Induced Types 2 and 3 Deiodinase in Non-Thyroidal Illness Syndrome and the Implications to Critical Illness-Induced Myopathy—A Prospective Cohort Study
Abstract
:1. Introduction
2. Results
2.1. Demographic and Clinical Findings
2.2. Muscle Ultrasound Measures
2.3. T3 Variations During the First Week in ICU
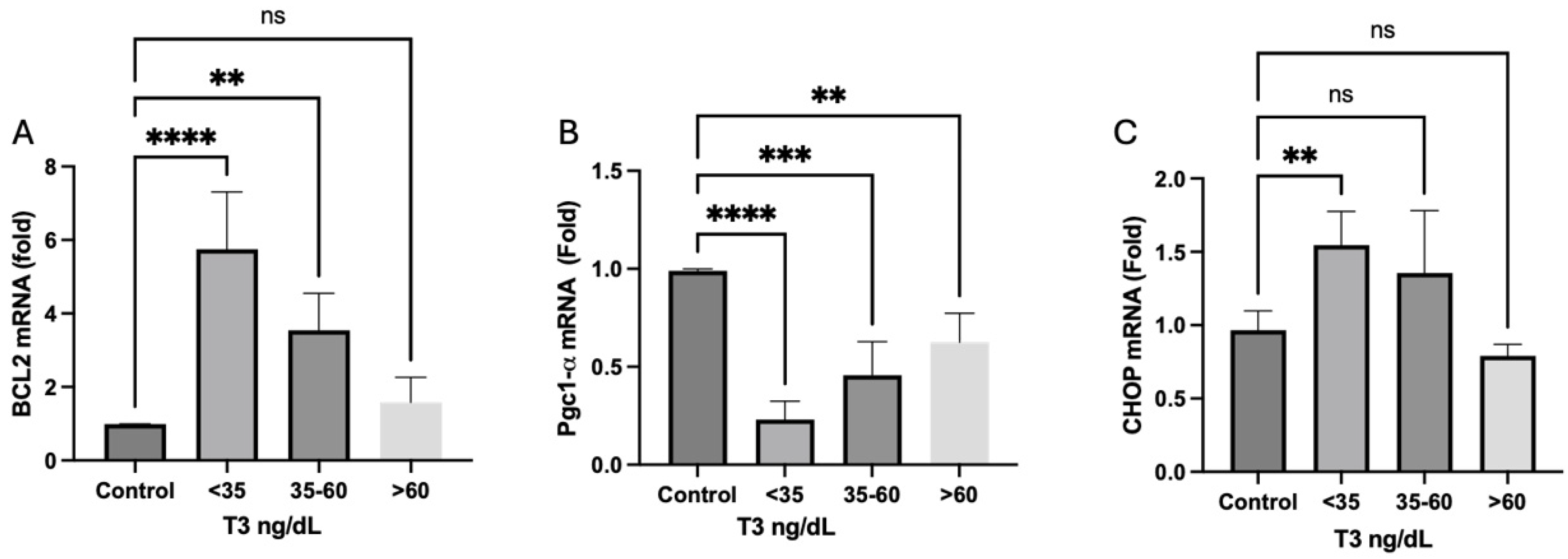
2.4. Analysis According to T3 Level
2.5. Muscle Biopsies
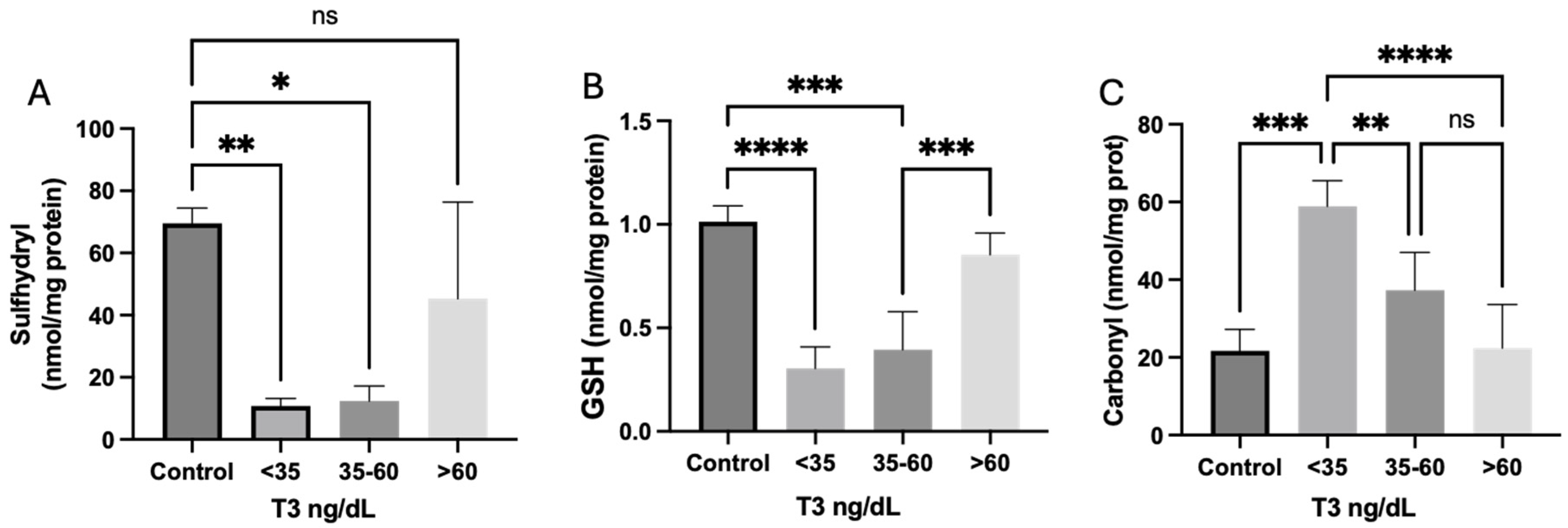
Redox Homeostasis
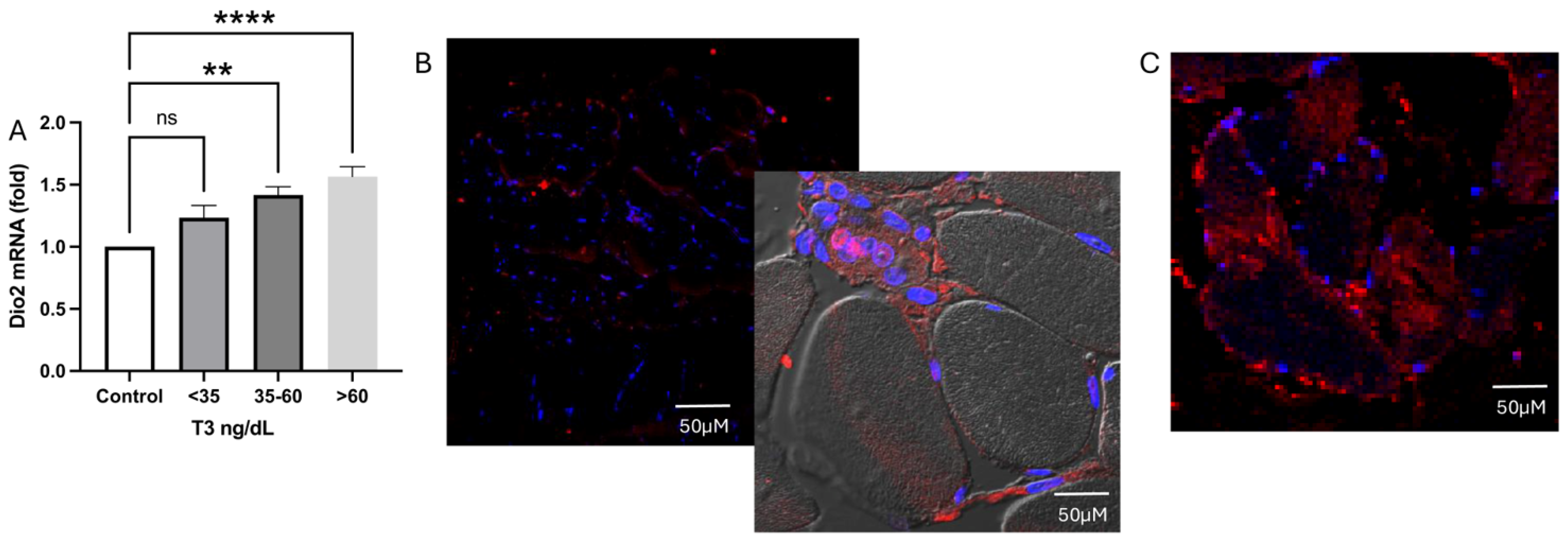
2.6. Deiodinase Expression
2.6.1. Type 2 Deiodinase Expression and Localization Inside the Muscle Tissue
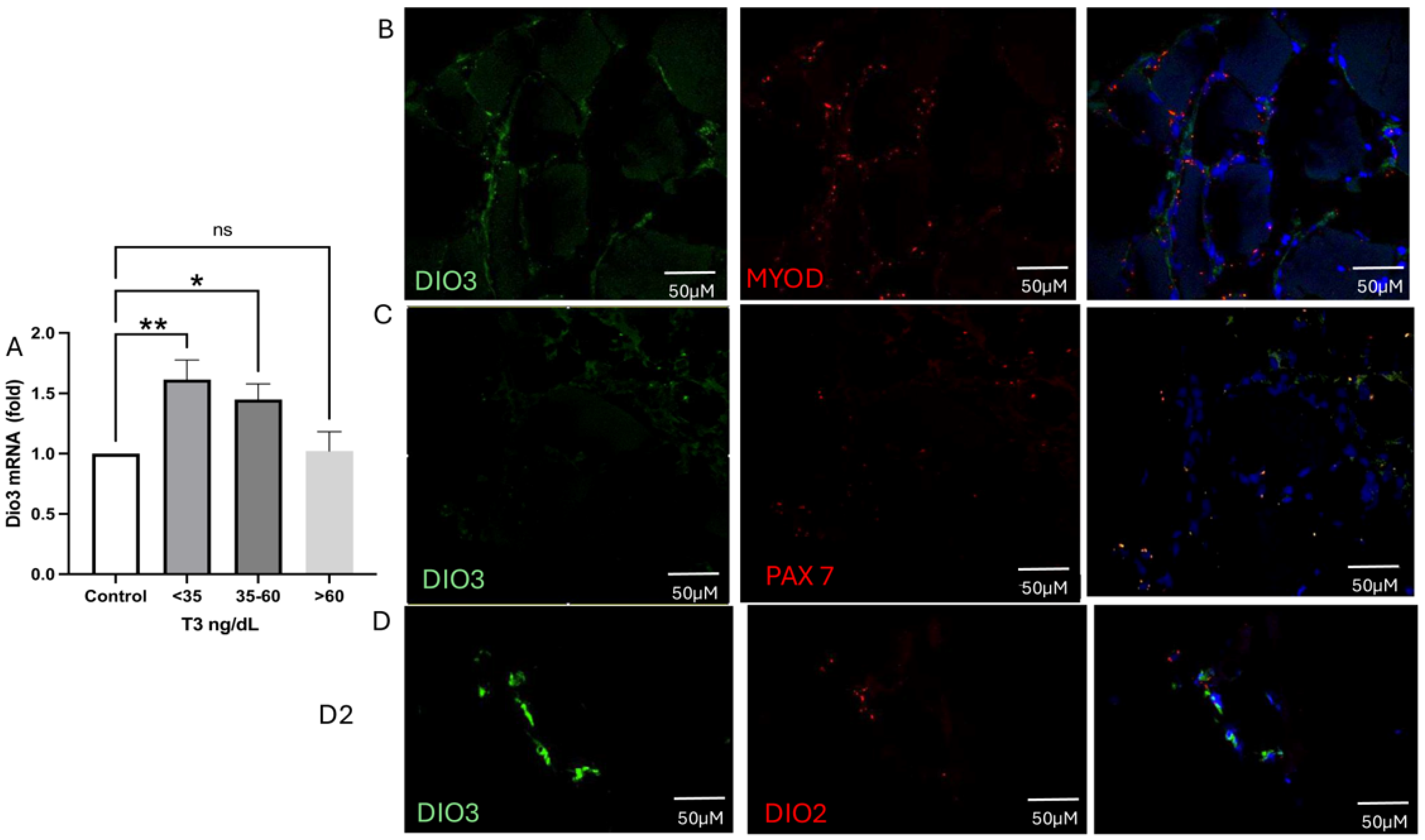
2.6.2. Type 3 Deiodinase mRNA Expression and Colocalization
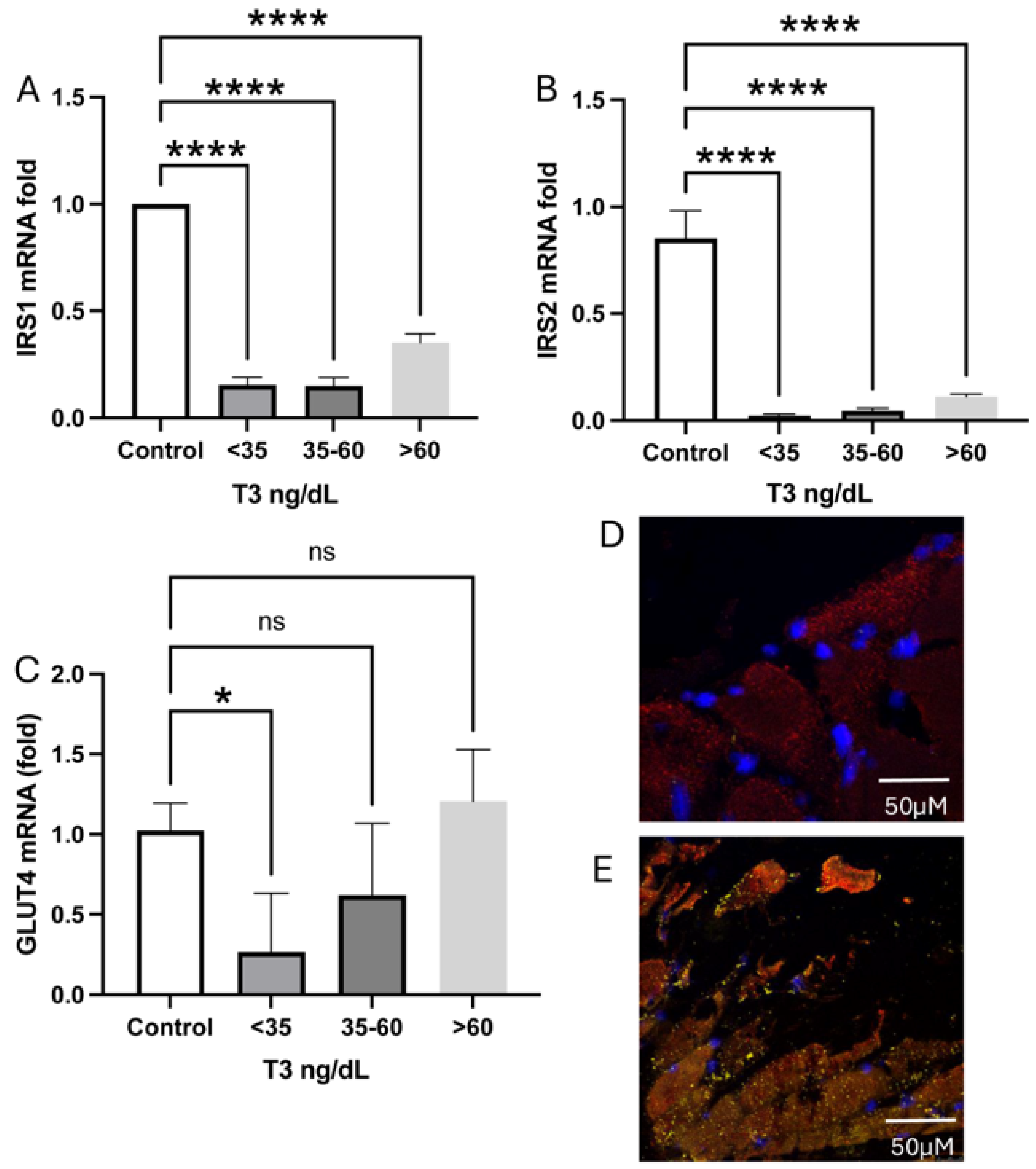
2.7. Glucose Receptors and Transporter
2.8. Mitochondrial Dynamics and Biogenesis
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Clinical, Laboratory, and Ultrasound Data
4.3. Muscle Biopsies
4.4. Oxidative Stress Parameters
4.4.1. Carbonyl Content
4.4.2. Sulfhydryl Content
4.5. Antioxidant Defenses
4.5.1. Reduced Glutathione Concentrations
4.5.2. Real-Time PCR
4.6. Western Blot Analyses
4.7. Immunofluorescence Studies
4.8. Measurement of T3 in Tissue
4.9. RNAscope In Situ Hybridization
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salvatore, D.; Simonides, W.S.; Dentice, M.; Zavacki, A.M.; Larsen, P.R. Thyroid hormones and skeletal muscle—New insights and potential implications. Nat. Rev. Endocrinol. 2014, 10, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Kelmenson, D.A.; Held, N.; Allen, R.R.; Quan, D.; Burnham, E.L.; Clark, B.J.; Ho, P.M.; Kiser, T.H.; Vandivier, R.W.; Moss, M. Outcomes of ICU Patients with a Discharge Diagnosis of Critical Illness Polyneuromyopathy: A Propensity-Matched Analysis. Crit. Care Med. 2017, 45, 2055–2060. [Google Scholar] [CrossRef]
- Latronico, N.; Herridge, M.; Hopkins, R.O.; Angus, D.; Hart, N.; Hermans, G.; Iwashyna, T.; Arabi, Y.; Citerio, G.; Ely, E.W.; et al. The ICM research agenda on intensive care unit-acquired weakness. Intensive Care Med. 2017, 43, 1270–1281. [Google Scholar] [CrossRef]
