Poliprotect®, a Medical Device Made of Substances, Potently Protects the Human Esophageal Mucosa Challenged by Multiple Agents: Evidence from In Vitro and Ex Vivo Electrophysiological Models
Abstract
:1. Introduction
2. Results
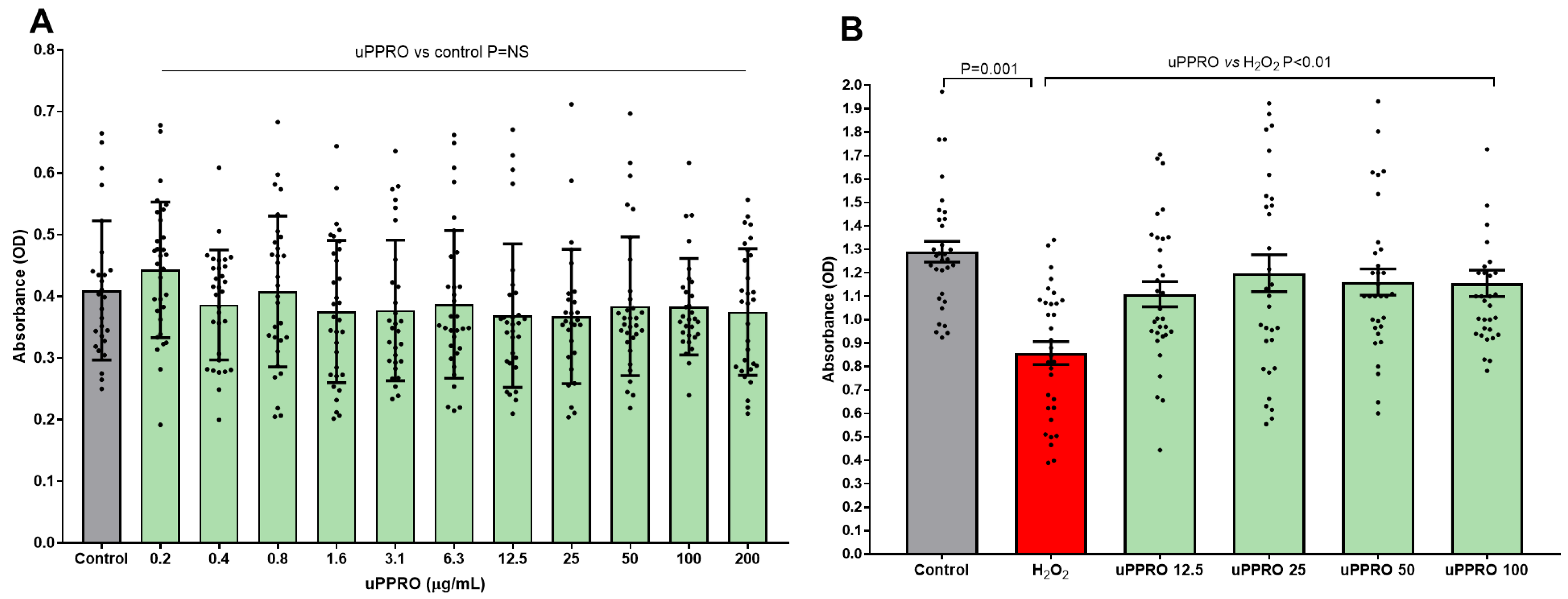
2.1. Protective Effect of uPPRO on Caco-2 Cell Viability Challenged with the Pro-Oxidant Agent H2O2
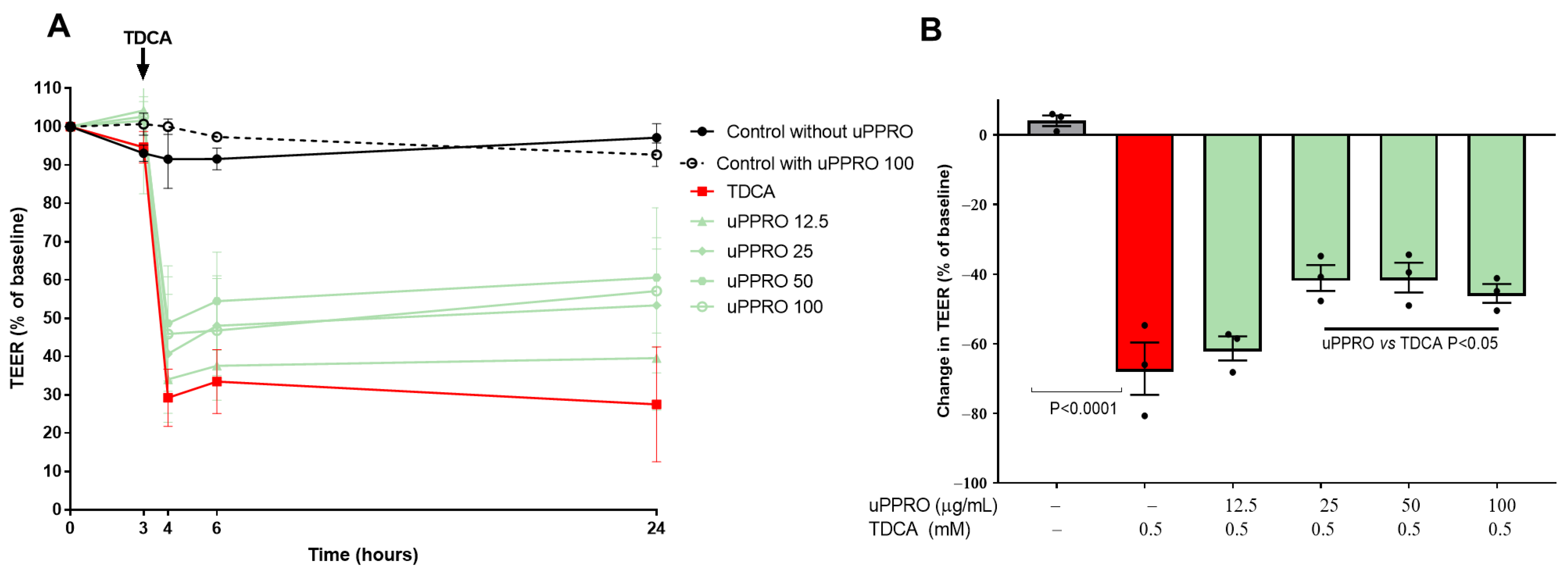
2.2. Preservation of the Permeability of a Caco-2 Cell Monolayer Treated with uPPRO and Challenged with TDCA: TEER Measurement
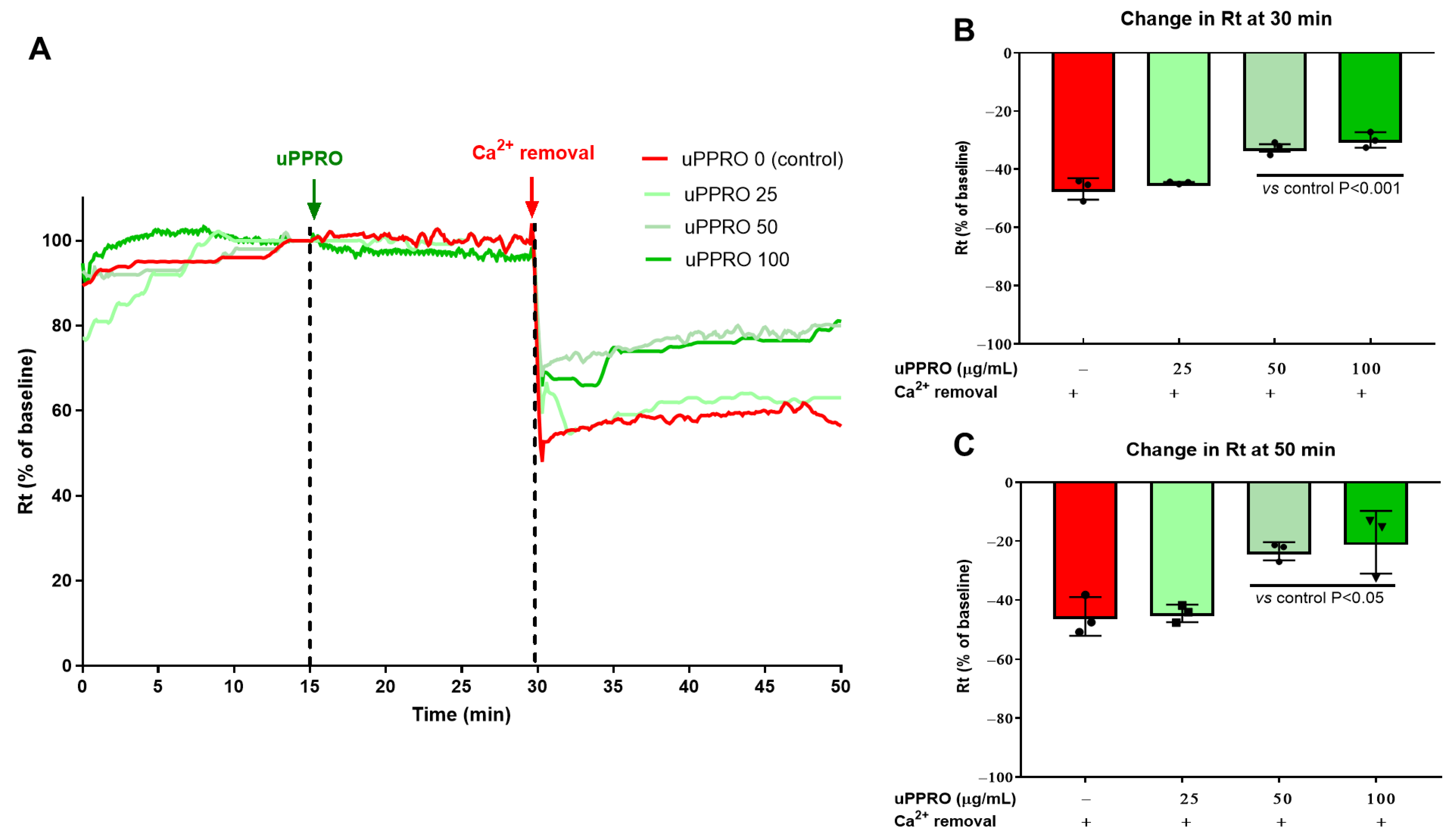
2.3. Protective Effect of uPPRO on Caco-2 Barrier Function upon the Ca2+ and TDCA Challenge
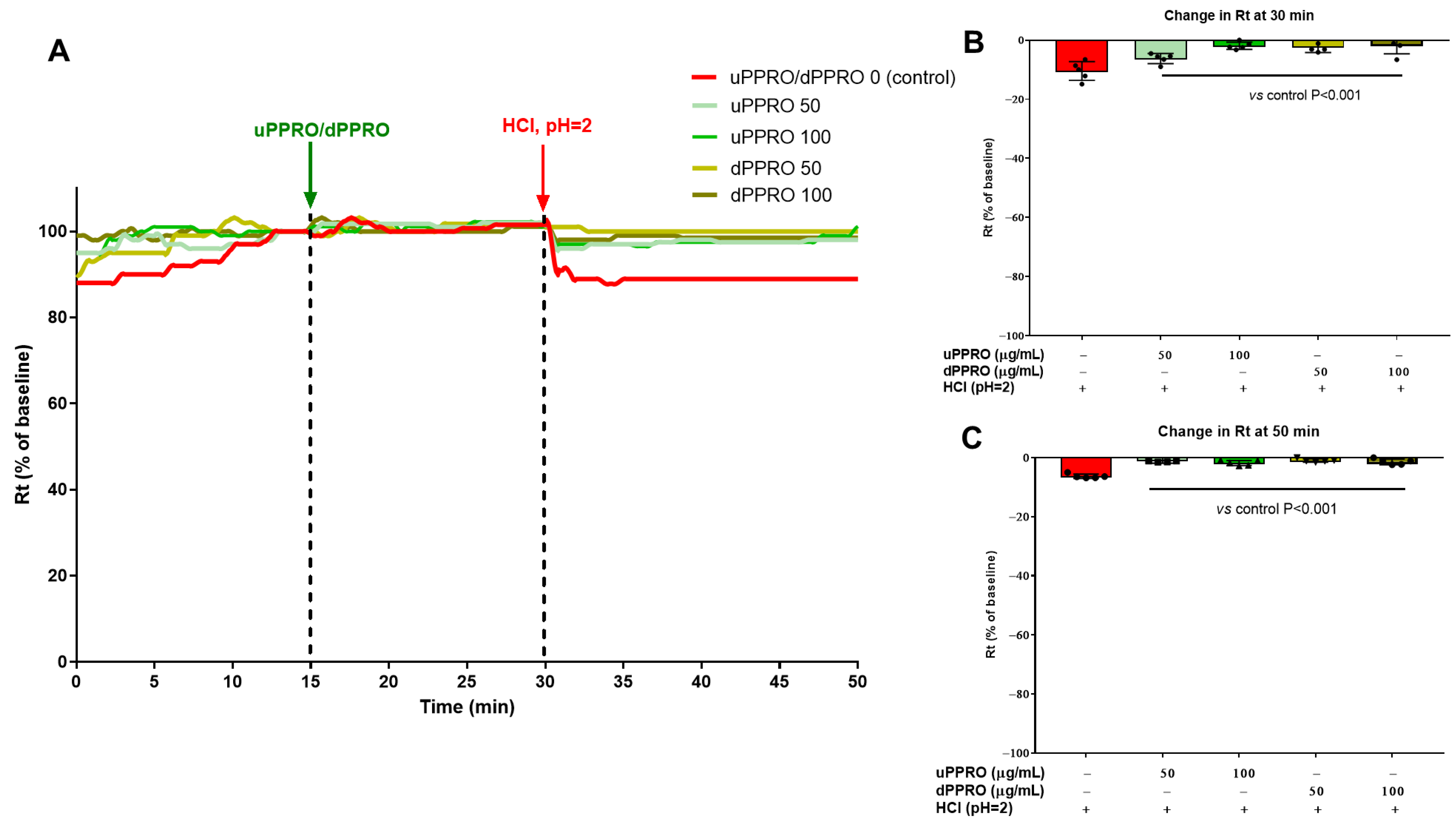
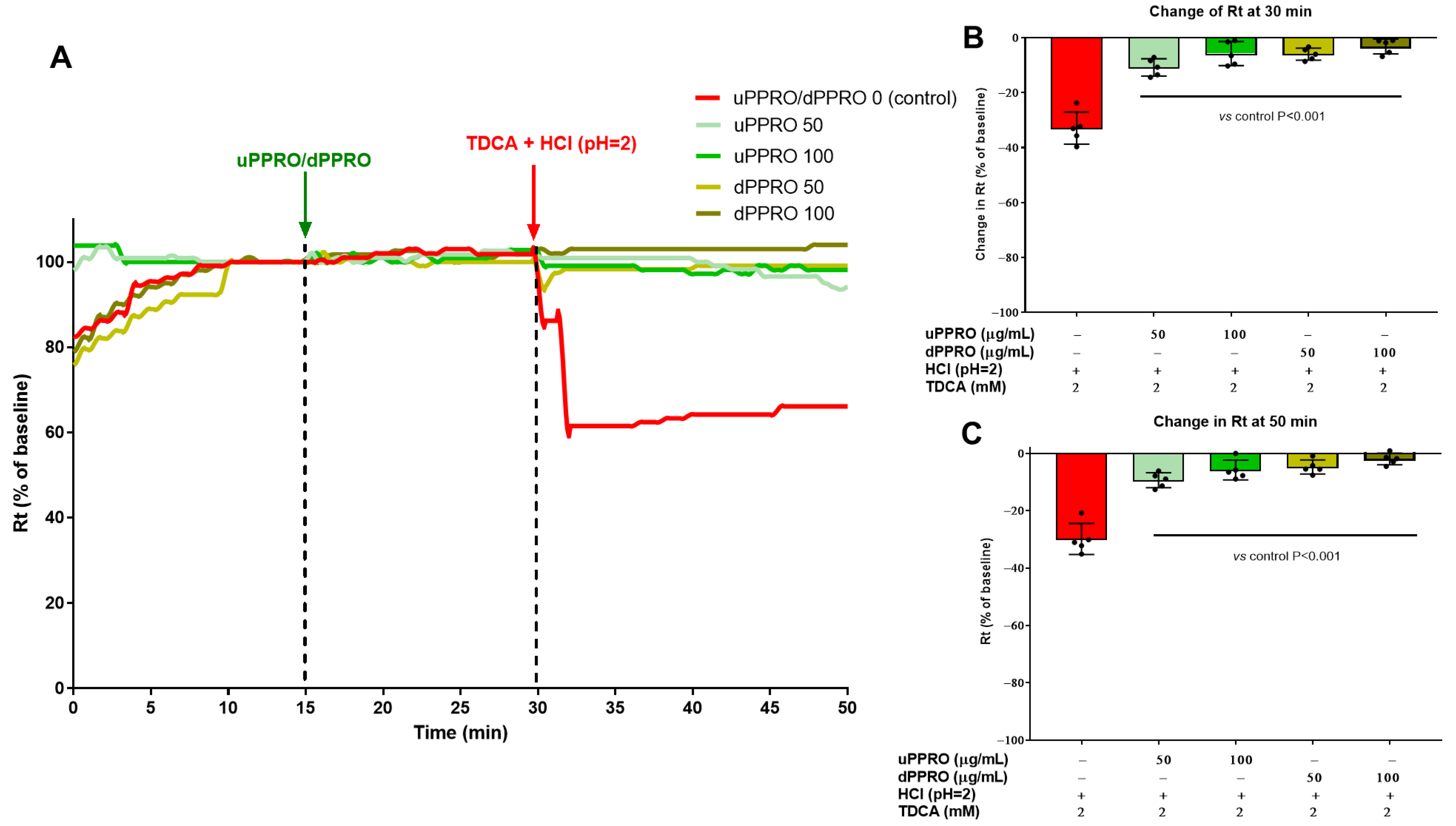
2.4. Effect of uPPRO and dPPRO on the Human Esophageal Barrier Following a HCl and TDCA Challenge in the Ussing Chamber
2.5. Histological Studies
3. Discussion
4. Materials and Methods
4.1. PPRO Preparation
- Undigested PPRO (uPPRO): One PPRO tablet was dissolved in 30 mL of double-distilled water (ddH2O). The solution was shaken for 10 min until PPRO was thoroughly dissolved and centrifuged at 14,000 rpm for 10 min. The supernatant was transferred to a sterile Eppendorf tube and stored at −20 °C for future use.
