HMGB1-Mediated Cell Death—A Crucial Element in Post-Hepatectomy Liver Failure
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
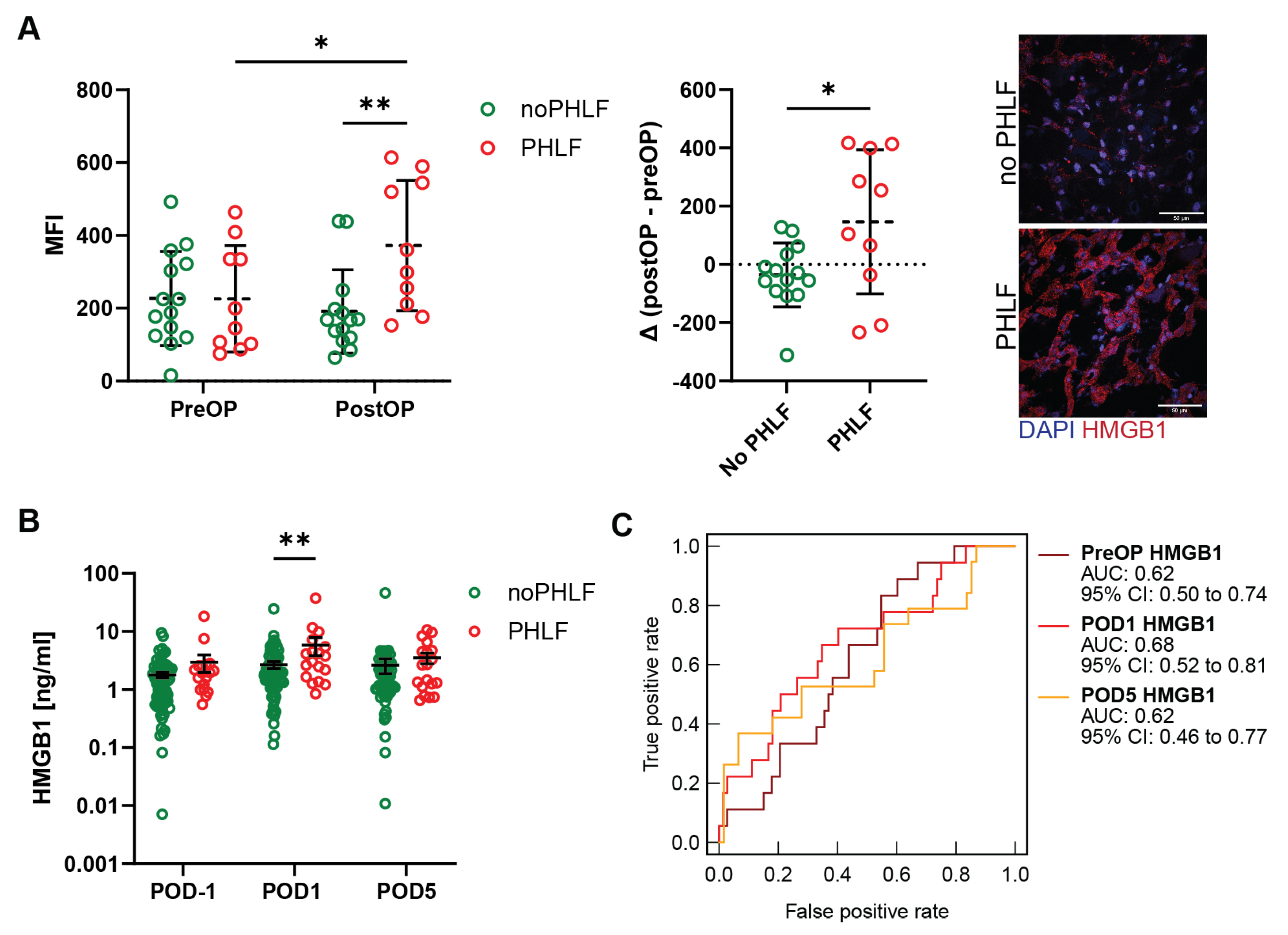
2.2. HMGB1 in the Regenerating Liver Is Elevated in Patients That Develop PHLF
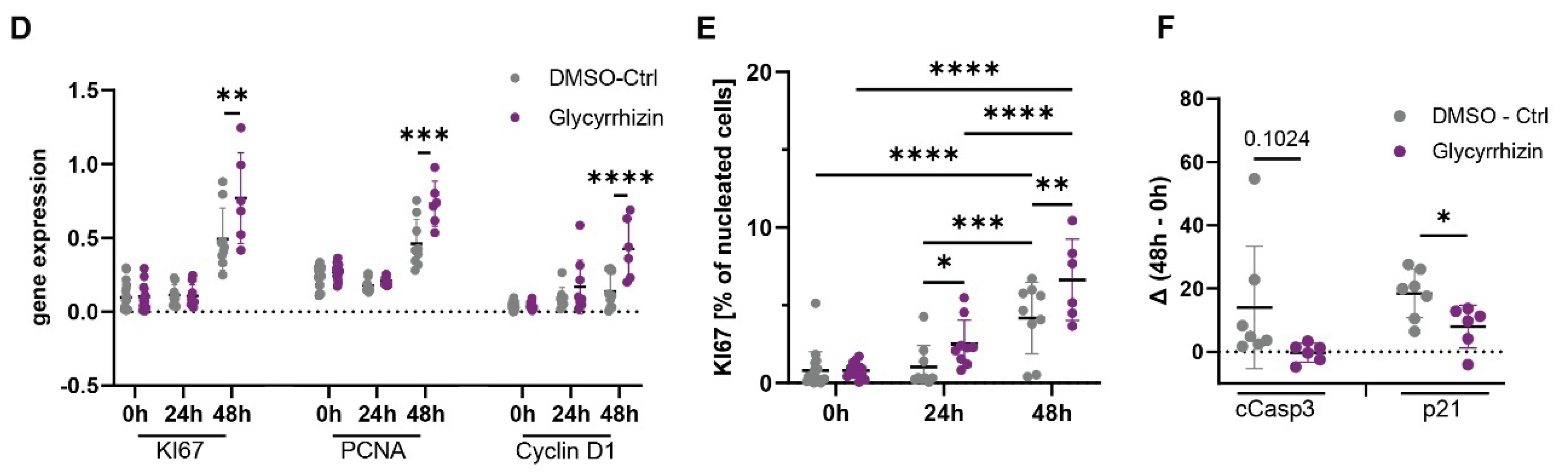
2.3. Inhibition of HMGB1 Improves Proliferation and Reduces Apoptosis in a LR Mouse Model
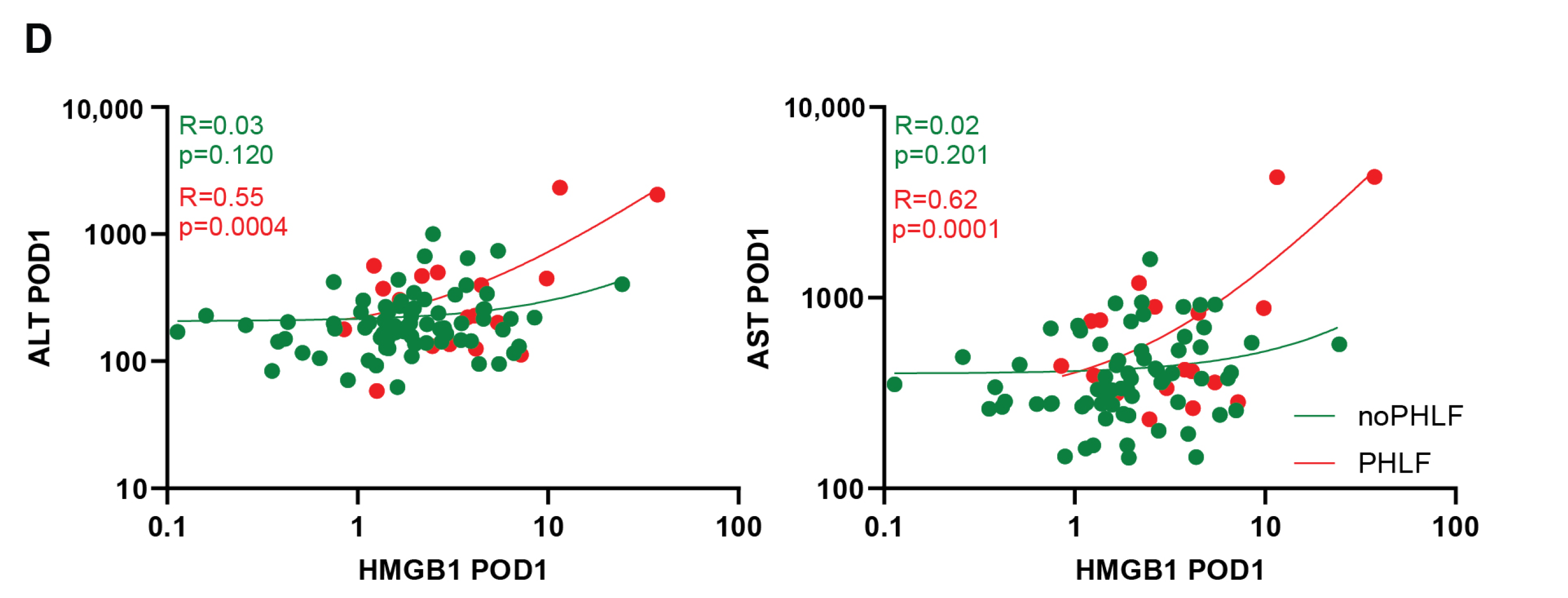
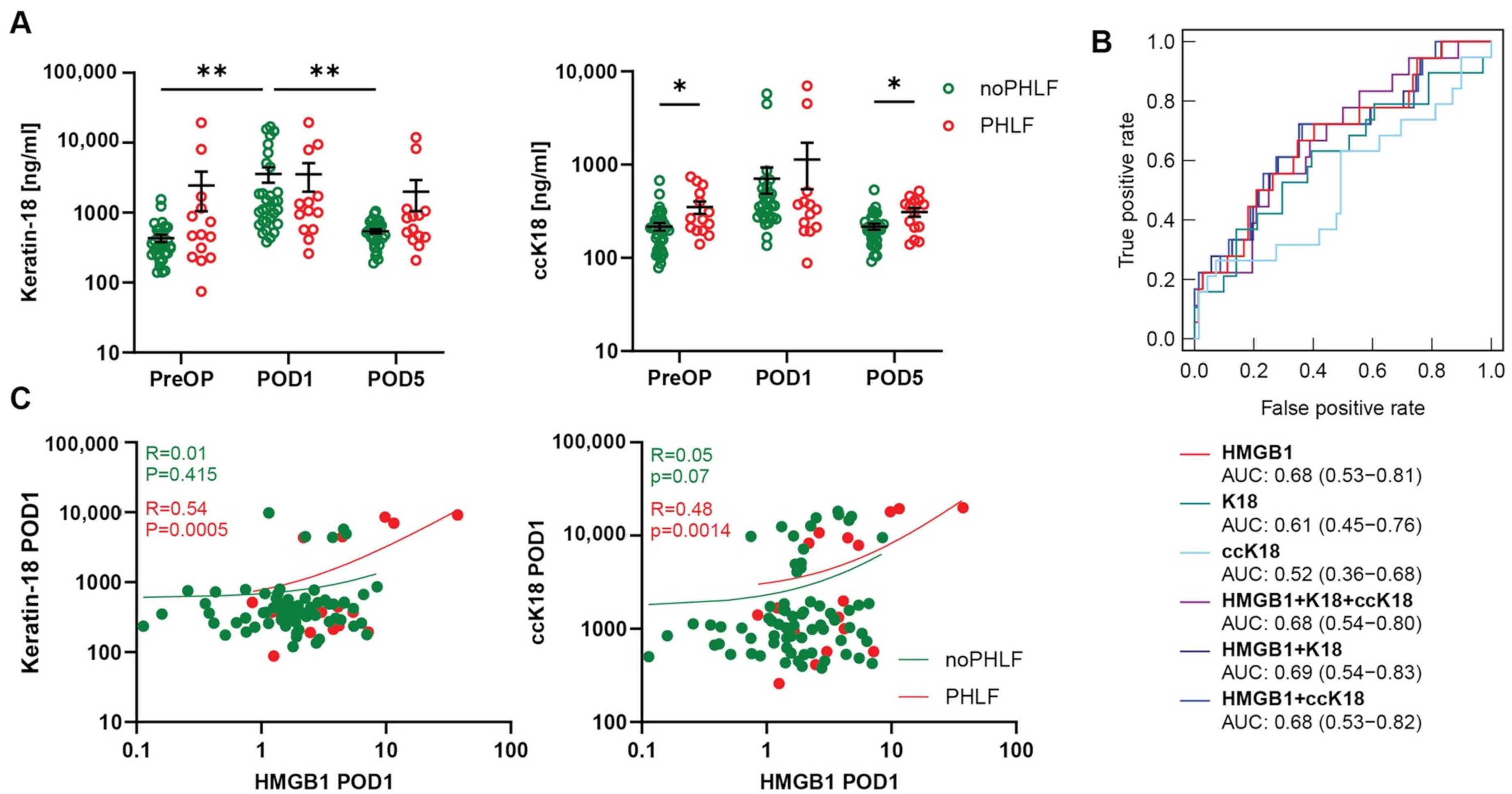
2.4. Cell Death Marker ccK-18 Is Associated with HMGB1 and PHLF Development in Patients
3. Discussion
4. Methods
4.1. Patient Cohort
4.2. Plasma Analysis
4.3. Animal Treatments
4.4. Immunofluorescence Staining
4.5. H&E and Oil Red O Staining
4.6. RNA Extraction and Real-Time qPCR Analysis
4.7. Statistical Evaluation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Yang, T.; Hu, L.Y.; Li, Z.L.; Liu, K.; Wu, H.; Xing, H.; Lau, W.Y.; Pawlik, T.M.; Zeng, Y.Y.; Zhou, Y.H.; et al. Liver Resection for Hepatocellular Carcinoma in Non-alcoholic Fatty Liver Disease: A Multicenter Propensity Matching Analysis with HBV-HCC. J. Gastrointest. Surg. 2020, 24, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Rühlmann, F.; Azizian, A.; Moosmann, C.; Bernhardt, M.; Keck, J.; Flebbe, H.; Al-Bourini, O.; Hosseini, A.S.A.; Grade, M.; Lorf, T.; et al. Perioperative LiMAx Test Analysis: Impact of Portal Vein Embolisation, Chemotherapy and Major Liver Resection. Biomedicines 2024, 12, 254. [Google Scholar] [CrossRef] [PubMed]