- Dres, M.; Jung, B.; Molinari, N.; Manna, F.; Dubé, B.-P.; Chanques, G.; Similowski, T.; Jaber, S.; Demoule, A. Respective contribution of intensive care unit-acquired limb muscle and severe diaphragm weakness on weaning outcome and mortality: A post hoc analysis of two cohorts. Crit. Care Lond. Engl. 2019, 23, 370. [Google Scholar] [CrossRef]
- Manning, E.M.; Shenkin, A. Nutritional assessment in the critically ill. Crit. Care Clin. 1995, 11, 603–634. [Google Scholar] [CrossRef]
- Chen, J.; Huang, M. Intensive casre unit-acquired weakness: Recent insights. J. Intensive Med. 2024, 4, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Chi-Fishman, G.; Hicks, J.E.; Cintas, H.M.; Sonies, B.C.; Gerber, L.H. Ultrasound imaging distinguishes between normal and weak muscle. Arch. Phys. Med. Rehabil. 2004, 85, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Freilich, R.J.; Kirsner, R.L.; Byrne, E. Isometric strength and thickness relationships in human quadriceps muscle. Neuromuscul. Disord. NMD 1995, 5, 415–422. [Google Scholar] [CrossRef]
- Miyatani, M.; Kanehisa, H.; Kuno, S.; Nishijima, T.; Fukunaga, T. Validity of ultrasonograph muscle thickness measurements for estimating muscle volume of knee extensors in humans. Eur. J. Appl. Physiol. 2002, 86, 203–208. [Google Scholar] [CrossRef]
- Paris, M.T.; Mourtzakis, M.; Day, A.; Leung, R.; Watharkar, S.; Kozar, R.; Earthman, C.; Kuchnia, A.; Dhaliwal, R.; Moisey, L.; et al. Validation of Bedside Ultrasound of Muscle Layer Thickness of the Quadriceps in the Critically Ill Patient (VALIDUM Study). JPEN J. Parenter. Enteral. Nutr. 2017, 41, 171–180. [Google Scholar] [CrossRef]
- Agyapong-Badu, S.; Warner, M.; Samuel, D.; Narici, M.; Cooper, C.; Stokes, M. Anterior thigh composition measured using ultrasound imaging to quantify relative thickness of muscle and non-contractile tissue: A potential biomarker for musculoskeletal health. Physiol. Meas. 2014, 35, 2165–2176. [Google Scholar] [CrossRef]