- Digested PPRO (dPPRO): One PPRO tablet was first pre-treated with 1 mL of human saliva from 3 healthy donors who had not taken drugs or probiotics for at least the last three months, then incubated for 5 min at 37 °C, and then dissolved in 29 mL of ddH2O. Then, the procedure was similar to that for uPPRO preparation.
4.2. In Vitro Model
4.2.1. Cell Culture
4.2.2. Cell Viability
4.2.3. Transepithelial Electrical Resistance (TEER)
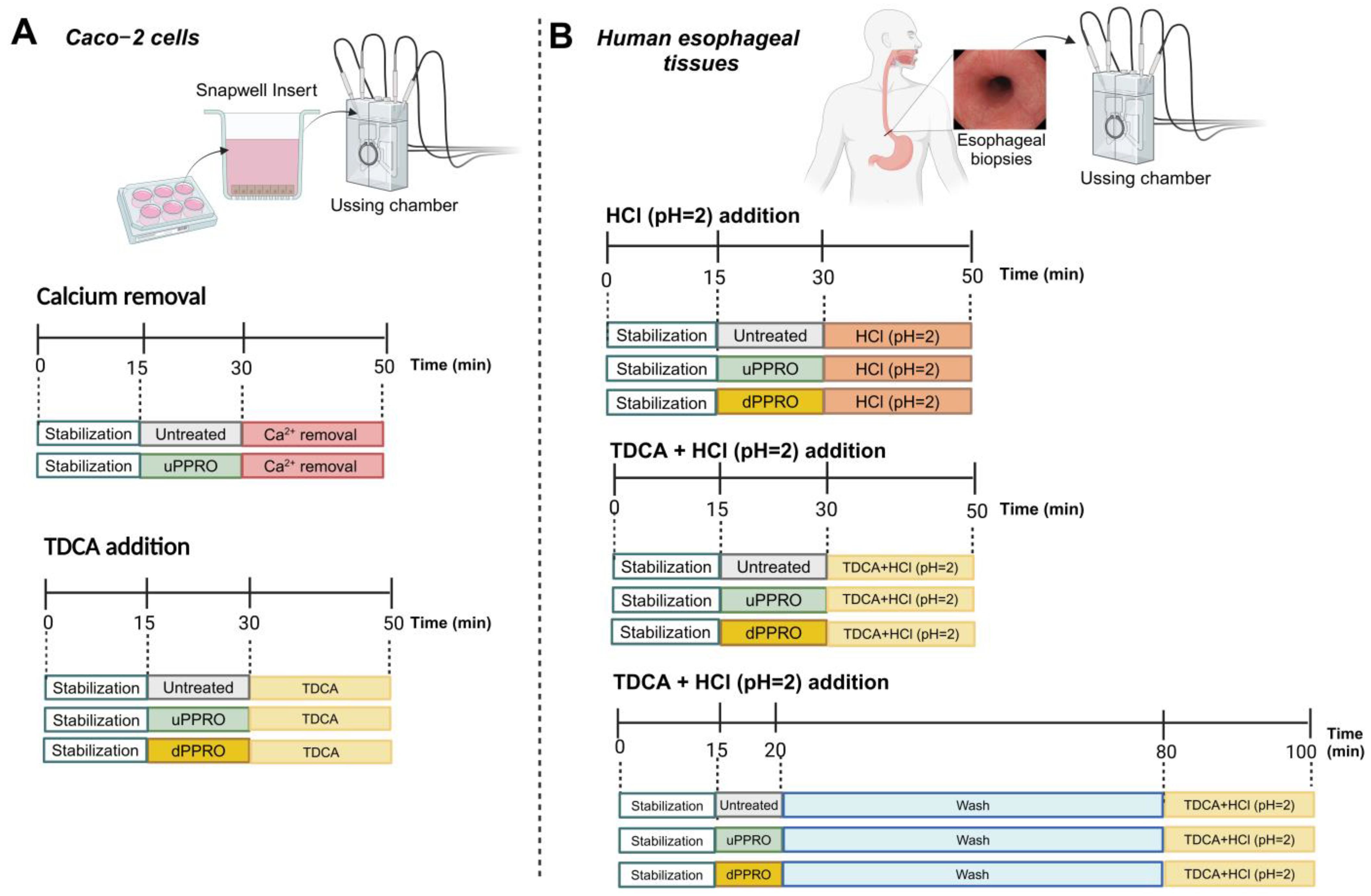
4.2.4. Ussing Chamber
4.3. Ex Vivo Model
4.3.1. Human Esophageal Tissues
4.3.2. Ussing Chamber
4.4. Histological Studies
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richter, J.E. Typical and atypical presentations of gastroesophageal reflux disease. The role of esophageal testing in diagnosis and management. Gastroenterol. Clin. N. Am. 1996, 25, 75–102. [Google Scholar] [CrossRef] [PubMed]
- Vakil, N.; van Zanten, S.V.; Kahrilas, P.; Dent, J.; Jones, R. The Montreal definition and classification of gastroesophageal reflux disease: A global evidence-based consensus. Am. J. Gastroenterol. 2006, 101, 1900–1920. [Google Scholar] [CrossRef] [PubMed]
- Tighe, M.; Afzal, N.A.; Bevan, A.; Hayen, A.; Munro, A.; Beattie, R.M. Pharmacological treatment of children with gastro-oesophageal reflux. Cochrane Database Syst. Rev. 2014, 2014, Cd008550. [Google Scholar] [CrossRef]
