Sodium Iodate-Induced Ferroptosis in Photoreceptor-Derived 661W Cells Through the Depletion of GSH
Abstract
1. Introduction
2. Results
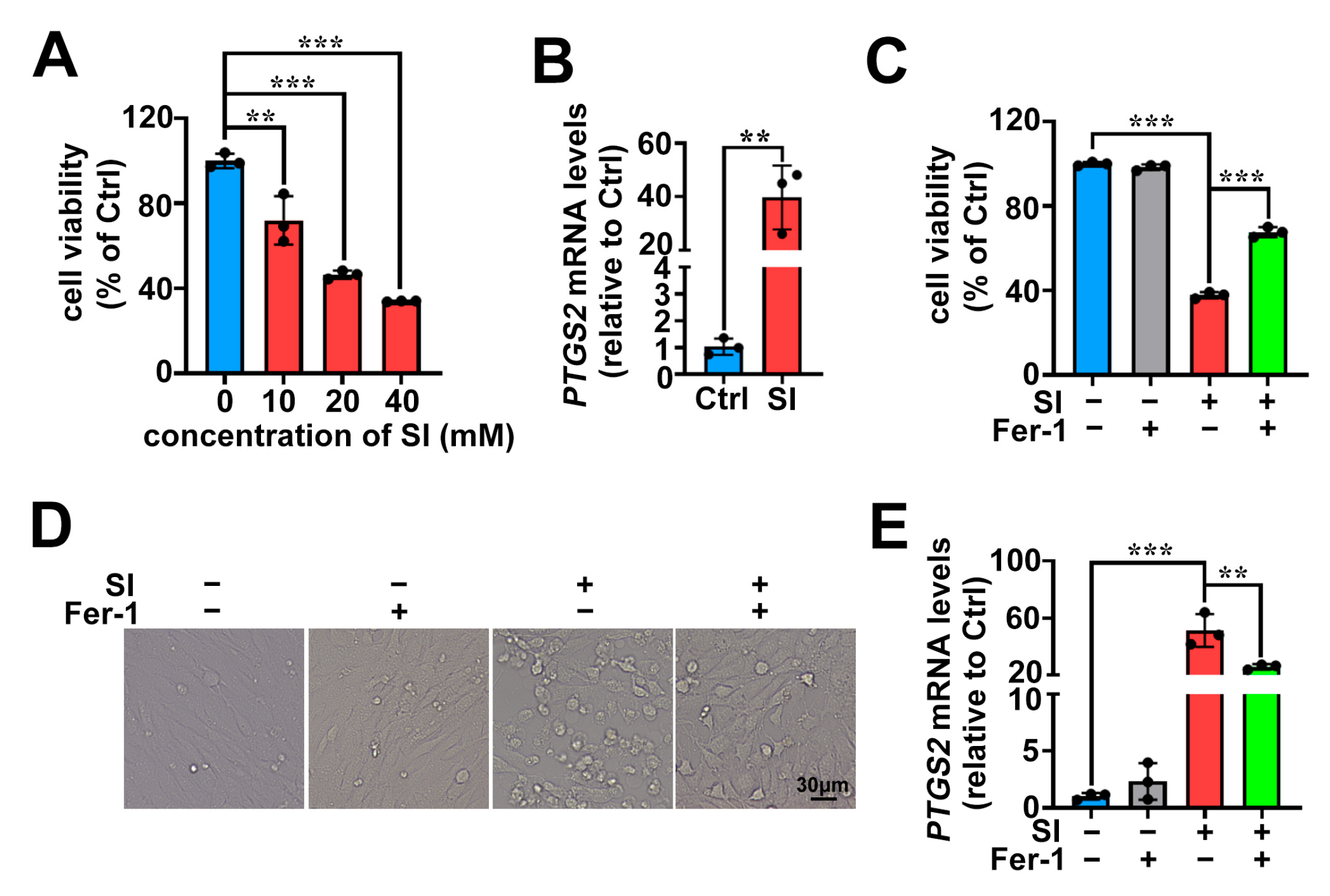
2.1. SI Induces Ferroptosis as One of the Cell Death Pathways in Photoreceptor-Derived 661W Cells
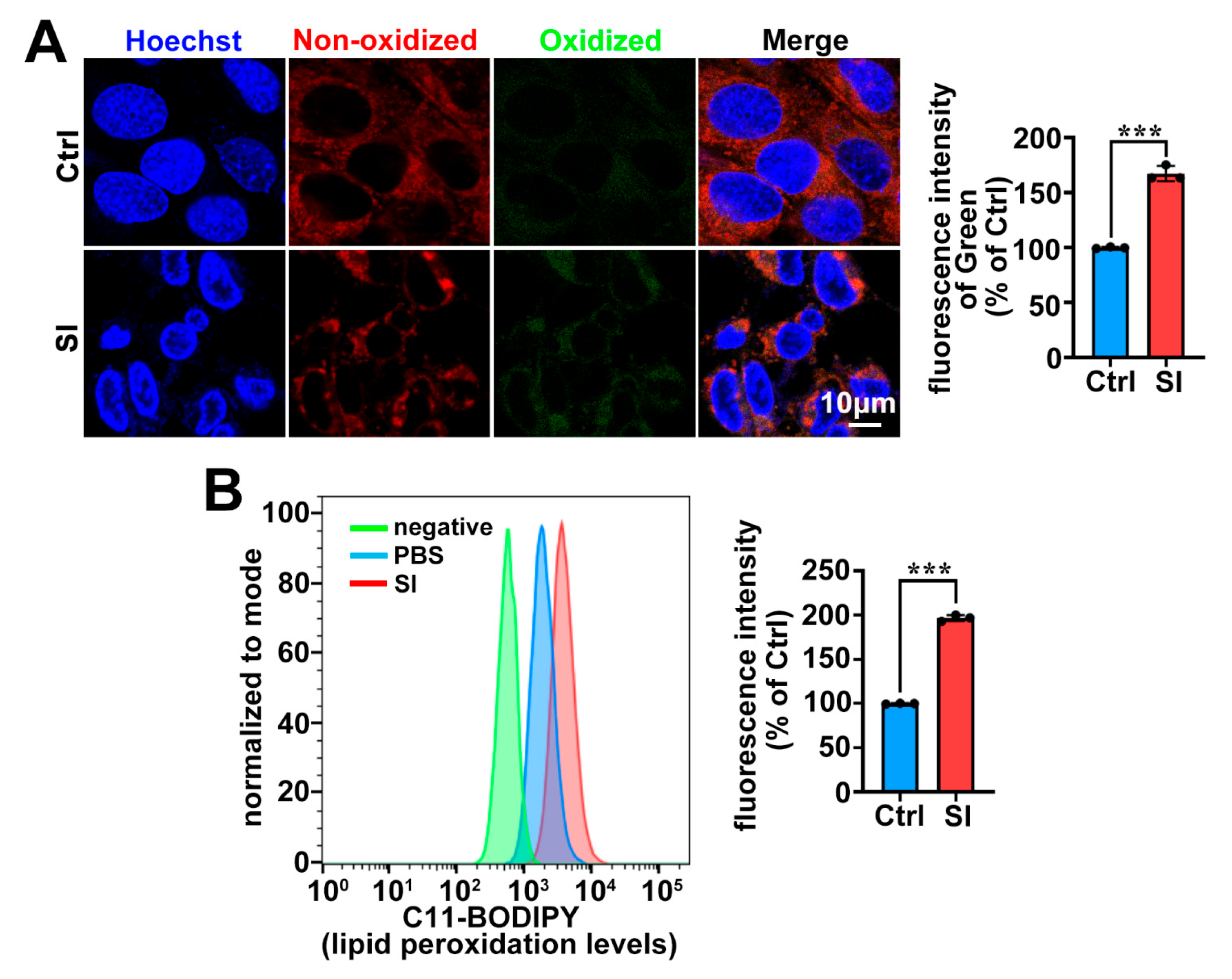
2.2. SI Treatment Leads to Lipid Peroxidation in Photoreceptor-Derived 661W Cells
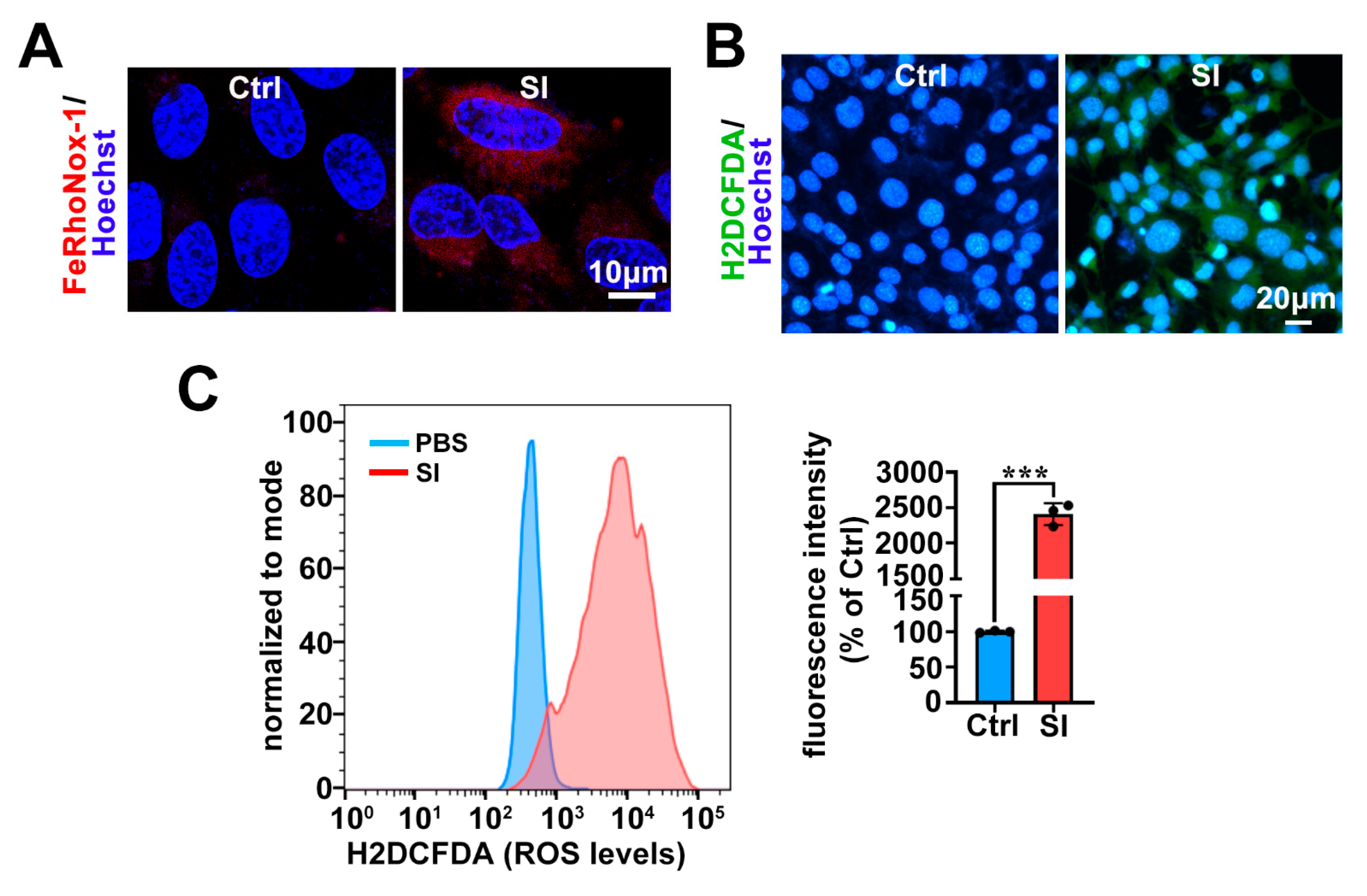
2.3. SI Elevates Intracellular Fe2+ and ROS Levels in Photoreceptor-Derived 661W Cells
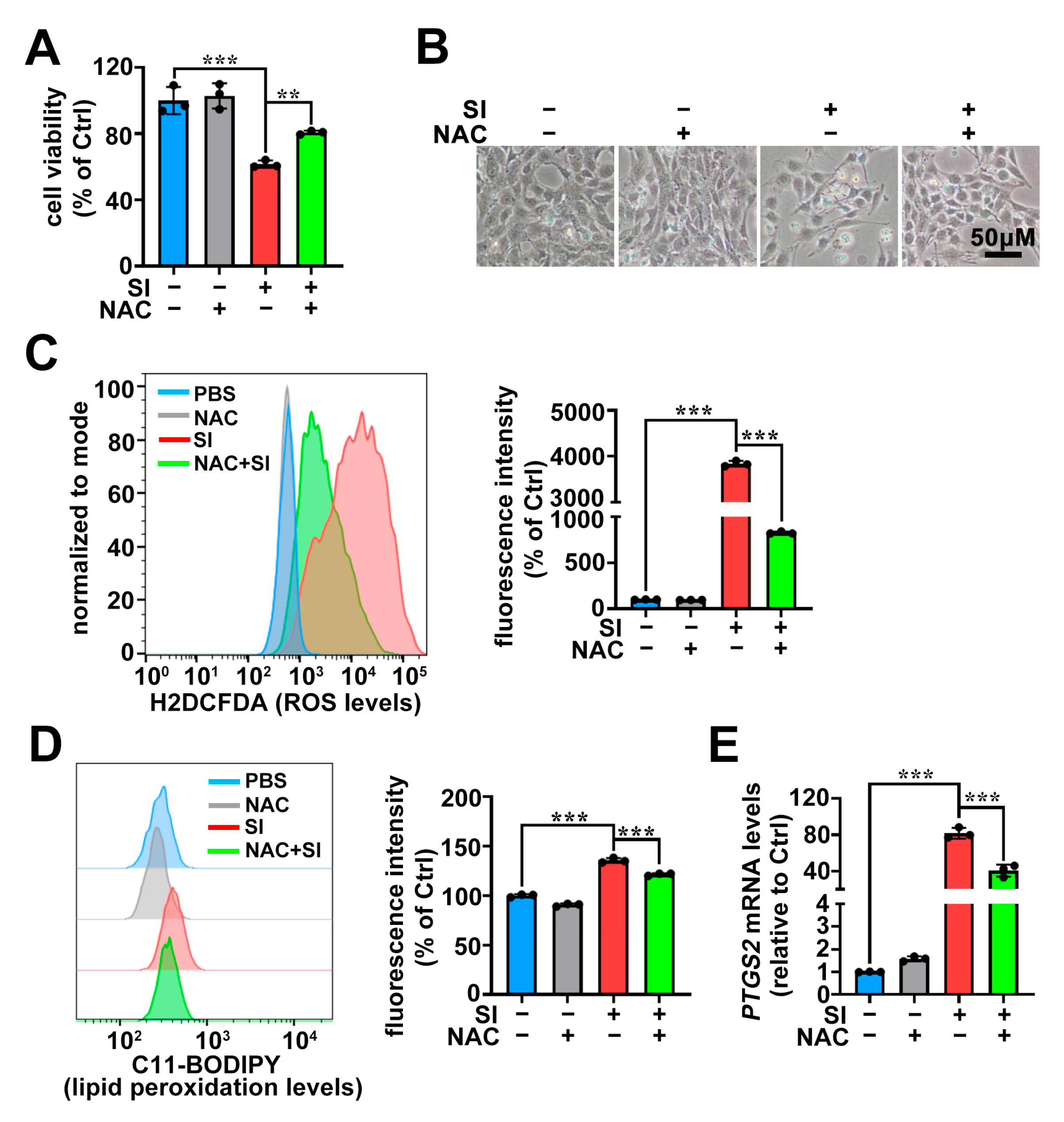
2.4. The Antioxidant NAC Protects Photoreceptor-Derived 661W Cells from SI-Induced Ferroptosis
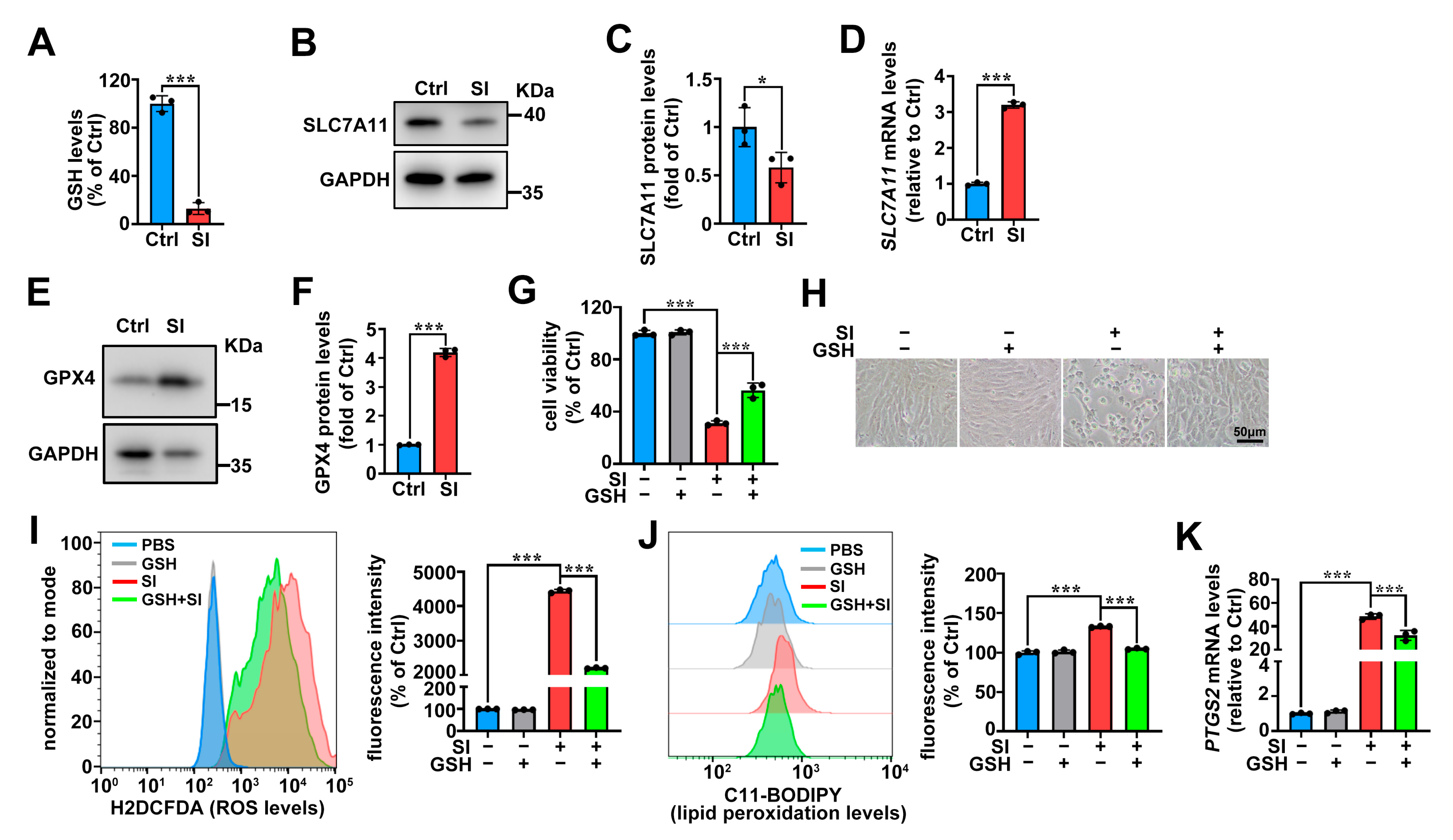
2.5. System Xc−/GSH/GPX4 Axis in SI-Induced Photoreceptor-Derived 661W Cell Ferroptosis
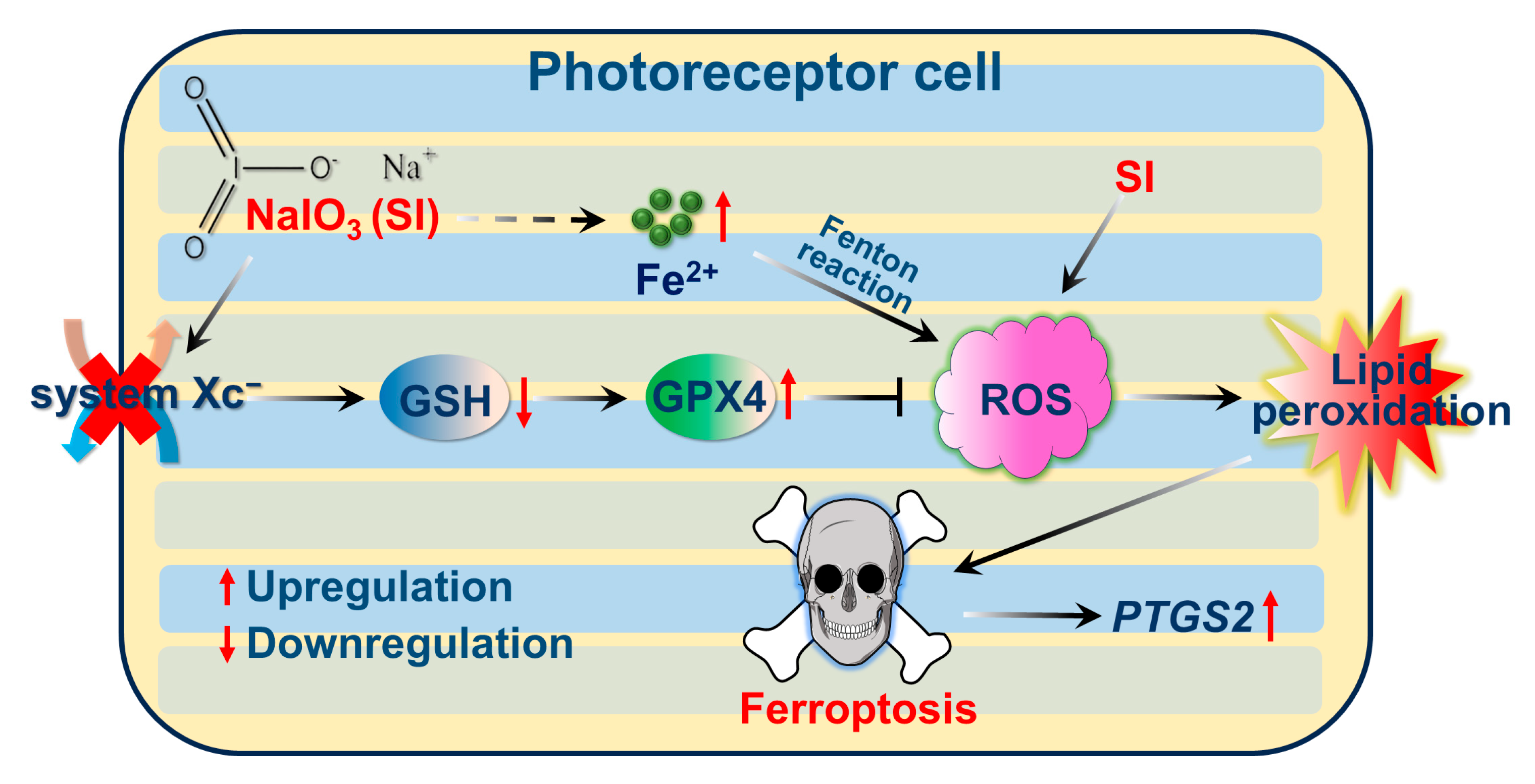
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture, Viability Assay, and Morphology Assessment
4.3. Measurement of GSH Levels
4.4. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
4.5. Western Blotting
4.6. Iron Assay
4.7. Lipid Peroxidation and ROS Levels Assay
4.8. Data Analysis and Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stahl, A. The Diagnosis and Treatment of Age-Related Macular Degeneration. Dtsch. Arztebl. Int. 2020, 117, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Qiao, L.; Du, M.; Qu, C.; Wan, L.; Li, J.; Huang, L. Age-related macular degeneration: Epidemiology, genetics, pathophysiology, diagnosis, and targeted therapy. Genes Dis. 2022, 9, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cui, K.; Li, J.; Tang, X.; Lin, J.; Lu, X.; Huang, R.; Yang, B.; Shi, Y.; Ye, D.; et al. Melatonin attenuates choroidal neovascularization by regulating macrophage/microglia polarization via inhibition of RhoA/ROCK signaling pathway. J. Pineal Res. 2020, 69, e12660. [Google Scholar] [CrossRef] [PubMed]
