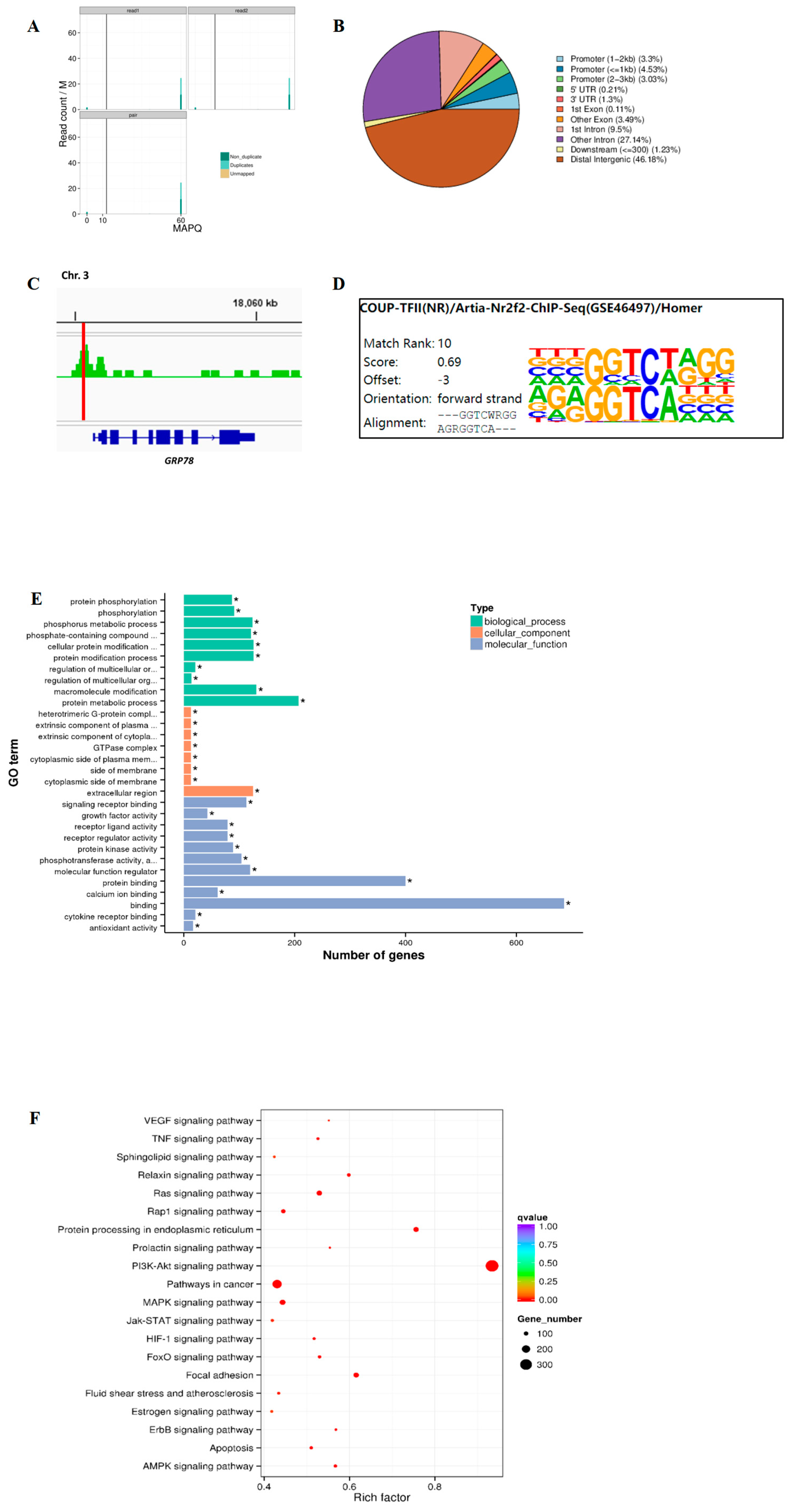
2.3. Genome-Wide Analysis of TRβ Binding and GRP78 Regulation Pathways in Rat Granulosa Cells
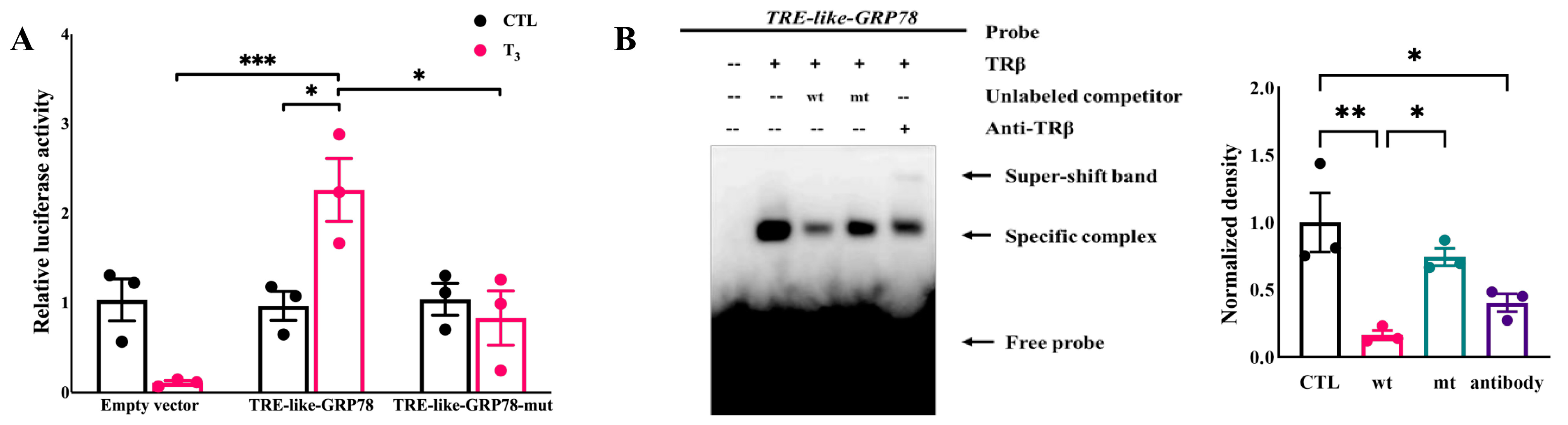
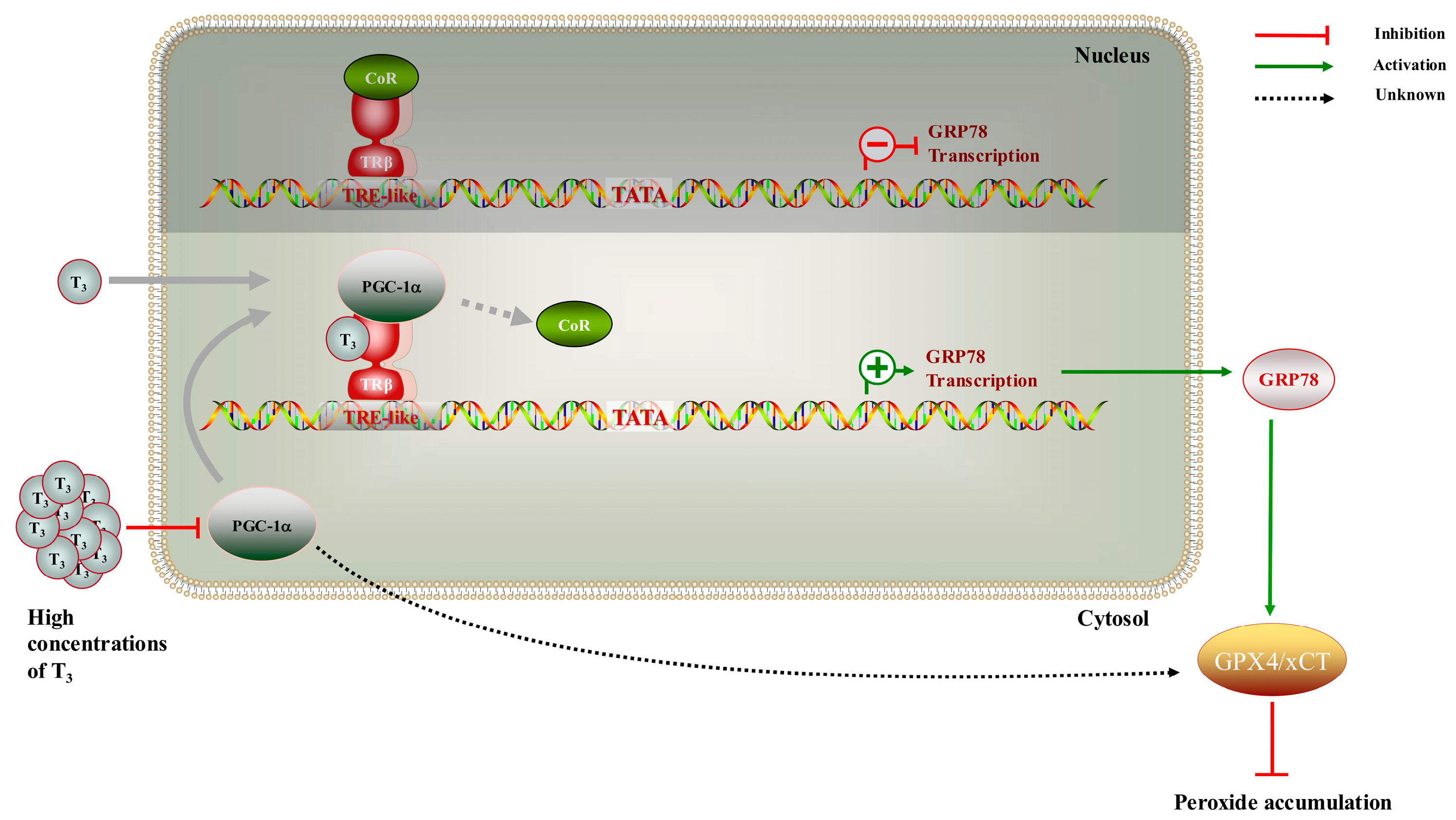
2.4. T3 Activates the Transcriptional Activity of GRP78 by the TRE-like Sequence Binding to TRβ
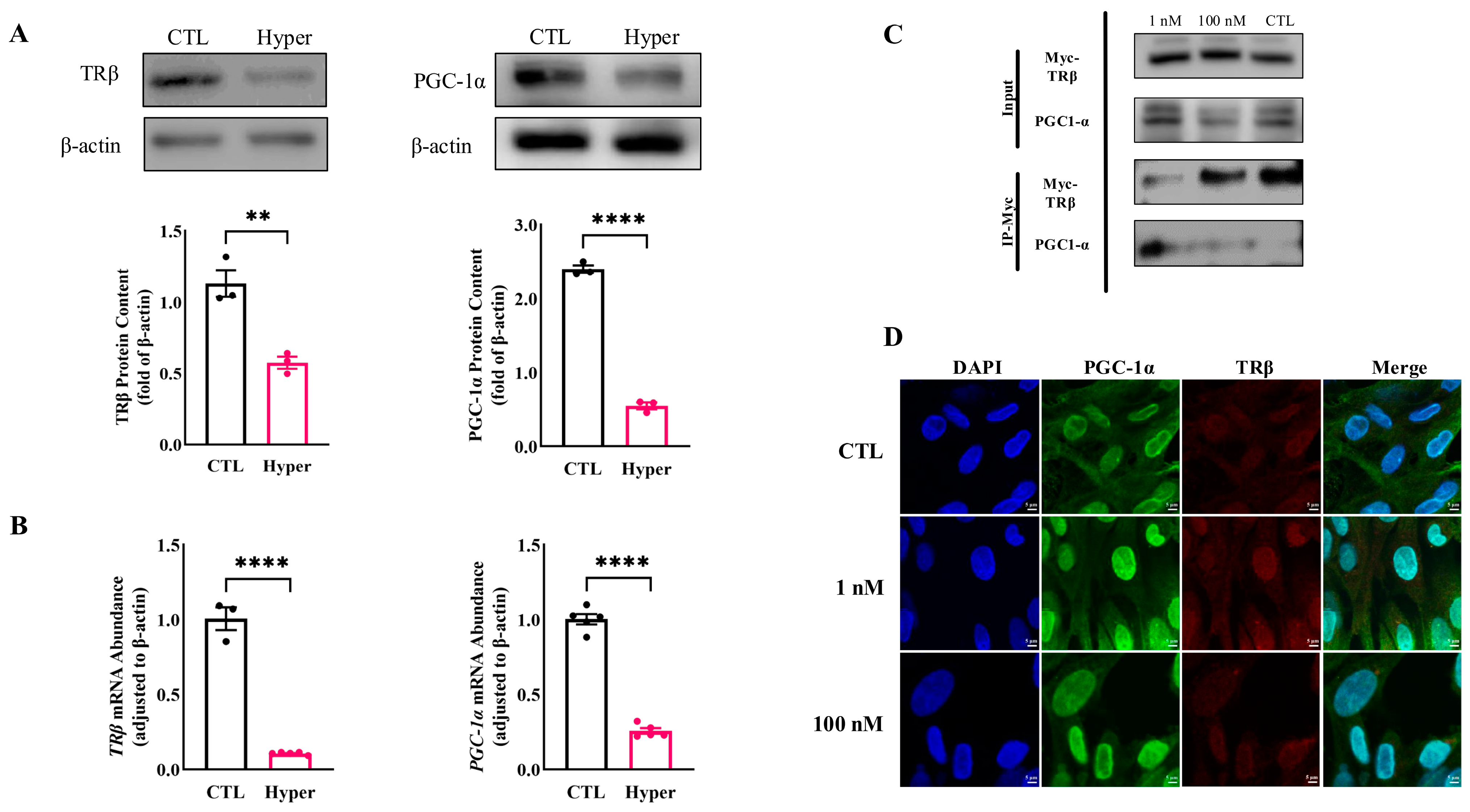
2.5. Interaction of TRβ with PGC-1α Involved in T3-Mediated Regulation of GRP78
3. Discussion
4. Materials and Methods
4.1. Animal Materials and Cell Collection
4.2. Cell Culture, Transfections, and Dual Luciferase Assay
4.3. Co-Immunoprecipitation (Co-IP)
4.4. Electrophoretic Mobility-Shift Assays (EMSAs)
4.5. Library Construction by CUT & Tag
4.6. Sequencing
4.7. Immunohistochemistry (IHC)
4.8. Immunofluorescence (IF)
4.9. Real-Time Quantitative Reverse Transcription PCR (RT-qPCR)
4.10. Immunoblotting (Western Blot)
4.11. ROS Measurement of GCs
4.12. Cell Viability Assays
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Han, Y.; Tian, Y.; Weng, X.; Hu, X.; Liu, W.; Heng, D.; Xu, K.; Yang, Y.; Zhang, C. Regulation by 3,5,3′-tri-iodothyronine and FSH of cytochrome P450 family 19 (CYP19) expression in mouse granulosa cells. Reprod. Fertil. Dev. 2018, 30, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tian, Y.; Ding, Y.; Heng, D.; Xu, K.; Liu, W.; Zhang, C. Role of CYP51 in the Regulation of T3 and FSH-Induced Steroidogenesis in Female Mice. Endocrinology 2017, 158, 3974–3987. [Google Scholar] [CrossRef]
- Xu, K.; Tian, Y.; Weng, X.; Hu, X.; Heng, D.; Xia, G.; Zhang, C. Effect of thyroid dysfunction on NOS expression in the female rat. Cell Tissue Res. 2020, 379, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Guo, L.; Zhu, B.; Feng, Y.; Yu, S.; An, N.; Wang, X. Effects of 3,5,3′-triiodothyronine (t3) and follicle stimulating hormone on apoptosis and proliferation of rat ovarian granulosa cells. Chin. J. Physiol. 2013, 56, 298–305. [Google Scholar]