- Dent, J.; El-Serag, H.B.; Wallander, M.A.; Johansson, S. Epidemiology of gastro-oesophageal reflux disease: A systematic review. Gut 2005, 54, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Dubois, D.; Coulie, B.; Jones, M.; Kahrilas, P.J.; Rentz, A.M.; Sonnenberg, A.; Stanghellini, V.; Stewart, W.F.; Tack, J.; et al. Prevalence and socioeconomic impact of upper gastrointestinal disorders in the United States: Results of the US Upper Gastrointestinal Study. Clin. Gastroenterol. Hepatol. 2005, 3, 543–552. [Google Scholar] [CrossRef]
- Visaggi, P.; Bertin, L.; Pasta, A.; Calabrese, F.; Ghisa, M.; Marabotto, E.; Ribolsi, M.; Savarino, V.; de Bortoli, N.; Savarino, E.V. Pharmacological management of gastro-esophageal reflux disease: State of the art in 2024. Expert Opin. Pharmacother. 2024, 25, 2077–2088. [Google Scholar] [CrossRef]
- Fass, R.; Sifrim, D. Management of heartburn not responding to proton pump inhibitors. Gut 2009, 58, 295. [Google Scholar] [CrossRef]
- Yibirin, M.; De Oliveira, D.; Valera, R.; Plitt, A.E.; Lutgen, S. Adverse Effects Associated with Proton Pump Inhibitor Use. Cureus 2021, 13, e12759. [Google Scholar] [CrossRef]
- Macke, L.; Schulz, C.; Koletzko, L.; Malfertheiner, P. Systematic review: The effects of proton pump inhibitors on the microbiome of the digestive tract-evidence from next-generation sequencing studies. Aliment. Pharmacol. Ther. 2020, 51, 505–526. [Google Scholar] [CrossRef]
