Dalbergia odorifera Trans-Nerolidol Protects Against Myocardial Ischemia via Downregulating Cytochrome- and Caspases-Signaling Pathways in Isoproterenol-Induced Rats
Abstract
1. Introduction
2. Results
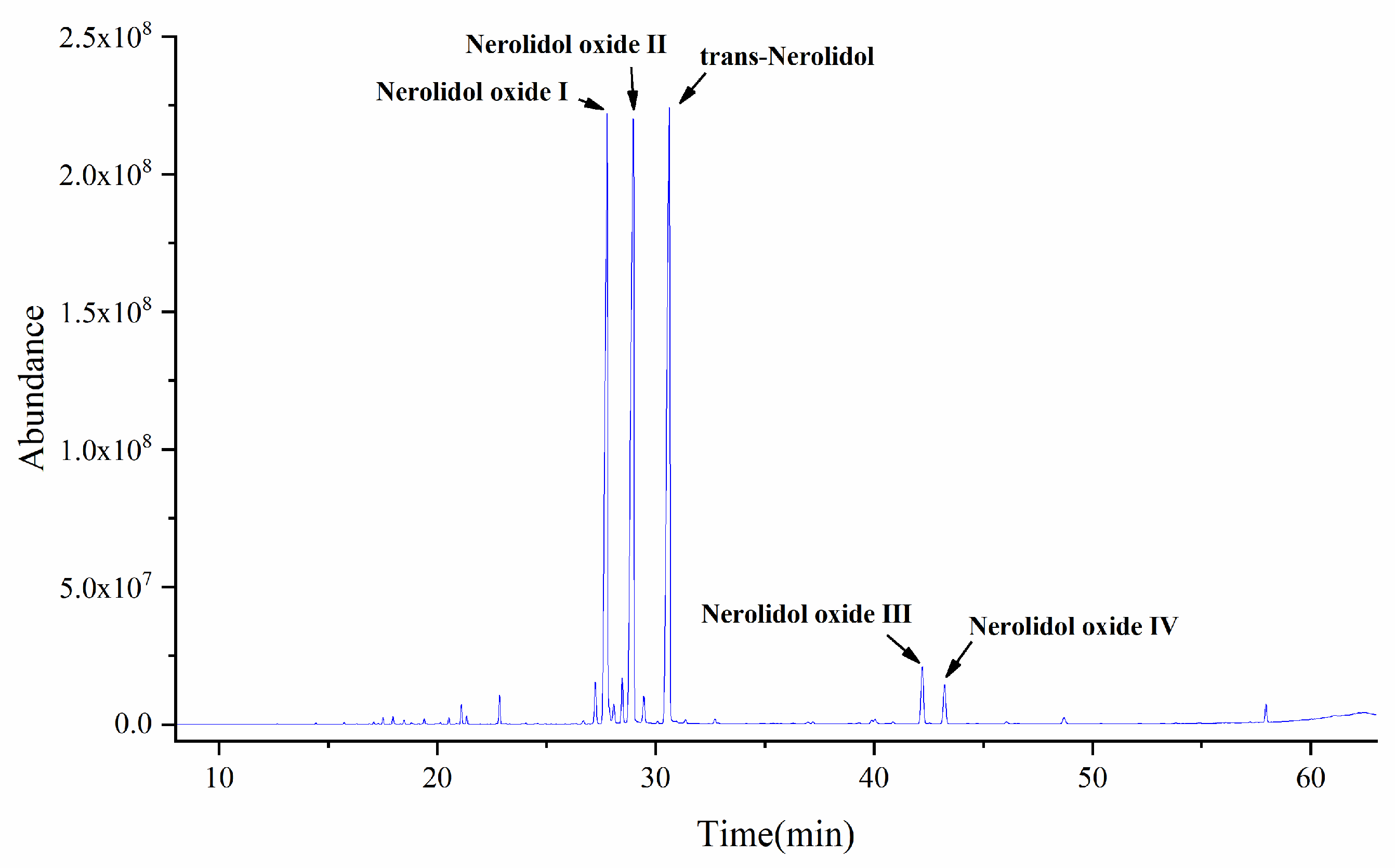
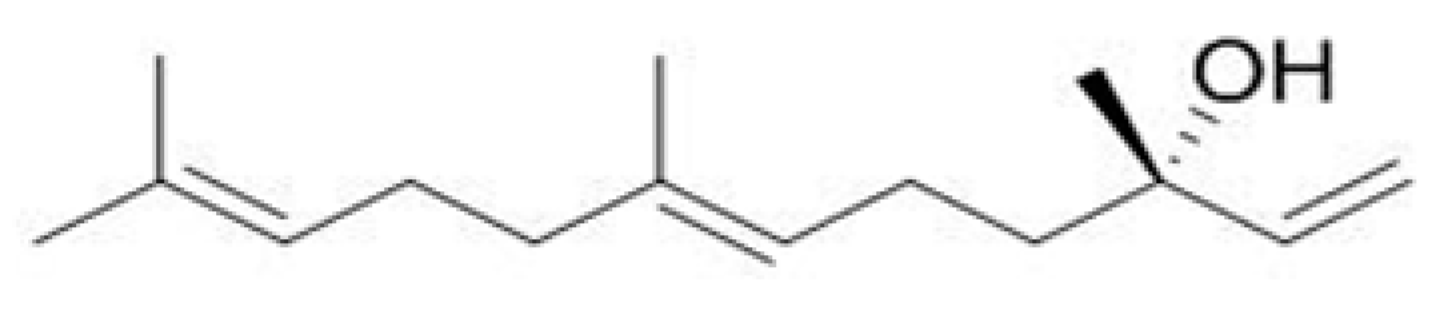
2.1. GC-MS Analysis of Essential Oil Components of D. odorifera
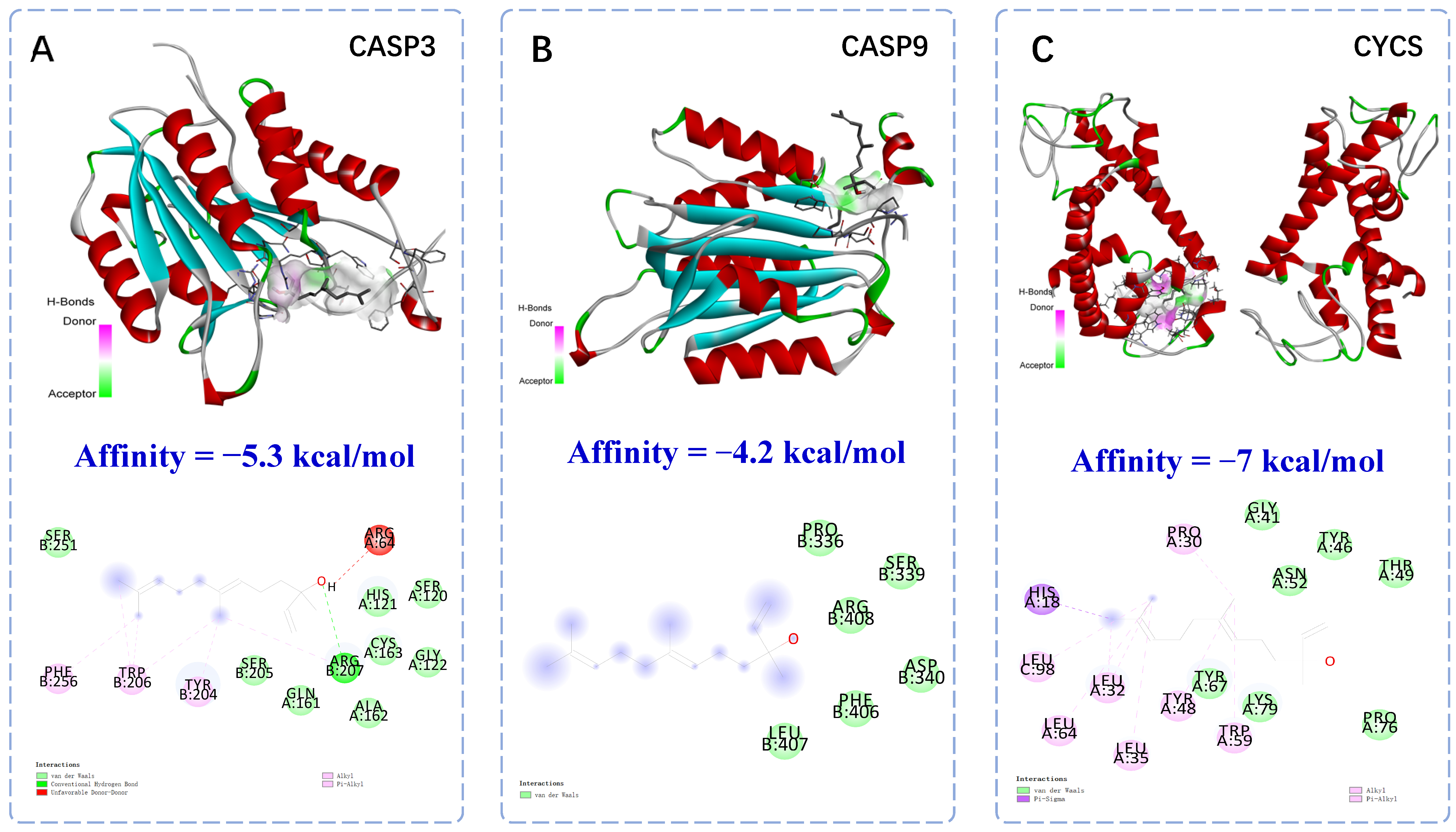
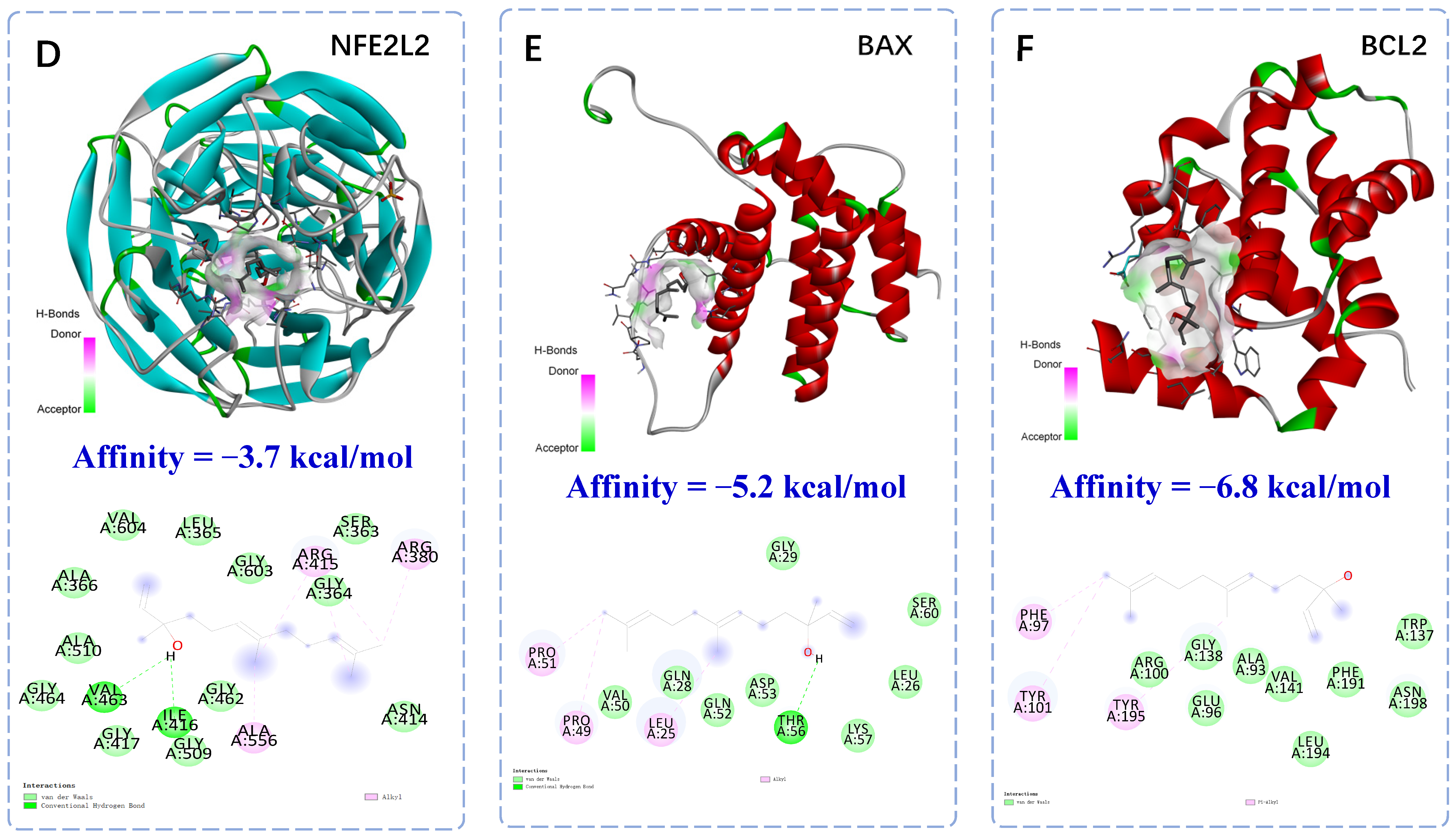
2.2. Molecular Docking of Anti-Apoptotic Mechanism of Trans-Nerolol
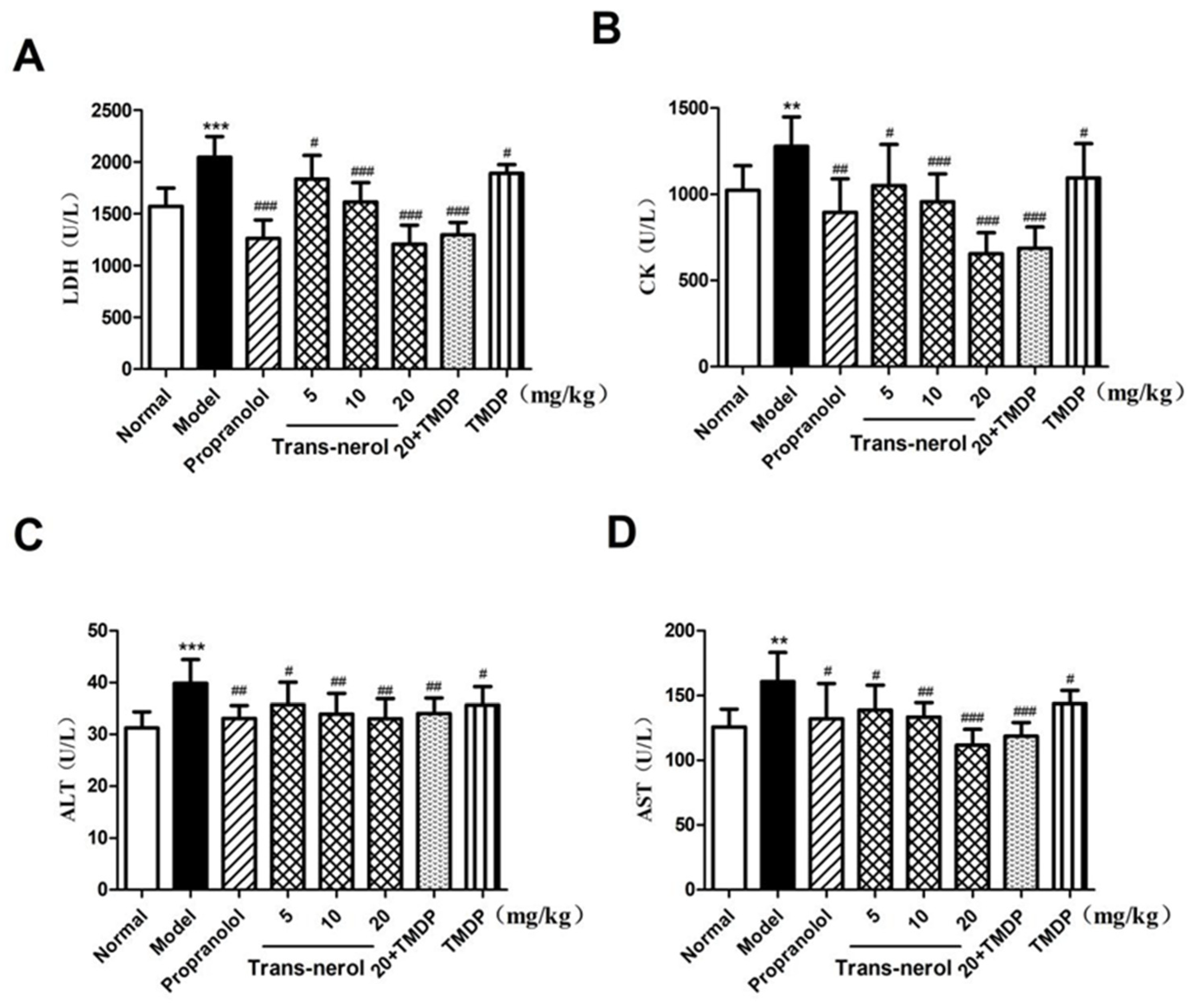
2.3. Effect of Trans-Nerolol on Myocardial Enzymes in Serum
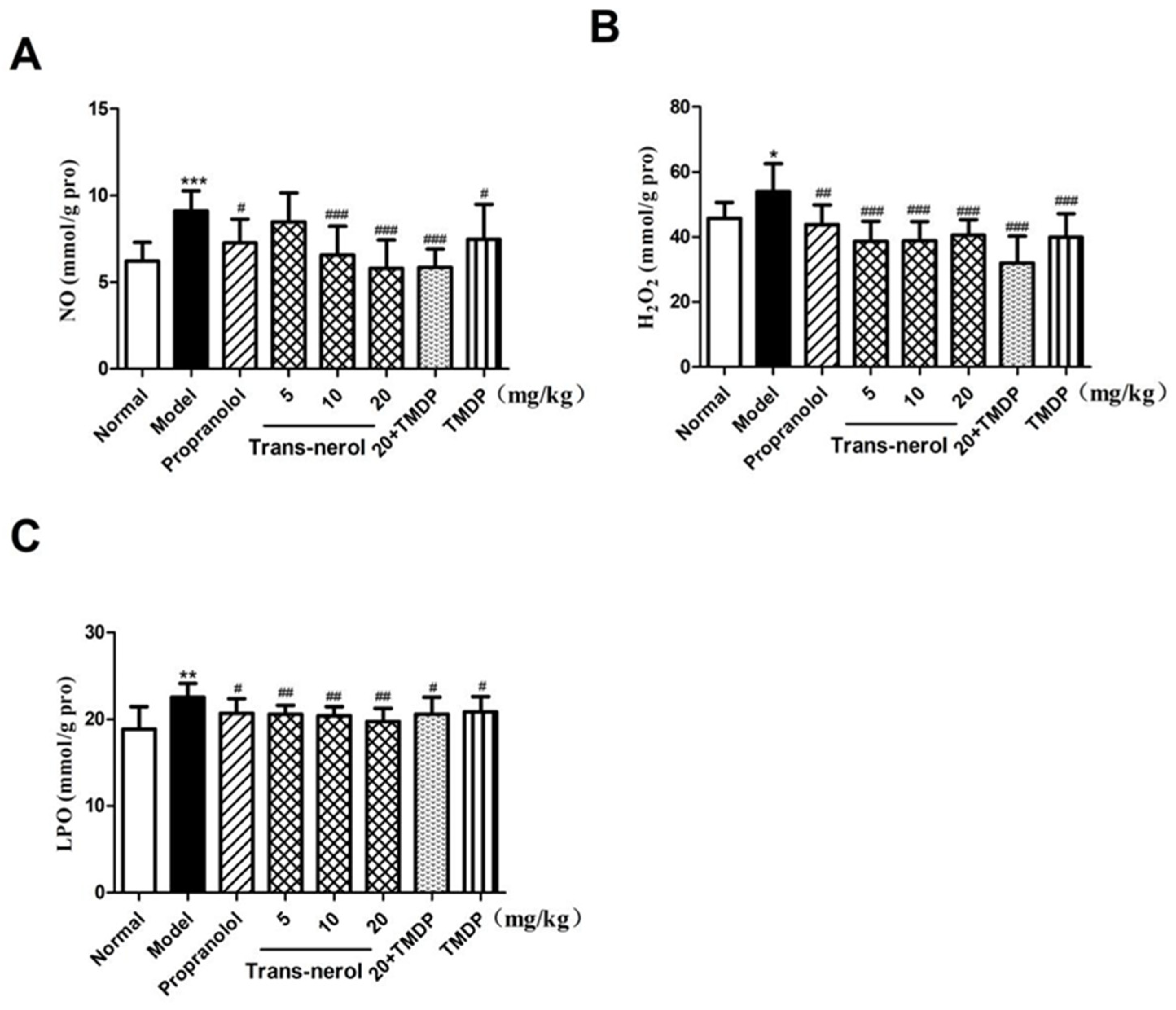
2.4. Effect of Trans-Nerolol on the Lipid Peroxidation in Homogenate Supernatant
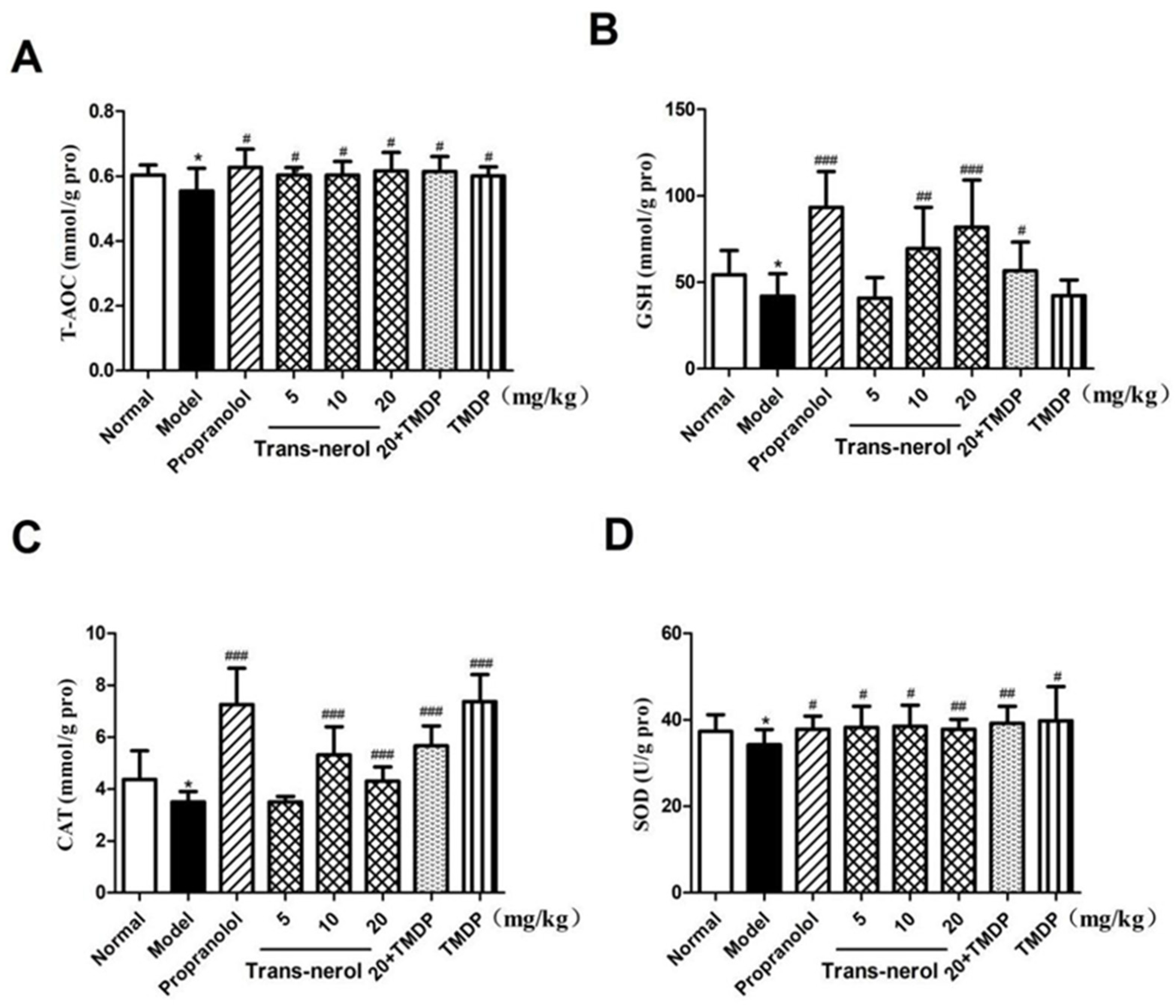
2.5. Effect of Trans-Nerolol on the Anti-Oxidation in Homogenate Supernatant
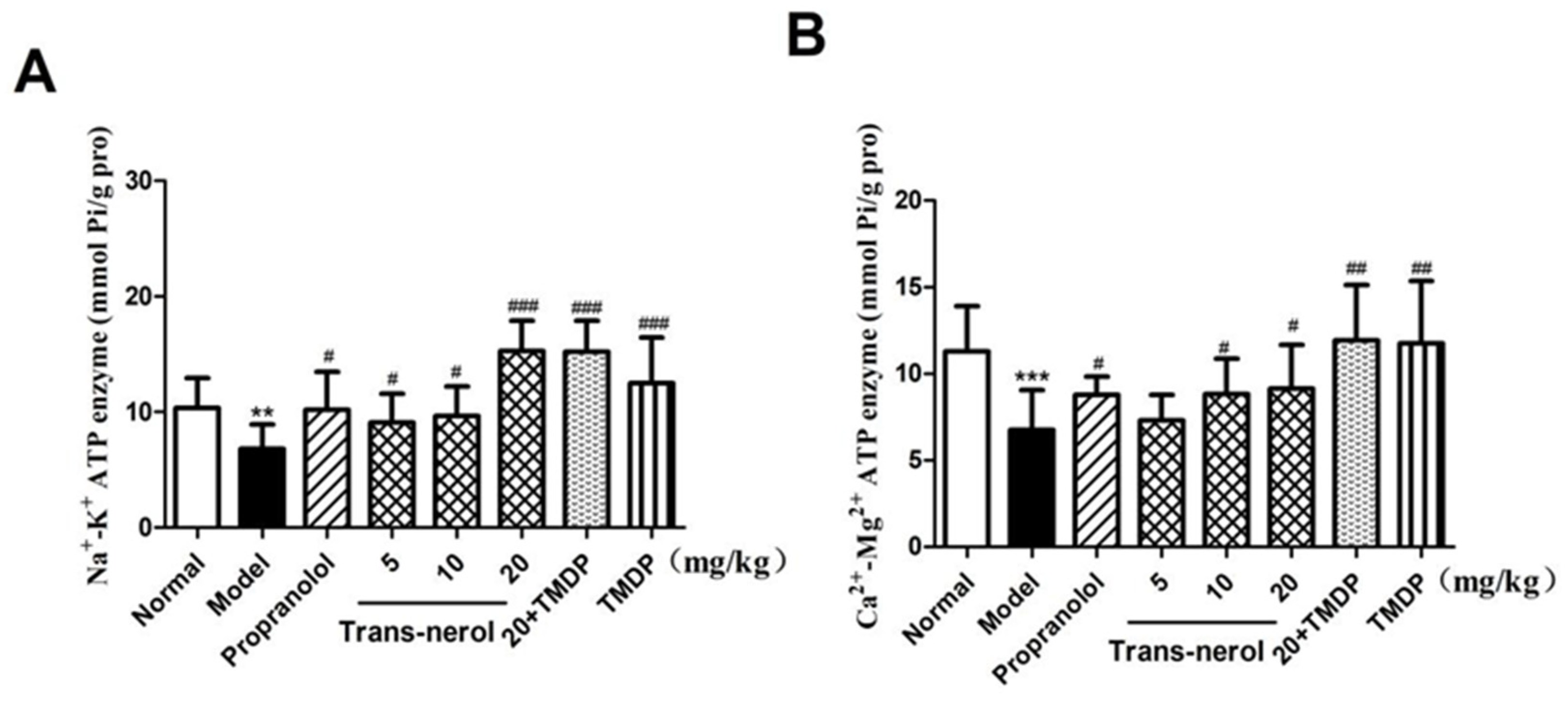
2.6. Effect of Trans-Nerolol on the ATPase in Homogenate Supernatant
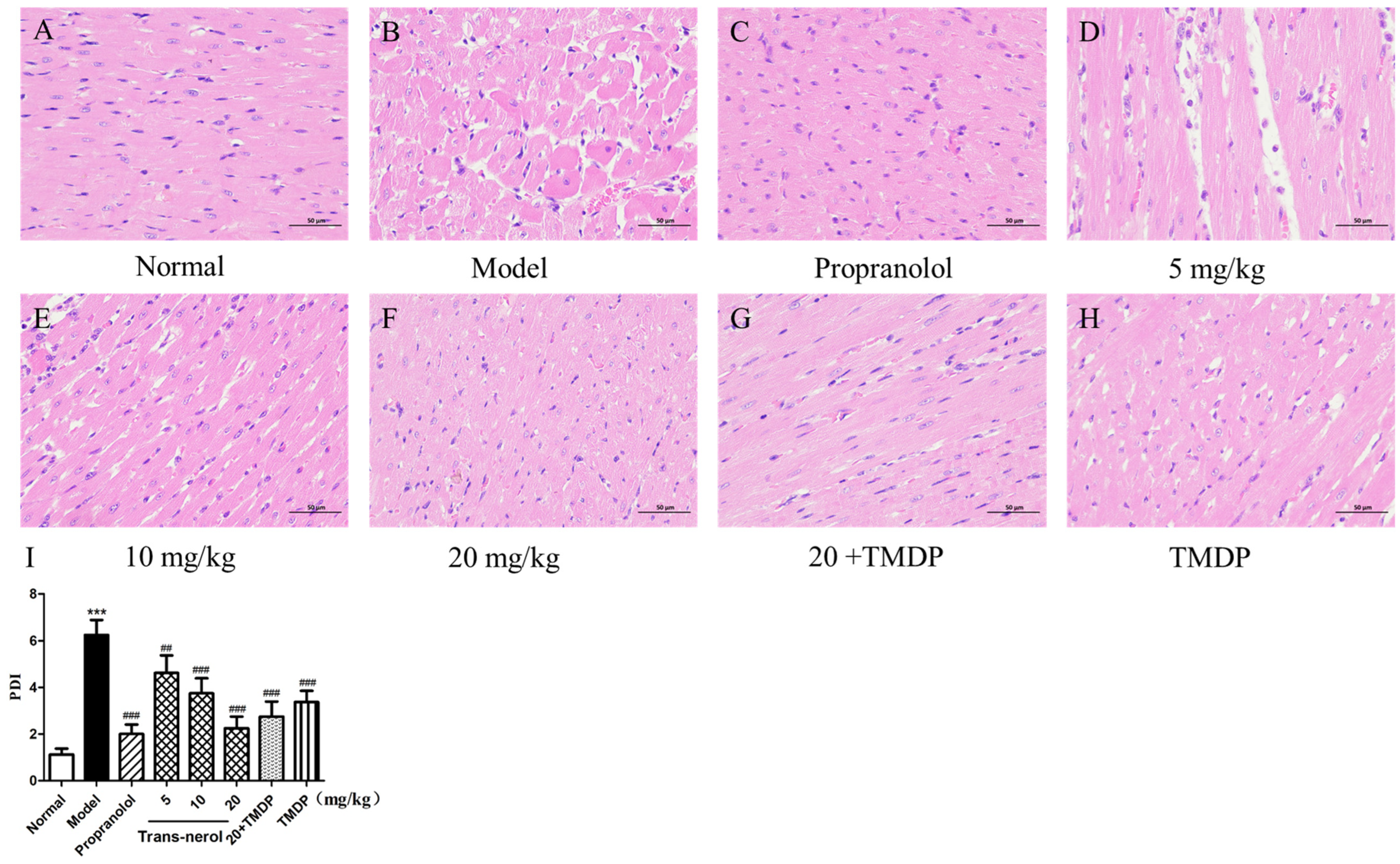
2.7. Myocardium Histopathological Examination
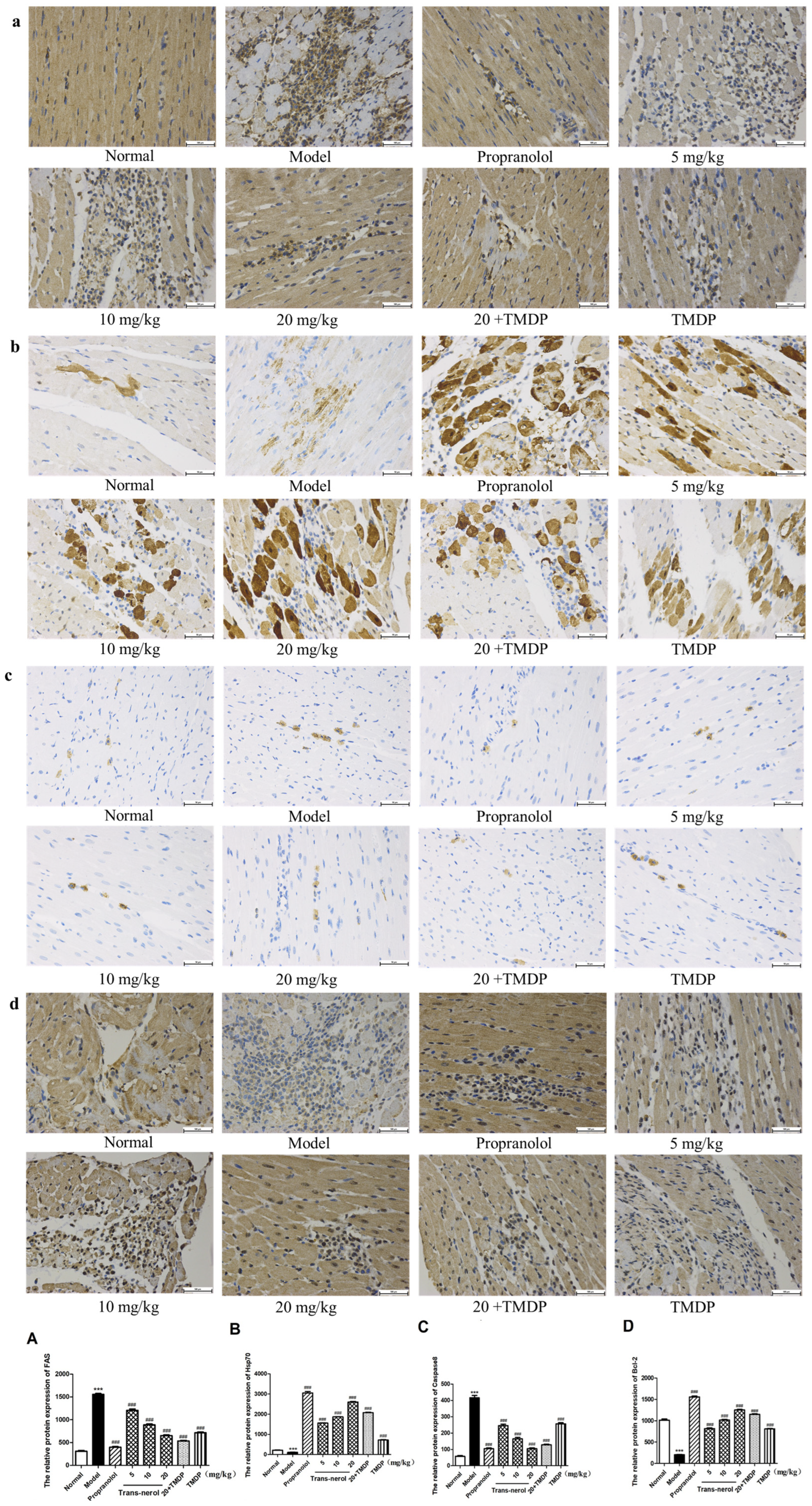
2.8. Effect of Trans-Nerolol on the Protein Expressions of Endogenous Apoptotic Pathway by IHC
2.9. Effect of Trans-Nerolol on the Protein Expressions of Exogenous Apoptosis Pathway by IHC
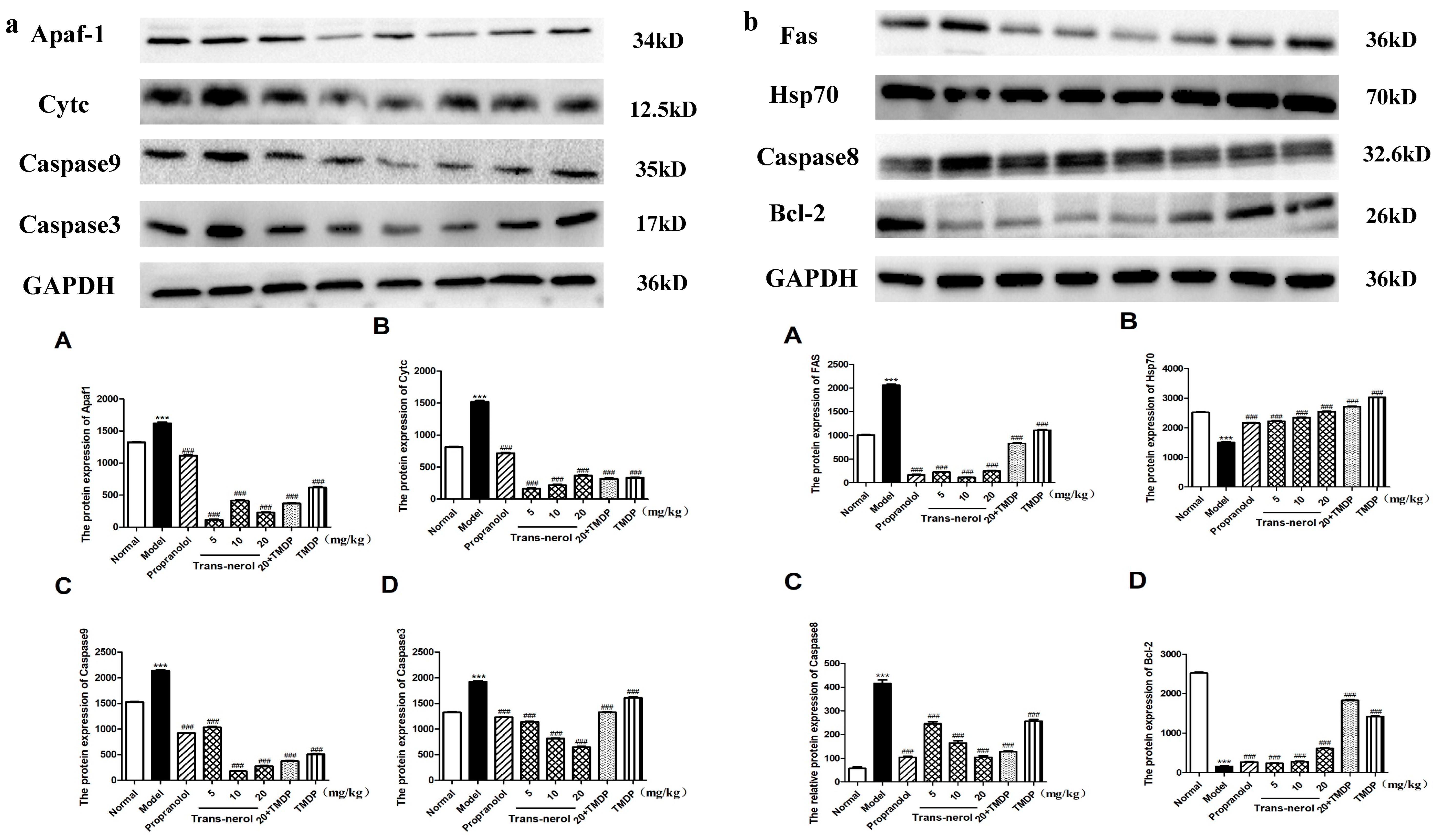
2.10. Effect of Trans-Nerolol on the Protein Expressions of Apoptosis Pathways by WB
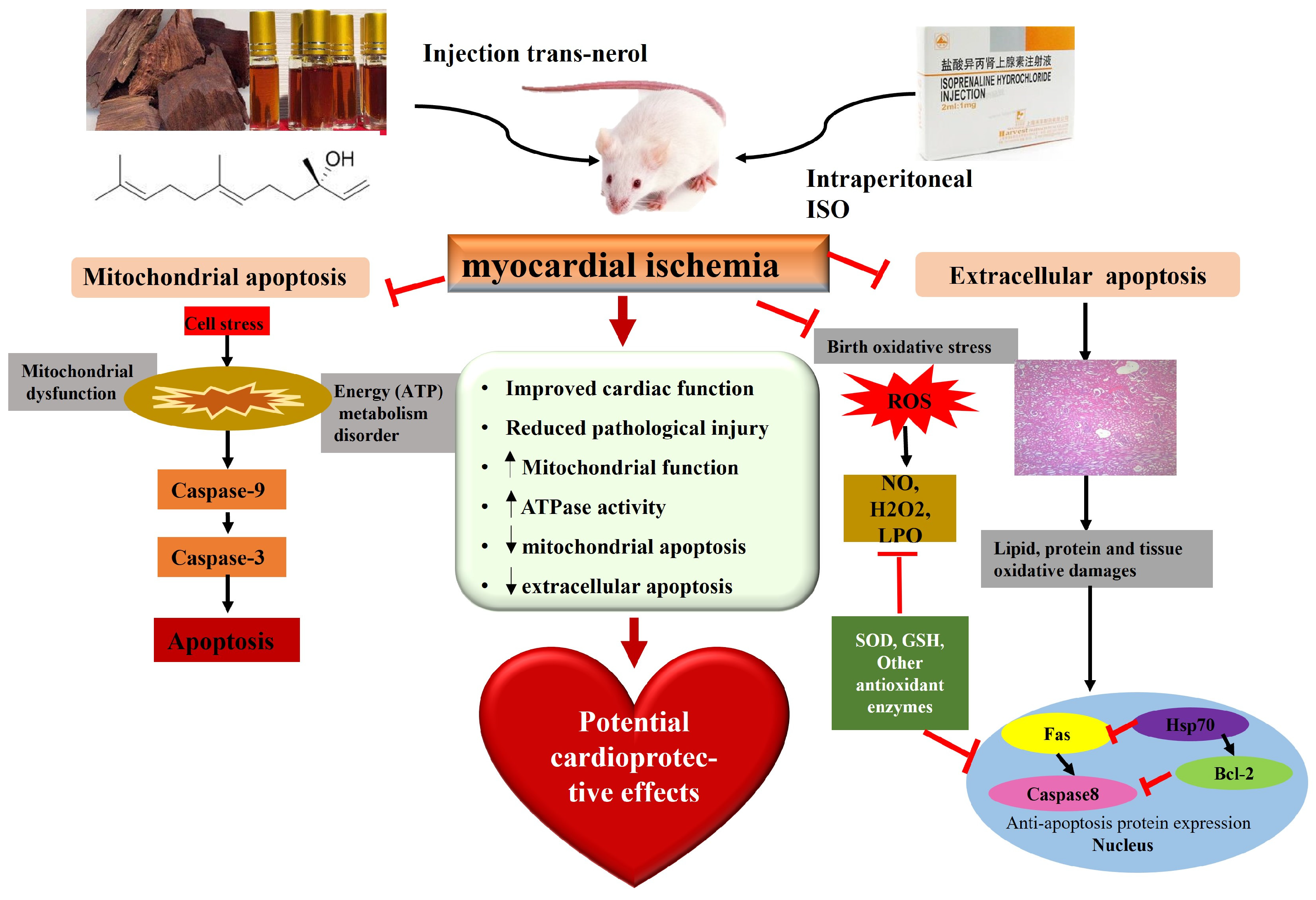
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Reagents
4.1.2. Drugs
4.2. Methods
4.2.1. Detection and Analysis of Essential Oil by GC-MS
GC-MS Analysis Condition
Preparation and Composition Detection of Volatile Oil
4.2.2. Component–Target Molecular Docking
4.2.3. Animal Experiments
4.2.4. Serum and Heart Tissue Homogenate Supernatant Preparation
