Temperature-Dependent Cytokine Neutralization Induced by Magnetoelectric Nanoparticles: An In Silico Study
Abstract
:1. Introduction
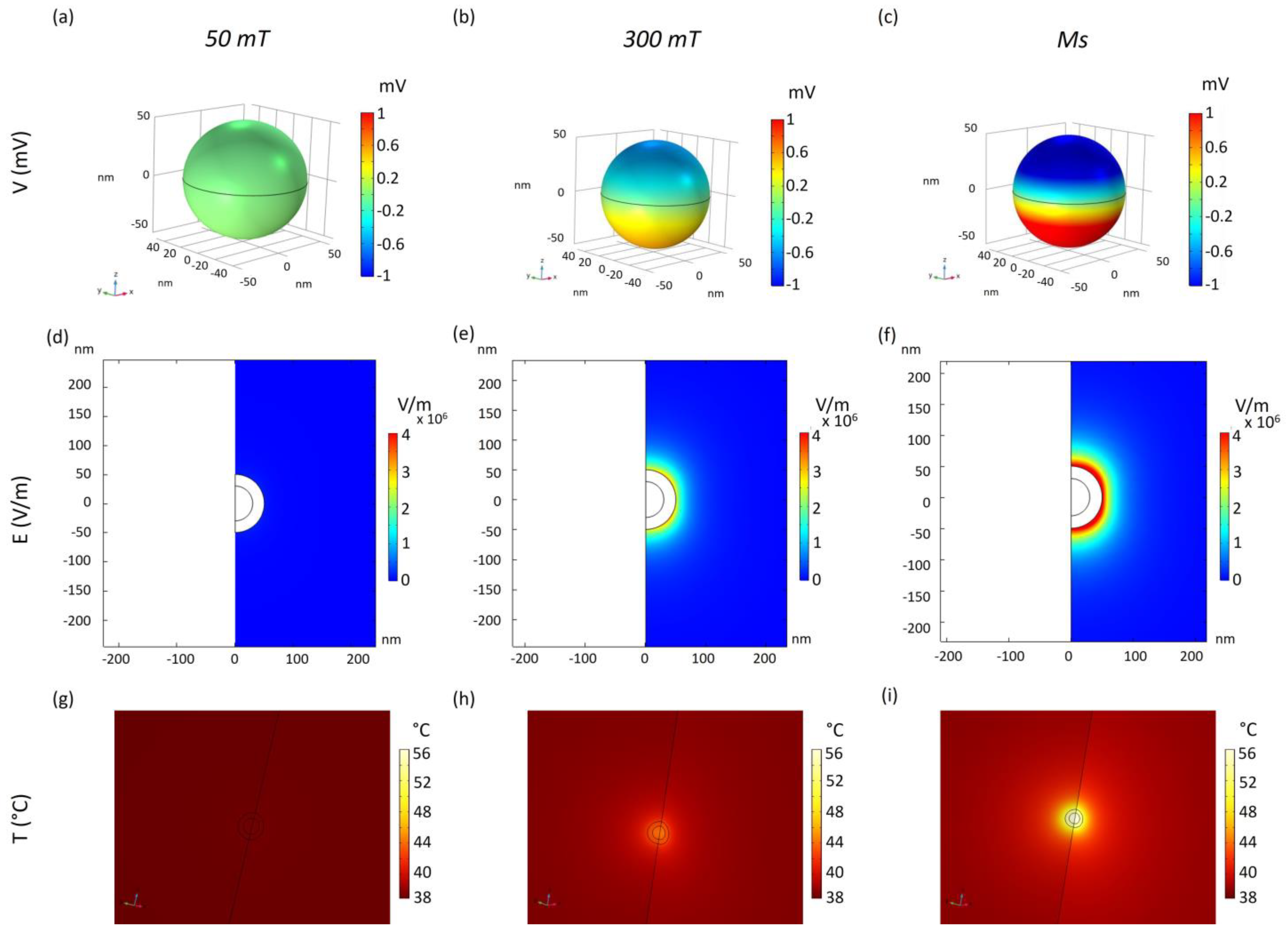
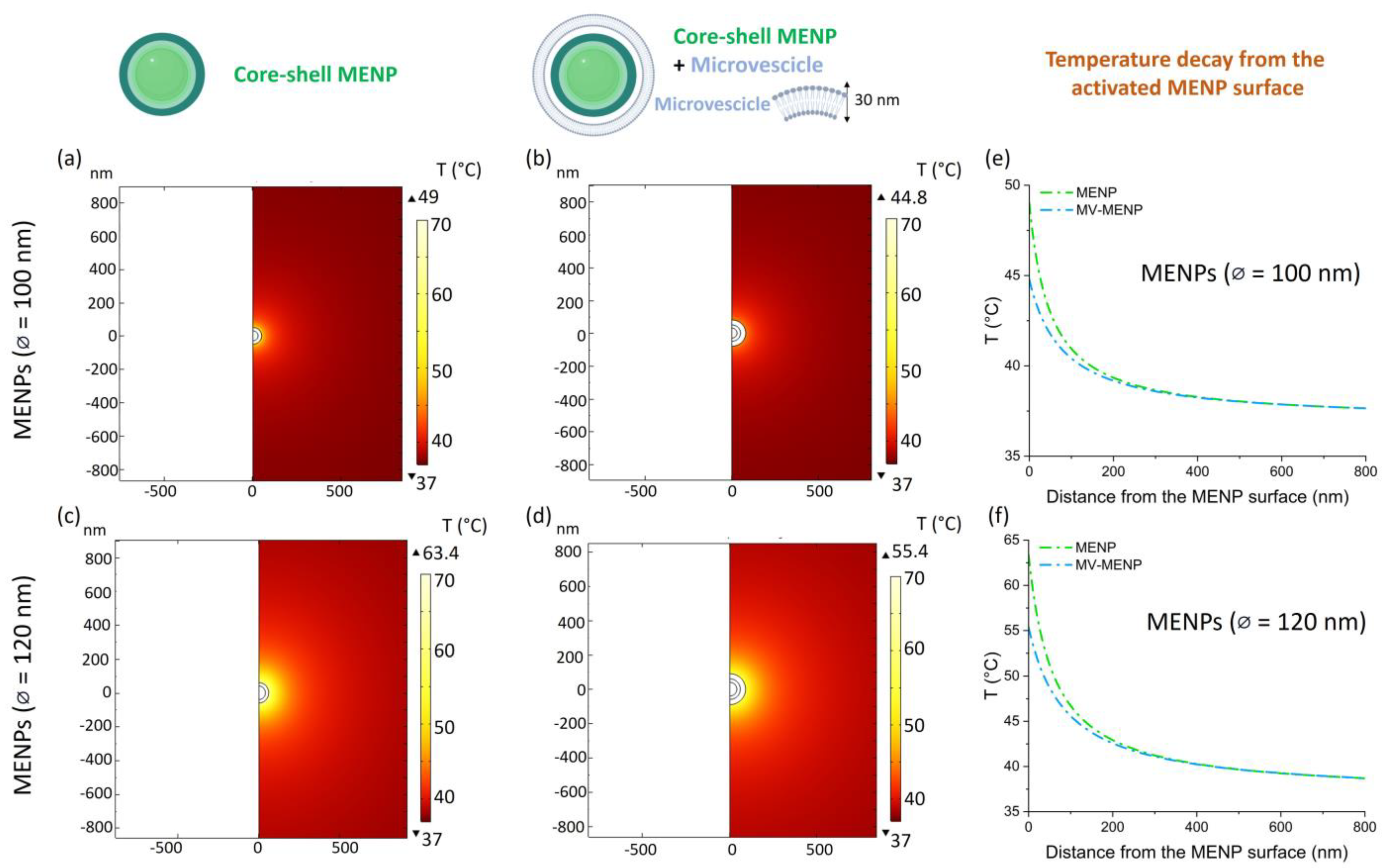
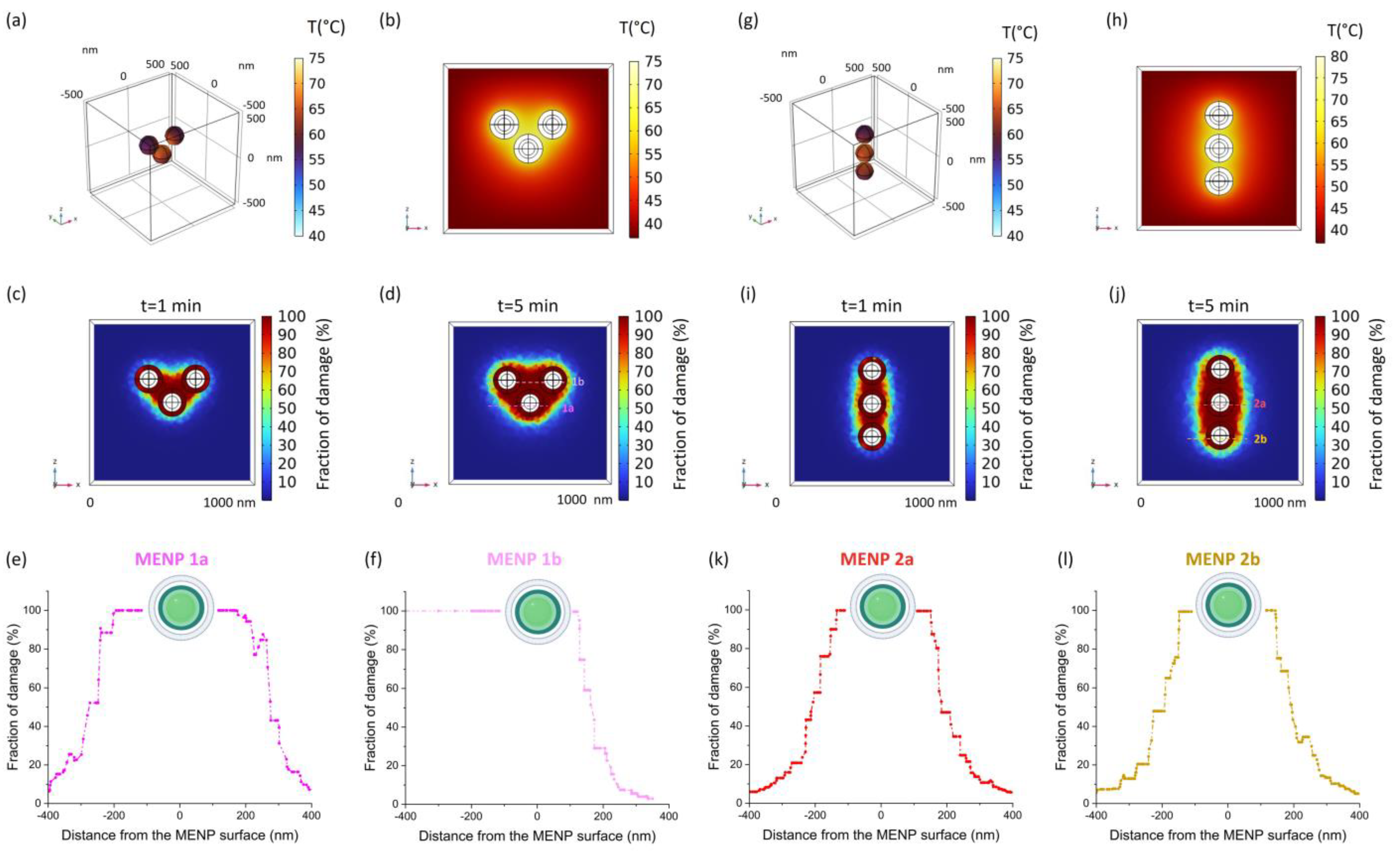
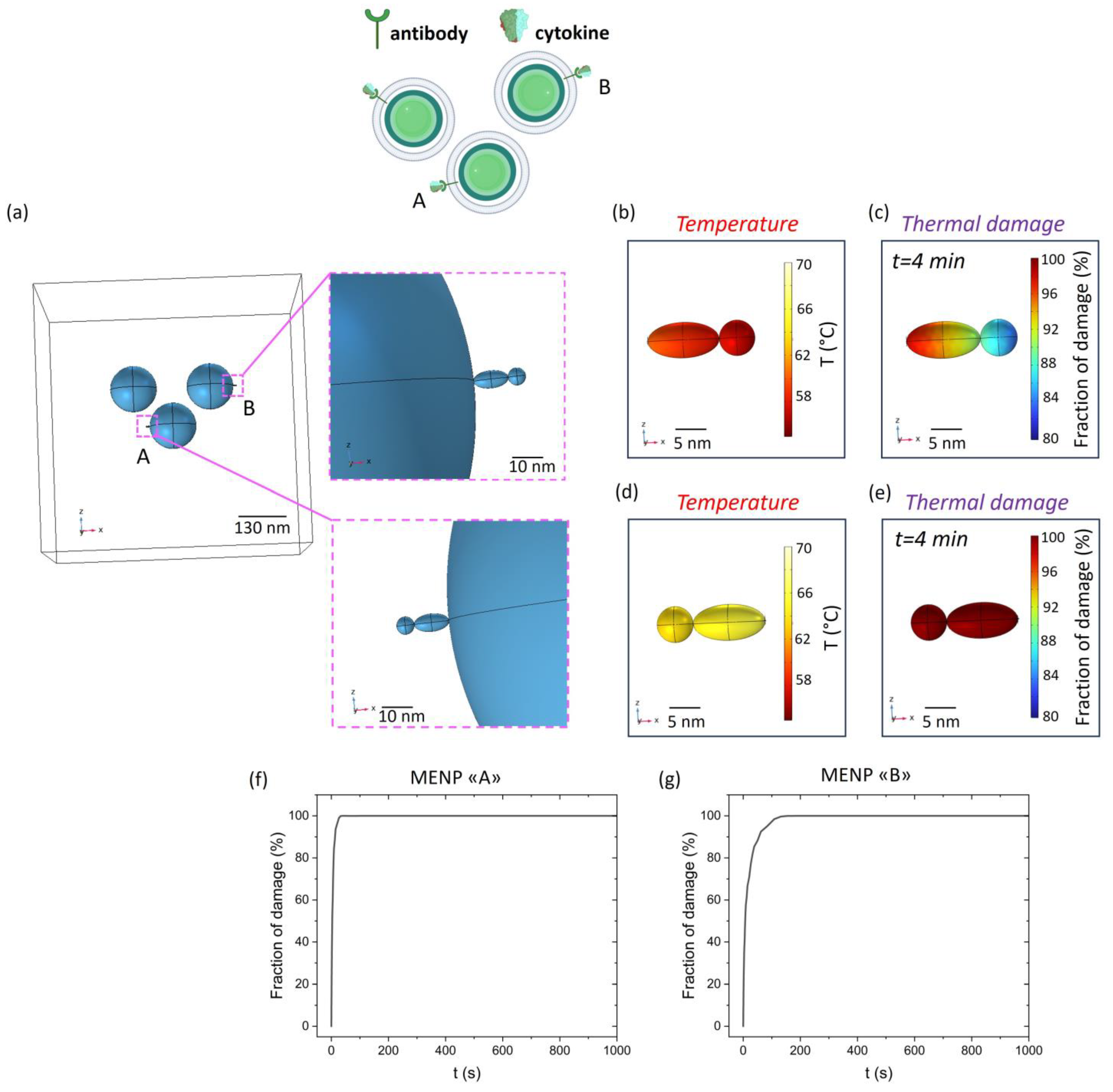
2. Results
3. Discussion
4. Materials and Methods
4.1. MENPs Multiphysics Model
4.1.1. Two-Dimensional MENPs Model
4.1.2. Three-Dimensional MENPs Cluster Model
4.1.3. Cytokine-Conjugated 3D Cluster Model
4.2. COMSOL Multiphysics Equations
4.2.1. Magnetoelectric Nanoparticles Model
4.2.2. Localized Temperature Distribution
| Domain | Electrical Conductivity (S/m) | Relative Permittivity | Density (kg/m3) | Thermal Conductivity (W/m·K) | Tissue-Specific Heat Capacity (J/(kg·K)) | Activation Energy (kJ/mol) | Frequency Factor (1/s) |
|---|---|---|---|---|---|---|---|
| Extracellular Fluid | 2 [54] | 109 [54] | 1006 [54] | 0.60 [54] | 3997 [16] | 281 [45] | 2.97 × 1042 [45] |
| MV-Coating | 0.3 [55] | 80 [56] | 1380 [57] | 0.547 [56] | 3890 [56] | 281 [45] | 2.97 × 1042 [45] |
| Antibody- Cytokine Complex | 0.16 [58] | 3.23 [59] | 1350 [60] | 0.3 [61] | 1200 [62] | 178 [45,63] | 1.42 × 1026 [45] |
4.2.3. Arrhenius Model
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanada, T.; Yoshimura, A. Regulation of Cytokine Signaling and Inflammation. Cytokine Growth Factor Rev. 2002, 13, 413–421. [Google Scholar] [CrossRef]
- Lima, A.C.; Cunha, C.; Carvalho, A.; Ferreira, H.; Neves, N.M. Interleukin-6 Neutralization by Antibodies Immobilized at the Surface of Polymeric Nanoparticles as a Therapeutic Strategy for Arthritic Diseases. ACS Appl. Mater. Interfaces 2018, 10, 13839–13850. [Google Scholar] [CrossRef] [PubMed]
- Ritz, M.A.; Jost, R. Severe Pneumococcal Pneumonia Following Treatment with Infliximab for Crohn’s Disease. Inflamm. Bowel Dis. 2001, 7, 327. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Letendre, S. Viral Pneumonia as a Serious Complication of Etanercept Therapy. Ann. Intern. Med. 2002, 136, 174. [Google Scholar] [CrossRef] [PubMed]