- Heloterä, H.; Kaarniranta, K. A Linkage between Angiogenesis and Inflammation in Neovascular Age-Related Macular Degeneration. Cells 2022, 11, 3453. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Westby, K.; Csaky, K.G.; Monés, J.; Pearlman, J.A.; Patel, S.S.; Joondeph, B.C.; Randolph, J.; Masonson, H.; Rezaei, K.A. C5 Inhibitor Avacincaptad Pegol for Geographic Atrophy Due to Age-Related Macular Degeneration: A Randomized Pivotal Phase 2/3 Trial. Ophthalmology 2021, 128, 576–586. [Google Scholar] [CrossRef]
- Colijn, J.M.; Buitendijk, G.H.S.; Prokofyeva, E.; Alves, D.; Cachulo, M.L.; Khawaja, A.P.; Cougnard-Gregoire, A.; Merle, B.M.J.; Korb, C.; Erke, M.G.; et al. Prevalence of Age-Related Macular Degeneration in Europe: The Past and the Future. Ophthalmology 2017, 124, 1753–1763. [Google Scholar] [CrossRef]
- Girgis, S.; Lee, L.R. Treatment of dry age-related macular degeneration: A review. Clin. Exp. Ophthalmol. 2023, 51, 835–852. [Google Scholar] [CrossRef]
- Shaw, L.T.; Mackin, A.; Shah, R.; Jain, S.; Jain, P.; Nayak, R.; Hariprasad, S.M. Risuteganib-a novel integrin inhibitor for the treatment of non-exudative (dry) age-related macular degeneration and diabetic macular edema. Expert. Opin. Investig. Drugs 2020, 29, 547–554. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Schmitz-Valckenberg, S.; Chakravarthy, U. Age-Related Macular Degeneration: A Review. JAMA 2024, 331, 147–157. [Google Scholar] [CrossRef]
- Thomas, C.J.; Mirza, R.G.; Gill, M.K. Age-Related Macular Degeneration. Med. Clin. N. Am. 2021, 105, 473–491. [Google Scholar] [CrossRef] [PubMed]
- Orozco, L.D.; Chen, H.H.; Cox, C.; Katschke, K.J., Jr.; Arceo, R.; Espiritu, C.; Caplazi, P.; Nghiem, S.S.; Chen, Y.J.; Modrusan, Z.; et al. Integration of eQTL and a Single-Cell Atlas in the Human Eye Identifies Causal Genes for Age-Related Macular Degeneration. Cell Rep. 2020, 30, 1246–1259.e6. [Google Scholar] [CrossRef] [PubMed]
