1. Introduction
Lamins, the main components of the nuclear lamina, are type V intermediate filament proteins that polymerize to form a highly organized meshwork between the inner nuclear membrane (INM) and the chromatin [
1]. Lamins are essential for the maintenance of nuclear structure and mechanics, gene regulation, chromatin organization, telomere homeostasis, DNA replication, and damage repair [
1,
2,
3]. Lamins are grouped into A-type and B-type depending on their structural and biochemical properties. A-type lamins bind to B-type lamins and to several structural proteins, including the integral INM protein emerin, the outer nuclear membrane (ONM) proteins’ nesprins, lamina-associated polypeptide 2 isoform alpha (LAP2α), NUP153, SUN-domain-containing proteins, nuclear actin, and protein 4.1R, thus forming a structural network essential for nuclear integrity and nucleo-cytoskeletal coupling [
4]. In mammals, the
LMNA gene gives rise to lamins A and C via alternative RNA splicing, and they are expressed in a tissue- and development–specific manner, whereas B-type lamins encoded by the
LMNB1 (lamin B1) and
LMNB2 (lamins B2 and B3) genes are ubiquitously expressed [
5,
6,
7,
8].
LMNA mutations cause a heterogeneous spectrum of rare human diseases commonly known as “laminopathies”, involving different tissues, such as striated muscle (including cardiac muscle, causing cardiomyopathies), adipose tissue (lypodystrophy syndromes), peripheral nerve (peripheral neuropathy), or multiple systems with features of accelerated aging [
9]. The most severe laminopathies are progeroid syndromes, including the premature aging disease, such as Hutchinson-Gilford progeria syndrome (HGPS-OMIM 176670), Atypical Werner’s syndrome (ORPHA79474), restrictive dermopathy (OMIM 619793), and mandibular acral dysplasia (OMIM 248370). The striated muscle laminopathies include disorders such as Emery-Dreifuss muscular dystrophy (EDMD-OMIM 181350) or limb-girdle muscular dystrophy type 1B, where cardiac muscle involvement represents the common feature coexisting without or with skeletal muscle disease. In addition, the EDMD may also occur as a result of mutations in the
EMD gene encoding emerin [
10]. While canonical Werner syndrome is a prototypical segmental progeroid syndrome characterized by multiple features consistent with accelerated aging, and is caused by null mutations of the
WRN gene and the atypical form derived by the mutation on the
LMNA gene, and follows an autosomal dominant pattern of inheritance. Atypical Werner’s syndrome shows accelerated aging characterized by short stature, thinning/graying of hair, a “bird-like” facial appearance, skin atrophy, lipodystrophy, and myopathy, along with other age-related disorders, such as osteoporosis and atherosclerosis. Compared to the canonical form, it has an earlier age of onset (early 20 s or earlier) and a more rapid rate of progression [
11]. With regard to the
LMNA-related congenital muscular dystrophy, this is a condition that primarily affects muscles used for movement (skeletal muscles) and is characterized by prominent axial hypotonia, predominantly proximal muscle weakness in upper limbs and distal in lower limbs, joint contractures, spinal rigidity, and progressive respiratory insufficiency, in the presence of moderately elevated serum creatine kinase. Cardiac arrhythmias and sudden death have also been reported [
12].
Previous reports have highlighted the pivotal role of post-translational modifications of lamins in maintaining their proper functions, distributions, and structural properties, such as farnesylation of the cysteine residue of the C-terminal CAAX motif, serine or tyrosine phosphorylation, SUMOylation, acetylation, O-Glc-NAcylation, and ubiquitination [
13,
14].
Src, the most representative member of the Src family of tyrosine kinases (SFKs), is involved in several cellular processes, such as cell proliferation, migration, and cell response to mechanical stimulation [
15,
16]. It is worth noting that, in addition to the most studied functions in the cytoplasmic compartment, Src kinases exert several roles in the nucleus, acting as kinase for targeted nuclear proteins and as cooperating protein in multi-complexes involved in regulating gene expression [
17].
Given this function of Src as a kinase of nuclear envelope proteins, as lamin A and emerin [
18,
19,
20], and that lamin A activity is regulated by phosphorylation [
21], in this work, we focused on the relationship between lamin A/C and Src proteins in healthy and laminopathic fibroblasts at the nanoscale level using confocal and super-resolution STED microscopy; we used advanced imaging tools, such as FRET after photobleaching (FRET-AB) and FLIM-FRET analyses to investigate their intermolecular distances and interactions.
