The Role of TLRs in Obesity and Its Related Metabolic Disorders
Abstract
1. Introduction
2. Search Strategy
3. Inflammation in Obesity
4. TLR
4.1. TLR1
4.2. TLR2
4.3. TLR3
4.4. TLR4
4.5. TLR5
4.6. TLR6
4.7. TLR7 y TLR8
4.8. TLR9
4.9. TLR10
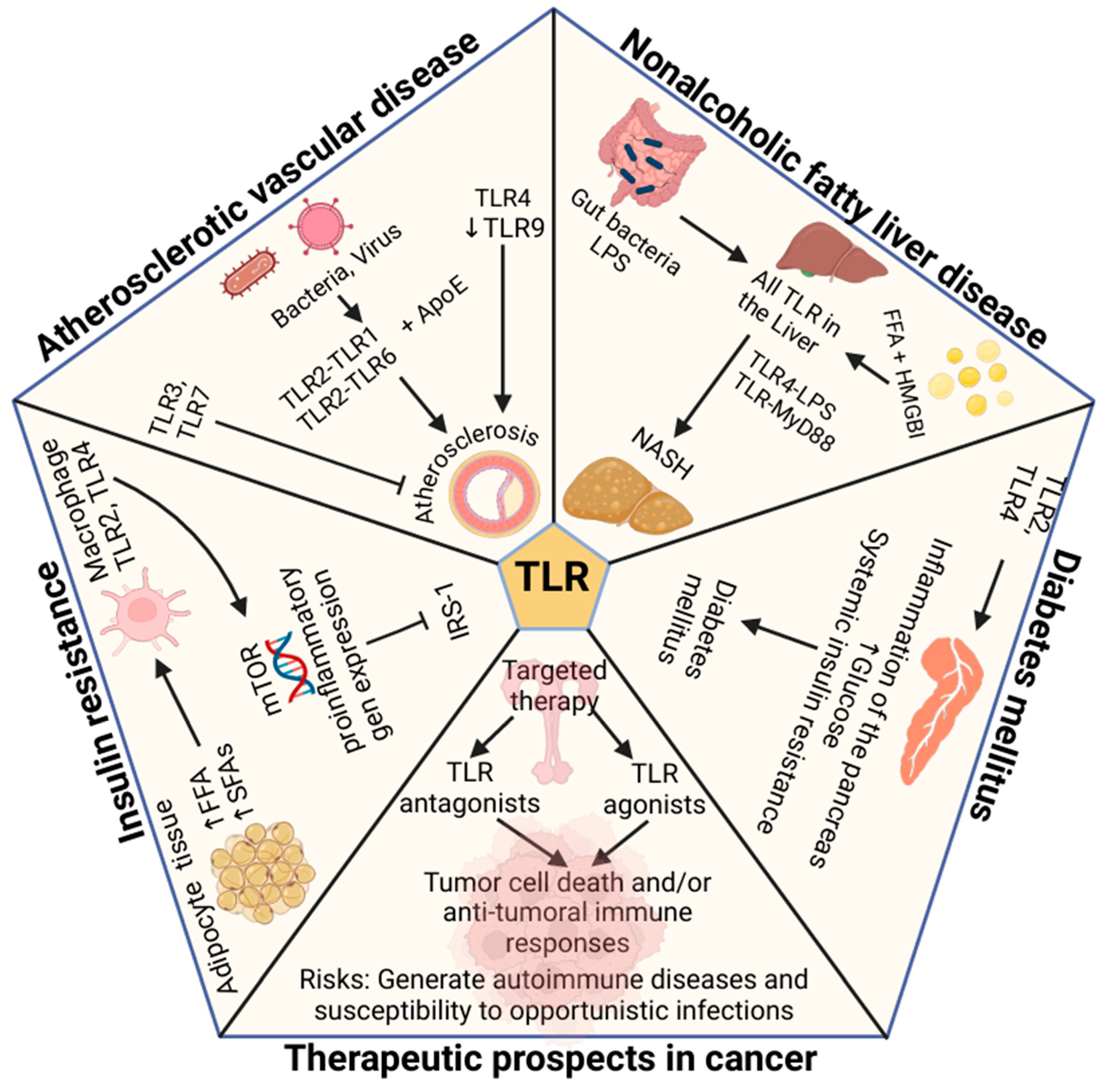
4.10. TLR in Atherosclerotic Vascular Disease
4.11. TLR in Insulin Resistance in Obesity
4.12. TLR in Diabetes and Obesity
4.13. TLR in NAFLD in Obesity
4.14. TLR in Cancer and Obesity
5. Potential Therapies
6. Conclusions
Funding
Conflicts of Interest
References
- Murray, C.J.L.; Aravkin, A.Y.; Zheng, P.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; et al. Global Burden of 87 Risk Factors in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global Epidemiology and Pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Singer, M.E.; Dorrance, K.A.; Oxenreiter, M.M.; Yan, K.R.; Close, K.L. The Type 2 Diabetes ‘Modern Preventable Pandemic’ and Replicable Lessons from the COVID-19 Crisis. Prev. Med. Rep. 2022, 25, 101636. [Google Scholar] [CrossRef] [PubMed]
- Pi-Sunyer, X. The medical risks of obesity. Postgrad. Med. 2009, 121, 21–33. [Google Scholar] [CrossRef]
- Perez, L.M.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Emanuele, E.; Lucia, A.; Galvez, B.G. “Adipaging”: Ageing and obesity share biological hallmarks related to a dysfunctional adipose tissue. J. Physiol. 2016, 594, 3187–3207. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Ahima, R.S.; Lazar, M.A. Adipokines and the peripheral and neural control of energy balance. Mol. Endocrinol. 2008, 22, 1023–1031. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Foundations of immunometabolism and implications for metabolic health and disease. Immunity 2017, 47, 406–420. [Google Scholar] [CrossRef]
- Lee, Y.S.; Li, P.; Huh, J.Y.; Hwang, I.J.; Lu, M.; Kim, J.I.; Ham, M.; Talukdar, S.; Chen, A.; Lu, W.J.; et al. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes 2011, 60, 2474–2483. [Google Scholar] [CrossRef]
- De Caterina, R.; Zampolli, A. From asthma to atherosclerosis–5-lipoxygenase, leukotrienes, and inflammation. N. Engl. J. Med. 2004, 350, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, J. Adipose tissue macrophages in the development of obesity-induced inflammation, insulin resistance and Type 2 Diabetes. Arch. Pharmacal Res. 2013, 36, 208–222. [Google Scholar] [CrossRef]
- Wallenius, V.; Wallenius, K.; Ahrén, B.; Rudling, M.; Carlsten, H.; Dickson, S.L.; Ohlsson, C.; Jansson, J.O. Interleukin-6-deficient mice develop mature-onset obesity. Nat. Med. 2002, 8, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Grosfeld, A.; Andre, J.; Hauguel-De Mouzon, S.; Berra, E.; Pouyssegur, J.; Guerre-Millo, M. Hypoxia-inducible factor 1 transactivates the human leptin gene promoter. J. Biol. Chem. 2002, 277, 42953–42957. [Google Scholar] [CrossRef]
- Gan, L.; Guo, K.; Cremona, M.L.; McGraw, T.E.; Leibel, R.L.; Zhang, Y. TNF-α up-regulates protein level and cell surface expression of the leptin receptor by stimulating its export via a PKC-dependent mechanism. Endocrinology 2012, 153, 5821–5833. [Google Scholar] [CrossRef]
- Myers, M.G.; Olson, D.P. Central nervous system control of metabolism. Nature 2012, 491, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, I.P.; Elliott, S.A.; Siervo, M.; Padwal, R.; Bertoli, S.; Battezzati, A.; Prado, C.M. Is obesity associated with altered energy expenditure? Adv. Nutr. 2016, 7, 476–487. [Google Scholar] [CrossRef]
- Oliveros, E.; Somers, V.K.; Sochor, O.; Goel, K.; Lopez-Jimenez, F. The concept of normal weight obesity. Prog. Cardiovasc. Dis. 2014, 56, 426–433. [Google Scholar] [CrossRef]
- Harman-Boehm, I.; Blüher, M.; Redel, H.; Sion-Vardy, N.; Ovadia, S.; Avinoach, E.; Shai, I.; Klöting, N.; Stumvoll, M.; Bashan, N. Macrophage infiltration into omental versus subcutaneous fat across different populations: Effect of regional adiposity and the comorbidities of obesity. J. Clin. Endocrinol. Metab. 2007, 92, 2240–2247. [Google Scholar] [CrossRef]
- Hardy, O.T.; Perugini, R.A.; Nicoloro, S.M.; Gallagher-Dorval, K.; Puri, V.; Straubhaar, J.; Czech, M.P. Body mass index-independent inflammation in omental adipose tissue associated with insulin resistance in morbid obesity. Surg. Obes. Relat. Dis. 2011, 7, 60–67. [Google Scholar] [CrossRef]
- Poulain-Godefroy, O.; Lecoeur, C.; Pattou, F.; Frühbeck, G.; Froguel, P. Inflammation Is Associated with a Decrease of Lipogenic Factors in Omental Fat in Women. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 295, R1–R7. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D.; Sarr, M.G.; Dumesic, D.A.; Southorn, P.A.; Levine, J.A. Regional Uptake of Meal Fatty Acids in Humans. Am. J. Physiol.-Endocrinol. Metab. 2003, 285, E1282–E1288. [Google Scholar] [CrossRef]
- Koutsari, C.; Ali, A.H.; Mundi, M.S.; Jensen, M.D. Storage of Circulating Free Fatty Acid in Adipose Tissue of Postabsorptive Humans: Quantitative Measures and Implications for Body Fat Distribution. Diabetes 2011, 60, 2032–2040. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in Obesity, Diabetes, and Related Disorders. Immunity 2022, 55, 31–55. [Google Scholar] [PubMed]
- Singh, G.M.; Danaei, G.; Farzadfar, F.; Stevens, G.A.; Woodward, M.; Wormser, D.; Kaptoge, S.; Whitlock, G.; Qiao, Q.; Lewington, S.; et al. The Age-Specific Quantitative Effects of Metabolic Risk Factors on Cardiovascular Diseases and Diabetes: A Pooled Analysis. PLoS ONE 2013, 8, e65174. [Google Scholar] [CrossRef]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose Tissue and Insulin Resistance in Obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin Signalling and the Regulation of Glucose and Lipid Metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic Inflammation in Fat Plays a Crucial Role in the Development of Obesity-Related Insulin Resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Moller, D.E. Potential Role of TNF-α in the Pathogenesis of Insulin Resistance and Type 2 Diabetes. Trends Endocrinol. Metab. 2000, 11, 212–217. [Google Scholar] [CrossRef]
- Berg, A.H.; Scherer, P.E. Adipose Tissue, Inflammation, and Cardiovascular Disease. Circ. Res. 2005, 96, 939–949. [Google Scholar] [CrossRef]
- Arner, P.; Rydén, M. Fatty Acids, Obesity and Insulin Resistance. Obes. Facts 2015, 8, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Urso, C.J.; Jadeja, V. Saturated Fatty Acids in Obesity-Associated Inflammation. J. Inflamm. Res. 2020, 13, 1–14. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K. Toll-like Receptor Signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Al-Mass, A.; Atizado, V.; Al-Hubail, A.; Al-Ghimlas, F.; Al-Arouj, M.; Bennakhi, A.; Dermime, S.; Behbehani, K. Elevated Expression of the Toll like Receptors 2 and 4 in Obese Individuals: Its Significance for Obesity-Induced Inflammation. J. Inflamm. 2012, 9, 48. [Google Scholar] [CrossRef]