- Savarino, V.; Marabotto, E.; Zentilin, P.; Furnari, M.; Bodini, G.; Giovanni Giannini, E.; Vincenzo Savarino, E. Gastrointestinal functional disorders can benefit from the use of medical devices made of substances. Front. Drug Saf. Regul. 2023, 3, 1119353. [Google Scholar] [CrossRef]
- Salehi, M.; Karegar-Borzi, H.; Karimi, M.; Rahimi, R. Medicinal Plants for Management of Gastroesophageal Reflux Disease: A Review of Animal and Human Studies. J. Altern. Complement. Med. 2017, 23, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, A.; Lankarani, K.B.; Mohagheghzadeh, A.; Long, C.; Pasalar, M. An Evidence-based Review of Medicinal Herbs for the Treatment of Gastroesophageal Reflux Disease (GERD). Curr. Drug Discov. Technol. 2018, 15, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Corazziari, E.S.; Gasbarrini, A.; D’Alba, L.; D’Ovidio, V.; Riggio, O.; Passaretti, S.; Annibale, B.; Cicala, M.; Repici, A.; Bassotti, G.; et al. Poliprotect vs Omeprazole in the Relief of Heartburn, Epigastric Pain, and Burning in Patients Without Erosive Esophagitis and Gastroduodenal Lesions: A Randomized, Controlled Trial. Am. J. Gastroenterol. 2023, 118, 2014–2024. [Google Scholar] [CrossRef] [PubMed]
- Cioeta, R.; Muti, P.; Rigoni, M.; Morlando, L.; Siragusa, F.; Cossu, A.; Giovagnoni, E. Effectiveness and tolerability of Poliprotect, a natural mucosal protective agent for gastroesophageal reflux disease and dyspepsia: Surveys from patients, physicians, and pharmacists. Front. Drug Saf. Regul. 2022, 2, 969831. [Google Scholar] [CrossRef]