FRET imaging is a non-radiative method of energy transfer from a donor fluorophore in the excited state to an acceptor fluorophore through long-range dipole–dipole interactions. However, FRET can occur only when donor and acceptor molecules are in close proximity (typically 1–10 nm) and the emission spectrum of a donor fluorophore significantly overlaps (>30%) the absorption spectrum of an acceptor [
22].
FLIM is a highly useful tool that allows for spatially resolving the lifetimes of one or more fluorophores. The fluorescence lifetime is the time expressed in pico- or nano-seconds in which the fluorophore remains in the excited state before returning to the ground state, and it is sensitive to environmental conditions (temperature, pH, oxygen content, ion concentration, etc.) and to excited state reactions, such as FRET, whereas it is not influenced by probe concentration, photobleaching, or internal settings (laser intensity, detector gain) of the instrument [
23,
24]. Because FRET reduces the donor lifetime in proportion to the energy transfer efficiency, FLIM analysis can be used for quantitative FRET detection. As a result, the combination of FRET and Fluorescence Lifetime (FRET-FLIM) methods provides high spatial (nanometer) and temporal (nanosecond) resolution by monitoring the change in donor lifetime in the presence and absence of an acceptor. FRET detection by time-domain FLIM has been applied to the characterization of protein–protein interactions with high spatial and temporal specificity (e.g., clustering), in the study of conformational changes, and in the analysis of binding sequences [
22,
24].
In this study, we provide evidence of the differential Src distribution in the dermal fibroblast nuclei, and its close relationship with lamin A/C, thereby strengthening the potential role of the Src–lamin A/C binding in pathogenesis and in the altered mechanosensitive processes linked to laminopathies.
2. Detailed Case Description
2.1. Patients’ Biological Samples
Two genetically confirmed LMNA patients have been the objective of this manuscript, followed by clinicians of Bambino Gesù Children’s Hospital, Rome, Italy (Patient 1) and at the National Rehabilitation Institute (INRPAC) in Santiago, Chile (Patient 2), respectively. Biological samples, including blood and skin biopsies, were obtained during the diagnostic investigation after obtaining signed informed consent. DNA was obtained from circulating leukocytes using standard procedures. Aged- and sex-matched controls of skin fibroblasts were also used. The study has been conducted according to the guidelines established in the Declaration of Helsinki. The collected data were stored in individual clinical records. Considering the retrospective nature of the analysis, the current study did not require the approval of the local ethics committee according to current legislation, but a notification was sent. Data were retrospectively analyzed in line with personal data protection policies.
2.2. Patients
2.2.1. Patient 1
The patient was born in 1985. At birth, this lady was surgically operated on for an umbilical hernia with expulsion of viscera. At the age of 8, she was reported to have acrogeria with lipodystrophy; later, at the age of 18, she was diagnosed with type II diabetes mellitus, and at the age of 25, she was admitted to the Endocrinology Department with the diagnosis of acrogeria to carry out laboratory control tests. The physical examination showed that she was in good general condition, having a weight of 39.4 kg (<3rd centile), height of 150 cm (3rd centile), HR of 79 bpm, and BP of 108/63 mmHg. She had characteristic facies with micrognathia, a pinched face, an “owl-eyed” appearance, and a beaked nose with marbled and thin skin. Diffuse hypertrichosis was observed, especially on the face and dorsal region, as well as hypotrophy of the muscle masses. Her abdomen was treatable; there was an umbilical hernia and no organomegaly. Abdomen ultrasound imaging only showed that her liver had a slightly heterogeneous echo structure but without focal lesions, and a cardiological examination with EKG and echocardiography showed no abnormalities. A laboratory examination showed normal CK and transaminases and an abnormal glycemic curve with slightly increased insulin. At a subsequent control, at the age of 26, she showed impaired glucose tolerance, increased triglycerides, and mitral valvular insufficiency, and two years later, a liver echo showed that it increased in size with a diffuse and homogeneous increase in echogenicity, suggesting hepatic steatosis. The patient was discharged with the diagnosis of type II diabetes and persistent valvular mitral insufficiency. The patient passed away at the age of 30 due to mandibular sarcoma that invaded surrounding tissues (tongue and palate). Mutational analysis by direct Sanger sequencing of the
LMNA gene (NM_170707.4), highlighted a heterozygous likely pathogenetic mutation c.1733A>T (p.Glu578Val) described elsewhere [
25], confirming the clinical presentation of a progeroid syndrome, specifically atypical Werner’s syndrome.