- Shwani, T.; Zhang, C.; Owen, L.A.; Shakoor, A.; Vitale, A.T.; Lillvis, J.H.; Barr, J.L.; Cromwell, P.; Finley, R.; Husami, N.; et al. Patterns of Gene Expression, Splicing, and Allele-Specific Expression Vary among Macular Tissues and Clinical Stages of Age-Related Macular Degeneration. Cells 2023, 12, 2668. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Ke, Q.; Nie, Q.; Qi, R.; Zhu, X.; Liu, W.; Hu, X.; Sun, Q.; Fu, J.L.; Tang, X.; et al. Inhibition of cGAS-STING by JQ1 alleviates oxidative stress-induced retina inflammation and degeneration. Cell Death Differ. 2022, 29, 1816–1833. [Google Scholar] [CrossRef]
- Tong, Y.; Wu, Y.; Ma, J.; Ikeda, M.; Ide, T.; Griffin, C.T.; Ding, X.Q.; Wang, S. Comparative mechanistic study of RPE cell death induced by different oxidative stresses. Redox Biol. 2023, 65, 102840. [Google Scholar] [CrossRef]
- Hanus, J.; Anderson, C.; Wang, S. RPE necroptosis in response to oxidative stress and in AMD. Ageing Res. Rev. 2015, 24 Pt B, 286–298. [Google Scholar] [CrossRef]
- Grimes, K.R.; Aloney, A.; Skondra, D.; Chhablani, J. Effects of systemic drugs on the development and progression of age-related macular degeneration. Surv. Ophthalmol. 2023, 68, 332–346. [Google Scholar] [CrossRef]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef]
- Kaufmann, M.; Han, Z. RPE melanin and its influence on the progression of AMD. Ageing Res. Rev. 2024, 99, 102358. [Google Scholar] [CrossRef]
