JAG1/Notch Pathway Inhibition Induces Ferroptosis and Promotes Cataractogenesis
Abstract
:1. Introduction
2. Results
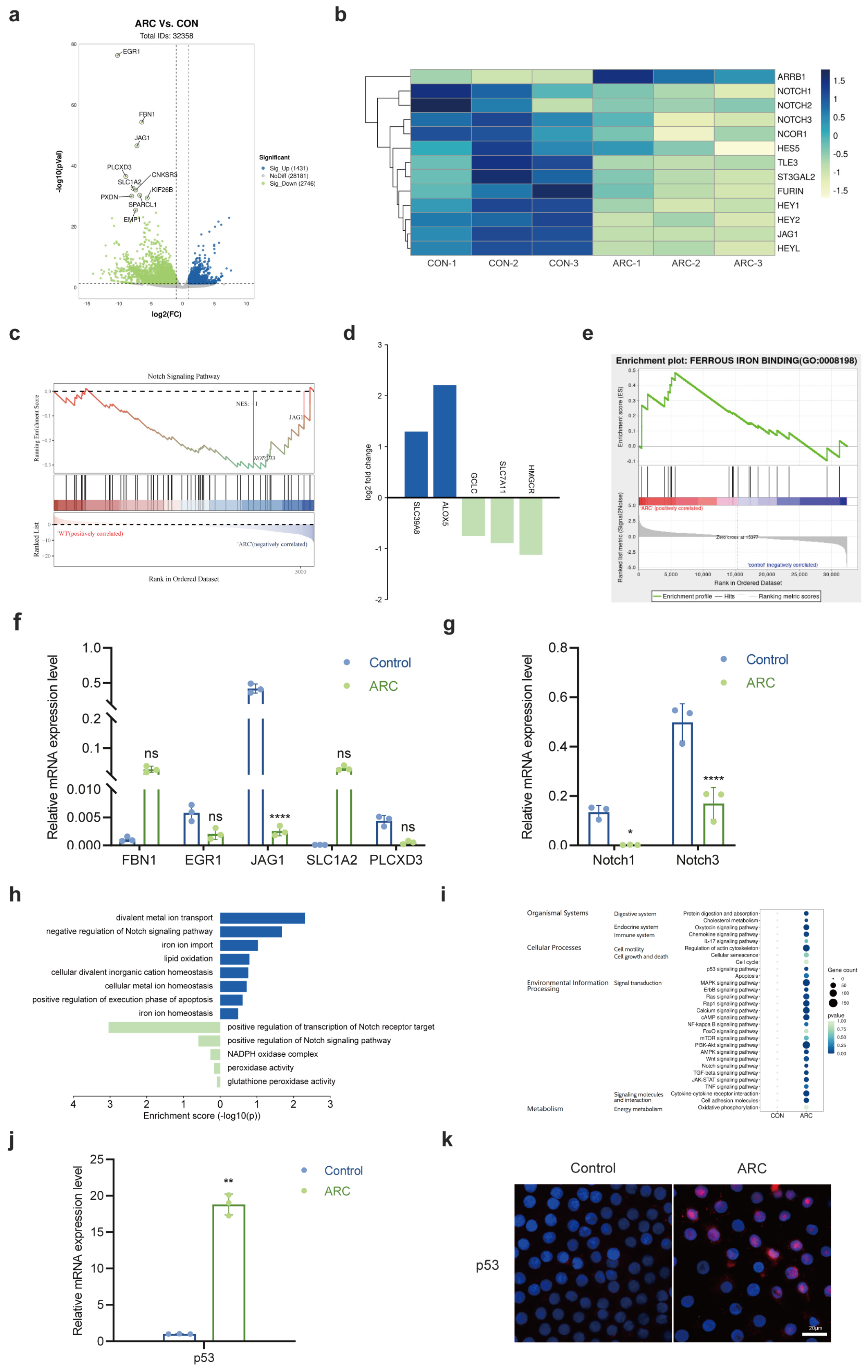
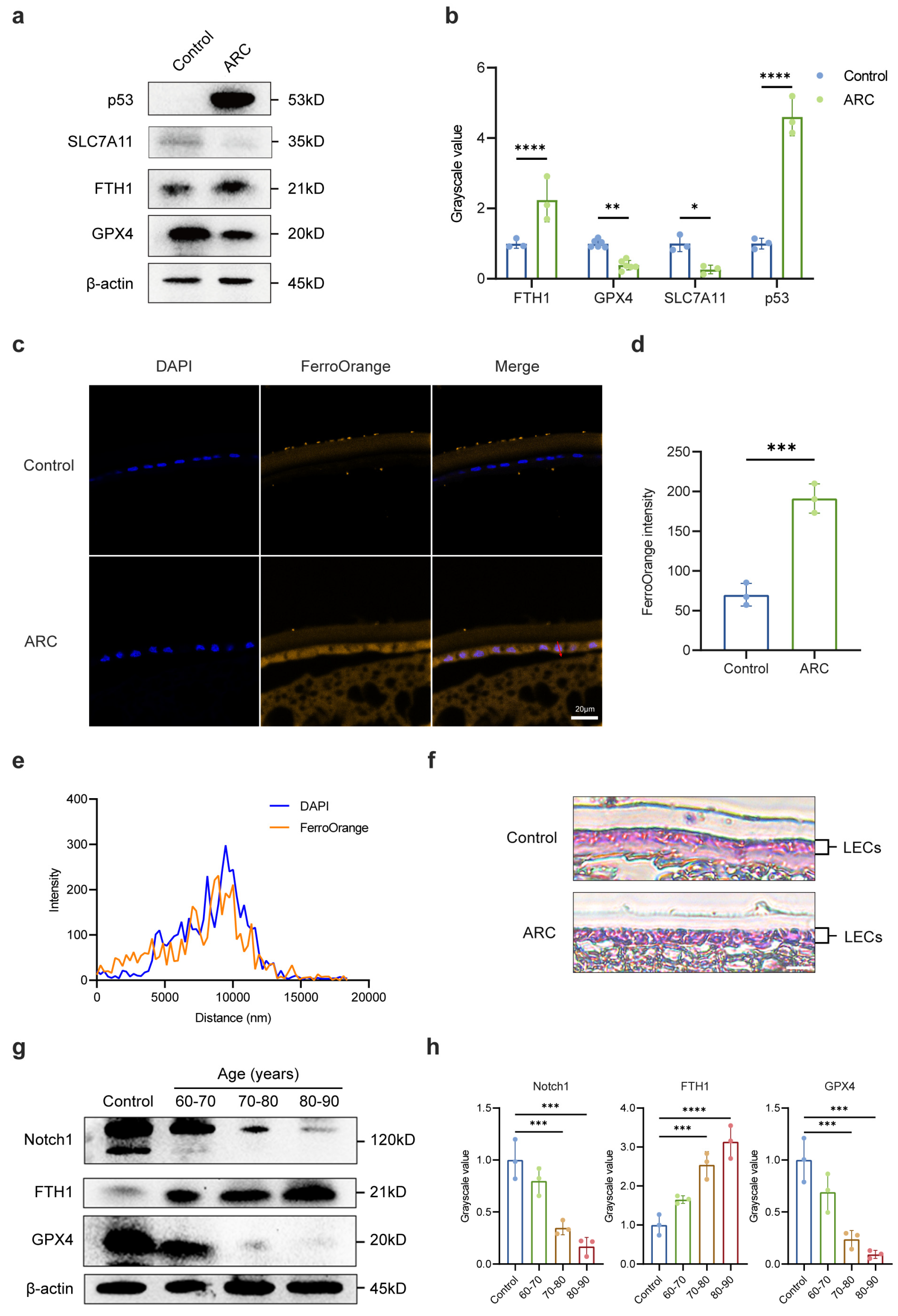
2.1. The Role of Notch Signaling Pathway in ARC Formation
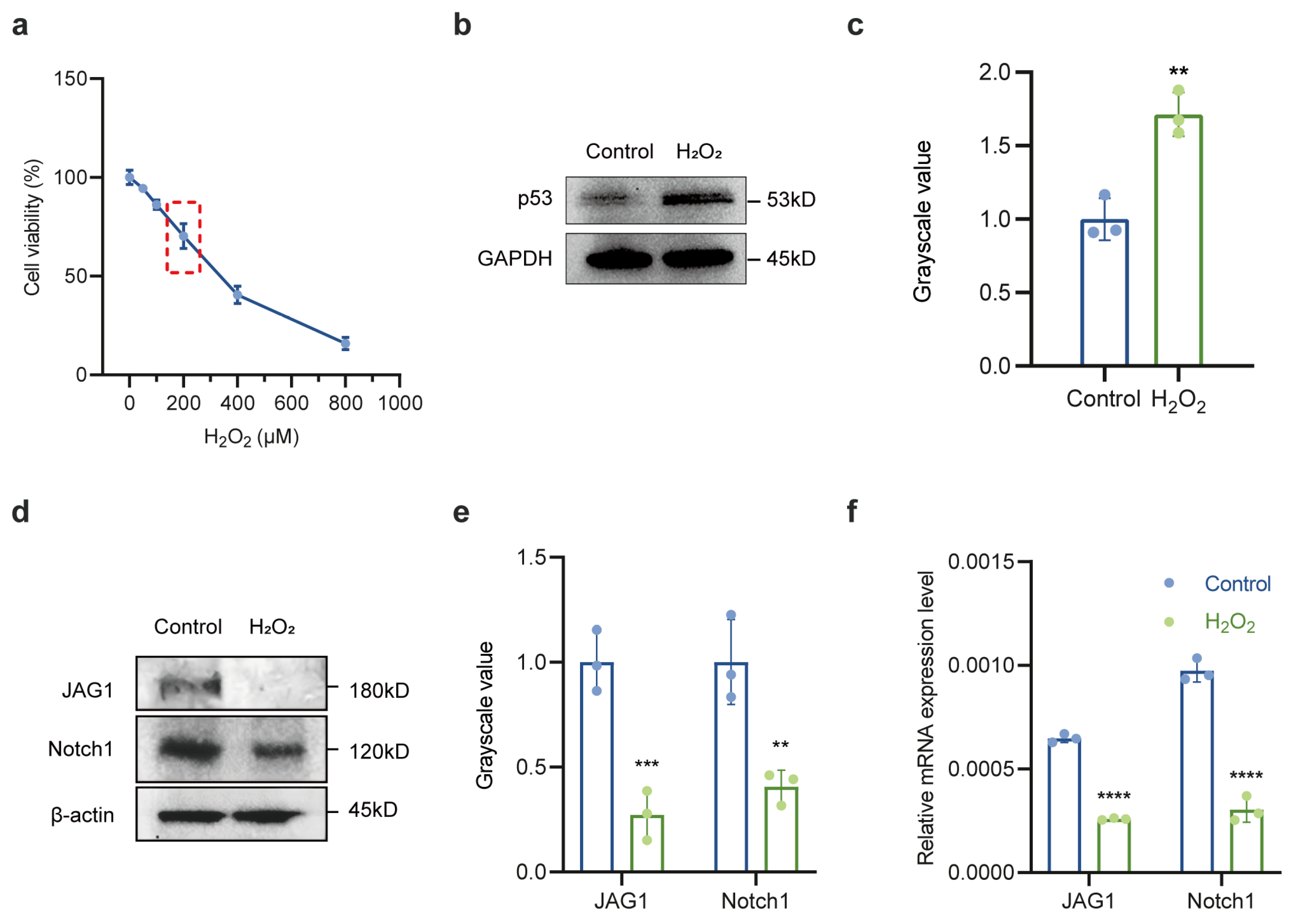
2.2. Downregulation of Notch Signaling Pathway in Oxidative Stress-Treated LECs
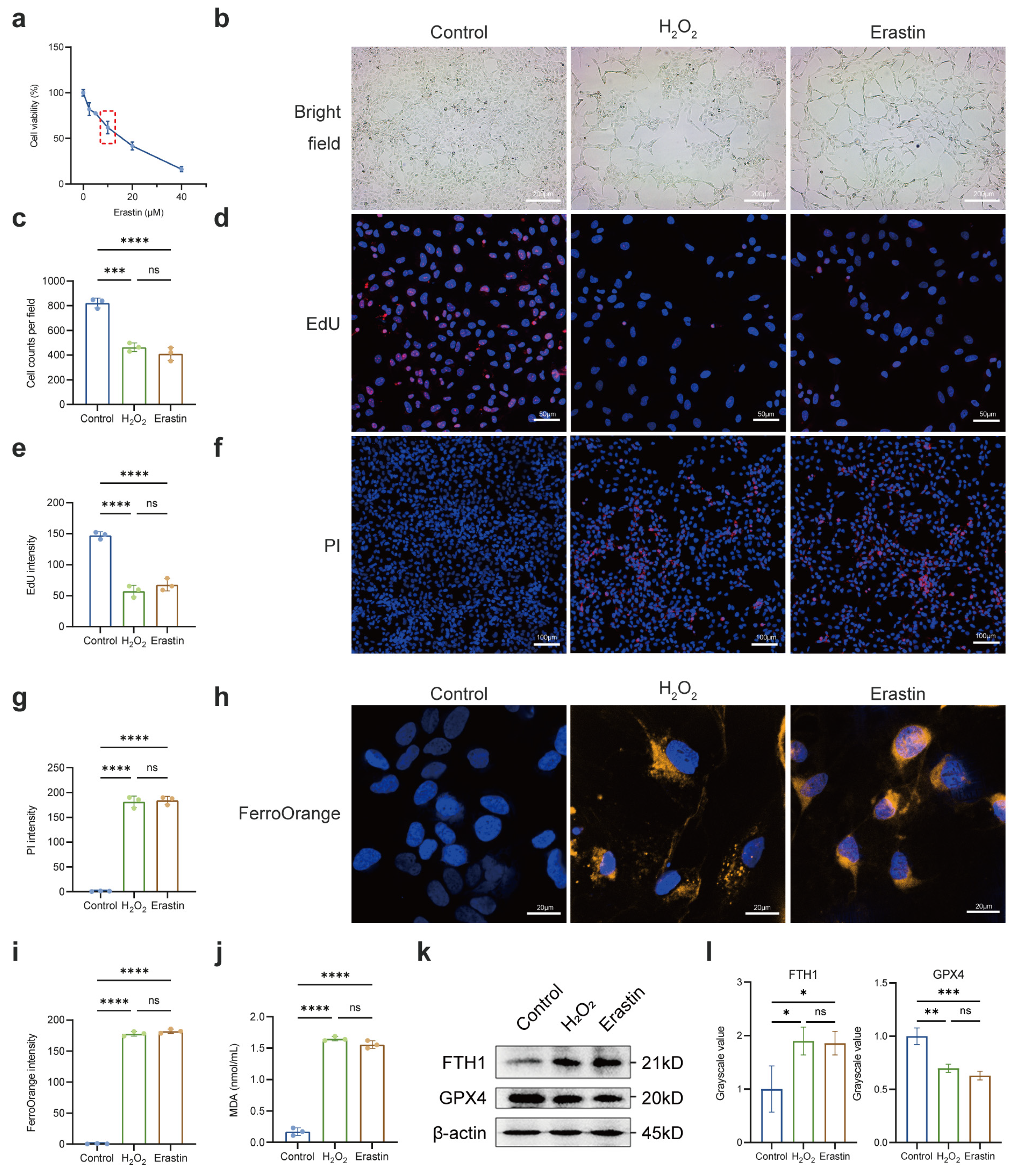
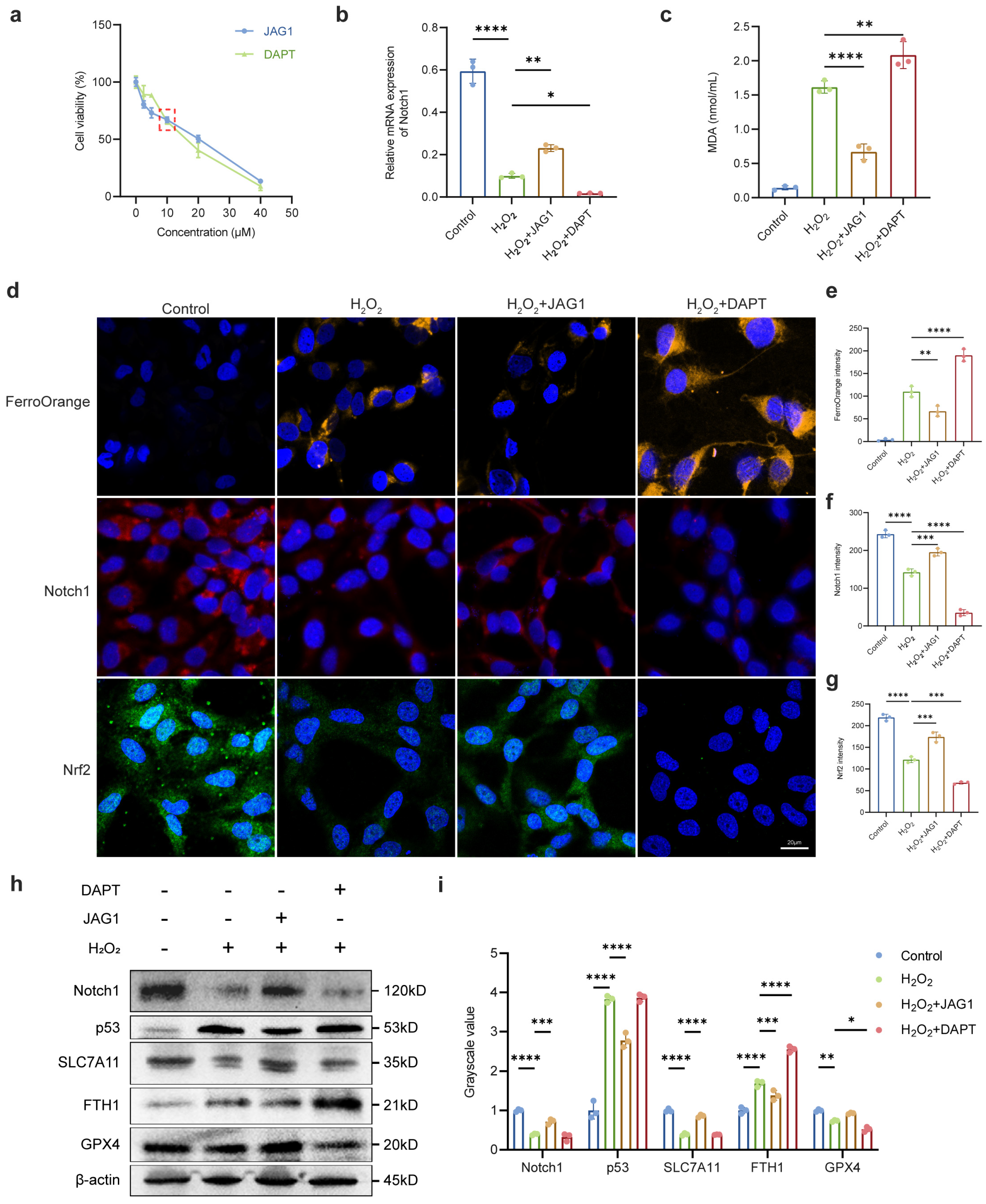
2.3. Regulation of Notch Signaling Pathway Affected Susceptibility of LECs to Oxidative Stress
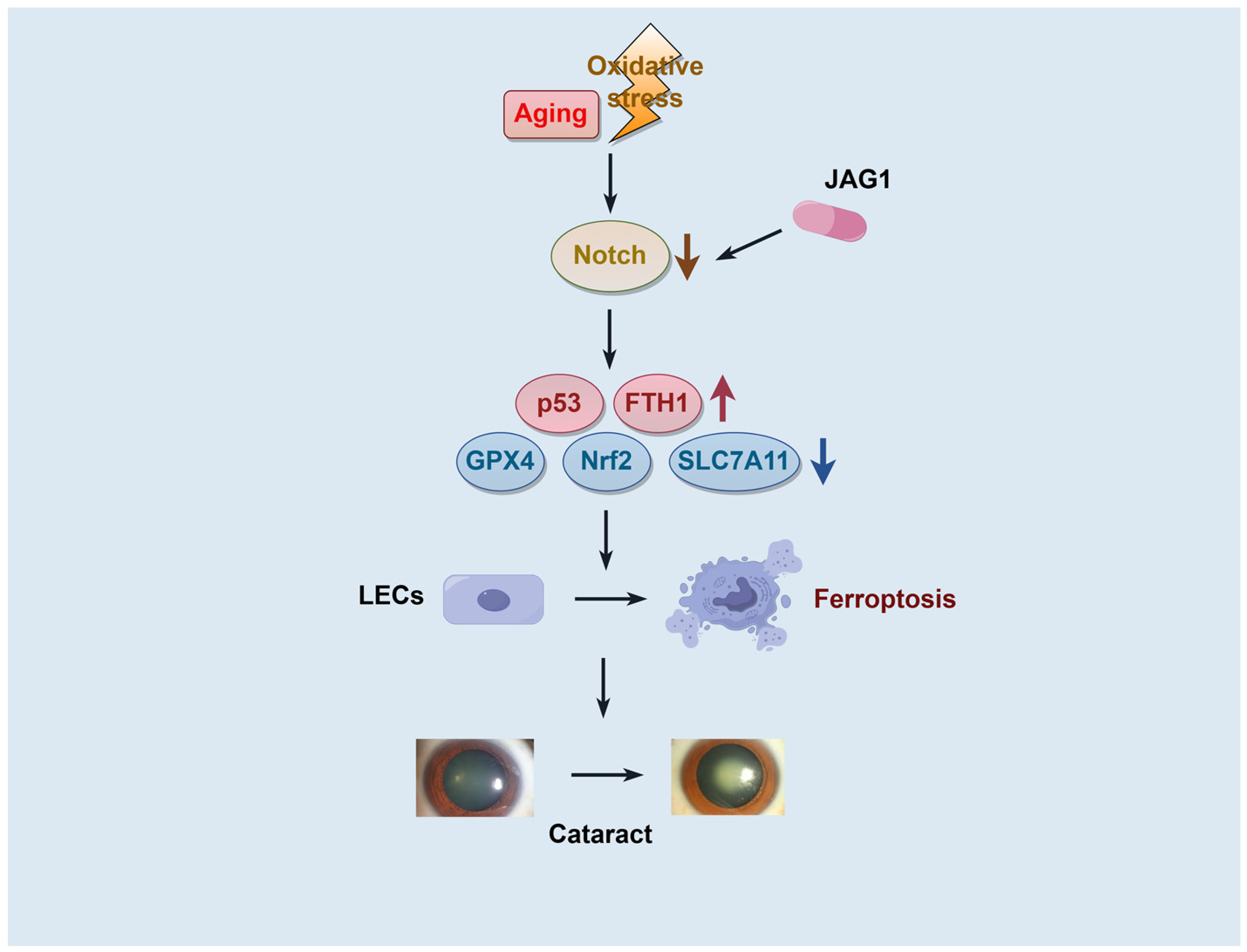
3. Discussion
4. Materials and Methods
4.1. Ethics Approval and Consent to Participate
4.2. Collection of Human Lens Samples
4.3. Western Blot Assay
4.4. Quantitative Real-Time Polymerase Chain Reaction
4.5. RNA Sequence Analysis
4.6. Cell Culture and Treatment
4.7. Immunofluorescence
4.8. Lillie Staining
4.9. FerroOrange
4.10. LOS Detection
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.-C.; Wilkins, M.; Kim, T.; Malyugin, B.; Mehta, J.S. Cataracts. Lancet 2017, 390, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Cicinelli, M.V.; Buchan, J.C.; Nicholson, M.; Varadaraj, V.; Khanna, R.C. Cataracts. Lancet 2023, 401, 377–389. [Google Scholar] [CrossRef]