2.2.2. Patient 2
The patient was the youngest of two siblings, born to non-consanguineous parents. She developed normally until 14 months. After she started walking, she began to have a waddling gait, fell frequently, and had difficulty getting up from the floor. Progressive proximal upper and lower limb weakness and serum creatine kinase levels were tenfold above the noted normal range. A muscle biopsy showed dystrophic changes. She lost the ability to walk at 31 months old and developed severe contractures in her elbows, wrists, hips, knees, elbows, and ankles. Over the years, she also developed hyperlordosis and a rigid spine. At the age of 15 years, she needed nocturnal non-invasive ventilation due to restrictive ventilatory insufficiency. When she was 16, she received an implantable defibrillator because of recurrent ventricular extrasystoles and a first-degree atrioventricular (AV) block. Sadly, she passed away at the age of 19 due to secondary heart failure. Mutational analysis via direct sequencing of the
LMNA gene (NM_170707.4) pointed out the heterozygous missense variant c.745C>T (p.Arg249Trp), previously described in other patients affected by congenital muscular dystrophy [
26].
2.3. Cell Cultures
Skin fibroblasts from laminopathic patients and two controls were grown in Dulbecco’s modified minimum essential medium (D-MEM), supplemented with 10% fetal bovine serum (Life Technologies, Segrate, Italy; a part of Thermo Fisher Scientific, Waltham, MA, USA), and 1% penicillin and streptomycin (Life Technologies, Segrate, Italy; a part of Thermo Fisher Scientific).
2.4. Immunofluorescence
In total, 5 × 103 cells were seeded into plastic-chambered glass microscope slides (BD Falcon), grown until sub-confluence, and fixed with 4% paraformaldehyde in PBS for 10 min followed by PBS/Triton 0.1% for 5 min. The following antibodies were used according to manufacturer instructions: primary rabbit anti-Src (sc-19) and mouse anti-lamin A/C (sc-7292) antibodies, purchased from Santa Cruz Biotechnology (Temecula, CA, USA); they were diluted in 1% PBS/BSA overnight, at 4 °C. Secondary goat immunoglobulins conjugated with Alexa Fluor 488 and 594 dyes (Life Technologies) have been used for confocal microscopy acquisitions. Slides were mounted with PBS/glycerol 1:1. In parallel experiments, primary anti-Src and lamin A/C were revealed using goat anti-rabbit and goat anti-mouse conjugated to STAR Orange and STAR Red dyes (Abberior, Göttingen, Germany), respectively, and, after PBS washes, slides were mounted using the mount solid antifade (Abberior) mounting medium and #1.5 thickness coverslips.