- Brown, E.E.; DeWeerd, A.J.; Ildefonso, C.J.; Lewin, A.S.; Ash, J.D. Mitochondrial oxidative stress in the retinal pigment epithelium (RPE) led to metabolic dysfunction in both the RPE and retinal photoreceptors. Redox Biol. 2019, 24, 101201. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, Y.; Yang, J.; Zhou, B.; Zhu, Y. Inhibiting the SARM1-NAD(+) axis reduces oxidative stress-induced damage to retinal and nerve cells. Int. Immunopharmacol. 2024, 134, 112193. [Google Scholar] [CrossRef] [PubMed]
- Cornebise, C.; Perus, M.; Hermetet, F.; Valls-Fonayet, J.; Richard, T.; Aires, V.; Delmas, D. Red Wine Extract Prevents Oxidative Stress and Inflammation in ARPE-19 Retinal Cells. Cells 2023, 12, 1408. [Google Scholar] [CrossRef] [PubMed]
- Hanus, J.; Zhang, H.; Wang, Z.; Liu, Q.; Zhou, Q.; Wang, S. Induction of necrotic cell death by oxidative stress in retinal pigment epithelial cells. Cell Death Dis. 2013, 4, e965. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Cheng, Q.; Hu, Y.; Fan, X.; Liang, C.; Niu, C.; Kang, Q.; Wei, T. Melatonin antagonizes oxidative stress-induced apoptosis in retinal ganglion cells through activating the thioredoxin-1 pathway. Mol. Cell Biochem. 2024, 479, 3393–3404. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Yang, Y.; Wu, G.; Kong, Y.; Zhang, Y.; Zha, X. Circ-CARD6 inhibits oxidative stress-induced apoptosis and autophagy in ARPE-19 cells via the miR-29b-3p/PRDX6/PI3K/Akt axis. Exp. Eye Res. 2024, 238, 109690. [Google Scholar] [CrossRef]
- Cai, B.; Liao, C.; He, D.; Chen, J.; Han, J.; Lu, J.; Qin, K.; Liang, W.; Wu, X.; Liu, Z.; et al. Gasdermin E mediates photoreceptor damage by all-trans-retinal in the mouse retina. J. Biol. Chem. 2022, 298, 101553. [Google Scholar] [CrossRef]
- Totsuka, K.; Ueta, T.; Uchida, T.; Roggia, M.F.; Nakagawa, S.; Vavvas, D.G.; Honjo, M.; Aihara, M. Oxidative stress induces ferroptotic cell death in retinal pigment epithelial cells. Exp. Eye Res. 2019, 181, 316–324. [Google Scholar] [CrossRef]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Chen, C.; Chen, J.; Wang, Y.; Liu, Z.; Wu, Y. Ferroptosis drives photoreceptor degeneration in mice with defects in all-trans-retinal clearance. J. Biol. Chem. 2021, 296, 100187. [Google Scholar] [CrossRef]