4.2.5. Levels of Myocardial Enzymes in Homogenate Supernatant of Heart Tissue
4.2.6. Determination of Oxidation Index Level in Homogenate Supernatant of Heart Tissue
4.2.7. Determination of ATP Level in Homogenate Supernatant of Heart Tissue
4.2.8. Histopathological Examination of Myocardium
4.2.9. Analyzing the Levels of Proteins Involved in Internal and External Apoptosis by IHC
4.2.10. Analysis the Levels of Proteins Involved in Internal and External Apoptosis by WB
4.2.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ham, S.A.; Wang, J.S.; Kang, E.S.; Yoo, T.; Lim, H.H.; Lee, W.J. Ethanol extract of protects skin keratinocytes against ultraviolet b-induced photoaging by suppressing production of reactive oxygen species. Biosci. Biotechnol. Biochem. 2015, 79, 760–766. [Google Scholar] [CrossRef]
- Pharmacopoeia of People’s Republic of China. Chinese Pharma-Copoeia Commission; Chemical Industry Press: Beijing, China, 2020. [Google Scholar]
- Lee, D.S.; Kim, Y.C.; Ko, W.; Kim, K.S.; Li, B.; Oh, H. The neoflavonoid latifolin isolated from MeOH extract of Dalbergia odorifera attenuates inflammatory responses by inhibiting NF-κB activation via Nrf2-mediated heme oxygenase-1 expression. Phytother. Res. 2014, 28, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Li, B.; Im, N.K.; Kim, Y.C.; Jeong, G.S. 4,2’,5’-Trihydroxy-4’-methoxychalcone from Dalbergia odorifera exhibits anti-inflammatory properties by inducing heme oxygenase-1 in murine macrophages. Int. Immunopharmacol. 2013, 16, 114–121. [Google Scholar] [CrossRef]
- Zheng, L.; Xing, H.; Li, W.; Chen, Z. Physicochemical properties, chemical composition and antioxidant activity of Dalbergia odorifera T. Chen seed oil. J. Am. Oil Chem. Soc. 2013, 89, 883–890. [Google Scholar]