- Medzhitov, R.; Preston-Hurlburt, P.; Janeway, C.A., Jr. A Human Homologue of the Drosophila Toll Protein Signals Activation of Adaptive Immunity. Nature 1997, 388, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. Toll-like Receptors in Innate Immunity. Int. Immunol. 2005, 17, 1–56. [Google Scholar] [CrossRef]
- Lee, J.Y.; Hwang, D.H. The Modulation of Inflammatory Gene Expression by Lipids: Mediation through Toll-like Receptors. Mol. Cells 2006, 21, 174–185. [Google Scholar] [CrossRef]
- Cook, D.N.; Pisetsky, D.S.; Schwartz, D.A. Toll-like Receptors in the Pathogenesis of Human Disease. Nat. Immunol. 2004, 5, 975–979. [Google Scholar] [CrossRef]
- Woolard, M.D.; Kevil, C.G. Paying the Toll for Glucose Regulation: A Central Role for TLR3. Diabetes 2015, 64, 3345–3346. [Google Scholar] [CrossRef]
- Pietsch, J.; Batra, A.; Stroh, T.; Fedke, I.; Glauben, R.; Okur, B.; Zeitz, M.; Siegmund, B. Toll-Like Receptor Expression and Response to Specific Stimulation in Adipocytes and Preadipocytes. Ann. N. Y. Acad. Sci. 2006, 1072, 407–409. [Google Scholar] [CrossRef]
- Könner, A.C.; Brüning, J.C. Toll-like Receptors: Linking Inflammation to Metabolism. Trends Endocrinol. Metab. 2011, 22, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Gay, N.J.; Symmons, M.F.; Gangloff, M.; Bryant, C.E. Assembly and Localization of Toll-like Receptor Signalling Complexes. Nat. Rev. Immunol. 2014, 14, 546–558. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity Is Associated with Macrophage Accumulation in Adipose Tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.-F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef]
- Kim, S.-J.; Choi, Y.; Choi, Y.-H.; Park, T. Obesity Activates Toll-like Receptor-Mediated Proinflammatory Signaling Cascades in the Adipose Tissue of Mice. J. Nutr. Biochem. 2012, 23, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Neyrinck, A.M.; Bäckhed, F.; Cani, P.D. Targeting Gut Microbiota in Obesity: Effects of Prebiotics and Probiotics. Nat. Rev. Endocrinol. 2011, 7, 639–646. [Google Scholar] [CrossRef]
- Lombardo, E.; DelaRosa, O.; Mancheño-Corvo, P.; Menta, R.; Ramírez, C.; Büscher, D. Toll-like Receptor–Mediated Signaling in Human Adipose-Derived Stem Cells: Implications for Immunogenicity and Immunosuppressive Potential. Tissue Eng. Part A 2009, 15, 1579–1589. [Google Scholar] [CrossRef]
- Fresno, M.; Alvarez, R.; Cuesta, N. Toll-like Receptors, Inflammation, Metabolism and Obesity. Arch. Physiol. Biochem. 2011, 117, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Baccala, R.; Hoebe, K.; Kono, D.H.; Beutler, B.; Theofilopoulos, A.N. TLR-Dependent and TLR-Independent Pathways of Type I Interferon Induction in Systemic Autoimmunity. Nat. Med. 2007, 13, 543–551. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Morrison, S.; McGee, S.L. 3T3-L1 Adipocytes Display Phenotypic Characteristics of Multiple Adipocyte Lineages. Adipocyte 2015, 4, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Fain, J.N.; Bahouth, S.W.; Madan, A.K. TNFalpha Release by the Nonfat Cells of Human Adipose Tissue. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Ajuwon, K.M.; Spurlock, M.E. Adiponectin Inhibits LPS-Induced NF-κB Activation and IL-6 Production and Increases PPARγ2 Expression in Adipocytes. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2005, 288, R1220–R1225. [Google Scholar] [CrossRef]
- Dandona, P.; Aljada, A.; Mohanty, P.; Ghanim, H.; Bandyopadhyay, A.; Chaudhuri, A. Insulin Suppresses Plasma Concentration of Vascular Endothelial Growth Factor and Matrix Metalloproteinase-9. Diabetes Care 2003, 26, 3310–3314. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, M.; Sayers, S.; D’Armiento, J.M.; Tall, A.R.; Welch, C.L. TLR3 Deficiency Protects against Collagen Degradation and Medial Destruction in Murine Atherosclerotic Plaques. Atherosclerosis 2013, 229, 52–61. [Google Scholar] [CrossRef]
- Kochumon, S.; Wilson, A.; Chandy, B.; Shenouda, S.; Tuomilehto, J.; Sindhu, S.; Ahmad, R. Palmitate Activates CCL4 Expression in Human Monocytic Cells via TLR4/MyD88 Dependent Activation of NF-κB/MAPK/ PI3K Signaling Systems. Cell Physiol. Biochem. 2018, 46, 953–964. [Google Scholar] [CrossRef]
- Kuo, L.-H.; Tsai, P.-J.; Jiang, M.-J.; Chuang, Y.-L.; Yu, L.; Lai, K.-T.A.; Tsai, Y.-S. Toll-like Receptor 2 Deficiency Improves Insulin Sensitivity and Hepatic Insulin Signalling in the Mouse. Diabetologia 2011, 54, 168–179. [Google Scholar] [CrossRef]
- Park, Y.; Park, S.; Yoo, E.; Kim, D.; Shin, H. Association of the Polymorphism for Toll-like Receptor 2 with Type 1 Diabetes Susceptibility. Ann. N. Y. Acad. Sci. 2004, 1037, 170–174. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen Recognition and Innate Immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Pålsson-McDermott, E.M.; O’Neill, L.A.J. The Potential of Targeting Toll-like Receptor 2 in Autoimmune and Inflammatory Diseases. Ir. J. Med. Sci. 2007, 176, 253–260. [Google Scholar] [CrossRef]
- Kang, J.Y.; Nan, X.; Jin, M.S.; Youn, S.-J.; Ryu, Y.H.; Mah, S.; Han, S.H.; Lee, H.; Paik, S.-G.; Lee, J.-O. Recognition of Lipopeptide Patterns by Toll-like Receptor 2-Toll-like Receptor 6 Heterodimer. Immunity 2009, 31, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, N.J.; Vladimer, G.I.; Stenvik, J.; Orning, M.P.A.; Zeid-Kilani, M.V.; Bugge, M.; Bergstroem, B.; Conlon, J.; Husebye, H.; Hise, A.G.; et al. A Role for the Adaptor Proteins TRAM and TRIF in Toll-like Receptor 2 Signaling*. J. Biol. Chem. 2015, 290, 3209–3222. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Sears, D.D. TLR4 and Insulin Resistance. Gastroenterol. Res. Pract. 2010, 2010, 212563. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Zhao, L.; Youn, H.S.; Weatherill, A.R.; Tapping, R.; Feng, L.; Lee, W.H.; Fitzgerald, K.A.; Hwang, D.H. Saturated Fatty Acid Activates but Polyunsaturated Fatty Acid Inhibits Toll-like Receptor 2 Dimerized with Toll-like Receptor 6 or 1*. J. Biol. Chem. 2004, 279, 16971–16979. [Google Scholar] [CrossRef]
- Bès-Houtmann, S.; Roche, R.; Hoareau, L.; Gonthier, M.-P.; Festy, F.; Caillens, H.; Gasque, P.; Lefebvre d’Hellencourt, C.; Cesari, M. Presence of Functional TLR2 and TLR4 on Human Adipocytes. Histochem. Cell Biol. 2007, 127, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Edfeldt, K. Toll To Be Paid at the Gateway to the Vessel Wall. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1085–1087. [Google Scholar] [CrossRef] [PubMed]
- Janssens, S.; Beyaert, R. Role of Toll-Like Receptors in Pathogen Recognition. Clin. Microbiol. Rev. 2003, 16, 637–646. [Google Scholar] [CrossRef]