- Gupta, U.; Ghosh, S.; Wallace, C.T.; Shang, P.; Xin, Y.; Nair, A.P.; Yazdankhah, M.; Strizhakova, A.; Ross, M.A.; Liu, H.; et al. Increased LCN2 (lipocalin 2) in the RPE decreases autophagy and activates inflammasome-ferroptosis processes in a mouse model of dry AMD. Autophagy 2023, 19, 92–111. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Li, L.; Zhao, Q.; Zeng, Y.; Shi, J.; Chen, Z.; Gao, G.; Lai, K. PEDF protects retinal pigment epithelium from ferroptosis and ameliorates dry AMD-like pathology in a murine model. Geroscience 2024, 46, 2697–2714. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, W.; Shah, A.; Yu, M.; Liu, Y.; He, L.; Dang, J.; Yang, L.; Yan, M.; Ying, Y.; et al. Sodium iodate induces ferroptosis in human retinal pigment epithelium ARPE-19 cells. Cell Death Dis. 2021, 12, 230. [Google Scholar] [CrossRef] [PubMed]
- Enzbrenner, A.; Zulliger, R.; Biber, J.; Pousa, A.M.Q.; Schäfer, N.; Stucki, C.; Giroud, N.; Berrera, M.; Kortvely, E.; Schmucki, R.; et al. Sodium Iodate-Induced Degeneration Results in Local Complement Changes and Inflammatory Processes in Murine Retina. Int. J. Mol. Sci. 2021, 22, 9218. [Google Scholar] [CrossRef]
- Goo, H.; Lee, M.Y.; Lee, Y.J.; Lee, S.; Ahn, J.C.; Hong, N. Multi-Wavelength Photobiomodulation Ameliorates Sodium Iodate-Induced Age-Related Macular Degeneration in Rats. Int. J. Mol. Sci. 2023, 24, 17394. [Google Scholar] [CrossRef]
- Upadhyay, M.; Bonilha, V.L. Regulated cell death pathways in the sodium iodate model: Insights and implications for AMD. Exp. Eye Res. 2024, 238, 109728. [Google Scholar] [CrossRef]