- Marotte, H.; Charrin, J.E.; Miossec, P. Infliximab-Induced Aseptic Meningitis. Lancet 2001, 358, 1784. [Google Scholar] [CrossRef] [PubMed]
- Baghai, M.; Osmon, D.R.; Wolk, D.M.; Wold, L.E.; Haidukewych, G.J.; Matteson, E.L. Fatal Sepsis in a Patient with Rheumatoid Arthritis Treated with Etanercept. Mayo Clin. Proc. 2001, 76, 653–656. [Google Scholar] [CrossRef]
- Riminton, S.; Pearce, N.; Antony, B. Tuberculosis and Treatment with Infliximab. N. Engl. J. Med. 2002, 346, 623–626. [Google Scholar]
- Warris, A.; Bjorneklett, A.; Gaustad, P. Invasive Pulmonary Aspergillosis Associated with Infliximab Therapy. N. Engl. J. Med. 2001, 344, 1099–1100. [Google Scholar]
- Thamphiwatana, S.; Angsantikul, P.; Escajadillo, T.; Zhang, Q.; Olson, J.; Luk, B.T.; Zhang, S.; Fang, R.H.; Gao, W.; Nizet, V. Macrophage-like Nanoparticles Concurrently Absorbing Endotoxins and Proinflammatory Cytokines for Sepsis Management. Proc. Natl. Acad. Sci. USA 2017, 114, 11488–11493. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Xia, S.; Xu, W.; Tian, R.; Yu, G.; Gu, C.; Pan, P.; Meng, Q.-F.; Cai, X.; Qu, D. Decoy Nanoparticles Protect against COVID-19 by Concurrently Adsorbing Viruses and Inflammatory Cytokines. Proc. Natl. Acad. Sci. USA 2020, 117, 27141–27147. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-M.J.; Fang, R.H.; Copp, J.; Luk, B.T.; Zhang, L. A Biomimetic Nanosponge that Absorbs Pore-Forming Toxins. Nat. Nanotechnol. 2013, 8, 336–340. [Google Scholar] [CrossRef]
- Hasan, M.; Choi, J.; Akter, H.; Kang, H.; Ahn, M.; Lee, S. Antibody-Conjugated Magnetic Nanoparticle Therapy for Inhibiting T-Cell Mediated Inflammation. Adv. Sci. 2024, 11, 2307148. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, E.; Toledo, D.; Smith, I.T.; Navarrete, B.; Furman, N.; Hernandez, A.F.; Telusma, M.; McDaniel, D.; Liang, P. Colossal Magnetoelectric Effect in Core–Shell Magnetoelectric Nanoparticles. Nano Lett. 2020, 20, 5765–5772. [Google Scholar] [CrossRef] [PubMed]