2.5. Confocal Microscopy Imaging and Data Analysis
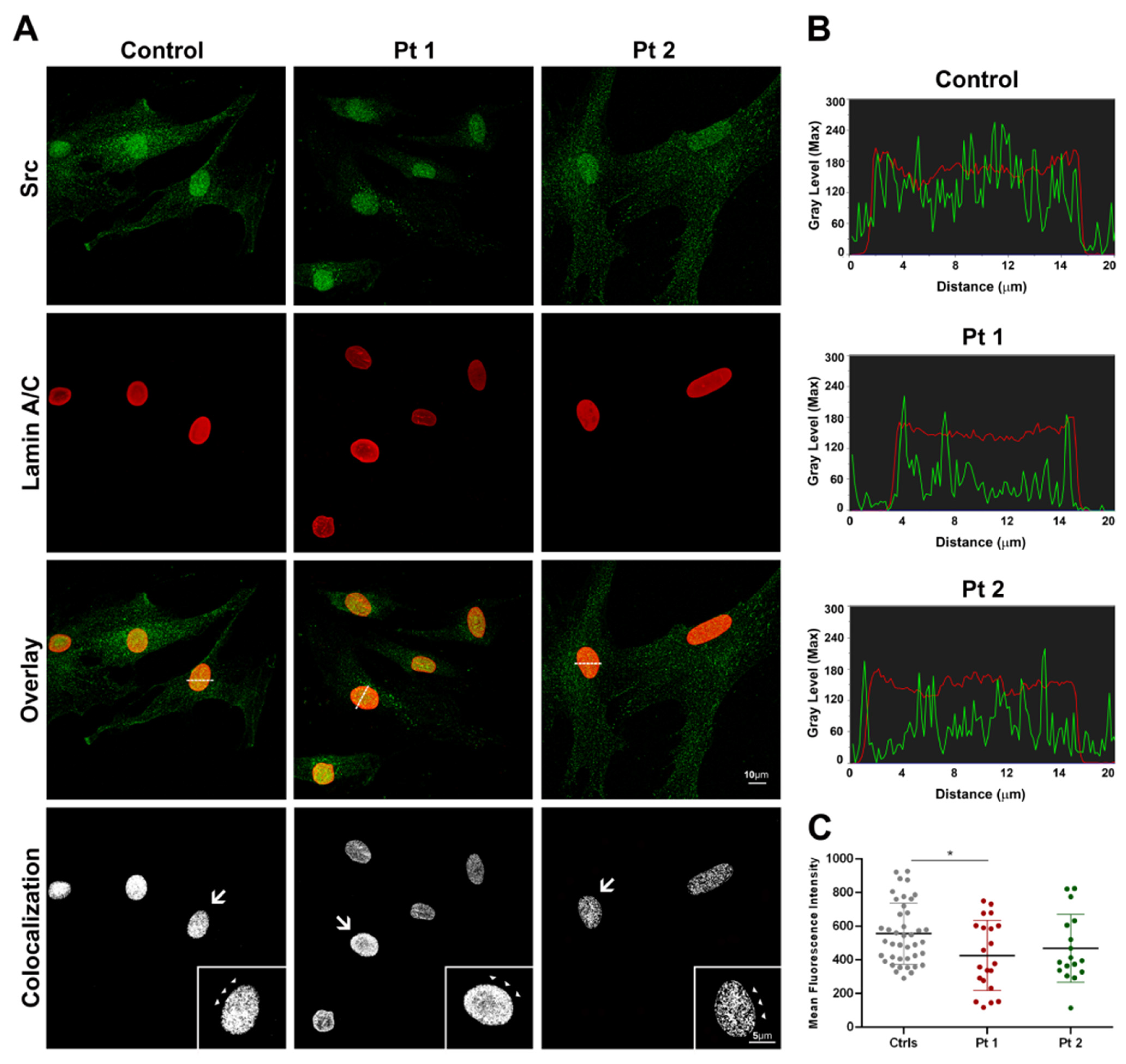
Confocal microscopy was performed on a Leica TCS-SP8X laser-scanning confocal microscope (Leica Microsystems, Mannheim, Germany) equipped with a tunable white light laser (WLL) source, a 405 nm diode laser, 3 internal spectral detector channels (PMTs), including 2 dedicated to single molecule detection (SMD), and 2 internal spectral detector channels (HyD) GaAsP. Sequential confocal images were acquired using HC PL APO 40x/oil immersion (1.30 numerical aperture, NA, Leica Microsystems) and 63x/oil immersion objectives (1.40 NA, Leica Microsystems). The mean fluorescence intensities (MFI) of Src and lamin A/C were manually measured in each nucleus, and then calculated using MetaMorph 7.8 (Molecular Devices, San Jose, CA, USA) software. From seven digital images (40x magnification), a number of nuclei >80 was randomly selected and analyzed for each cell sample, and the experiments were repeated twice. Colocalization analysis was performed using the Huygens Colocalization Analyzer (Scientific Volume Imaging, SVI, Hilversum, The Netherlands) to obtain the overlap coefficient value between the fluorophore pair, and to build the colocalized pixel distribution map.