- Barlow, W.J.; Orlando, R.C. The pathogenesis of heartburn in nonerosive reflux disease: A unifying hypothesis. Gastroenterology 2005, 128, 771–778. [Google Scholar] [CrossRef]
- Harer, K.N.; Hasler, W.L. Functional dyspepsia: A review of the symptoms, evaluation, and treatment options. Gastroenterol. Hepatol. 2020, 16, 66. [Google Scholar]
- Scally, B.; Emberson, J.R.; Spata, E.; Reith, C.; Davies, K.; Halls, H.; Holland, L.; Wilson, K.; Bhala, N.; Hawkey, C.; et al. Effects of gastroprotectant drugs for the prevention and treatment of peptic ulcer disease and its complications: A meta-analysis of randomised trials. Lancet Gastroenterol. Hepatol. 2018, 3, 231–241. [Google Scholar] [CrossRef]
- Murugan, S.K.; Bethapudi, B.; Raghunandhakumar, S.; Purusothaman, D.; Nithyanantham, M.; Mundkinajeddu, D.; Talkad, M.S. A flavonoid rich standardized extract of Glycyrrhiza glabra protects intestinal epithelial barrier function and regulates the tight-junction proteins expression. BMC Complement. Med. Ther. 2022, 22, 38. [Google Scholar] [CrossRef]
- Sebai, H.; Jabri, M.A.; Souli, A.; Hosni, K.; Rtibi, K.; Tebourbi, O.; El-Benna, J.; Sakly, M. Chemical composition, antioxidant properties and hepatoprotective effects of chamomile (Matricaria recutita L.) decoction extract against alcohol-induced oxidative stress in rat. Gen. Physiol. Biophys. 2015, 34, 263–275. [Google Scholar] [CrossRef]