- Santol, J.; Kim, S.; Gregory, L.A.; Baumgartner, R.; Murtha-Lemekhova, A.; Birgin, E.; Gloor, S.; Braunwarth, E.; Ammann, M.; Starlinger, J.; et al. An APRI+ALBI Based Multivariable Model as Preoperative Predictor for Posthepatectomy Liver Failure. Ann. Surg. 2023. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Stockmann, M.; Lock, J.F.; Malinowski, M.; Niehues, S.M.; Seehofer, D.; Neuhaus, P. The LiMAx test: A new liver function test for predicting postoperative outcome in liver surgery. HPB 2010, 12, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Starlinger, P.; Brunnthaler, L.; McCabe, C.; Pereyra, D.; Santol, J.; Steadman, J.; Hackl, M.; Skalicky, S.; Hackl, H.; Gronauer, R.; et al. Transcriptomic landscapes of effective and failed liver regeneration in humans. JHEP Rep. 2023, 5, 100683. [Google Scholar] [CrossRef] [PubMed]
- Brunnthaler, L.; Pereyra, D.; Brenner, M.; Santol, J.; Herrmann, L.; Schrottmaier, W.C.; Pirabe, A.; Schmuckenschlager, A.; Kim, S.; Kern, A.E.; et al. Intrahepatic neutrophil accumulation and extracellular trap formation are associated with posthepatectomy liver failure. Hepatol. Commun. 2023, 8, e0348. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.W.; Jiang, W.; Reich, C.F., 3rd; Pisetsky, D.S. The extracellular release of HMGB1 during apoptotic cell death. Am. J. Physiol. Cell Physiol. 2006, 291, C1318–C1325. [Google Scholar] [CrossRef] [PubMed]
- Khambu, B.; Yan, S.; Huda, N.; Yin, X.M. Role of High-Mobility Group Box-1 in Liver Pathogenesis. Int. J. Mol. Sci. 2019, 20, 5314. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ochani, M.; Li, J.; Qiang, X.; Tanovic, M.; Harris, H.E.; Susarla, S.M.; Ulloa, L.; Wang, H.; DiRaimo, R.; et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc. Natl. Acad. Sci. USA 2004, 101, 296–301. [Google Scholar] [CrossRef]
- Chen, R.; Hou, W.; Zhang, Q.; Kang, R.; Fan, X.G.; Tang, D. Emerging role of high-mobility group box 1 (HMGB1) in liver diseases. Mol. Med. 2013, 19, 357–366. [Google Scholar] [CrossRef]
- Hernandez, C.; Huebener, P.; Pradere, J.P.; Antoine, D.J.; Friedman, R.A.; Schwabe, R.F. HMGB1 links chronic liver injury to progenitor responses and hepatocarcinogenesis. J. Clin. Investig. 2018, 128, 2436–2451. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, M.; Sadamori, H.; Nishibori, M.; Sato, Y.; Tazawa, H.; Shinoura, S.; Umeda, Y.; Yoshida, R.; Nobuoka, D.; Utsumi, M.; et al. Anti-high mobility group box 1 monoclonal antibody improves ischemia/reperfusion injury and mode of liver regeneration after partial hepatectomy. Am. J. Surg. 2016, 211, 179–188. [Google Scholar] [CrossRef]