2.6. Super-Resolution STED Microscopy
STED imaging was performed on a STED microscope platform (STEDYCON, Abberior Instruments GmbH, Göttingen, Germany) equipped with an upright Zeiss Axioimager Z.2 microscope (Zeiss Microscopy GmbH, Oberkochen, Germany) and a pulsed STED laser (Abberior Instruments GmbH) with a depletion wavelength of 775 nm working at a repetition rate of 40 MHz, a pulse duration of 1 ns, using STEDYCON SmartControl software version 9. Skin fibroblasts were immunostained using Src and lamin A/C antibodies, revealing specific goat secondary antibodies conjugated to the dyes STAR Red and STAR Orange (Abberior Instruments GmbH), respectively. STAR RED was imaged with a pulsed source at a wavelength of 640 nm (detection range between 650 and 700 nm, gated detection between 1 and 7 ns), whereas STAR Orange was excited with a pulsed source at 561 nm (detection range between 575 and 625 nm, gated detection between 1 and 7 ns). The pinhole was set to 1.1 Airy units at 650 nm. For STED imaging, an oil-immersion objective lens was used (100x Plan Apochromat oil immersion objective, 1.46 NA, Zeiss, Oberkochen, Germany). Colocalization analysis was performed using the Huygens Colocalization Analyzer (SVI) to evaluate the overlap coefficient (n = 10 of fibroblast nuclei for each sample were analyzed).
2.7. Fluorescence Lifetime Imaging Microscopy-FRET Analysis
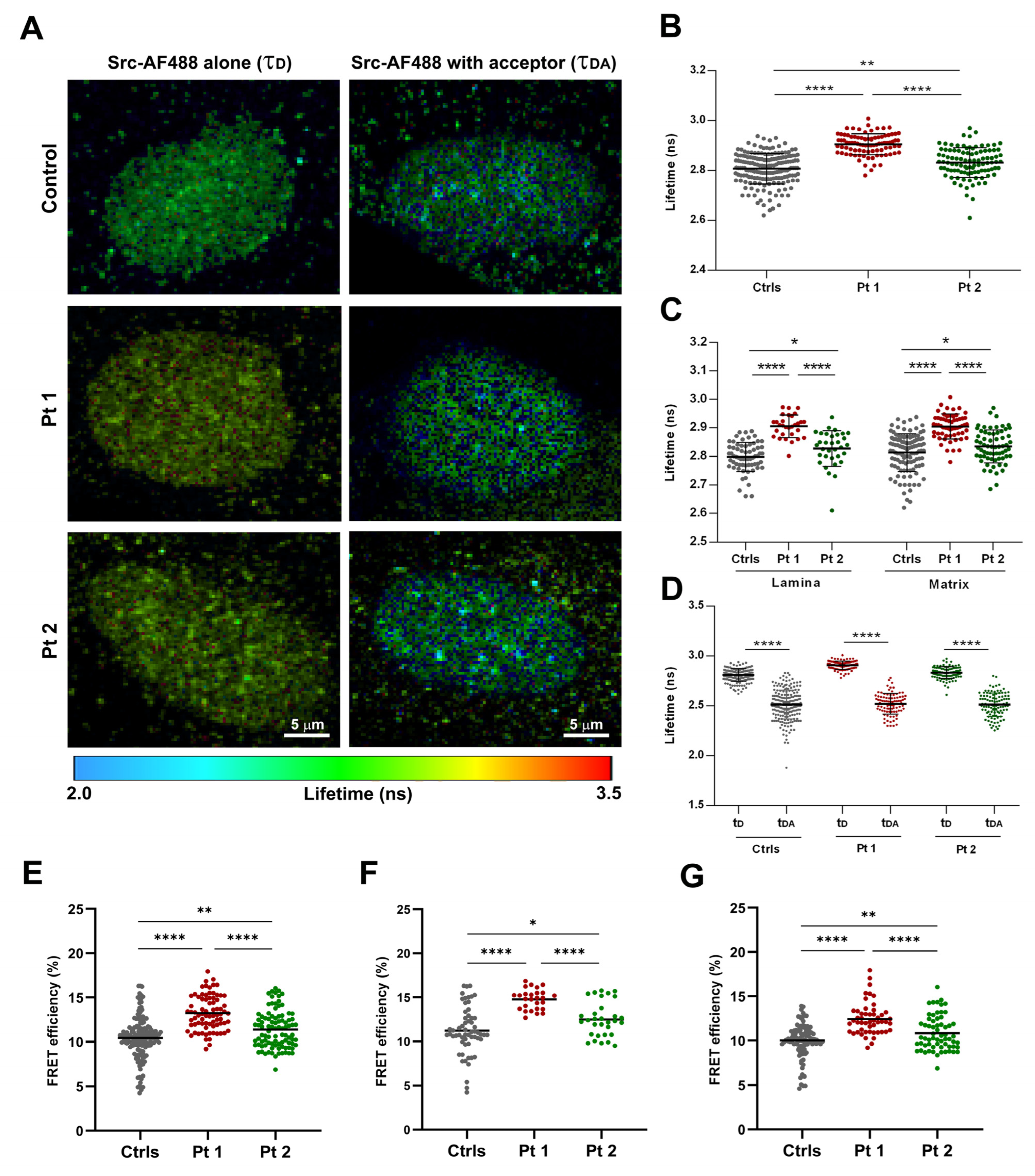
Fluorescence lifetime (FLIM) imaging was carried out with a Leica TCS-SP8 FLIM (Leica Microsystems, Manheim, Germany) confocal microscope, with a time-correlated single photon counting (TCSPC) module PicoHarp 300 time-resolved unit (PicoQuant, Berlin, Germany), with a pulsed WLL tunable in the range of 470–670 nm, 2 internal SMD spectral detector channels (PMTs), through a HCX PL APO 63x/1.30 glycerol immersion CS2 objective. FLIM recordings were performed with a 40 MHz repetition rate, an image size of 256 × 256 pixels, and a zoom factor of 5x. A minimum of 1000 photons per pixel was acquired for all FLIM acquisitions. In all experiments, the laser power was adjusted to achieve average photon counting rates of ≤105 photons/s and peak rates close to 106 photons/s when recording FLIM images, thus preventing pile-up effects. The donor fluorescent protein, Alexa Fluor 488 (AF488), was excited with a 488 nm (laser power 3%), and fluorescence was detected by a PMT-FLIM detector in the 500–550 nm range. In the presence of the AF594 acceptor, the donor emission