- Hwang, S.H.; Wang, Z.; Guillen Quispe, Y.N.; Lim, S.S.; Yu, J.M. Evaluation of Aldose Reductase, Protein Glycation, and Antioxidant Inhibitory Activities of Bioactive Flavonoids in Matricaria recutita L. and Their Structure-Activity Relationship. J. Diabetes Res. 2018, 2018, 3276162. [Google Scholar] [CrossRef]
- Jabri, M.A.; Aissani, N.; Tounsi, H.; Sakly, M.; Marzouki, L.; Sebai, H. Protective effect of chamomile (Matricaria recutita L.) decoction extract against alcohol-induced injury in rat gastric mucosa. Pathophysiology 2017, 24, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Moschetta, A.; Palasciano, G. Cholesterol gallstone disease. Lancet 2006, 368, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Khalifeh, H.; Baldini, F.; Serale, N.; Parodi, A.; Voci, A.; Vergani, L.; Daher, A. Antitumor Activity of Ethanolic Extract from Thymbra Spicata L. aerial Parts: Effects on Cell Viability and Proliferation, Apoptosis Induction, STAT3, and NF-kB Signaling. Nutr. Cancer 2020, 73, 1193–1206. [Google Scholar] [CrossRef] [PubMed]
- Aleman, R.S.; Page, R.; Cedillos, R.; Montero-Fernández, I.; Fuentes, J.A.M.; Olson, D.W.; Aryana, K. Influences of Yogurt with Functional Ingredients from Various Sources That Help Treat Leaky Gut on Intestinal Barrier Dysfunction in Caco-2 Cells. Pharmaceuticals 2023, 16, 1511. [Google Scholar] [CrossRef] [PubMed]