- Shishido, T.; Nozaki, N.; Takahashi, H.; Arimoto, T.; Niizeki, T.; Koyama, Y.; Abe, J.; Takeishi, Y.; Kubota, I. Central Role of Endogenous Toll-like Receptor-2 Activation in Regulating Inflammation, Reactive Oxygen Species Production, and Subsequent Neointimal Formation after Vascular Injury. Biochem. Biophys. Res. Commun. 2006, 345, 1446–1453. [Google Scholar] [CrossRef]
- Favre, J.; Musette, P.; Douin-Echinard, V.; Laude, K.; Henry, J.-P.; Arnal, J.-F.; Thuillez, C.; Richard, V. Toll-Like Receptors 2-Deficient Mice Are Protected Against Postischemic Coronary Endothelial Dysfunction. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1064–1071. [Google Scholar] [CrossRef]
- Creely, S.J.; McTernan, P.G.; Kusminski, C.M.; Fisher, F.M.; Da Silva, N.F.; Khanolkar, M.; Evans, M.; Harte, A.L.; Kumar, S. Lipopolysaccharide Activates an Innate Immune System Response in Human Adipose Tissue in Obesity and Type 2 Diabetes. Am. J. Physiol.-Endocrinol. Metab. 2007, 292, E740–E747. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.A.; Favelyukis, S.; Nguyen, A.-K.; Reichart, D.; Scott, P.A.; Jenn, A.; Liu-Bryan, R.; Glass, C.K.; Neels, J.G.; Olefsky, J.M. A Subpopulation of Macrophages Infiltrates Hypertrophic Adipose Tissue and Is Activated by Free Fatty Acids via Toll-like Receptors 2 and 4 and JNK-Dependent Pathways. J. Biol. Chem. 2007, 282, 35279–35292. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, N.; Fernández-Veledo, S.; Punzón, C.; Moreno, C.; Barrocal, B.; Sreeramkumar, V.; Desco, M.; Fresno, M. Opposing Actions of TLR2 and TLR4 in Adipocyte Differentiation and Mature-Onset Obesity. Int. J. Mol. Sci. 2022, 23, 15682. [Google Scholar] [CrossRef] [PubMed]
- Shechter, R.; London, A.; Kuperman, Y.; Ronen, A.; Rolls, A.; Chen, A.; Schwartz, M. Hypothalamic Neuronal Toll-like Receptor 2 Protects against Age-Induced Obesity. Sci. Rep. 2013, 3, 1254. [Google Scholar] [CrossRef]
- Poulain-Godefroy, O.; Le Bacquer, O.; Plancq, P.; Lecœur, C.; Pattou, F.; Frühbeck, G.; Froguel, P. Inflammatory Role of Toll-Like Receptors in Human and Murine Adipose Tissue. Mediat. Inflamm. 2010, 2010, 823486. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B. Neo-Ligands for Innate Immune Receptors and the Etiology of Sterile Inflammatory Disease. Immunol. Rev. 2007, 220, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Strodthoff, D.; Ma, Z.; Wirström, T.; Strawbridge, R.J.; Ketelhuth, D.F.J.; Engel, D.; Clarke, R.; Falkmer, S.; Hamsten, A.; Hansson, G.K.; et al. Toll-Like Receptor 3 Influences Glucose Homeostasis and β-Cell Insulin Secretion. Diabetes 2015, 64, 3425–3438. [Google Scholar] [CrossRef]
- Jiang, S.-Y.; Wei, C.-C.; Shang, T.-T.; Lian, Q.; Wu, C.-X.; Deng, J.-Y. High Glucose Induces Inflammatory Cytokine through Protein Kinase C-Induced Toll-like Receptor 2 Pathway in Gingival Fibroblasts. Biochem. Biophys. Res. Commun. 2012, 427, 666–670. [Google Scholar] [CrossRef]
- Waterman, R.S.; Tomchuck, S.L.; Henkle, S.L.; Betancourt, A.M. A New Mesenchymal Stem Cell (MSC) Paradigm: Polarization into a Pro-Inflammatory MSC1 or an Immunosuppressive MSC2 Phenotype. PLoS ONE 2010, 5, e10088. [Google Scholar] [CrossRef]
- Latorre, J.; Moreno-Navarrete, J.M.; Sabater, M.; Buxo, M.; Rodriguez-Hermosa, J.I.; Girones, J.; Fort, J.M.; Vilallonga, R.; Ricart, W.; Simo, R.; et al. Decreased TLR3 in Hyperplastic Adipose Tissue, Blood and Inflamed Adipocytes Is Related to Metabolic Inflammation. Cell Physiol. Biochem. 2018, 51, 1051–1068. [Google Scholar] [CrossRef]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 Links Innate Immunity and Fatty Acid–Induced Insulin Resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef]
- Kolek, M.J.; Carlquist, J.F.; Muhlestein, J.B.; Whiting, B.M.; Horne, B.D.; Bair, T.L.; Anderson, J.L. Toll–like Receptor 4 Gene Asp299Gly Polymorphism Is Associated with Reductions in Vascular Inflammation, Angiographic Coronary Artery Disease, and Clinical Diabetes. Am. Heart J. 2004, 148, 1034–1040. [Google Scholar] [CrossRef]
- Tarkowski, A.; Bjersing, J.; Shestakov, A.; Bokarewa, M.I. Resistin Competes with Lipopolysaccharide for Binding to Toll-like Receptor 4. J. Cell. Mol. Med. 2010, 14, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Kiechl, S.; Lorenz, E.; Reindl, M.; Wiedermann, C.J.; Oberhollenzer, F.; Bonora, E.; Willeit, J.; Schwartz, D.A. Toll-like Receptor 4 Polymorphisms and Atherogenesis. N. Engl. J. Med. 2002, 347, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.H.; Shah, P.K.; Faure, E.; Equils, O.; Thomas, L.; Fishbein, M.C.; Luthringer, D.; Xu, X.-P.; Rajavashisth, T.B.; Yano, J.; et al. Toll-Like Receptor-4 Is Expressed by Macrophages in Murine and Human Lipid-Rich Atherosclerotic Plaques and Upregulated by Oxidized LDL. Circulation 2001, 104, 3103–3108. [Google Scholar] [CrossRef] [PubMed]
- Edfeldt, K.; Swedenborg, J.; Hansson, G.K.; Yan, Z. Expression of Toll-Like Receptors in Human Atherosclerotic Lesions. Circulation 2002, 105, 1158–1161. [Google Scholar] [CrossRef]
- Suganami, T.; Tanimoto-Koyama, K.; Nishida, J.; Itoh, M.; Yuan, X.; Mizuarai, S.; Kotani, H.; Yamaoka, S.; Miyake, K.; Aoe, S.; et al. Role of the Toll-like Receptor 4/NF-κB Pathway in Saturated Fatty Acid–Induced Inflammatory Changes in the Interaction Between Adipocytes and Macrophages. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 84–91. [Google Scholar] [CrossRef]
- Frantz, S.; Kobzik, L.; Kim, Y.-D.; Fukazawa, R.; Medzhitov, R.; Lee, R.T.; Kelly, R.A. Toll4 (TLR4) Expression in Cardiac Myocytes in Normal and Failing Myocardium. J. Clin. Investig. 1999, 104, 271–280. [Google Scholar] [CrossRef]
- Suganami, T.; Mieda, T.; Itoh, M.; Shimoda, Y.; Kamei, Y.; Ogawa, Y. Attenuation of Obesity-Induced Adipose Tissue Inflammation in C3H/HeJ Mice Carrying a Toll-like Receptor 4 Mutation. Biochem. Biophys. Res. Commun. 2007, 354, 45–49. [Google Scholar] [CrossRef]
- Li, B.; Leung, J.C.K.; Chan, L.Y.Y.; Yiu, W.H.; Tang, S.C.W. A Global Perspective on the Crosstalk between Saturated Fatty Acids and Toll-like Receptor 4 in the Etiology of Inflammation and Insulin Resistance. Prog. Lipid Res. 2020, 77, 101020. [Google Scholar] [CrossRef]
- Schaeffler, A.; Gross, P.; Buettner, R.; Bollheimer, C.; Buechler, C.; Neumeier, M.; Kopp, A.; Schoelmerich, J.; Falk, W. Fatty Acid-Induced Induction of Toll-like Receptor-4/Nuclear Factor-κB Pathway in Adipocytes Links Nutritional Signalling with Innate Immunity. Immunology 2009, 126, 233–245. [Google Scholar] [CrossRef]
- Park, B.S.; Lee, J.-O. Recognition of Lipopolysaccharide Pattern by TLR4 Complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Peng, Y.; Wu, J.; Wang, Y.; Yao, L. Toll-like Receptor 2/4 Links to Free Fatty Acid-Induced Inflammation and β-Cell Dysfunction. J. Leukoc. Biol. 2014, 95, 47–52. [Google Scholar] [CrossRef]