- Fan, Z.M.; Wang, D.Y.; Yang, J.M.; Lin, Z.X.; Lin, Y.X.; Yang, A.L. Dalbergia odorifera extract promotes angiogenesis through upregulation of vegfrs and pi3k/mapk signaling pathways. J. Ethnopharmacol. 2017, 204, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dong, W.H.; Zuo, W.J.; Liu, S.; Zhong, H.M.; Mei, W.L. Five new sesquiterpenoids from Dalbergia odorifera. Fitoterapia 2014, 95, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Park, K.R.; Yun, H.M.; Quang, T.H.; Oh, H.; Lee, D.S.; Auh, Q. 4-methoxydalbergione suppresses growth and induces apoptosis in human osteosarcoma cells in vitro and in vivo xenograft model through down-regulation of the jak2/stat3 pathway. Oncotarget 2016, 7, 6960–6971. [Google Scholar] [CrossRef]
- Wang, H.; Dong, W.H.; Zuo, W.J.; Wang, H.; Zhong, H.M.; Mei, W.L. Three new phenolic compounds from Dalbergia odorifera. J. Asian Nat. Prod. Res. 2014, 16, 1109–1118. [Google Scholar] [CrossRef]
- Zhao, X.; Mei, W.; Gong, M.; Zuo, W.; Bai, H.; Dai, H. Antibacterial activity of the flavonoids from Dalbergia odorifera on Ral-stonia solanacearum. Molecules 2011, 16, 9775–9782. [Google Scholar] [CrossRef]
- Cheung, S.; Fang, W.; Li, X.; Wang, R.; Luo, W.; Muhammad, I. Chemical constituents of Dalbergia odorifera. Chem. Nat. Compd. 2021, 57, 1122–1124. [Google Scholar] [CrossRef]
- Wang, C.H.; Gong, B.; Meng, H.; Wu, Y.L.; Zhao, X.S.; Wei, J.H. Dalbergia odorifera essential oil protects against myocardial ischemia through upregulating Nrf2 and inhibiting Caspases signaling pathways in isoproterenol-induced rats. World J. Trad. Chin. Med. 2023, 8, 338–347. [Google Scholar] [CrossRef]