- Pongkorpsakol, P.; Satianrapapong, W.; Wongkrasant, P.; Steinhagen, P.R.; Tuangkijkul, N.; Pathomthongtaweechai, N.; Muanprasat, C. Establishment of Intestinal Epithelial Cell Monolayers and Their Use in Calcium Switch Assay for Assessment of Intestinal Tight Junction Assembly. Methods Mol. Biol. 2021, 2367, 273–290. [Google Scholar] [CrossRef]
- Khalil, M.; Piccapane, F.; Vacca, M.; Celano, G.; Mahdi, L.; Perniola, V.; Apa, C.A.; Annunziato, A.; Iacobellis, I.; Procino, G.; et al. Nutritional and Physiological Properties of Thymbra spicata: In Vitro Study Using Fecal Fermentation and Intestinal Integrity Models. Nutrients 2024, 16, 588. [Google Scholar] [CrossRef]
- Woodland, P.; Batista-Lima, F.; Lee, C.; Preston, S.L.; Dettmar, P.; Sifrim, D. Topical protection of human esophageal mucosal integrity. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G975–G980. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, M.; Perniola, V.; Lanza, E.; Mahdi, L.; Sallustio, P.; Idone, V.; Semeraro, D.; Mastrodonato, M.; Testini, M.; Desaphy, J.-F.; et al. Poliprotect®, a Medical Device Made of Substances, Potently Protects the Human Esophageal Mucosa Challenged by Multiple Agents: Evidence from In Vitro and Ex Vivo Electrophysiological Models. Int. J. Mol. Sci. 2025, 26, 791. https://doi.org/10.3390/ijms26020791