- McKernan, K.; Varghese, M.; Patel, R.; Singer, K. Role of TLR4 in the Induction of Inflammatory Changes in Adipocytes and Macrophages. Adipocyte 2020, 9, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Francisco, S.; Billod, J.-M.; Merino, J.; Punzón, C.; Gallego, A.; Arranz, A.; Martin-Santamaria, S.; Fresno, M. Induction of TLR4/TLR2 Interaction and Heterodimer Formation by Low Endotoxic Atypical LPS. Front. Immunol. 2022, 12, 748303. [Google Scholar] [CrossRef]
- Takeda, K.; Kaisho, T.; Akira, S. Toll-Like Receptors. Annu. Rev. Immunol. 2003, 21, 335–376. [Google Scholar] [CrossRef]
- Lin, Y.; Lee, H.; Berg, A.H.; Lisanti, M.P.; Shapiro, L.; Scherer, P.E. The Lipopolysaccharide-Activated Toll-like Receptor (TLR)-4 Induces Synthesis of the Closely Related Receptor TLR-2 in Adipocytes*. J. Biol. Chem. 2000, 275, 24255–24263. [Google Scholar] [CrossRef] [PubMed]
- Georgel, P.; Jiang, Z.; Kunz, S.; Janssen, E.; Mols, J.; Hoebe, K.; Bahram, S.; Oldstone, M.B.A.; Beutler, B. Vesicular Stomatitis Virus Glycoprotein G Activates a Specific Antiviral Toll-like Receptor 4-Dependent Pathway. Virology 2007, 362, 304–313. [Google Scholar] [CrossRef]
- Dasu, M.R.; Devaraj, S.; Park, S.; Jialal, I. Increased Toll-Like Receptor (TLR) Activation and TLR Ligands in Recently Diagnosed Type 2 Diabetic Subjects. Diabetes Care 2010, 33, 861–868. [Google Scholar] [CrossRef]
- Rocha, D.M.; Caldas, A.P.; Oliveira, L.L.; Bressan, J.; Hermsdorff, H.H. Saturated Fatty Acids Trigger TLR4-Mediated Inflammatory Response. Atherosclerosis 2016, 244, 211–215. [Google Scholar] [CrossRef]
- Ellenbroek, G.H.J.M.; van Puijvelde, G.H.M.; Anas, A.A.; Bot, M.; Asbach, M.; Schoneveld, A.; van Santbrink, P.J.; Foks, A.C.; Timmers, L.; Doevendans, P.A.; et al. Leukocyte TLR5 Deficiency Inhibits Atherosclerosis by Reduced Macrophage Recruitment and Defective T-Cell Responsiveness. Sci. Rep. 2017, 7, 42688. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y. Pterostilbene, a Novel Natural Plant Conduct, Inhibits High Fat-Induced Atherosclerosis Inflammation via NF-κB Signaling Pathway in Toll-like Receptor 5 (TLR5) Deficient Mice. Biomed. Pharmacother. 2016, 81, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Arias-Loste, M.T.; Iruzubieta, P.; Puente, Á.; Ramos, D.; Santa Cruz, C.; Estébanez, Á.; Llerena, S.; Alonso-Martín, C.; Segundo, D.S.; Álvarez, L.; et al. Increased Expression Profile and Functionality of TLR6 in Peripheral Blood Mononuclear Cells and Hepatocytes of Morbidly Obese Patients with Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2016, 17, 1878. [Google Scholar] [CrossRef] [PubMed]
- Bønnelykke, K.; Matheson, M.C.; Pers, T.H.; Granell, R.; Strachan, D.P.; Alves, A.C.; Linneberg, A.; Curtin, J.A.; Warrington, N.M.; Standl, M.; et al. Meta-Analysis of Genome-Wide Association Studies Identifies Ten Loci Influencing Allergic Sensitization. Nat. Genet. 2013, 45, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Hemmi, H.; Kaisho, T.; Takeuchi, O.; Sato, S.; Sanjo, H.; Hoshino, K.; Horiuchi, T.; Tomizawa, H.; Takeda, K.; Akira, S. Small Anti-Viral Compounds Activate Immune Cells via the TLR7 MyD88–Dependent Signaling Pathway. Nat. Immunol. 2002, 3, 196–200. [Google Scholar] [CrossRef]
- Ahmad, R.; Kochumon, S.; Thomas, R.; Atizado, V.; Sindhu, S. Increased Adipose Tissue Expression of TLR8 in Obese Individuals with or without Type-2 Diabetes: Significance in Metabolic Inflammation. J. Inflamm. 2016, 13, 38. [Google Scholar] [CrossRef][Green Version]
- Ashkar, A.A.; Rosenthal, K.L. Toll-like Receptor 9, CpG DNA and Innate Immunity. Curr. Mol. Med. 2002, 2, 545–556. [Google Scholar] [CrossRef]
- Means, T.K.; Luster, A.D. Toll-Like Receptor Activation in the Pathogenesis of Systemic Lupus Erythematosus. Ann. N. Y. Acad. Sci. 2005, 1062, 242–251. [Google Scholar] [CrossRef]
- Zipris, D.; Lien, E.; Nair, A.; Xie, J.X.; Greiner, D.L.; Mordes, J.P.; Rossini, A.A. TLR9-Signaling Pathways Are Involved in Kilham Rat Virus-Induced Autoimmune Diabetes in the Biobreeding Diabetes-Resistant Rat1. J. Immunol. 2007, 178, 693–701. [Google Scholar] [CrossRef]
- Sindhu, S.; Akhter, N.; Kochumon, S.; Thomas, R.; Wilson, A.; Shenouda, S.; Tuomilehto, J.; Ahmad, R. Increased Expression of the Innate Immune Receptor TLR10 in Obesity and Type-2 Diabetes: Association with ROS-Mediated Oxidative Stress. Cell Physiol. Biochem. 2018, 45, 572–590. [Google Scholar] [CrossRef]
- Lazarus, R.; Raby, B.A.; Lange, C.; Silverman, E.K.; Kwiatkowski, D.J.; Vercelli, D.; Klimecki, W.J.; Martinez, F.D.; Weiss, S.T. TOLL-like Receptor 10 Genetic Variation Is Associated with Asthma in Two Independent Samples. Am. J. Respir. Crit. Care Med. 2004, 170, 594–600. [Google Scholar] [CrossRef]
- Morgan, A.R.; Lam, W.-J.; Han, D.-Y.; Fraser, A.G.; Ferguson, L.R. Genetic Variation within TLR10 Is Associated with Crohn’s Disease in a New Zealand Population. Hum. Immunol. 2012, 73, 416–420. [Google Scholar] [CrossRef]
- Lockhart, P.B.; Bolger, A.F.; Papapanou, P.N.; Osinbowale, O.; Trevisan, M.; Levison, M.E.; Taubert, K.A.; Newburger, J.W.; Gornik, H.L.; Gewitz, M.H.; et al. Periodontal Disease and Atherosclerotic Vascular Disease: Does the Evidence Support an Independent Association? Circulation 2012, 125, 2520–2544. [Google Scholar] [CrossRef]
- Charo, I.F.; Taub, R. Anti-Inflammatory Therapeutics for the Treatment of Atherosclerosis. Nat. Rev. Drug Discov. 2011, 10, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Lichtman, A.H. Monocyte-Macrophages and T Cells in Atherosclerosis. Immunity 2017, 47, 621–634. [Google Scholar] [CrossRef]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Doyle, B.; Caplice, N. Plaque Neovascularization and Antiangiogenic Therapy for Atherosclerosis. J. Am. Coll. Cardiol. 2007, 49, 2073–2080. [Google Scholar] [CrossRef]
- Pothineni, N.V.K.; Subramany, S.; Kuriakose, K.; Shirazi, L.F.; Romeo, F.; Shah, P.K.; Mehta, J.L. Infections, Atherosclerosis, and Coronary Heart Disease. Eur. Heart J. 2017, 38, 3195–3201. [Google Scholar] [CrossRef]
- Velsko, I.M.; Chukkapalli, S.S.; Rivera, M.F.; Lee, J.-Y.; Chen, H.; Zheng, D.; Bhattacharyya, I.; Gangula, P.R.; Lucas, A.R.; Kesavalu, L. Active Invasion of Oral and Aortic Tissues by Porphyromonas Gingivalis in Mice Causally Links Periodontitis and Atherosclerosis. PLoS ONE 2014, 9, e97811. [Google Scholar] [CrossRef]
- Li, B.; Xia, Y.; Hu, B. Infection and Atherosclerosis: TLR-Dependent Pathways. Cell. Mol. Life Sci. 2020, 77, 2751–2769. [Google Scholar] [CrossRef]
- Sharma, S.; Garg, I.; Ashraf, M.Z. TLR Signalling and Association of TLR Polymorphism with Cardiovascular Diseases. Vasc. Pharmacol. 2016, 87, 30–37. [Google Scholar] [CrossRef]
- Adamczak, D.M. The Role of Toll-Like Receptors and Vitamin D in Cardiovascular Diseases—A Review. Int. J. Mol. Sci. 2017, 18, 2252. [Google Scholar] [CrossRef]