- Wang, C.H.; Gong, B.; Wu, Y.L.; Bai, C.W.; Yang, M.H.; Zhao, X.S.; Wei, J.H. Pharmacokinetics and molecular docking of the cardioprotective flavonoids in Dalbergia odorifera. J. Sep. Sci. 2023, 47, e2300614. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.; Basu, A.; Ghosh, A. Involvement of oxidative stress in ischemic heart disease (IHD) in patients admitted in a tertiary care hospital, West Bengal, India. India. Asian J. Pharm. Clin. Res. 2013, 6, 161–166. [Google Scholar]
- Panchal, S.K.S. Quercetin ameliorates cardiovascular, hepatic, and metabolic changes in diet-induced metabolic syndrome in rats. J. Nutrit 2012, 142, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, S.B.; Yuan, T.Y.; Wu, Y.J.; Yan, Y.; Li, L. Coptisine protects rat heart against myocardial ischemia/reperfusion injury by suppressing myocardial apoptosis and inflammation. Atherosclerosis 2013, 231, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Bangalore, S.; Pursnani, S.; Kumar, S.; Bagos, P.G. Percutaneous coronary intervention versus optimal medical therapy for prevention of spontaneous myocardial infarction in subjects with stable ischemic heart disease. Circulation 2013, 127, 769–781. [Google Scholar] [CrossRef]
- Frank, A.; Bonney, M.; Bonney, S.; Weitzel, L.; Koeppen, M.; Eckle, T.M. Cardial ischemia reperfusion injury: From basic science to clinical bedside, Seminars in Cardiothoracic and Vascular. Anesthesia 2012, 16, 123–132. [Google Scholar]
- Arslan, F.; Dominique, P.; Kleijn, D.; Timmers, L.; Pieter, A.; Pasterkamp, D.G. Bridging innate immunity and myocardial ischemia/reperfusion injury: The search for therapeutic targets. Curr. Pharm. Design 2008, 14, 1205–1216. [Google Scholar] [CrossRef]
- Lin, Z.; Li, X.; Meng, J. The Cardiomyocytes Injury Efficiency Induced by Hydrogen Peroxide. Pro Biotechnol. 2004, 24, 81–85. [Google Scholar]