- Zhang, C.; Wang, X.; Wang, Z.; Niu, W.; Zhu, B.; Xia, G. Effect of different culture systems and 3, 5, 3′-triiodothyronine/follicle-stimulating hormone on preantral follicle development in mice. PLoS ONE 2013, 8, e61947. [Google Scholar] [CrossRef] [PubMed]
- Krassas, G.E.; Poppe, K.; Glinoer, D. Thyroid function and human reproductive health. Endocr. Rev. 2010, 31, 702–755. [Google Scholar] [CrossRef]
- Fedail, J.S.; Zheng, K.; Wei, Q.; Kong, L.; Shi, F. Roles of thyroid hormones in follicular development in the ovary of neonatal and immature rats. Endocrine 2014, 46, 594–604. [Google Scholar] [CrossRef]
- Zhang, J.; Lazar, M.A. The mechanism of action of thyroid hormones. Annu. Rev. Physiol. 2000, 62, 439–466. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-Y.; Leonard, J.L.; Davis, P.J. Molecular Aspects of Thyroid Hormone Actions. Endocr. Rev. 2010, 31, 139–170. [Google Scholar] [CrossRef]
- Cheng, S.Y. Multiple mechanisms for regulation of the transcriptional activity of thyroid hormone receptors. Rev. Endocr. Metab. Disord. 2000, 1, 9–18. [Google Scholar] [CrossRef]
- Sap, J.; Munoz, A.; Damm, K.; Goldberg, Y.; Ghysdael, J.; Leutz, A.; Beug, H.; Vennstrom, B. The C-Erb-a Protein Is a High-Affinity Receptor for Thyroid-Hormone. Nature 1986, 324, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, C.; Thompson, C.C.; Ong, E.S.; Lebo, R.; Gruol, D.J.; Evans, R.M. The c-erb-A gene encodes a thyroid hormone receptor. Nature 1986, 324, 641–646. [Google Scholar] [CrossRef]
- Aghajanova, L.; Lindeberg, M.; Carlsson, I.B.; Stavreus-Evers, A.; Zhang, P.; Scott, J.E.; Hovatta, O.; Skjoldebrand-Sparre, L. Receptors for thyroid-stimulating hormone and thyroid hormones in human ovarian tissue. Reprod. Biomed. Online 2009, 18, 337–347. [Google Scholar] [CrossRef]
- Yen, P.M. Physiological and molecular basis of thyroid hormone action. Physiol. Rev. 2001, 81, 1097–1142. [Google Scholar] [CrossRef]
- Wagner, R.L.; Apriletti, J.W.; McGrath, M.E.; West, B.L.; Baxter, J.D.; Fletterick, R.J. A structural role for hormone in the thyroid hormone receptor. Nature 1995, 378, 690–697. [Google Scholar] [CrossRef]