- Braakhuis, A.J.; Donaldson, C.I.; Lim, J.C.; Donaldson, P.J. Nutritional strategies to prevent lens cataract: Current status and future strategies. Nutrients 2019, 11, 1186. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.C.; Suzuki-Kerr, H.; Nguyen, T.X.; Lim, C.J.J.; Poulsen, R.C. Redox homeostasis in ocular tissues: Circadian regulation of glutathione in the lens? Antioxidants 2022, 11, 1516. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Qu, C.; Zhao, N.; Li, S.; Pu, N.; Song, Z.; Tao, Y. The significance of precisely regulating heme oxygenase-1 expression: Another avenue for treating age-related ocular disease? Ageing Res. Rev. 2024, 97, 102308. [Google Scholar] [CrossRef]
- Kador, P.F.; Salvi, R. Multifunctional redox modulators protect auditory, visual, and cognitive function. Antioxid. Redox Signal. 2021, 36, 1136–1157. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- He, R.; Liu, B.; Xiong, R.; Geng, B.; Meng, H.; Lin, W.; Hao, B.; Zhang, L.; Wang, W.; Jiang, W.; et al. Itaconate inhibits ferroptosis of macrophage via Nrf2 pathways against sepsis-induced acute lung injury. Cell Death Discov. 2022, 8, 43. [Google Scholar] [CrossRef]
- Liu, K.; Li, H.; Wang, F.; Su, Y. Ferroptosis: Mechanisms and advances in ocular diseases. Mol. Cell Biochem. 2023, 478, 2081–2095. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Hao, C.; Huangfu, J.; Srinivasagan, R.; Zhang, X.; Fan, X. Aging lens epithelium is susceptible to ferroptosis. Free Radic. Biol. Med. 2021, 167, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tang, P.; Li, S.; Qin, X.; Yang, H.; Wu, C.; Liu, Y. Notch signaling pathway networks in cancer metastasis: A new target for cancer therapy. Med. Oncol. 