- Koshinuma, S.; Miyamae, M.; Kaneda, K.; Kotani, J.; Figueredoet, V.M. Combination of necroptosis and apoptosis inhibition enhances cardioprotection against myocardial ischemia reperfusion injury. J. Anesthesia 2014, 28, 235–241. [Google Scholar] [CrossRef]
- Wang, Z.G.; Wang, Y.; Ye, J.M.; Lu, X.H.; Cheng, Y.; Xiang, L.J. BFGF attenuates endoplasmic reticulum stress and mitochondrial injury on myocardial ischaemia/reperfusion via activation of PI3K/Akt/ERK1/2 pathway. J. Cell Mol. Med. 2015, 19, 595–607. [Google Scholar] [CrossRef]
- Wang, C.H.; Peng, D.Q.; Liu, Y.Y.; Yu, Z.X.; Wei, J.H. Agarwood alcohol extract ameliorates I soproterenol-induced myocardial ischemia by inhibiting oxidation and apoptosis. Cardiol. Res. Pract. 2020, 3, 3640815. [Google Scholar]
- Anandan, R.; Mathew, S.; Sankar, T.; Viswanathan, N.P. Protective effect of n-3 polyunsaturated fatty acids concentrate on isoproterenol-induced myocardial infarction in rats. Prostaglandins Leukot. Essent. Fat. Acids 2007, 76, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, A.; Zhu, B.M.; Takahara, A.; Satoh, Y.; Hashimoto, K. Cardiac effects of Salvia miltiorrhiza/Dalbergia odorifera mixture, an intravenously applicable Chinese medicine widely used for patients with ischemic heart disease in China. Circ. J. 2002, 66, 182–184. [Google Scholar] [CrossRef]
- Bi, Y.F.; Wang, X.L.; Zhang, X.; Hou, Y.Z.; Zhao, Z.Q.; Ren, X.Y. Protocol to study the effects of Traditional Chinese Medicine on patients with coronary heart disease showing phlegm-heat-stasis symptom pattern. J. Tradit. Chin. Med. 2021, 41, 826–832. [Google Scholar] [PubMed]
- Shang, H.C.; Zhang, J.H.; Yao, C.; Liu, B.Y. Qi-Shen-Yi-Qi dripping pills for the secondary prevention of myocardial infarction: A randomised clinical trial. Evid. Based Compl. Alt. 2013, 7329, 738391. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Li, Q.; Pan, C.S.; Yan, L.; Fan, J.Y.; He, K. QiShenYiQi Pills, a compound in Chinese medicine, protects against pressure overload-induced cardiac hypertrophy through a multi-component and multi-target mode. Sci. Rep. 2015, 5, 11802. [Google Scholar] [CrossRef]