- Wu, Y.; Koenig, R.J. Gene regulation by thyroid hormone. Trends Endocrinol. Metab. 2000, 11, 207–211. [Google Scholar] [CrossRef]
- Alexandre, S.; Nakaki, T.; Vanhamme, L.; Lee, A.S. A binding site for the cyclic adenosine 3′,5′-monophosphate-response element-binding protein as a regulatory element in the grp78 promoter. Mol. Endocrinol. 1991, 5, 1862–1872. [Google Scholar] [CrossRef]
- Li, W.W.; Alexandre, S.; Cao, X.; Lee, A.S. Transactivation of the grp78 promoter by Ca2+ depletion. A comparative analysis with A23187 and the endoplasmic reticulum Ca2+-ATPase inhibitor thapsigargin. J. Biol. Chem. 1993, 268, 12003–12009. [Google Scholar] [CrossRef]
- Wooden, S.K.; Li, L.J.; Navarro, D.; Qadri, I.; Pereira, L.; Lee, A.S. Transactivation of the grp78 promoter by malfolded proteins, glycosylation block, and calcium ionophore is mediated through a proximal region containing a CCAAT motif which interacts with CTF/NF-I. Mol. Cell Biol. 1991, 11, 5612–5623. [Google Scholar]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, X.; Zhang, C. Effects of thyroid hormone on ovarian cell apoptosis in the rat. Reprod. Fertil. Dev. 2020, 32, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Kogure, K.; Nakamura, K.; Ikeda, S.; Kitahara, Y.; Nishimura, T.; Iwamune, M.; Minegishi, T. Glucose-regulated protein, 78-kilodalton is a modulator of luteinizing hormone receptor expression in luteinizing granulosa cells in rats. Biol. Reprod. 2013, 88, 8. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, P.; Chen, F.; Wang, A.; Lan, X.; Song, Y.; Jin, Y. Luman recruiting factor regulates endoplasmic reticulum stress in mouse ovarian granulosa cell apoptosis. Theriogenology 2013, 79, 633–639.e3. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Patel, D.N.; Welsch, M.; Skouta, R.; Lee, E.D.; Hayano, M.; Thomas, A.G.; Gleason, C.E.; Tatonetti, N.P.; Slusher, B.S.; et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife 2014, 3, e02523. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mi, Y.; Zhang, X.; Ma, Q.; Song, Y.; Zhang, L.; Wang, D.; Xing, J.; Hou, B.; Li, H.; et al. Dihydroartemisinin-induced unfolded protein response feedback attenuates ferroptosis via PERK/ATF4/HSPA5 pathway in glioma cells. J. Exp. Clin. Cancer Res. 2019, 38, 402. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, Q.; Sun, X.; Zeh, H.J., 3rd; Lotze, M.T.; Kang, R.; Tang, D. HSPA5 Regulates Ferroptotic Cell Death in Cancer Cells. Cancer Res. 2017, 77, 2064–2077. [Google Scholar] [CrossRef]
- Sengupta, A.; Lichti, U.F.; Carlson, B.A.; Cataisson, C.; Ryscavage, A.O.; Mikulec, C.; Conrad, M.; Fischer, S.M.; Hatfield, D.L.; Yuspa, S.H. Targeted disruption of glutathione peroxidase 4 in mouse skin epithelial cells impairs postnatal hair follicle morphogenesis that is partially rescued through inhibition of COX-2. J. Investig. Dermatol. 2013, 133, 1731–1741. [Google Scholar] [CrossRef]
- Ueta, T.; Inoue, T.; Furukawa, T.; Tamaki, Y.; Nakagawa, Y.; Imai, H.; Yanagi, Y. Glutathione peroxidase 4 is required for maturation of photoreceptor cells. J. Biol. Chem. 2012, 287, 7675–7682. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef]
- Yoo, S.E.; Chen, L.; Na, R.; Liu, Y.; Rios, C.; Van Remmen, H.; Richardson, A.; Ran, Q. Gpx4 ablation in adult mice results in a lethal phenotype accompanied by neuronal loss in brain. Free Radic. Biol. Med. 2012, 52, 1820–1827. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Morgan, E.H. Plasma Iron Binding Capacity and Iron Stores in Altered Thyroid Metabolism in the Rat. Q. J. Exp. Physiol. Cogn. Med. Sci. 1963, 48, 176–180. [Google Scholar] [CrossRef]
- Rivlin, R.S.; Wagner, H.N., Jr. Anemia in hyperthyroidism. Ann. Intern. Med. 1969, 70, 507–516. [Google Scholar] [CrossRef]
- Venditti, P.; Di Meo, S. Thyroid hormone-induced oxidative stress. Cell Mol. Life Sci. 2006, 63, 414–434. [Google Scholar] [CrossRef]