- Puthucheary, Z.A.; Rawal, J.; McPhail, M.; Connolly, B.; Ratnayake, G.; Chan, P.; Hopkinson, N.S.; Phadke, R.; Dew, T.; Sidhu, P.S.; et al. Acute skeletal muscle wasting in critical illness. JAMA 2013, 310, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Dupont, A.C.; Sauerbrei, E.E.; Fenton, P.V.; Shragge, P.C.; Loeb, G.E.; Richmond, F.J. Real-time sonography to estimate muscle thickness: Comparison with MRI and CT. J. Clin. Ultrasound JCU 2001, 29, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Bloise, F.F.; Cordeiro, A.; Ortiga-Carvalho, T.M. Role of thyroid hormone in skeletal muscle physiology. J. Endocrinol. 2018, 236, R57–R68. [Google Scholar] [CrossRef]
- Mebis, L.; Paletta, D.; Debaveye, Y.; Ellger, B.; Langouche, L.; D’Hoore, A.; Darras, V.M.; Visser, T.J.; Berghe, G.V.D. Expression of thyroid hormone transporters during critical illness. Eur. J. Endocrinol. 2009, 161, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Brunetto, E.L.; Teixeira, S.D.S.; Giannocco, G.; Machado, U.F.; Nunes, M.T. T3 rapidly increases SLC2A4 gene expression and GLUT4 trafficking to the plasma membrane in skeletal muscle of rat and improves glucose homeostasis. Thyroid. Off J. Am. Thyroid. Assoc. 2012, 22, 70–79. [Google Scholar] [CrossRef]
- Zanquetta, M.M.; Alves-Wagner, A.B.; Mori, R.C.; Campello, R.S.; Machado, U.F. Recovery of insulin sensitivity and Slc2a4 mRNA expression depend on T3 hormone during refeeding. Metabolism 2014, 63, 328–334. [Google Scholar] [CrossRef]
- Weinstein, S.P.; O’Boyle, E.; Haber, R.S. Thyroid hormone increases basal and insulin-stimulated glucose transport in skeletal muscle: The role of GLUT4 glucose transporter expression. Diabetes 1994, 43, 1185–1189. [Google Scholar] [CrossRef]
- Vidart, J.; Jaskulski, P.; Kunzler, A.L.; Marschner, R.A.; Ferreira de Azeredo da Silva, A.; Wajner, S.M. Non-thyroidal illness syndrome predicts outcome in adult critically ill patients: A systematic review and meta-analysis. Endocr. Connect. 2022, 11, e210504. [Google Scholar] [CrossRef]