2017, 34, 180. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, H.; Yamada, T.; Hasegawa, S.; Miyachi, K.; Ishii, Y.; Hasebe, Y.; Inoue, Y.; Tanaka, H.; Iwata, Y.; Arima, M.; et al. Senescent cell removal via JAG1-NOTCH1 signalling in the epidermis. Exp. Dermatol. 2021, 30, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Turajane, K.; Ji, G.; Chinenov, Y.; Chao, M.; Ayturk, U.; Suhardi, V.J.; Greenblatt, M.B.; Ivashkiv, L.B.; Bostrom, M.P.G.; Yang, X. RNA-seq analysis of peri-implant tissue shows differences in immune, Notch, Wnt, and angiogenesis pathways in aged versus young mice. JBMR Plus 2021, 5, e10535. [Google Scholar] [CrossRef]
- Jo, H.R.; Hwang, J.; Jeong, J.H. MicroRNA miR-214-5p induces senescence of microvascular endothelial cells by targeting the JAG1/Notch signaling pathway. Noncoding RNA Res. 2023, 8, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, P.; Chen, P.; Kang, J.; Lee, S.H.; Leone, S.; Shen, J. Human Presenilin-1 delivered by AAV9 rescues impaired gamma-secretase activity, memory deficits, and neurodegeneration in Psen mutant mice. Proc. Natl. Acad. Sci. USA 2023, 120, e2306714120. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Yang, X.; Han, S.; Guo, H.; Zheng, Z.; Wang, H.; Guan, H.; Jia, Y.; Gao, J.; Yang, T.; et al. Notch1 pathway protects against burn-induced myocardial injury by repressing reactive oxygen species production through JAK2/STAT3 signaling. Oxid. Med. Cell. Longev. 2016, 2016, 5638943. [Google Scholar] [CrossRef]