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998, 92, 829–839. [Google Scholar] [CrossRef]
- Wu, Y.; Delerive, P.; Chin, W.W.; Burris, T.P. Requirement of helix 1 and the AF-2 domain of the thyroid hormone receptor for coactivation by PGC-1. J. Biol. Chem. 2002, 277, 8898–8905. [Google Scholar] [CrossRef]
- Zhang, G.; Wan, Y.; Zhang, Y.; Lan, S.; Jia, R.; Wang, Z.; Fan, Y.; Wang, F. Expression of Mitochondria-Associated Genes (PPARGC1A, NRF-1, BCL-2 and BAX) in Follicular Development and Atresia of Goat Ovaries. Reprod. Domest. Anim. 2015, 50, 465–473. [Google Scholar] [CrossRef]
- Lin, M.L.; Chen, S.S.; Ng, S.H. CHM-1 Suppresses Formation of Cell Surface-associated GRP78-p85alpha Complexes, Inhibiting PI3K-AKT Signaling and Inducing Apoptosis of Human Nasopharyngeal Carcinoma Cells. Anticancer. Res. 2015, 35, 5359–5368. [Google Scholar]
- Zhao, S.; Li, H.; Wang, Q.; Su, C.; Wang, G.; Song, H.; Zhao, L.; Luan, Z.; Su, R. The role of c-Src in the invasion and metastasis of hepatocellular carcinoma cells induced by association of cell surface GRP78 with activated alpha2M. BMC Cancer 2015, 15, 389. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Pan, H.J. Thyroid hormone direct repeat 4 response element is a positive regulatory element for the human TR2 orphan receptor, a member of steroid receptor superfamily. Mol. Cell Biochem. 1998, 189, 195–200. [Google Scholar] [CrossRef]
- Zavacki, A.M.; Harney, J.W.; Brent, G.A.; Larsen, P.R. Structural features of thyroid hormone response elements that increase susceptibility to inhibition by an RTH mutant thyroid hormone receptor. Endocrinology 1996, 137, 2833–2841. [Google Scholar] [CrossRef]
- Canipari, R.; Mangialardo, C.; Di Paolo, V.; Alfei, F.; Ucci, S.; Russi, V.; Santaguida, M.G.; Virili, C.; Segni, M.; Misiti, S.; et al. Thyroid hormones act as mitogenic and pro survival factors in rat ovarian follicles. J. Endocrinol. Investig. 2019, 42, 271–282. [Google Scholar] [CrossRef]