- Tobias, P.; Curtiss, L.K. Thematic Review Series: The Immune System and Atherogenesis. Paying the Price for Pathogen Protection: Toll Receptors in Atherogenesis. J. Lipid Res. 2005, 46, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Subramanian, S.; Montes, V.N.; Goodspeed, L.; Wang, S.; Han, C.; Teresa, A.S., III; Kim, J.; O’Brien, K.D.; Chait, A. Toll-Like Receptor 4 Deficiency Decreases Atherosclerosis But Does Not Protect Against Inflammation in Obese Low-Density Lipoprotein Receptor–Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1596–1604. [Google Scholar] [CrossRef]
- Karper, J.C.; Ewing, M.M.; Habets, K.L.L.; de Vries, M.R.; Peters, E.A.B.; van Oeveren-Rietdijk, A.M.; de Boer, H.C.; Hamming, J.F.; Kuiper, J.; Kandimalla, E.R.; et al. Blocking Toll-Like Receptors 7 and 9 Reduces Postinterventional Remodeling via Reduced Macrophage Activation, Foam Cell Formation, and Migration. Arterioscler. Thromb. Vasc. Biol. 2012, 32, e72–e80. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.E.; Navin, T.J.; Cross, A.J.; Goddard, M.E.; Alexopoulou, L.; Mitra, A.T.; Davies, A.H.; Flavell, R.A.; Feldmann, M.; Monaco, C. Unexpected Protective Role for Toll-like Receptor 3 in the Arterial Wall. Proc. Natl. Acad. Sci. USA 2011, 108, 2372–2377. [Google Scholar] [CrossRef] [PubMed]
- Salagianni, M.; Galani, I.E.; Lundberg, A.M.; Davos, C.H.; Varela, A.; Gavriil, A.; Lyytikäinen, L.-P.; Lehtimäki, T.; Sigala, F.; Folkersen, L.; et al. Toll-Like Receptor 7 Protects From Atherosclerosis by Constraining “Inflammatory” Macrophage Activation. Circulation 2012, 126, 952–962. [Google Scholar] [CrossRef]
- Koulis, C.; Chen, Y.-C.; Hausding, C.; Ahrens, I.; Kyaw, T.S.; Tay, C.; Allen, T.; Jandeleit-Dahm, K.; Sweet, M.J.; Akira, S.; et al. Protective Role for Toll-Like Receptor-9 in the Development of Atherosclerosis in Apolipoprotein E–Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 516–525. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms Linking Obesity to Insulin Resistance and Type 2 Diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Lee, B.-C.; Lee, J. Cellular and Molecular Players in Adipose Tissue Inflammation in the Development of Obesity-Induced Insulin Resistance. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2014, 1842, 446–462. [Google Scholar] [CrossRef]
- White, M.F. Insulin Signaling in Health and Disease. Science 2003, 302, 1710–1711. [Google Scholar] [CrossRef] [PubMed]
- Osborn, O.; Olefsky, J.M. The Cellular and Signaling Networks Linking the Immune System and Metabolism in Disease. Nat. Med. 2012, 18, 363–374. [Google Scholar] [CrossRef]
- Taskinen, M.-R. Type 2 Diabetes as a Lipid Disorder. Curr. Mol. Med. 2005, 5, 297–308. [Google Scholar] [CrossRef]
- Artwohl, M.; Graier, W.F.; Roden, M.; Bischof, M.; Freudenthaler, A.; Waldhäusl, W.; Baumgartner-Parzer, S.M. Diabetic LDL Triggers Apoptosis in Vascular Endothelial Cells. Diabetes 2003, 52, 1240–1247. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hartstra, A.V.; Bouter, K.E.C.; Bäckhed, F.; Nieuwdorp, M. Insights Into the Role of the Microbiome in Obesity and Type 2 Diabetes. Diabetes Care 2014, 38, 159–165. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Lyroni, K.; Patsalos, A.; Daskalaki, M.G.; Doxaki, C.; Soennichsen, B.; Helms, M.; Liapis, I.; Zacharioudaki, V.; Kampranis, S.C.; Tsatsanis, C. Epigenetic and Transcriptional Regulation of IRAK-M Expression in Macrophages. J. Immunol. 2017, 198, 1297–1307. [Google Scholar] [CrossRef]
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, Regional, and National Burden of Diabetes from 1990 to 2021, with Projections of Prevalence to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Donath, M.Y.; Shoelson, S.E. Type 2 Diabetes as an Inflammatory Disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and Metabolic Disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.-A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Oguntibeju, O.O. Type 2 Diabetes Mellitus, Oxidative Stress and Inflammation: Examining the Links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a Link between Obesity, Metabolic Syndrome and Type 2 Diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Caterina, R.D. Endothelial Dysfunctions: Common Denominators in Vascular Disease. Curr. Opin. Lipidol. 2000, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in Atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Cubbon, R.M.; Rajwani, A.; Wheatcroft, S.B. The Impact of Insulin Resistance on Endothelial Function, Progenitor Cells and Repair. Diabetes Vasc. Dis. Res. 2007, 4, 103–111. [Google Scholar] [CrossRef]
- Goligorsky, M.S.; Chen, J.; Brodsky, S. Workshop: Endothelial Cell Dysfunction Leading to Diabetic Nephropathy. Hypertension 2001, 37, 744–748. [Google Scholar] [CrossRef]
- Flyvbjerg, A. Putative Pathophysiological Role of Growth Factors and Cytokines in Experimental Diabetic Kidney Disease. Diabetologia 2000, 43, 1205–1223. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Vascular Complications in Diabetes Mellitus: The Role of Endothelial Dysfunction. Clin. Sci. 2005, 109, 143–159. [Google Scholar] [CrossRef]
- Group, B.M.J.P. United Kingdom Prospective Diabetes Study (UKPDS) 13: Relative Efficacy of Randomly Allocated Diet, Sulphonylurea, Insulin, or Metformin in Patients with Newly Diagnosed Non-Insulin Dependent Diabetes Followed for Three Years. BMJ 1995, 310, 83–88. [Google Scholar]
- Calles-Escandon, J.; Cipolla, M. Diabetes and Endothelial Dysfunction: A Clinical Perspective. Endocr. Rev. 2001, 22, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.S.; Wen, L. Toll-Like Receptors and Diabetes. Ann. N. Y. Acad. Sci. 2008, 1150, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Sepehri, Z.; Kiani, Z.; Nasiri, A.A.; Kohan, F. Toll-like Receptor 2 and Type 2 Diabetes. Cell. Mol. Biol. Lett. 2016, 21, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, C.H.; Liu, Y.L. Toll-like Receptor (TLR)-2/4 Expression in Retinal Ganglion Cells in a High-Glucose Environment and Its Implications. Genet. Mol. Res. 2016, 15, 23–41. [Google Scholar] [CrossRef]
- Mukhuty, A.; Fouzder, C.; Kundu, R. Blocking TLR4-NF-κB Pathway Protects Mouse Islets from the Combinatorial Impact of High Fat and Fetuin-A Mediated Dysfunction and Restores Ability for Insulin Secretion. Mol. Cell. Endocrinol. 2021, 532, 111314. [Google Scholar] [CrossRef]
- Zu, L.; He, J.; Jiang, H.; Xu, C.; Pu, S.; Xu, G. Bacterial Endotoxin Stimulates Adipose Lipolysis via Toll-Like Receptor 4 and Extracellular Signal-Regulated Kinase Pathway*. J. Biol. Chem. 2009, 284, 5915–5926. [Google Scholar] [CrossRef]
- Devaraj, S.; Dasu, M.R.; Park, S.H.; Jialal, I. Increased Levels of Ligands of Toll-like Receptors 2 and 4 in Type 1 Diabetes. Diabetologia 2009, 52, 1665–1668. [Google Scholar] [CrossRef]