Khalil M, Perniola V, Lanza E, Mahdi L, Sallustio P, Idone V, Semeraro D, Mastrodonato M, Testini M, Desaphy J-F, et al. Poliprotect®, a Medical Device Made of Substances, Potently Protects the Human Esophageal Mucosa Challenged by Multiple Agents: Evidence from In Vitro and Ex Vivo Electrophysiological Models. International Journal of Molecular Sciences. 2025; 26(2):791. https://doi.org/10.3390/ijms26020791
Chicago/Turabian StyleKhalil, Mohamad, Valeria Perniola, Elisa Lanza, Laura Mahdi, Pierluca Sallustio, Valeria Idone, Daniela Semeraro, Maria Mastrodonato, Mario Testini, Jean-Francois Desaphy, and et al. 2025. "Poliprotect®, a Medical Device Made of Substances, Potently Protects the Human Esophageal Mucosa Challenged by Multiple Agents: Evidence from In Vitro and Ex Vivo Electrophysiological Models" International Journal of Molecular Sciences 26, no. 2: 791. https://doi.org/10.3390/ijms26020791
APA StyleKhalil, M., Perniola, V., Lanza, E., Mahdi, L., Sallustio, P., Idone, V., Semeraro, D., Mastrodonato, M., Testini, M., Desaphy, J.-F., & Portincasa, P. (2025). Poliprotect®, a Medical Device Made of Substances, Potently Protects the Human Esophageal Mucosa Challenged by Multiple Agents: Evidence from In Vitro and Ex Vivo Electrophysiological Models. International Journal of Molecular Sciences, 26(2), 791. https://doi.org/10.3390/ijms26020791