- Li, L.-J.; Chang, P.M.-H.; Li, C.-H.; Chang, Y.-C.; Lai, T.-C.; Su, C.-Y.; Chen, C.-L.; Chang, W.-M.; Hsiao, M.; Feng, S.-W. FAS receptor regulates NOTCH activity through ERK-JAG1 axis activation and controls oral cancer stemness ability and pulmonary metastasis. Cell Death Discov. 2022, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Kopan, R.; Ilagan, M.X.G. The canonical Notch signaling pathway: Unfolding the activation mechanism. Cell 2009, 137, 216–233. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-L.; Wu, X.; Zhu, R.-R.; Xu, H.; Li, Y.-Y.; Xu, Q.-R.; Liu, S.; Lai, S.-Q.; Xu, X.; Wan, L.; et al. Notch1–Nrf2 signaling crosstalk provides myocardial protection by reducing ROS formation. Biochem. Cell Biol. 2020, 98, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Ye, T.; Yang, L.; Shen, Y.; Li, H. Ferroptosis signaling and regulators in atherosclerosis. Front. Cell Dev. Biol. 2021, 9, 809457. [Google Scholar] [CrossRef]
- Xing, M.; Ma, X.; Wang, X.; Wang, H.; Xie, M.; Zhang, Z.; Zhou, J. Emodin disrupts the Notch1/Nrf2/GPX4 antioxidant system and promotes renal cell ferroptosis. J. Appl. Toxicol. 2023, 43, 1702–1718. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Zhang, F.; Yang, L.; He, Y.; Kong, X.; Guo, K.; Xie, Y.; Xie, H.; Xie, B.; Jiang, Y.; et al. Bromodomain-containing protein 4 knockdown promotes neuronal ferroptosis in a mouse model of subarachnoid hemorrhage. Neural Regener. Res. 2024. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhang, D.; Dong, Y.; Shu, Y.; Wu, X.; Ni, Y.; Zhao, R.; Ma, W. Sulfiredoxin-1 accelerates erastin-induced ferroptosis in HT-22 hippocampal neurons by driving heme Oxygenase-1 activation. Free Radic. Biol. Med. 2024, 223, 430–442. [Google Scholar] [CrossRef]
- Sui, Y.; Geng, X.; Wang, Z.; Zhang, J.; Yang, Y.; Meng, Z. Targeting the regulation of iron homeostasis as a potential therapeutic strategy for nonalcoholic fatty liver disease. Metabolism 2024, 157, 155953. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mao, W.; Zhang, Y.; Feng, W.; Bai, B.; Ji, B.; Chen, J.; Cheng, B.; Yan, F. NOX1 triggers ferroptosis and ferritinophagy, contributes to Parkinson’s disease. Free Radic. Biol. Med. 2024, 222, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Olzmann, J.A. The cell biology of ferroptosis. Nat. Rev. Mol. Cell Biol. 2024, 25, 424–442. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Lu, L.; Zhu, D.; Zhang, H.; Fu, Y.; Tan, Y.; Tan, X.; Guo, M.; Zhang, Y.; Yang, H.; et al. MafG/MYH9-LCN2 axis promotes liver fibrosis through inhibiting ferroptosis of hepatic stellate cells. Cell Death Differ. 2024, 31, 1127–1139. [Google Scholar] [CrossRef]
- Gao, M.; Yi, J.; Zhu, J.; Minikes, A.M.; Monian, P.; Thompson, C.B.; Jiang, X. Role of mitochondria in ferroptosis. Mol. Cell 2019, 73, 354–363.e3. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Liu, Y.; Li, L.; Wang, H.; Li, K.; Zheng, G. Astaxanthin ameliorates oxidative stress in lens epithelial cells by regulating GPX4 and ferroptosis. Chem. Biol. Interact. 2023, 383, 110684. [Google Scholar] [CrossRef] [PubMed]