- Hussey, S.E.; Liang, H.; Costford, S.R.; Klip, A.; DeFronzo, R.A.; Sanchez-Avila, A.; Ely, B.; Musi, N. TAK-242, a Small-Molecule Inhibitor of Toll-like Receptor 4 Signalling, Unveils Similarities and Differences in Lipopolysaccharide- and Lipidinduced Inflammation and Insulin Resistance in Muscle Cells. Biosci. Rep. 2012, 33, e00004. [Google Scholar] [CrossRef]
- Ballak, D.B.; van Asseldonk, E.J.P.; van Diepen, J.A.; Jansen, H.; Hijmans, A.; Joosten, L.A.B.; Tack, C.J.; Netea, M.G.; Stienstra, R. TLR-3 Is Present in Human Adipocytes, but Its Signalling Is Not Required for Obesity-Induced Inflammation in Adipose Tissue In Vivo. PLoS ONE 2015, 10, e0123152. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease—Meta-analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 2016, 64, 73. [Google Scholar] [CrossRef]
- Portillo-Sanchez, P.; Bril, F.; Maximos, M.; Lomonaco, R.; Biernacki, D.; Orsak, B.; Subbarayan, S.; Webb, A.; Hecht, J.; Cusi, K. High Prevalence of Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes Mellitus and Normal Plasma Aminotransferase Levels. J. Clin. Endocrinol. Metab. 2015, 100, 2231–2238. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E. Nonalcoholic Fatty Liver Disease: A Systematic Review. JAMA 2015, 313, 2263–2273. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Calzadilla-Bertot, L.; Wong, V.W.-S.; Castellanos, M.; la Fuente, R.A.; Metwally, M.; Eslam, M.; Gonzalez-Fabian, L.; Sanz, M.A.-Q.; Conde-Martin, A.F.; et al. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology 2018, 155, 443–457.e17. [Google Scholar] [CrossRef]
- Adams, L.A.; Anstee, Q.M.; Tilg, H.; Targher, G. Non-Alcoholic Fatty Liver Disease and Its Relationship with Cardiovascular Disease and Other Extrahepatic Diseases. Gut 2017, 66, 1138–1153. [Google Scholar] [CrossRef]
- Wicherska-Pawłowska, K.; Wróbel, T.; Rybka, J. Toll-Like Receptors (TLRs), NOD-Like Receptors (NLRs), and RIG-I-Like Receptors (RLRs) in Innate Immunity. TLRs, NLRs, and RLRs Ligands as Immunotherapeutic Agents for Hematopoietic Diseases. Int. J. Mol. Sci. 2021, 22, 13397. [Google Scholar] [CrossRef]
- Seki, E.; Schnabl, B. Role of Innate Immunity and the Microbiota in Liver Fibrosis: Crosstalk between the Liver and Gut. J. Physiol. 2012, 590, 447–458. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The Role of Pattern-Recognition Receptors in Innate Immunity: Update on Toll-like Receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Kanuri, G.; Ladurner, R.; Skibovskaya, J.; Spruss, A.; Königsrainer, A.; Bischoff, S.C.; Bergheim, I. Expression of Toll-like Receptors 1–5 but Not TLR 6–10 Is Elevated in Livers of Patients with Non-Alcoholic Fatty Liver Disease. Liver Int. 2015, 35, 562–568. [Google Scholar] [CrossRef]
- Wagnerberger, S.; Spruss, A.; Kanuri, G.; Volynets, V.; Stahl, C.; Bischoff, S.C.; Bergheim, I. Toll-like Receptors 1–9 Are Elevated in Livers with Fructose-Induced Hepatic Steatosis. Br. J. Nutr. 2012, 107, 1727–1738. [Google Scholar] [CrossRef]
- Khan, A.; Ding, Z.; Ishaq, M.; Bacha, A.S.; Khan, I.; Hanif, A.; Li, W.; Guo, X. Understanding the Effects of Gut Microbiota Dysbiosis on Nonalcoholic Fatty Liver Disease and the Possible Probiotics Role: Recent Updates. Int. J. Biol. Sci. 2021, 17, 818–833. [Google Scholar] [CrossRef]
- Tarantino, G.; Citro, V.; Capone, D. Nonalcoholic Fatty Liver Disease: A Challenge from Mechanisms to Therapy. J. Clin. Med. 2020, 9, 15. [Google Scholar] [CrossRef]
- Gehrke, N.; Schattenberg, J.M. Metabolic Inflammation—A Role for Hepatic Inflammatory Pathways as Drivers of Comorbidities in Nonalcoholic Fatty Liver Disease? Gastroenterology 2020, 158, 1929–1947.e6. [Google Scholar] [CrossRef] [PubMed]
- Roh, Y.S.; Seki, E. Toll-like Receptors in Alcoholic Liver Disease, Non-Alcoholic Steatohepatitis and Carcinogenesis. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. 1), 38–42. [Google Scholar] [CrossRef]
- Seki, E.; Brenner, D.A. Toll-like Receptors and Adaptor Molecules in Liver Disease: Update. Hepatology 2008, 48, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.-B.; Pimentel-Nunes, P.; Roncon-Albuquerque, R.; Leite-Moreira, A. The Role of Lipopolysaccharide/Toll-like Receptor 4 Signaling in Chronic Liver Diseases. Hepatol. Int. 2010, 4, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Kodama, Y.; Inokuchi, S.; Schnabl, B.; Aoyama, T.; Ohnishi, H.; Olefsky, J.M.; Brenner, D.A.; Seki, E. Toll-Like Receptor 9 Promotes Steatohepatitis by Induction of Interleukin-1β in Mice. Gastroenterology 2010, 139, 323–334.e7. [Google Scholar] [CrossRef]
- Miura, K.; Seki, E.; Ohnishi, H.; Brenner, D.A. Role of Toll-Like Receptors and Their Downstream Molecules in the Development of Nonalcoholic Fatty Liver Disease. Gastroenterol. Res. Pract. 2010, 2010, 362847. [Google Scholar] [CrossRef]
- Jiang, Z.; Georgel, P.; Du, X.; Shamel, L.; Sovath, S.; Mudd, S.; Huber, M.; Kalis, C.; Keck, S.; Galanos, C.; et al. CD14 Is Required for MyD88-Independent LPS Signaling. Nat. Immunol. 2005, 6, 565–570. [Google Scholar] [CrossRef]
- Schromm, A.B.; Lien, E.; Henneke, P.; Chow, J.C.; Yoshimura, A.; Heine, H.; Latz, E.; Monks, B.G.; Schwartz, D.A.; Miyake, K.; et al. Molecular Genetic Analysis of an Endotoxin Nonresponder Mutant Cell Line: A Point Mutation in a Conserved Region of Md-2 Abolishes Endotoxin-Induced Signaling. J. Exp. Med. 2001, 194, 79–88. [Google Scholar] [CrossRef]
- Kim, H.M.; Park, B.S.; Kim, J.-I.; Kim, S.E.; Lee, J.; Oh, S.C.; Enkhbayar, P.; Matsushima, N.; Lee, H.; Yoo, O.J.; et al. Crystal Structure of the TLR4-MD-2 Complex with Bound Endotoxin Antagonist Eritoran. Cell 2007, 130, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Hoebe, K.; Georgel, P.; Rutschmann, S.; Du, X.; Mudd, S.; Crozat, K.; Sovath, S.; Shamel, L.; Hartung, T.; Zähringer, U.; et al. CD36 Is a Sensor of Diacylglycerides. Nature 2005, 433, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Vitez, R.; Der Mevlen, V.; Zemvez, B. Uptake by Liver Cells of Endotoxin Following Its Intravenous Injection. Lab. Investig. J. Tech. Methods Pathol. 1981, 45, 38–45. [Google Scholar]
- van Egmond, M.; van Garderen, E.; van Spriel, A.B.; Damen, C.A.; van Amersfoort, E.S.; van Zandbergen, G.; van Hattum, J.; Kuiper, J.; van de Winkel, J.G.J. FcαRI-Positive Liver Kupffer Cells: Reappraisal of the Function of Immunoglobulin A in Immunity. Nat. Med. 2000, 6, 680–685. [Google Scholar] [CrossRef]