- Maldonado, L.S.; Murata, G.H.; Hershman, J.M.; Braunstein, G.D. Do thyroid function tests independently predict survival in the critically ill? Thyroid. Off. J. Am. Thyroid. Assoc. 1992, 2, 119–123. [Google Scholar] [CrossRef]
- Sagliocchi, S.; Cicatiello, A.G.; Di Cicco, E.; Ambrosio, R.; Miro, C.; Di Girolamo, D.; Nappi, A.; Mancino, G.; De Stefano, M.A.; Luongo, C.; et al. The thyroid hormone activating enzyme, type 2 deiodinase, induces myogenic differentiation by regulating mitochondrial metabolism and reducing oxidative stress. Redox Biol. 2019, 24, 101228. [Google Scholar] [CrossRef] [PubMed]
- Gereben, B.; Zavacki, A.M.; Ribich, S.; Kim, B.W.; Huang, S.A.; Simonides, W.S.; Zeold, A.; Bianco, A.C. Cellular and Molecular Basis of Deiodinase-Regulated Thyroid Hormone Signaling1. Endocr. Rev. 2008, 29, 898–938. [Google Scholar] [CrossRef] [PubMed]
- Peeters, R.P.; van der Geyten, S.; Wouters, P.J.; Darras, V.M.; van Toor, H.; Kaptein, E.; Visser, T.J.; Berghe, G.V.D. Tissue thyroid hormone levels in critical illness. J. Clin. Endocrinol. Metab. 2005, 90, 6498–6507. [Google Scholar] [CrossRef]
- da Silva Rosa, S.C.; Nayak, N.; Caymo, A.M.; Gordon, J.W. Mechanisms of muscle insulin resistance and the cross-talk with liver and adipose tissue. Physiol. Rep. 2020, 8, e14607. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, J.D.; Kaufman, R.J. The endoplasmic reticulum and the unfolded protein response. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2007; Volume 18, pp. 716–731. [Google Scholar]
- Parry, S.M.; El-Ansary, D.; Cartwright, M.S.; Sarwal, A.; Berney, S.; Koopman, R.; Annoni, R.; Puthucheary, Z.; Gordon, I.R.; Morris, P.E.; et al. Ultrasonography in the intensive care setting can be used to detect changes in the quality and quantity of muscle and is related to muscle strength and function. J. Crit. Care. 2015, 30, 1151.e9–1151.e14. [Google Scholar] [CrossRef]
- Lepper, C.; Partridge, T.A.; Fan, C.M. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Dev. Camb. Engl. 2011, 138, 3639–3646. [Google Scholar] [CrossRef]