- Marrella, A.; Suarato, G.; Fiocchi, S.; Chiaramello, E.; Bonato, M.; Parazzini, M.; Ravazzani, P. Magnetoelectric Nanoparticles Shape Modulates Their Electrical Output. Front. Bioeng. Biotechnol. 2023, 11, 1219777. [Google Scholar] [CrossRef]
- Fiocchi, S.; Chiaramello, E.; Marrella, A.; Suarato, G.; Bonato, M.; Parazzini, M.; Ravazzani, P. Modeling of Core-Shell Magneto-Electric Nanoparticles for Biomedical Applications: Effect of Composition, Dimension, and Magnetic Field Features on Magnetoelectric Response. PLoS ONE 2022, 17, e0274676. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Borbolla, A.; García-Hevia, L.; Fanarraga, M.L. Cell Membrane-Coated Nanoparticles for Precision Medicine: A Comprehensive Review of Coating Techniques for Tissue-Specific Therapeutics. Int. J. Mol. Sci. 2024, 25, 2071. [Google Scholar] [CrossRef] [PubMed]
- Ben-Akiva, E.; Meyer, R.A.; Yu, H.; Smith, J.T.; Pardoll, D.M.; Green, J.J. Biomimetic Anisotropic Polymeric Nanoparticles Coated with Red Blood Cell Membranes for Enhanced Circulation and Toxin Removal. Sci. Adv. 2020, 6, eaay9035. [Google Scholar] [CrossRef]
- Dewey, W.C. Arrhenius Relationships from the Molecule and Cell to the Clinic. Int. J. Hyperth. 1994, 10, 457–483. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The Pathogenesis of Rheumatoid Arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef]
- Bracaglia, C.; Prencipe, G.; De Benedetti, F. Macrophage Activation Syndrome: Different Mechanisms Leading to a One Clinical Syndrome. Pediatr. Rheumatol. 2017, 15, 1–7. [Google Scholar] [CrossRef]
- Deane, S.; Selmi, C.; Teuber, S.S.; Gershwin, M.E. Macrophage Activation Syndrome in Autoimmune Disease. Int. Arch. Allergy Immunol. 2010, 153, 109–120. [Google Scholar] [CrossRef]