- Tirone, M.; Tran, N.L.; Ceriotti, C.; Gorzanelli, A.; Canepari, M.; Bottinelli, R.; Raucci, A.; Di Maggio, S.; Santiago, C.; Mellado, M.; et al. High mobility group box 1 orchestrates tissue regeneration via CXCR4. J. Exp. Med. 2018, 215, 303–318. [Google Scholar] [CrossRef]
- Hou, W.; Wei, X.; Liang, J.; Fang, P.; Ma, C.; Zhang, Q.; Gao, Y. HMGB1-Induced Hepatocyte Pyroptosis Expanding Inflammatory Responses Contributes to the Pathogenesis of Acute-on-Chronic Liver Failure (ACLF). J. Inflamm. Res. 2021, 14, 7295–7313. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekaran, V.; Seth, R.K.; Dattaroy, D.; Alhasson, F.; Ziolenka, J.; Carson, J.; Berger, F.G.; Kalyanaraman, B.; Diehl, A.M.; Chatterjee, S. HMGB1-RAGE pathway drives peroxynitrite signaling-induced IBD-like inflammation in murine nonalcoholic fatty liver disease. Redox Biol. 2017, 13, 8–19. [Google Scholar] [CrossRef]
- Song, F.; Hurtado del Pozo, C.; Rosario, R.; Zou, Y.S.; Ananthakrishnan, R.; Xu, X.; Patel, P.R.; Benoit, V.M.; Yan, S.F.; Li, H.; et al. RAGE regulates the metabolic and inflammatory response to high-fat feeding in mice. Diabetes 2014, 63, 1948–1965. [Google Scholar] [CrossRef]