- Wei, Q.; Fedail, J.S.; Kong, L.; Zheng, K.; Meng, C.; Fadlalla, M.B.; Shi, F. Thyroid hormones alter estrous cyclicity and antioxidative status in the ovaries of rats. Anim. Sci. J. 2018, 89, 513–526. [Google Scholar] [CrossRef]
- Yu, Y.; Yao, Y.; Liu, Y.; Sun, Y.; Feng, H.; Kong, N.; Chen, R.; Wu, M.; Guo, S.; Tian, S.; et al. Effects of Thyroid Hormones on Cellular Development in Human Ovarian Granulosa Tumor Cells (KGN). Reprod. Sci. 2024; ahead of print. [Google Scholar]
- Xu, W.; Hou, D.; Jiang, X.; Lu, Z.; Guo, T.; Liu, Y.; Wang, D.; Zen, K.; Yu, B.; Zhang, C.Y. The protective role of peroxisome proliferator-activated receptor gamma coactivator-1alpha in hyperthyroid cardiac hypertrophy. J. Cell Physiol. 2012, 227, 3243–3253. [Google Scholar] [CrossRef]
- Zhang, C.; Xia, G.; Tsang, B.K. Interactions of thyroid hormone and FSH in the regulation of rat granulosa cell apoptosis. Front. Biosci. (Elite Ed.) 2011, 3, 1401–1413. [Google Scholar] [CrossRef]
- Filatov, M.; Khramova, Y.; Parshina, E.; Bagaeva, T.; Semenova, M. Influence of gonadotropins on ovarian follicle growth and development in vivo and in vitro. Zygote 2017, 25, 235–243. [Google Scholar] [CrossRef]
- Glass, C.K.; Holloway, J.M.; Devary, O.V.; Rosenfeld, M.G. The thyroid hormone receptor binds with opposite transcriptional effects to a common sequence motif in thyroid hormone and estrogen response elements. Cell 1988, 54, 313–323. [Google Scholar] [CrossRef]
- Gething, M.J.; Sambrook, J. Protein folding in the cell. Nature 1992, 355, 33–45. [Google Scholar] [CrossRef]
- Sanders, S.L.; Whitfield, K.M.; Vogel, J.P.; Rose, M.D.; Schekman, R.W. Sec61p and BiP directly facilitate polypeptide translocation into the ER. Cell 1992, 69, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Hendershot, L.M. Immunoglobulin heavy chain and binding protein complexes are dissociated in vivo by light chain addition. J. Cell Biol. 1990, 111, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C. Roles of Grp78 in Female Mammalian Reproduction. Adv. Anat. Embryol. Cell Biol. 2017, 222, 129–155. [Google Scholar] [PubMed]