- Murphy, M.M.; Lawson, J.A.; Mathew, S.J.; Hutcheson, D.A.; Kardon, G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Dev. Camb. Engl. 2011, 138, 3625–3637. [Google Scholar]
- Lassar, A.B.; Paterson, B.M.; Weintraub, H. Transfection of a DNA locus that mediates the conversion of 10T1/2 fibroblasts to myoblasts. Cell 1986, 47, 649–656. [Google Scholar] [CrossRef]
- Wosczyna, M.N.; Rando, T.A. A Muscle Stem Cell Support Group: Coordinated Cellular Responses in Muscle Regeneration. Dev. Cell 2018, 46, 135–143. [Google Scholar] [CrossRef]
- Peeters, R.P.; Wouters, P.J.; Kaptein, E.; van Toor, H.; Visser, T.J.; Van den Berghe, G. Reduced activation and increased inactivation of thyroid hormone in tissues of critically ill patients. J. Clin. Endocrinol. Metab. 2003, 88, 3202–3211. [Google Scholar] [CrossRef]
- Lehnen, T.E.; Marschner, R.; Dias, F.; Maia, A.L.; Wajner, S.M. Oxidative remote induction of type 3 deiodinase impacts nonthyroidal illness syndrome. J. Endocrinol. 2020, 246, 237–246. [Google Scholar] [CrossRef]
- Ogawa-Wong, A.; Carmody, C.; Le, K.; Marschner, R.A.; Larsen, P.R.; Zavacki, A.M.; Wajner, S.M. Modulation of Deiodinase Types 2 and 3 during Skeletal Muscle Regeneration. Metabolites 2022, 12, 612. [Google Scholar] [CrossRef] [PubMed]
- Andrés, V.; Walsh, K. Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J. Cell Biol. 1996, 132, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Rocheteau, P.; Chatre, L.; Briand, D.; Mebarki, M.; Jouvion, G.; Bardon, J.; Crochemore, C.; Serrani, P.; Lecci, P.P.; Latil, M.; et al. Sepsis induces long-term metabolic and mitochondrial muscle stem cell dysfunction amenable by mesenchymal stem cell therapy. Nat. Commun. 2015, 6, 10145. [Google Scholar] [CrossRef] [PubMed]
- Lado-Abeal, J.; Romero, A.; Castro-Piedras, I.; Rodriguez-Perez, A.; Alvarez-Escudero, J. Thyroid hormone receptors are down-regulated in skeletal muscle of patients with non-thyroidal illness syndrome secondary to non-septic shock. Eur. J. Endocrinol. 2010, 163, 765–773. [Google Scholar] [CrossRef]