- Aierken, Y.; He, H.; Li, R.; Lin, Z.; Xu, T.; Zhang, L.; Wu, Y.; Liu, Y. Inhibition of Slc39a14/Slc39a8 reduce vascular calcification via alleviating iron overload induced ferroptosis in vascular smooth muscle cells. Cardiovasc. Diabetol. 2024, 23, 186. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xu, X.; Li, J.; Bai, M.; Zhu, W.; Liu, Y.; Liu, S.; Zhao, Z.; Li, T.; Jiang, N.; et al. ALOX5 deficiency contributes to bladder cancer progression by mediating ferroptosis escape. Cell Death Dis. 2023, 14, 800. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Guo, Y.; Wang, T.; Wang, L.; Yan, Y.; Xia, L.; Bam, R.; Yang, Z.; Lee, H.; Iwawaki, T.; et al. IRE1α determines ferroptosis sensitivity through regulation of glutathione synthesis. Nat. Commun. 2024, 15, 4114. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Jia, F.; Wang, S.; Jiao, Q.; Du, X.; Chen, X.; Jiang, H. The involvement of IRP2-induced ferroptosis through the p53-SLC7A11-ALOX12 pathway in Parkinson’s disease. Free Radic. Biol. Med. 2024, 222, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shu, L.; Lv, C.; Liu, N.; Long, Y.; Peng, X.; Ling, H.; Tao, T.; Tang, J.; Cheng, Y.; et al. BRCC36 deubiquitinates HMGCR to regulate the interplay between ferroptosis and pyroptosis. Adv. Sci. 2024, 11, e2304263. [Google Scholar] [CrossRef]