was detected at 550–550 nm and 600–650 nm (FRET channel). For each experiment, at least 10 individual cells were imaged, and all the experiments were repeated at least two times. Lifetime was determined in manually selected, specific regions of interest (ROIs), corresponding to two nuclear districts in which Src showed a co-distribution with lamin A/C (at the nuclear rim where lamin A/C was polymerized and in matrix regions where Src showed a dotted distribution). The best fitting results were obtained for estimating the donor fluorescence lifetime in the absence of the acceptor (τD, unquenched donor lifetime), by applying a mono-exponential decay model using the n-exponential reconvolution fitting approach, and the quality of the fit was judged by the residual distribution and the goodness of the χ2 value (close to 1). The lifetime τ and the relative amplitude A of the individual exponential components were obtained from the SymPhoTime64 software 2.4 (PicoQuant, Berlin, Germany). By applying a bi-exponential decay, the donor fluorescence lifetime in the presence of the acceptor, using the donor lifetimes only, the values of τAV AMP (amplitude-weighted average lifetime) were obtained using SymPhoTime64 software.
The FRET efficiency (E) was calculated for each ROI using the equation:
E = 1 − (τ
AV AMP/τ
D), where τ
AV AMP is the amplitude-weighted average lifetime of the donor in the presence of the acceptor, and τ
D is the donor in the absence of the acceptor. The Donor-Acceptor (D-A) fluorophore distance (calculated as R-value) was obtained assuming a random orientation of both fluorophores using the following formula [
27]:
To obtain R-values, the Förster distance (R
0) was defined as the D–A distance when E = 50% was applied. The R
0 distance for the Alexa Fluor 488 and Alexa Fluor 594 (A-D) FRET pair was assumed to be 58.89 angstroms, as reported in the fluorescent biosensor database (FPbase FRET Calculator;
https://www.fpbase.org/fret/). The tables of the images were processed using Adobe Photoshop CS4 software (Adobe Systems Inc., San Jose, CA, USA).
2.8. Statistical Analysis
The differences between the means of data containing two groups were analyzed using unpaired two-tailed Student’s t-test, and the results were considered significant when p < 0.05. GraphPad Prism was used for statistical analyses. Significance levels were p < 0.05 (*), p < 0.01 (**), p < 0.0001 (****). In the figures, error bars indicate the standard deviation of the mean (SD).