- Lerkvaleekul, B.; Vilaiyuk, S. Macrophage Activation Syndrome: Early Diagnosis Is Key. Open Access Rheumatol. Res. Rev. 2018, 10, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Siddall, E.; Khatri, M.; Radhakrishnan, J. Capillary Leak Syndrome: Etiologies, Pathophysiology, and Management. Kidney Int. 2017, 92, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, E.; Guan, X.; Li, J.; Qin, A.; Wang, C.; Fu, X.; Huang, C.; Li, J.; Tang, Y. Magnetically Controlled Nanorobots Induced Oriented and Rapid Clearance of the Cytokine Storm for Acute Lung Injury Therapy. Colloids Surf. B Biointerfaces 2024, 234, 113731. [Google Scholar] [CrossRef] [PubMed]
- Mhambi, S.; Fisher, D.; Tchokonte, M.B.T.; Dube, A. Permeation Challenges of Drugs for Treatment of Neurological Tuberculosis and HIV and the Application of Magneto-Electric Nanoparticle Drug Delivery Systems. Pharmaceutics 2021, 13, 1479. [Google Scholar] [CrossRef]
- Tang, Q.; Sun, S.; Wang, P.; Sun, L.; Wang, Y.; Zhang, L.; Xu, M.; Chen, J.; Wu, R.; Zhang, J. Genetically Engineering Cell Membrane-Coated BTO Nanoparticles for MMP2-Activated Piezocatalysis-Immunotherapy. Adv. Mater. 2023, 35, 2300964. [Google Scholar] [CrossRef]
- Wu, M.; Le, W.; Mei, T.; Wang, Y.; Chen, B.; Liu, Z.; Xue, C. Cell Membrane Camouflaged Nanoparticles: A New Biomimetic Platform for Cancer Photothermal Therapy. Int. J. Nanomed. 2019, 14, 4431–4448. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tu, Q.; Xie, D.; Chen, S.; Gao, K.; Xu, X.; Zhang, Z.; Mei, X. Triamcinolone Acetonide-Loaded Nanoparticles Encapsulated by CD90+ MCSs-Derived Microvesicles Drive Anti-Inflammatory Properties and Promote Cartilage Regeneration after Osteoarthritis. J. Nanobiotechnol. 2022, 20, 150. [Google Scholar] [CrossRef]
- Li, R.; He, Y.; Zhu, Y.; Jiang, L.; Zhang, S.; Qin, J.; Wu, Q.; Dai, W.; Shen, S.; Pang, Z. Route to Rheumatoid Arthritis by Macrophage-Derived Microvesicle-Coated Nanoparticles. Nano Lett. 2018, 19, 124–134. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, K.; Li, C.; Guo, Q.; Chen, Q.; He, X.; Liu, L.; Zhang, Y.; Lu, Y.; Chen, X.; et al. Macrophage-Membrane-Coated Nanoparticles for Tumor-Targeted Chemotherapy. Nano Lett. 2018, 18, 1908–1915. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Jia, M.; Zheng, X.; Liu, Y.; Wang, C.; Lei, F.; Niu, H.; Li, C. ZIF-8 Nanoparticles Coated with Macrophage-Derived Microvesicles for Sustained, Targeted Delivery of Dexamethasone to Arthritic Joints. J. Drug Target. 2022, 30, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Zhang, X.; Yuan, L.; Li, T.; Chen, Y.; Tian, H.; Ma, D.; Deng, J.; Qi, X.; Yin, X. Integrated Endotoxin-Adsorption and Antibacterial Properties of Platelet-Membrane-Coated Copper Silicate Hollow Microspheres for Wound Healing. J. Nanobiotechnol. 2021, 19, 383. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, B.; Wei, Y.; Liu, Y.; Yang, L.; Qiu, Y.; Su, J.; Qiu, M. Bioinspired Ginsenoside Rg3 PLGA Nanoparticles Coated with Tumor-Derived Microvesicles to Improve Chemotherapy Efficacy and Alleviate Toxicity. Biomater. Sci. 2024, 12, 2672–2688. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, S.; Xue, N. Research Progress of Cancer Cell Membrane Coated Nanoparticles for the Diagnosis and Therapy of Breast Cancer. Front. Oncol. 2023, 13, 1270407. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Jha, L.A.; Hasan, N.; Shrestha, J.; Pangeni, R.; Parvez, N.; Mohammed, Y.; Jha, S.K.; Paudel, K.R. “Nanodecoys”—Future of Drug Delivery by Encapsulating Nanoparticles in Natural Cell Membranes. Int. J. Pharm. 2022, 621, 121790. [Google Scholar] [CrossRef] [PubMed]
- Rampado, R.; Caliceti, P.; Agostini, M. Latest Advances in Biomimetic Cell Membrane-Coated and Membrane-Derived Nanovectors for Biomedical Applications. Nanomaterials 2022, 12, 1543. [Google Scholar] [CrossRef]
- Zhang, H.; Chiao, M. Anti-Fouling Coatings of Poly(Dimethylsiloxane) Devices for Biological and Biomedical Applications. J. Med. Biol. Eng. 2015, 35, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, C.; Lee, I.; Cowan, K.R.; Suh, J. Coating Barium Titanate Nanoparticles with Polyethylenimine Improves Cellular Uptake and Allows for Coupled Imaging and Gene Delivery. Colloids Surf. B Biointerfaces 2013, 112, 108–112. [Google Scholar] [CrossRef]