- Lin, M.J.; Deng, M.J.; Billiar, T.R.; Scott, M.J. Hepatocyte-specific high-mobility group box 1 (HC-HMGB1) protects against liver fat accumulation and cellular stress during high fat diet feeding. FASEB J. 2018, 32, 150–157. [Google Scholar] [CrossRef]
- Terry, A.Q.; Kojima, H.; Sosa, R.A.; Kaldas, F.M.; Chin, J.L.; Zheng, Y.; Naini, B.V.; Noguchi, D.; Nevarez-Mejia, J.; Jin, Y.P.; et al. Disulfide-HMGB1 signals through TLR4 and TLR9 to induce inflammatory macrophages capable of innate-adaptive crosstalk in human liver transplantation. Am. J. Transplant. 2023, 23, 1858–1871. [Google Scholar] [CrossRef] [PubMed]
- Rahbari, N.N.; Garden, O.J.; Padbury, R.; Brooke-Smith, M.; Crawford, M.; Adam, R.; Koch, M.; Makuuchi, M.; Dematteo, R.P.; Christophi, C.; et al. Posthepatectomy liver failure: A definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011, 149, 713–724. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Mitchell, C.; Willenbring, H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat. Protoc. 2008, 3, 1167–1170. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Cohort (n = 96) | PHLF Cohort (n = 22) | no PHLF Cohort (n = 74) | Missing Values | |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 58 (60.4%) | 13 (59.1%) | 45 (60.8%) | ||
| Female | 38 (39.6%) | 9 (40.9%) | 29 (39.2%) | ||