- Tang, Z.; Ju, Y.; Dai, X.; Ni, N.; Liu, Y.; Zhang, D.; Gao, H.; Sun, H.; Zhang, J.; Gu, P. HO-1-mediated ferroptosis as a target for protection against retinal pigment epithelium degeneration. Redox Biol. 2021, 43, 101971. [Google Scholar] [CrossRef]
- Henning, Y.; Blind, U.S.; Larafa, S.; Matschke, J.; Fandrey, J. Hypoxia aggravates ferroptosis in RPE cells by promoting the Fenton reaction. Cell Death Dis. 2022, 13, 662. [Google Scholar] [CrossRef]
- Yang, M.; Tsui, M.G.; Tsang, J.K.W.; Goit, R.K.; Yao, K.M.; So, K.F.; Lam, W.C.; Lo, A.C.Y. Involvement of FSP1-CoQ(10)-NADH and GSH-GPx-4 pathways in retinal pigment epithelium ferroptosis. Cell Death Dis. 2022, 13, 468. [Google Scholar] [CrossRef]
- Wang, J.; Iacovelli, J.; Spencer, C.; Saint-Geniez, M. Direct effect of sodium iodate on neurosensory retina. Invest. Ophthalmol. Vis. Sci. 2014, 55, 1941–1953. [Google Scholar] [CrossRef]
- Miotto, G.; Rossetto, M.; Di Paolo, M.L.; Orian, L.; Venerando, R.; Roveri, A.; Vučković, A.M.; Bosello Travain, V.; Zaccarin, M.; Zennaro, L.; et al. Insight into the mechanism of ferroptosis inhibition by ferrostatin-1. Redox Biol. 2020, 28, 101328. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Tonade, D.; Kern, T.S. Photoreceptor cells and RPE contribute to the development of diabetic retinopathy. Prog. Retin. Eye Res. 2021, 83, 100919. [Google Scholar] [CrossRef]
- Kawamura, S.; Tachibanaki, S. Molecular bases of rod and cone differences. Prog. Retin. Eye Res. 2022, 90, 101040. [Google Scholar] [CrossRef]
- Kaplan, L.; Drexler, C.; Pfaller, A.M.; Brenna, S.; Wunderlich, K.A.; Dimitracopoulos, A.; Merl-Pham, J.; Perez, M.T.; Schlötzer-Schrehardt, U.; Enzmann, V.; et al. Retinal regions shape human and murine Müller cell proteome profile and functionality. Glia 2023, 71, 391–414. [Google Scholar] [CrossRef]