- De Creus, A.; Abe, M.; Lau, A.H.; Hackstein, H.; Raimondi, G.; Thomson, A.W. Low TLR4 Expression by Liver Dendritic Cells Correlates with Reduced Capacity to Activate Allogeneic T Cells in Response to Endotoxin1. J. Immunol. 2005, 174, 2037–2045. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity Alters Gut Microbial Ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Brun, P.; Castagliuolo, I.; Leo, V.D.; Buda, A.; Pinzani, M.; Palù, G.; Martines, D. Increased Intestinal Permeability in Obese Mice: New Evidence in the Pathogenesis of Nonalcoholic Steatohepatitis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2007, 292, G518–G525. [Google Scholar] [CrossRef]
- Yang, S.Q.; Lin, H.Z.; Lane, M.D.; Clemens, M.; Diehl, A.M. Obesity Increases Sensitivity to Endotoxin Liver Injury: Implications for the Pathogenesis of Steatohepatitis. Proc. Natl. Acad. Sci. USA 1997, 94, 2557–2562. [Google Scholar] [CrossRef]
- Rivera, C.A.; Adegboyega, P.; van Rooijen, N.; Tagalicud, A.; Allman, M.; Wallace, M. Toll-like Receptor-4 Signaling and Kupffer Cells Play Pivotal Roles in the Pathogenesis of Non-Alcoholic Steatohepatitis. J. Hepatol. 2007, 47, 571–579. [Google Scholar] [CrossRef]
- Szabo, G.; Velayudham, A.; Romics, L., Jr.; Mandrekar, P. Modulation of Non-Alcoholic Steatohepatitis by Pattern Recognition Receptors in Mice: The Role of Toll-Like Receptors 2 and 4. Alcohol. Clin. Exp. Res. 2005, 29 (Suppl. 2), 140S–145S. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, S.; Lin, H.; Huang, J.; Watkins, P.A.; Moser, A.B.; DeSimone, C.; Song, X.; Diehl, A.M. Probiotics and Antibodies to TNF Inhibit Inflammatory Activity and Improve Nonalcoholic Fatty Liver Disease. Hepatology 2003, 37, 343. [Google Scholar] [CrossRef]
- Lanuza, F.; Sapunar, J.; Hofmann, E.; Lanuza, F.; Sapunar, J.; Hofmann, E. Análisis Crítico Del Tratamiento de La Enfermedad Hepática Grasa No Alcohólica. Rev. Médica Chile 2018, 146, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Moore, J.B. Practical Lifestyle Management of Nonalcoholic Fatty Liver Disease for Busy Clinicians. Diabetes Spectr. 2024, 37, 39–47. [Google Scholar] [CrossRef]
- Dyerberg, J.; Bang, H.O. Haemostatic function and platelet polyunsaturated fatty acids in Eskimos. Lancet 1979, 314, 433–435. [Google Scholar] [CrossRef]
- Watanabe, Y.; Tatsuno, I. Prevention of Cardiovascular Events with Omega-3 Polyunsaturated Fatty Acids and the Mechanism Involved. J. Atheroscler. Thromb. 2020, 27, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Poggioli, R.; Hirani, K.; Jogani, V.G.; Ricordi, C. Modulation of inflammation and immunity by omega-3 fatty acids: A possible role for prevention and to halt disease progression in autoimmune, viral, and age-related disorders. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 7380–7400. [Google Scholar]
- Salman, H.B.; Salman, M.A.; Yildiz Akal, E. The Effect of Omega-3 Fatty Acid Supplementation on Weight Loss and Cognitive Function in Overweight or Obese Individuals on Weight-Loss Diet. Nutr. Hosp. 2022, 39, 803–813. [Google Scholar] [CrossRef]
- Musazadeh, V.; Karimi, A.; Malekahmadi, M.; Ahrabi, S.S.; Dehghan, P. Omega-3 Polyunsaturated Fatty Acids in the Treatment of Non-Alcoholic Fatty Liver Disease: An Umbrella Systematic Review and Meta-Analysis. Clin. Exp. Pharmacol. Physiol. 2023, 50, 327–334. [Google Scholar] [CrossRef]
- Kaser, S.; Ebenbichler, C.F.; Tilg, H. Pharmacological and Non-Pharmacological Treatment of Non-Alcoholic Fatty Liver Disease. Int. J. Clin. Pract. 2010, 64, 968–983. [Google Scholar] [CrossRef]
- Baumann, A.; Nier, A.; Hernández-Arriaga, A.; Brandt, A.; Pisarello, M.J.L.; Jin, C.J.; Pilar, E.; Camarinha-Silva, A.; Schattenberg, J.M.; Bergheim, I. Toll-like Receptor 1 as a Possible Target in Non-Alcoholic Fatty Liver Disease. Sci. Rep. 2021, 11, 17815. [Google Scholar] [CrossRef] [PubMed]
- Brunner, K.T.; Henneberg, C.J.; Wilechansky, R.M.; Long, M.T. Nonalcoholic Fatty Liver Disease and Obesity Treatment. Curr. Obes. Rep. 2019, 8, 220–228. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Bhaskaran, K.; Douglas, I.; Forbes, H.; dos-Santos-Silva, I.; Leon, D.A.; Smeeth, L. Body-Mass Index and Risk of 22 Specific Cancers: A Population-Based Cohort Study of 5.24 Million UK Adults. Lancet 2014, 384, 755–765. [Google Scholar] [CrossRef]
- Prado, C.M.; Cushen, S.J.; Orsso, C.E.; Ryan, A.M. Sarcopenia and Cachexia in the Era of Obesity: Clinical and Nutritional Impact. Proc. Nutr. Soc. 2016, 75, 188–198. [Google Scholar] [CrossRef]
- Kapoor, N.D.; Twining, P.K.; Groot, O.Q.; Pielkenrood, B.J.; Bongers, M.E.R.; Newman, E.T.; Verlaan, J.J.; Schwab, J.H. Adipose Tissue Density on CT as a Prognostic Factor in Patients with Cancer: A Systematic Review. Acta Oncol. 2020, 59, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.P.; Sabatini, D.M. Cancer Cell Metabolism: Warburg and Beyond. Cell 2008, 134, 703–707. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Gunter, M.J.; Hoover, D.R.; Yu, H.; Wassertheil-Smoller, S.; Rohan, T.E.; Manson, J.E.; Li, J.; Ho, G.Y.F.; Xue, X.; Anderson, G.L.; et al. Insulin, Insulin-Like Growth Factor-I, and Risk of Breast Cancer in Postmenopausal Women. JNCI J. Natl. Cancer Inst. 2009, 101, 48–60. [Google Scholar] [CrossRef]
- Kalaany, N.Y.; Sabatini, D.M. Tumours with PI3K Activation Are Resistant to Dietary Restriction. Nature 2009, 458, 725–731. [Google Scholar] [CrossRef]
- Guo, J.Y.; White, E. Autophagy Is Required for Mitochondrial Function, Lipid Metabolism, Growth, and Fate of KRASG12D-Driven Lung Tumors. Autophagy 2013, 9, 1636–1638. [Google Scholar] [CrossRef] [PubMed]
- Nieman, K.M.; Kenny, H.A.; Penicka, C.V.; Ladanyi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.L.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes Promote Ovarian Cancer Metastasis and Provide Energy for Rapid Tumor Growth. Nat. Med. 2011, 17, 1498–1503. [Google Scholar] [CrossRef]
- Nieman, K.M.; Romero, I.L.; Van Houten, B.; Lengyel, E. Adipose Tissue and Adipocytes Support Tumorigenesis and Metastasis. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2013, 1831, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Harris, F.T.; Rahman, S.M.J.; Hassanein, M.; Qian, J.; Hoeksema, M.D.; Chen, H.; Eisenberg, R.; Chaurand, P.; Caprioli, R.M.; Shiota, M.; et al. Acyl-Coenzyme A–Binding Protein Regulates Beta-Oxidation Required for Growth and Survival of Non–Small Cell Lung Cancer. Cancer Prev. Res. 2014, 7, 748–757. [Google Scholar] [CrossRef]
- Nomura, S.; Sakamaki, S.; Hongu, M.; Kawanishi, E.; Koga, Y.; Sakamoto, T.; Yamamoto, Y.; Ueta, K.; Kimata, H.; Nakayama, K.; et al. Discovery of Canagliflozin, a Novel C-Glucoside with Thiophene Ring, as Sodium-Dependent Glucose Cotransporter 2 Inhibitor for the Treatment of Type 2 Diabetes Mellitus. J. Med. Chem. 2010, 53, 6355–6360. [Google Scholar] [CrossRef] [PubMed]
- Hussein, W.M.; Liu, T.-Y.; Skwarczynski, M.; Toth, I. Toll-like Receptor Agonists: A Patent Review (2011–2013). Expert. Opin. Ther. Pat. 2014, 24, 453–470. [Google Scholar] [CrossRef]