- Du, K.; Wang, L.; Jun, J.H.; Dutta, R.K.; Maeso-Díaz, R.; Oh, S.H.; Ko, D.C.; Diehl, A.M. Aging promotes metabolic dysfunction-associated steatotic liver disease by inducing ferroptotic stress. Nat. Aging 2024, 4, 949–968. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gu, S.; Chen, J.; Yuan, Z.; Liang, P.; Cui, H. Mechanism of notch signaling pathway in malignant progression of glioblastoma and targeted therapy. Biomolecules 2024, 14, 480. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wei, J.; Xiao, W.; Xie, W.; Ru, Q.; Chen, L.; Wu, Y.; Mobasheri, A.; Li, Y. Insights into the Notch signaling pathway in degenerative musculoskeletal disorders: Mechanisms and perspectives. Biomed. Pharmacother. 2023, 169, 115884. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.-y.; Liu, J.-x.; Wang, L.-d.; Zhi, X.-y.; Luo, L.; Zhao, J.-y.; Qin, Y. GSK-3β-dependent Nrf2 antioxidant response modulates ferroptosis of lens epithelial cells in age-related cataract. Free Radical Biol. Med. 2023, 204, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gu, W. p53 in ferroptosis regulation: The new weapon for the old guardian. Cell Death Differ. 2022, 29, 895–910. [Google Scholar] [CrossRef]
- Abouelezz, H.M.; El-Kashef, D.H.; Abdelaziz, R.R.; Nader, M.A. Tenofovir alone or combined with doxorubicin abrogates DMBA-induced mammary cell carcinoma: An insight into its modulatory impact on oxidative/Notch/apoptotic signaling. Life Sci. 2023, 326, 121798. [Google Scholar] [CrossRef] [PubMed]