- Huang, M.; Wang, Y.; Wu, X.; Li, W. Crosstalk between Endoplasmic Reticulum Stress and Ferroptosis in Liver Diseases. Front. Biosci. (Landmark Ed.) 2024, 29, 221. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Zuo, Y.X.; Chang, X.Q.; Chi, H.T. Potential intervention target of atherosclerosis: Ferroptosis (Review). Mol. Med. Rep. 2022, 26, 343. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, B.Q.; Ma, S.; Xu, Y.; Zhao, D.H.; Zhang, J.S.; Li, C.J.; Zhou, X.; Zheng, L.W. Broadening horizons: The role of ferroptosis in polycystic ovary syndrome. Front. Endocrinol. 2024, 15, 1390013. [Google Scholar] [CrossRef]
- Tsai, Y.L.; Ha, D.P.; Zhao, H.; Carlos, A.J.; Wei, S.; Pun, T.K.; Wu, K.; Zandi, E.; Kelly, K.; Lee, A.S. Endoplasmic reticulum stress activates SRC, relocating chaperones to the cell surface where GRP78/CD109 blocks TGF-beta signaling. Proc. Natl. Acad. Sci. USA 2018, 115, E4245–E4254. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Y.F.; Liu, X.T.; Li, Y.C.; Zhu, H.M.; Sun, M.R.; Li, P.; Liu, B.; Yang, H. Songorine promotes cardiac mitochondrial biogenesis via Nrf2 induction during sepsis. Redox Biol. 2021, 38, 101771. [Google Scholar] [CrossRef]
- Zhao, T.; Yu, Z.; Zhou, L.; Wang, X.; Hui, Y.; Mao, L.; Fan, X.; Wang, B.; Zhao, X.; Sun, C. Regulating Nrf2-GPx4 axis by bicyclol can prevent ferroptosis in carbon tetrachloride-induced acute liver injury in mice. Cell Death Discov. 2022, 8, 380. [Google Scholar] [CrossRef]
- Nishi, Y.; Yanase, T.; Mu, Y.; Oba, K.; Ichino, I.; Saito, M.; Nomura, M.; Mukasa, C.; Okabe, T.; Goto, K.; et al. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology 2001, 142, 437–445. [Google Scholar] [CrossRef]