- Pa̧zik, R.; Andersson, R.; Kȩpiński, L.; Nedelec, J.-M.; Kessler, V.G.; Seisenbaeva, G.A. Surface Functionalization of the Metal Oxide Nanoparticles with Biologically Active Molecules Containing Phosphonate Moieties. Case Study of BaTiO3. J. Phys. Chem. C 2011, 115, 9850–9860. [Google Scholar] [CrossRef]
- Sood, A.; Desseigne, M.; Dev, A.; Maurizi, L.; Kumar, A.; Millot, N.; Han, S.S. A Comprehensive Review on Barium Titanate Nanoparticles as a Persuasive Piezoelectric Material for Biomedical Applications: Prospects and Challenges. Small 2023, 19, 2206401. [Google Scholar] [CrossRef]
- Munir, A.; Zhu, Z.; Wang, J.; Zhou, H.S. FEM Analysis of Magnetic Agitation for Tagging Biomolecules with Magnetic Nanoparticles in a Microfluidic System. Sens. Actuators B Chem. 2014, 197, 1–12. [Google Scholar] [CrossRef]
- Narhi, L.O.; Philo, J.S.; Li, T.; Zhang, M.; Samal, B.; Arakawa, T. Induction of α-Helix in the β-Sheet Protein Tumor Necrosis Factor-α: Thermal-and Trifluoroethanol-Induced Denaturation at Neutral pH. Biochemistry 1996, 35, 11447–11453. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.C.; Erdös, E.A.; Chiang, H.-S.; Calderwood, T.; Tsai, K.; Visor, G.C.; Duffy, J.; Hsu, W.-C.; Foster, L.C. Stability of Interleukin 1β (IL-1β) in Aqueous Solution: Analytical Methods, Kinetics, Products, and Solution Formulation Implications. Pharm. Res. 1991, 8, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Schön, A.; Clarkson, B.R.; Jaime, M.; Freire, E. Temperature Stability of Proteins: Analysis of Irreversible Denaturation Using Isothermal Calorimetry. Proteins Struct. Funct. Bioinform. 2017, 85, 2009–2016. [Google Scholar] [CrossRef]
- Qin, Z.; Balasubramanian, S.K.; Wolkers, W.F.; Pearce, J.A.; Bischof, J.C. Correlated Parameter Fit of Arrhenius Model for Thermal Denaturation of Proteins and Cells. Ann. Biomed. Eng. 2014, 42, 2392–2404. [Google Scholar] [CrossRef]