- Yager, J.D.; Davidson, N.E. Estrogen Carcinogenesis in Breast Cancer. N. Engl. J. Med. 2006, 354, 270–282. [Google Scholar] [CrossRef]
- Cleary, M.P.; Grossmann, M.E. Obesity and Breast Cancer: The Estrogen Connection. Endocrinology 2009, 150, 2537–2542. [Google Scholar] [CrossRef]
- Kaaks, R.; Lukanova, A.; Kurzer, M.S. Obesity, Endogenous Hormones, and Endometrial Cancer Risk: A Synthetic Review. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1531–1543. [Google Scholar]
- Shaw, K.A.; Gennat, H.C.; O’Rourke, P.; Mar, C.D. Exercise for Overweight or Obesity. Cochrane Database Syst. Rev. 2006, 2006, CD003817. [Google Scholar] [CrossRef]
- Neal, E.G.; Chaffe, H.; Schwartz, R.H.; Lawson, M.S.; Edwards, N.; Fitzsimmons, G.; Whitney, A.; Cross, J.H. The Ketogenic Diet for the Treatment of Childhood Epilepsy: A Randomised Controlled Trial. Lancet Neurol. 2008, 7, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Alarim, R.A.; Alasmre, F.A.; Alotaibi, H.A.; Alshehri, M.A.; Hussain, S.A.; Alarim, R.A.; Alasmre, F.A.; Alotaibi, H.A.; Alshehri, M.A.; Hussain, S.A. Effects of the Ketogenic Diet on Glycemic Control in Diabetic Patients: Meta-Analysis of Clinical Trials. Cureus 2020, 12, e10796. [Google Scholar] [CrossRef] [PubMed]
- Dajon, M.; Iribarren, K.; Cremer, I. Toll-like Receptor Stimulation in Cancer: A pro- and Anti-Tumor Double-Edged Sword. Immunobiology 2017, 222, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Gilliet, M.; Conrad, C.; Geiges, M.; Cozzio, A.; Thürlimann, W.; Burg, G.; Nestle, F.O.; Dummer, R. Psoriasis Triggered by Toll-like Receptor 7 Agonist Imiquimod in the Presence of Dermal Plasmacytoid Dendritic Cell Precursors. Arch. Dermatol. 2004, 140, 1490–1495. [Google Scholar] [CrossRef]
- Makkouk, A.; Abdelnoor, A.M. The Potential Use of Toll-like Receptor (TLR) Agonists and Antagonists as Prophylactic and/or Therapeutic Agents. Immunopharmacol. Immunotoxicol. 2009, 31, 331–338. [Google Scholar] [CrossRef]
- Hainer, V.; Toplak, H.; Mitrakou, A. Treatment Modalities of Obesity: What Fits Whom? Diabetes Care 2008, 31 (Suppl. 2), S269–S277. [Google Scholar] [CrossRef]

| Studied TLR | Disease Model | Method | Results | Cite |
|---|---|---|---|---|
| TLR1, TLR2, TLR6 | Atherosclerosis | TLR1- or TLR6-deficient mice fed a high-fat diet (HFD) for 10 weeks. | TlR2 interacts with either TLR1 or TLR6 to promote atherosclerosis in LDL receptors. Deficiency of TLR1 or TLR6 did not diminish HFD-driven disease. | [125] |
| TLR4 | Atherosclerosis | TLR4 and LDL receptor double knockout (TLR4−/−Ldlr−/−) mice and Ldlr−/− mice were fed either a standard chow or a diabetogenic diet for 24 weeks. | TLR4 deficiency markedly decreased atherosclerosis in obese Tlr4−/−Ldlr−/− mice. | [126] |
| TLR3 | Atherosclerosis | Hypercholesterolemia-induced arterial injury in mice and human atheroma-derived smooth muscle cells. | Genetic deletion of TLR3 dramatically enhanced the development of elastic lamina damage after collar-induced injury. Deficiency of TLR3 accelerated the onset of atherosclerosis. | [128] |
| TLR9 | Atherosclerosis | Double-knockout ApoE−/−:TLR9−/− mice and control ApoE−/− mice were fed a high-fat diet from 8 weeks. | Genetic deletion of the innate immune receptor TLR9 exacerbated atherosclerosis lesion severity. | [130] |
| TLR2, TLR4 | Diabetic retinopathy | Human retinal ganglion cell culture (control, high-glucose, and siRNA-transfected groups). | TLR2/4, TNF-α, and IL-8 were significantly increased in retinal ganglion cells treated with high-glucose. | [156] |
| TLR4 | Diabetes mellitus type 1 | Healthy controls (n = 37) and type 1 diabetic patients (n = 34) were recruited, and a fasting blood sample was obtained. | TLR4 ligand and endotoxin were significantly elevated in type 1 diabetic patients compared to matched controls. Hsp60 and HMGB1 concentrations were also considerably increased in the patients. | [159] |
| TLR4 | Non-alcoholic steatohepatitis | Male control and TLR4 mutant mice with fed control or methionine/choline-deficient diet (MCDD). | Histological evidence is typical of steatohepatitis, portal endotoxemia, and enhanced TLR4 expression in wild-type mice fed MCDD. | [192] |
| TLR 1-5 | Non-alcoholic steatohepatitis | 11 patients with NAFLD that underwent either a partial liver resection or 11 controls. | Livers of NAFLD patients had significantly higher Hepatic TLR 1-5 mRNAs expression, increased lipid peroxidation, and alterations in insulin signaling. | [169] |
| TLR9 | Non-alcoholic steatohepatitis | Type Mice and mutant TLR9 mice were steatohepatitis induced by a choline-deficient amino acid-defined diet. | Mutant TLR9 mice showed less steatohepatitis and liver fibrosis than wild-type mice. | [179] |
| TLR4 | Non-alcoholic steatohepatitis | Male control and TLR4, TLR2 mutant mice with steatohepatitis induced. | TLR4 mutant mice had lower injury and lipid accumulation markers than control. | [194] |
| TLR4, TLR2 | Non-alcoholic steatohepatitis | Male control and TLR4, TLR2 mutant mice with nonalcoholic steatohepatitis-induced. | TLR4 wild-type and TLR2 deficient mice had more liver injury in nonalcoholic steatohepatitis. | [193] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos-Bayardo, T.I.; Román-Rojas, D.; García-Sánchez, A.; Cardona-Muñoz, E.G.; Sánchez-Lozano, D.I.; Totsuka-Sutto, S.; Gómez-Hermosillo, L.F.; Casillas-Moreno, J.; Andrade-Sierra, J.; Pazarín-Villaseñor, L.; et al. The Role of TLRs in Obesity and Its Related Metabolic Disorders. Int. J. Mol. Sci. 2025, 26, 2229. https://doi.org/10.3390/ijms26052229
Campos-Bayardo TI, Román-Rojas D, García-Sánchez A, Cardona-Muñoz EG, Sánchez-Lozano DI, Totsuka-Sutto S, Gómez-Hermosillo LF, Casillas-Moreno J, Andrade-Sierra J, Pazarín-Villaseñor L, et al. The Role of TLRs in Obesity and Its Related Metabolic Disorders. International Journal of Molecular Sciences. 2025; 26(5):2229. https://doi.org/10.3390/ijms26052229
Chicago/Turabian StyleCampos-Bayardo, Tannia Isabel, Daniel Román-Rojas, Andrés García-Sánchez, Ernesto Germán Cardona-Muñoz, Daniela Itzel Sánchez-Lozano, Sylvia Totsuka-Sutto, Luis Francisco Gómez-Hermosillo, Jorge Casillas-Moreno, Jorge Andrade-Sierra, Leonardo Pazarín-Villaseñor, and et al. 2025. "The Role of TLRs in Obesity and Its Related Metabolic Disorders" International Journal of Molecular Sciences 26, no. 5: 2229. https://doi.org/10.3390/ijms26052229
APA StyleCampos-Bayardo, T. I., Román-Rojas, D., García-Sánchez, A., Cardona-Muñoz, E. G., Sánchez-Lozano, D. I., Totsuka-Sutto, S., Gómez-Hermosillo, L. F., Casillas-Moreno, J., Andrade-Sierra, J., Pazarín-Villaseñor, L., Campos-Pérez, W., Martínez-López, E., & Miranda-Díaz, A. G. (2025). The Role of TLRs in Obesity and Its Related Metabolic Disorders. International Journal of Molecular Sciences, 26(5), 2229. https://doi.org/10.3390/ijms26052229