- Ayala, C.; Valdez, S.R.; Morero, M.L.; Soaje, M.; Carreno, N.B.; Sanchez, M.S.; Bittencourt, J.C.; Jahn, G.A.; Celis, M.E. Hypo- and hyperthyroidism affect NEI concentration in discrete brain areas of adult male rats. Peptides 2011, 32, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Tian, Y.; Guo, M.; Liu, J.; Heng, D.; Zhu, B.; Yang, Y.; Zhang, C. Regulation of glucose transport by thyroid hormone in rat ovary. Cell Tissue Res. 2016, 366, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Kaya-Okur, H.S.; Wu, S.J.; Codomo, C.A.; Pledger, E.S.; Bryson, T.D.; Henikoff, J.G.; Ahmad, K.; Henikoff, S. CUT&Tag for efficient epigenomic profiling of small samples and single cells. Nat. Commun. 2019, 10, 1930. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chang, S.C.; Wooden, S.K.; Nakaki, T.; Kim, Y.K.; Lin, A.Y.; Kung, L.; Attenello, J.W.; Lee, A.S. Rat gene encoding the 78-kDa glucose-regulated protein GRP78: Its regulatory sequences and the effect of protein glycosylation on its expression. Proc. Natl. Acad. Sci. USA 1987, 84, 680–684. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yao, Y.; Yu, Y.; Sun, Y.; Wu, M.; Chen, R.; Feng, H.; Guo, S.; Yang, Y.; Zhang, C. Role of T3 in the Regulation of GRP78 on Granulosa Cells in Rat Ovaries. Int. J. Mol. Sci. 2025, 26, 4196. https://doi.org/10.3390/ijms26094196
Liu Y, Yao Y, Yu Y, Sun Y, Wu M, Chen R, Feng H, Guo S, Yang Y, Zhang C. Role of T3 in the Regulation of GRP78 on Granulosa Cells in Rat Ovaries. International Journal of Molecular Sciences. 2025; 26(9):4196. https://doi.org/10.3390/ijms26094196
Chicago/Turabian StyleLiu, Yan, Yilin Yao, Yakun Yu, Ying Sun, Mingqi Wu, Rui Chen, Haoyuan Feng, Shuaitian Guo, Yanzhou Yang, and Cheng Zhang. 2025. "Role of T3 in the Regulation of GRP78 on Granulosa Cells in Rat Ovaries" International Journal of Molecular Sciences 26, no. 9: 4196. https://doi.org/10.3390/ijms26094196
APA StyleLiu, Y., Yao, Y., Yu, Y., Sun, Y., Wu, M., Chen, R., Feng, H., Guo, S., Yang, Y., & Zhang, C. (2025). Role of T3 in the Regulation of GRP78 on Granulosa Cells in Rat Ovaries. International Journal of Molecular Sciences, 26(9), 4196. https://doi.org/10.3390/ijms26094196