- Erickson, H.P. Size and Shape of Protein Molecules at the Nanometer Level Determined by Sedimentation, Gel Filtration, and Electron Microscopy. Biol. Proced. Online 2009, 11, 32–51. [Google Scholar] [CrossRef] [PubMed]
- You, D.; Chen, T.; Liu, G. Multiphysics Modeling of Plasmonic Photothermal Therapy. Therm. Sci. Eng. Prog. 2023, 46, 102240. [Google Scholar] [CrossRef]
- Lio, G.E.; Palermo, G.; De Luca, A.; Caputo, R. Numerical Modeling of Active Thermo-Plasmonics Experiments. arXiv 2020, arXiv:2001.05039. [Google Scholar]
- Kolishetti, N.; Alexis, F.; Pridgen, E.M.; Farokhzad, O.C. Biodistribution and Pharmacokinetics of Nanoprobes. Nanoplatfor-Based Mol. Imaging 2011, 1, 75–106. [Google Scholar]
- Kaushik, A.; Jayant, R.D.; Nikkhah-Moshaie, R.; Bhardwaj, V.; Roy, U.; Huang, Z.; Ruiz, A.; Yndart, A.; Atluri, V.; El-Hage, N. Magnetically Guided Central Nervous System Delivery and Toxicity Evaluation of Magneto-Electric Nanocarriers. Sci. Rep. 2016, 6, 25309. [Google Scholar] [CrossRef]
- Kozielski, K.L.; Jahanshahi, A.; Gilbert, H.B.; Yu, Y.; Erin, Ö.; Francisco, D.; Alosaimi, F.; Temel, Y.; Sitti, M. Nonresonant Powering of Injectable Nanoelectrodes Enables Wireless Deep Brain Stimulation in Freely Moving Mice. Sci. Adv. 2021, 7, eabc4189. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Gao, J.; Wang, P.; Nagesetti, A.; Andrews, P.; Masood, S.; Vriesman, Z.; Liang, P.; Khizroev, S.; Jin, X. In Vivo Wireless Brain Stimulation via Non-Invasive and Targeted Delivery of Magnetoelectric Nanoparticles. Neurotherapeutics 2021, 18, 2091–2106. [Google Scholar] [CrossRef] [PubMed]
- Kolishetti, N.; Vashist, A.; Arias, A.Y.; Atluri, V.; Dhar, S.; Nair, M. Recent Advances, Status, and Opportunities of Magneto-Electric Nanocarriers for Biomedical Applications. Mol. Asp. Med. 2022, 83, 101046. [Google Scholar] [CrossRef]
- IT’S Foundation Tissue Properties Database 4.0; IT’IS Foundation: Zurich, Switzerland, 2018.