- Wang, X.L.; Zhang, X.R.; Gong, T.; Zhang, Z.R.; Laura, W.B.; Wei, X. Urinary metabolomics study of Xiangdan submicron emulsions on a rat model of myocardial ischaemia. J. Trad. Chin. Med. 2018, 5, 139–150. [Google Scholar] [CrossRef]
- Wang, H.; Mei, W.L.; Guo, Z.K.; Xia, Z.F.; Dai, H.F. Chemical constituents of Dalbergia odorifera. China J. Chin. Mater. Med. 2014, 39, 1625–1629. [Google Scholar]
- Mo, Y.; Tang, L.; Ma, Y.; Wu, S. Pramipexole pretreatment attenuates myocardial ischemia/reperfusion injury through upregulation of autophagy. Biochem. Biophy Res. Commun. 2016, 473, 1119–1124. [Google Scholar] [CrossRef]
- Haramaki, N.; Stewart, D.; Aggarwal, S.; Ikeda, H.; Reznick, A.; Packer, L. Networking antioxidants in the isolated rat heart are selectively depleted by ischemia-reperfusion. Free Rad. Biol. Med. 1998, 25, 329–339. [Google Scholar] [CrossRef]
- De Ávila, P.H.M.; Ávila, R.I.; Xavier, D.S.F.E.; Cunha Bastos, C.C.; Batista, A.C.; Mendonca, E.F. Mucoadhesive formulation of Bidens pilosa L. (Asteraceae) reduces intestinal injury from 5-fluorouracil-induced mucositis in mice. Toxicol. Rep. 2015, 2, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Van Sebille, Y.Z.A.; Stansborough, R.; Wardill, H.R.; Bateman, E.; Gibson, R.J.; Keefe, D.M. Management of mucositis during chemotherapy: From pathophysiology to pragmatic therapeutics. Curr. Oncol. Rep. 2015, 17, 50–58. [Google Scholar] [CrossRef]