- Rodriguez-Perez, A.; Palos-Paz, F.; Kaptein, E.; Visser, T.J.; Dominguez-Gerpe, L.; Alvarez-Escudero, J.; Lado-Abeal, J. Identification of molecular mechanisms related to nonthyroidal illness syndrome in skeletal muscle and adipose tissue from patients with septic shock. Clin. Endocrinol. 2008, 68, 821–827. [Google Scholar] [CrossRef]
- Dentice, M.; Marsili, A.; Zavacki, A.; Larsen, P.R.; Salvatore, D. The deiodinases and the control of intracellular thyroid hormone signaling during cellular differentiation. Biochim. Biophys. Acta 2013, 1830, 3937–3945. [Google Scholar] [CrossRef]
- Mishra, P.; Varuzhanyan, G.; Pham, A.H.; Chan, D.C. Mitochondrial Dynamics is a Distinguishing Feature of Skeletal Muscle Fiber Types and Regulates Organellar Compartmentalization. Cell Metab. 2015, 22, 1033–1044. [Google Scholar] [CrossRef]
- Schnyder, S.; Handschin, C. Skeletal muscle as an endocrine organ: PGC-1α, myokines and exercise. Bone 2015, 80, 115–125. [Google Scholar] [CrossRef]
- Rius-Pérez, S.; Torres-Cuevas, I.; Millán, I.; Ortega, Á.L.; Pérez, S. PGC-1α, Inflammation, and Oxidative Stress: An Integrative View in Metabolism. Oxid. Med. Cell Longev. 2020, 2020, 1452696. [Google Scholar] [CrossRef]
- Kny, M.; Fielitz, J. Hidden Agenda—The Involvement of Endoplasmic Reticulum Stress and Unfolded Protein Response in Inflammation-Induced Muscle Wasting. Front. Immunol. 2022, 13, 878755. [Google Scholar] [CrossRef]
- Vidart, J.; Axelrud, L.; Braun, A.C.; Marschner, R.A.; Wajner, S.M. Relationship among low T3 levels, type 3 deiodinase, oxidative stress, and mortality in sepsis and septic shock: Defining patient outcomes. Int. J. Mol. Sci. 2023, 24, 3935. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, Â.; Viegas, C.M.; Tonin, A.M.; Busanello, E.N.B.; Grings, M.; Moura, A.P.; Leipnitz, G.; Wajner, M. Disturbance of redox homeostasis by ornithine and homocitrulline in rat cerebellum: A possible mechanism of cerebellar dysfunction in HHH syndrome. Life Sci. 2013, 93, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Aksenov, M.Y.; Markesbery, W.R. Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci. Lett. 2001, 302, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Browne, R.W.; Armstrong, D. Reduced glutathione and glutathione disulfide. Free. Radic. Antioxid. Protoc. 1998, 108, 347–352. [Google Scholar]
- Fuziwara, C.S.; Kimura, E.T. High iodine blocks a Notch/miR-19 loop activated by the BRAF(V600E) oncoprotein and restores the response to TGFβ in thyroid follicular cells. Thyroid. Off J. Am. Thyroid. Assoc. 2014, 24, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Asakura, A.; Kikyo, N. Immunofluorescence analysis of myogenic differentiation. Methods Cell Biol. 2022, 170, 117–125. [Google Scholar]


| Total (n = 96) | Survivors (n = 68) | Non-Survivors (n = 28) | |
|---|---|---|---|
| Demographic Characteristics on Day One—Means (±sd) | |||
| Age (y) | 59.65 | 59.3 ± 13.6 | 60.5 ± 16.1 |
| Male (%) | 56% | 56.5 | 53.6 |
| Sepsis or septic shock (%) | 67 | 69.3 | 64.6 |
| Predictive Scores on Admission—Means (±sd) | |||
| SAPS | 67.2 ± 17.3 | 65.7 ± 16.8 | 71.1 ± 18.4 |
| SOFA | 7.3 ± 4 | 7.36 ± 4 | 7.47 ± 4 |
| Laboratory Findings on admission—Median (IQR) | |||
| Reactive C-protein | 168 (77–281) | 139 (51–300) | 187 (90–276) |
| White blood cell | 12,140 (7860–17,155) | 11,530 (7660–16,680) | 13,185 (9310–19,620) |
| Thyroid Hormones—Median (IQR) | |||
| NTIS (%) | 88.7 | 88.4 | 89.3 |
| TSH | 0.85 (0.37–1.61) | 0.87 (0.40–1.43) | 0.82 (0.27–2.35) |
| Total T4 | 5.93 (4.53–6.99) | 5.96 (4.64–7.00) | 5.65 (3.51–6.91) |
| Free T4 | 0.89 (0.75–1.06) | 0.93 (0.79–1.07) | 0.83 (0.72–0.97) |
| T3 | 34.2 (30.0–48.2) | 32.5 (30.0–46.8) | 36.0 (30.0–53.1) |
| Ultrasound Measurements—Means (±sd) | |||
| Quadriceps thickness (cm) | 2.22 ± 0.78 | 2.17 ± 0.8 | 2.33 ± 0.74 |
| Clinical Findings on Day 7 | ||||
|---|---|---|---|---|
| Variable | Total (n = 59) | Survivors (n = 44) | Non-Survivors (n = 15) | |
| Median T3 (ng/dL) * | 42.4 (30.0–53.0) | 45.0 (31.3–56.5) | 39.5 (30.0–47.8) | p = 0.085 |