- To, H.T.N.; Park, J.-H.; Kim, J.W.; Kang, D. Delta/Notch-like epidermal growth factor-related receptor (DNER), a potential prognostic marker of gastric cancer regulates cell survival and cell cycle progression. Int. J. Mol. Sci. 2023, 24, 10077. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Qin, Y.; Yang, Y.; Li, Z.; Cui, B.; Ju, R.; Wu, M. BMP-4 and BMP-7 Inhibit EMT in a Model of Anterior Subcapsular Cataract in Part by Regulating the Notch Signaling Pathway. Investig. Ophthalmol. Visual Sci. 2023, 64, 12. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, Y.; Liu, L.; Jiang, F.; Wu, M.; Qin, Y. JAG1/Notch Pathway Inhibition Induces Ferroptosis and Promotes Cataractogenesis. Int. J. Mol. Sci. 2025, 26, 307. https://doi.org/10.3390/ijms26010307
Ni Y, Liu L, Jiang F, Wu M, Qin Y. JAG1/Notch Pathway Inhibition Induces Ferroptosis and Promotes Cataractogenesis. International Journal of Molecular Sciences. 2025; 26(1):307. https://doi.org/10.3390/ijms26010307
Chicago/Turabian StyleNi, Yan, Liangping Liu, Fanying Jiang, Mingxing Wu, and Yingyan Qin. 2025. "JAG1/Notch Pathway Inhibition Induces Ferroptosis and Promotes Cataractogenesis" International Journal of Molecular Sciences 26, no. 1: 307. https://doi.org/10.3390/ijms26010307
APA StyleNi, Y., Liu, L., Jiang, F., Wu, M., & Qin, Y. (2025). JAG1/Notch Pathway Inhibition Induces Ferroptosis and Promotes Cataractogenesis. International Journal of Molecular Sciences, 26(1), 307. https://doi.org/10.3390/ijms26010307