- Obradovic, M.; Bjelogrlic, P.; Rizzo, M.; Katsiki, N.; Haidara, M.; Stewart, A.J. Effects of obesity and estradiol on Na+/K+-ATPase and their relevance to cardiovascular diseases. J. Endocrinol. 2013, 218, R13–R23. [Google Scholar] [CrossRef]
- David, B.; Richard, M.; Scott, T.; Hari, L.; Joseph, S.; Komal, S. The Role of Na/K-ATPase Signaling in Oxidative Stress Related to Aging: Implications in Obesity and Cardiovascular Disease. Int. J. Mol. Sci. 2018, 19, 2139. [Google Scholar] [CrossRef]
- Lam, A.; Galione, A. The endoplasmic reticulum and junctional membrane communication during calcium signaling. Biochim. Biophys. Acta 2013, 1833, 2542–2559. [Google Scholar] [CrossRef] [PubMed]
- Zalvidea, S.; André, L.; Loyer, X.; Cassan, C.; Sainte-Marie, Y.; Thireau, J. ACE inhibition prevents diastolic Ca2+ overload and loss of myofilament Ca2+ sensitivity after myocardial infarction. Curr. Mol. Med. 2012, 12, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, S.; Wang, Y.L.; Miao, L.; Liu, Y.X. Protective effects of circulating microvesicles derived from myocardial ischemic rats on apoptosis of cardiomyocytes in myocardial ischemia/reperfusion injury. Oncotarget 2017, 8, 54572–54582. [Google Scholar] [CrossRef]
- Dorn, G. Apoptotic and non-apoptotic programmed cardiomyocyte death in ventricular remodeling. Cardio Res. 2009, 3, 465–473. [Google Scholar]
- Zhang, S.W.; Liu, Y.; Wang, F.; Qiang, J.; Liu, P.; Zhang, J. Ilexsaponin A attenuates ischemia-reperfusion-induced myocardial injury through anti-apoptotic pathway. PLoS ONE 2017, 12, e0170984. [Google Scholar] [CrossRef]
- Huang, L.H.; Li, J.P.; Gu, M.X.; Qu, Y.; Wang, Z.Y. Butorphanol attenuates myocardial ischemia reperfusion injury through inhibiting mitochondria-mediated apoptosis in mice. Eur. Rev. Med. Pharmaco Sci. 2018, 22, 1819–1824. [Google Scholar]


| No | Name | Gene Name | Gene Symbol |
|---|---|---|---|