| Mean quadriceps thickness (cm) * | 1.82 ± 0.63 | 1.85 ± 0.52 | 2.1 ± 0.8 | p = 0.165 |
| Proposed caloric intake achieved % ** | 75 | 79 | 64 | p = 0.296 |
| Protein intake achieved (g/kg/d) | 1.50 (1.24–1.75) | 1.53 (1.31–1.75) | 1.50 (0.72–1.71) | p = 0.333 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braun, A.C.; Oliveira, T.C.; Thomazini, L.C.D.; Argenti, G.; Kotzian, B.J.; Machado, V.; Conte, J.H.M.; Zanfir, C.; Souto, A.C.A.; Ulian, B.; et al. Induced Types 2 and 3 Deiodinase in Non-Thyroidal Illness Syndrome and the Implications to Critical Illness-Induced Myopathy—A Prospective Cohort Study. Int. J. Mol. Sci. 2025, 26, 2410. https://doi.org/10.3390/ijms26062410
Braun AC, Oliveira TC, Thomazini LCD, Argenti G, Kotzian BJ, Machado V, Conte JHM, Zanfir C, Souto ACA, Ulian B, et al. Induced Types 2 and 3 Deiodinase in Non-Thyroidal Illness Syndrome and the Implications to Critical Illness-Induced Myopathy—A Prospective Cohort Study. International Journal of Molecular Sciences. 2025; 26(6):2410. https://doi.org/10.3390/ijms26062410
Chicago/Turabian StyleBraun, André Cardoso, Thaliane Carvalho Oliveira, Ludmilla C. D. Thomazini, Gustavo Argenti, Bruno Jaskulski Kotzian, Valentina Machado, João Henrique M. Conte, Carolina Zanfir, Amanda C. A. Souto, Bruna Ulian, and et al. 2025. "Induced Types 2 and 3 Deiodinase in Non-Thyroidal Illness Syndrome and the Implications to Critical Illness-Induced Myopathy—A Prospective Cohort Study" International Journal of Molecular Sciences 26, no. 6: 2410. https://doi.org/10.3390/ijms26062410
APA StyleBraun, A. C., Oliveira, T. C., Thomazini, L. C. D., Argenti, G., Kotzian, B. J., Machado, V., Conte, J. H. M., Zanfir, C., Souto, A. C. A., Ulian, B., Vidart, J., & Wajner, S. M. (2025). Induced Types 2 and 3 Deiodinase in Non-Thyroidal Illness Syndrome and the Implications to Critical Illness-Induced Myopathy—A Prospective Cohort Study. International Journal of Molecular Sciences, 26(6), 2410. https://doi.org/10.3390/ijms26062410