- Terao, R.; Lee, T.J.; Colasanti, J.; Pfeifer, C.W.; Lin, J.B.; Santeford, A.; Hase, K.; Yamaguchi, S.; Du, D.; Sohn, B.S.; et al. LXR/CD38 activation drives cholesterol-induced macrophage senescence and neurodegeneration via NAD(+) depletion. Cell Rep. 2024, 43, 114102. [Google Scholar] [CrossRef]
- Zhi, X.; Lu, H.; Ma, D.; Liu, J.; Luo, L.; Wang, L.; Qin, Y. Melatonin protects photoreceptor cells against ferroptosis in dry AMD disorder by inhibiting GSK-3B/Fyn-dependent Nrf2 nuclear translocation. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166969. [Google Scholar] [CrossRef]
- Qin, S.; Lu, Y.; Rodrigues, G.A. Resveratrol protects RPE cells from sodium iodate by modulating PPARα and PPARδ. Exp. Eye Res. 2014, 118, 100–108. [Google Scholar] [CrossRef]
- Balmer, J.; Zulliger, R.; Roberti, S.; Enzmann, V. Retinal Cell Death Caused by Sodium Iodate Involves Multiple Caspase-Dependent and Caspase-Independent Cell-Death Pathways. Int. J. Mol. Sci. 2015, 16, 15086–15103. [Google Scholar] [CrossRef]
- Park, E.; Chung, S.W. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 2019, 10, 822. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Han, X.; Lan, X.; Gao, Y.; Wan, J.; Durham, F.; Cheng, T.; Yang, J.; Wang, Z.; Jiang, C.; et al. Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight 2017, 2, e90777. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Y.; Du, T.; Yang, H.; Lei, L.; Guo, M.; Ding, H.F.; Zhang, J.; Wang, H.; Chen, X.; et al. ATF3 promotes erastin-induced ferroptosis by suppressing system Xc−. Cell Death Differ. 2020, 27, 662–675. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Kang, R.; Klionsky, D.J.; Tang, D. Ferroptosis: Machinery and regulation. Autophagy 2021, 17, 2054–2081. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, M. PM2.5 induces ferroptosis in human endothelial cells through iron overload and redox imbalance. Environ. Pollut. 2019, 254 Pt A, 112937. [Google Scholar] [CrossRef]