| 1 | Nrf2 | NFE2L2 | Nfe2l2 nuclear factor, erythroid derived 2, like 2 |
| 2 | Cytochrome C | Cycs | cytochrome c, somatic |
| 3 | Caspase9 | Casp9 | caspase 9 |
| 4 | Caspase3 | Casp3 | caspase 3 |
| 5 | Bax | BAX | BCL2 associated X, apoptosis regulator |
| 6 | Bcl-2 | Bcl2 | B cell leukemia/lymphoma 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Wu, Y.; Gong, B.; Zhao, X.; Meng, H.; Mou, J.; Cheng, X.; Tan, Y.; Wei, J. Dalbergia odorifera Trans-Nerolidol Protects Against Myocardial Ischemia via Downregulating Cytochrome- and Caspases-Signaling Pathways in Isoproterenol-Induced Rats. Int. J. Mol. Sci. 2025, 26, 2251. https://doi.org/10.3390/ijms26052251
Wang C, Wu Y, Gong B, Zhao X, Meng H, Mou J, Cheng X, Tan Y, Wei J. Dalbergia odorifera Trans-Nerolidol Protects Against Myocardial Ischemia via Downregulating Cytochrome- and Caspases-Signaling Pathways in Isoproterenol-Induced Rats. International Journal of Molecular Sciences. 2025; 26(5):2251. https://doi.org/10.3390/ijms26052251
Chicago/Turabian StyleWang, Canhong, Yulan Wu, Bao Gong, Xiangsheng Zhao, Hui Meng, Junyu Mou, Xiaoling Cheng, Yinfeng Tan, and Jianhe Wei. 2025. "Dalbergia odorifera Trans-Nerolidol Protects Against Myocardial Ischemia via Downregulating Cytochrome- and Caspases-Signaling Pathways in Isoproterenol-Induced Rats" International Journal of Molecular Sciences 26, no. 5: 2251. https://doi.org/10.3390/ijms26052251
APA StyleWang, C., Wu, Y., Gong, B., Zhao, X., Meng, H., Mou, J., Cheng, X., Tan, Y., & Wei, J. (2025). Dalbergia odorifera Trans-Nerolidol Protects Against Myocardial Ischemia via Downregulating Cytochrome- and Caspases-Signaling Pathways in Isoproterenol-Induced Rats. International Journal of Molecular Sciences, 26(5), 2251. https://doi.org/10.3390/ijms26052251