- Towhidi, L.; Khodadadi, D.; Maimari, N.; Pedrigi, R.M.; Ip, H.; Kis, Z.; Kwak, B.R.; Petrova, T.W.; Delorenzi, M.; Krams, R. Comparison between Direct and Reverse Electroporation of Cells in Situ: A Simulation Study. Physiol. Rep. 2016, 4, e12673. [Google Scholar] [CrossRef]
- Yan, Z.; Hao, C.; Yin, L.; Liu, K.; Qiu, J. Simulation of the Influence of Temperature on the Dynamic Process of Electroporation Based on Finite Element Analysis. IEEE Trans. Plasma Sci. 2021, 49, 2839–2850. [Google Scholar] [CrossRef]
- Yurinskaya, V.E.; Vereninov, I.A.; Vereninov, A.A. A Tool for Computation of Changes in Na+, K+, Cl− Channels and Transporters Due to Apoptosis by Data on Cell Ion and Water Content Alteration. Front. Cell Dev. Biol. 2019, 7, 448871. [Google Scholar] [CrossRef]
- Ayadi, M.; Leuliet, J.; Chopard, F.; Berthou, M.; Lebouche, M. Electrical Conductivity of Whey Protein Deposit: Xanthan Gum Effect on Temperature Dependency. Food Bioprod. Process. 2004, 82, 320–325. [Google Scholar] [CrossRef]
- Amin, M.; Küpper, J. Variations in Proteins Dielectric Constants. ChemistryOpen 2020, 9, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.; Polikarpov, I.; Craievich, A.F. Average Protein Density Is a Molecular-weight-dependent Function. Protein Sci. 2004, 13, 2825–2828. [Google Scholar] [CrossRef] [PubMed]
- Yamato, T.; Wang, T.; Sugiura, W.; Laprévote, O.; Katagiri, T. Computational Study on the Thermal Conductivity of a Protein. J. Phys. Chem. B 2022, 126, 3029–3036. [Google Scholar] [CrossRef]
- Högg, E.; Rauh, C. Towards a Better Understanding of Texturization during High-Moisture Extrusion (HME)—Part I: Modeling the Texturability of Plant-Based Proteins. Foods 2023, 12, 1955. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Hwang, A.; Phillips, J. Effect of Temperature on Microbial Growth Rate–Mathematical Analysis: The Arrhenius and Eyring–Polanyi Connections. J. Food Sci. 2011, 76, E553–E560. [Google Scholar] [CrossRef] [PubMed]
- Chinnasamy, C.N.; Senoue, M.; Jeyadevan, B.; Perales-Perez, O.; Shinoda, K.; Tohji, K. Synthesis of size-controlled cobalt ferrite particles with high coercivity and squareness ratio. J. Colloid Interface Sci. 2003, 263, 80–83. [Google Scholar] [CrossRef]
- Betal, S.; Shrestha, B.; Dutta, M.; Cotica, L.F.; Khachatryan, E.; Nash, K.; Tang, L.; Bhalla, A.S.; Guo, R. Magneto-elasto-electroporation (MEEP): In-Vitro visualization and numerical characteristics. Sci Rep 2016, 6, 32019. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Feng, M.; Liu, M.; Hua, J.; Ma, J.; Wu, L.; Xu, H.; Wang, A.P.; Li, H.B. Electric-field tuning of magnetic anisotropy in the artificial multiferroic Fe3O4/PMN–PT heterostructure. Mater. Res. Lett. 2018, 6, 592–597. [Google Scholar] [CrossRef]
- Kurian, M.; Thankachan, S.; Nair, D.S.; EK, A.; Babu, A.; Thomas, A.; Krishna KT, B. Structural, magnetic, and acidic properties of cobalt ferrite nanoparticles synthesised by wet chemical methods. J. Adv. Ceram. 2015, 4, 199–205. [Google Scholar] [CrossRef]
- Ananth Subray, P.V.; Hanumagowda, B.N.; Raju CS, K.; Varma SV, K.; Jagdish, P.; Yook, S.J.; Shah, N.A. Analytical analysis of inclined three-layered composite channel with cobalt ferrite nanoparticles and Hall current in Darcy medium. Propuls. Power Res. 2023, 12, 523–538. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marrella, A.; Giannoni, P.; Lenzuni, M.; Suarato, G.; Fiocchi, S.; Chiaramello, E.; Ravazzani, P. Temperature-Dependent Cytokine Neutralization Induced by Magnetoelectric Nanoparticles: An In Silico Study. Int. J. Mol. Sci. 2024, 25, 13591. https://doi.org/10.3390/ijms252413591
Marrella A, Giannoni P, Lenzuni M, Suarato G, Fiocchi S, Chiaramello E, Ravazzani P. Temperature-Dependent Cytokine Neutralization Induced by Magnetoelectric Nanoparticles: An In Silico Study. International Journal of Molecular Sciences. 2024; 25(24):13591. https://doi.org/10.3390/ijms252413591
Chicago/Turabian StyleMarrella, Alessandra, Paolo Giannoni, Martina Lenzuni, Giulia Suarato, Serena Fiocchi, Emma Chiaramello, and Paolo Ravazzani. 2024. "Temperature-Dependent Cytokine Neutralization Induced by Magnetoelectric Nanoparticles: An In Silico Study" International Journal of Molecular Sciences 25, no. 24: 13591. https://doi.org/10.3390/ijms252413591
APA StyleMarrella, A., Giannoni, P., Lenzuni, M., Suarato, G., Fiocchi, S., Chiaramello, E., & Ravazzani, P. (2024). Temperature-Dependent Cytokine Neutralization Induced by Magnetoelectric Nanoparticles: An In Silico Study. International Journal of Molecular Sciences, 25(24), 13591. https://doi.org/10.3390/ijms252413591