- Liao, C.; Cai, B.; Feng, Y.; Chen, J.; Wu, Y.; Zhuang, J.; Liu, Z.; Wu, Y. Activation of JNK signaling promotes all-trans-retinal-induced photoreceptor apoptosis in mice. J. Biol. Chem. 2020, 295, 6958–6971. [Google Scholar] [CrossRef]
- Xie, B.S.; Wang, Y.Q.; Lin, Y.; Mao, Q.; Feng, J.F.; Gao, G.Y.; Jiang, J.Y. Inhibition of ferroptosis attenuates tissue damage and improves long-term outcomes after traumatic brain injury in mice. CNS Neurosci. Ther. 2019, 25, 465–475. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Wang, H.; Yang, J.; Zhao, B.; Lei, Y.; Li, H.; Yang, K.; Liu, B.; Diao, Y. Sodium Iodate-Induced Ferroptosis in Photoreceptor-Derived 661W Cells Through the Depletion of GSH. Int. J. Mol. Sci. 2025, 26, 2334. https://doi.org/10.3390/ijms26052334
Chen C, Wang H, Yang J, Zhao B, Lei Y, Li H, Yang K, Liu B, Diao Y. Sodium Iodate-Induced Ferroptosis in Photoreceptor-Derived 661W Cells Through the Depletion of GSH. International Journal of Molecular Sciences. 2025; 26(5):2334. https://doi.org/10.3390/ijms26052334
Chicago/Turabian StyleChen, Chao, Han Wang, Jiuyu Yang, Bi Zhao, Yutian Lei, Hanqiao Li, Kunhuan Yang, Benying Liu, and Yong Diao. 2025. "Sodium Iodate-Induced Ferroptosis in Photoreceptor-Derived 661W Cells Through the Depletion of GSH" International Journal of Molecular Sciences 26, no. 5: 2334. https://doi.org/10.3390/ijms26052334
APA StyleChen, C., Wang, H., Yang, J., Zhao, B., Lei, Y., Li, H., Yang, K., Liu, B., & Diao, Y. (2025). Sodium Iodate-Induced Ferroptosis in Photoreceptor-Derived 661W Cells Through the Depletion of GSH. International Journal of Molecular Sciences, 26(5), 2334. https://doi.org/10.3390/ijms26052334