| Age (years) | 63.3 (23.0–86.1) | 67.6 (35,0–82.4) | 62.5 (23.0–86.1) | ||
| Hepatic resection | |||||
| Minor (<3 segments) | 23 (24.0%) | 2 (9.1%) | 21 (28.4%) | ||
| Major (≥3 segments) | 73 (76.0%) | 20 (90.9%) | 53 (71.6%) | ||
| Tumor type | |||||
| CRLM | 39 (40.6%) | 7 (31.8%) | 32 (43.2%) | ||
| HCC | 17 (17.7%) | 5 (22.7%) | 12 (16.2%) | ||
| CCA | 18 (18.8%) | 7 (31.8%) | 11 (14.9%) | ||
| Pancreas related | 10 (10.4%) | 1 (4.5%) | 9 (12.2%) | ||
| Others | 12 (12.5%) | 2 (9.1%) | 10 (13.5%) | ||
| Hepatic comorbidities | |||||
| Steatosis (%) | 15.0 (0.0–100.0%) | 6.0 (0.0–40.0%) | 17 (0.0–100.0%) | ||
| Steatohepatitis | 44 (45.8%) | 7 (31.8%) | 37 (50.0%) | 3 (3.1%) | |
| Fibrosis | 40 (41.7%) | 7 (31.8%) | 33 (44.6%) | 3 (3.1%) | |
| CASH | 12 (12.5%) | 4 (18.2%) | 8 (10.8%) | 2 (2.1%) | |
| Treatment | |||||
| Intraoperative RBCs transfusion | 16 (16.7%) | 7 (31.8%) | 9 (11.8%) | ||
| Neoadjuvant CTx | 38 (39.6%) | 8 (36.4%) | 30 (40.5%) | 4 (4.2%) | |
| Preoperative parameters | |||||
| PDR (%) | 20.99 (9.7–40.0) | 20.0 (9.7–40.0) | 21.3 (12.5–31-4) | 27 (28.1%) | |
| Platelets (×103/µL) | 238.0 (92.0–465.0) | 238.4 (129.0–345.0) | 237.8 (92.0–465.0) | 17 (17.7%) | |
| SB (mg/dL) | 0.5 (0.1–6.6) | 0.7 (0.2–6.6) | 0.5 (0.1–2.3) | 16 (16.7%) | |
| PT (%) | 103.0 (64.0–150.0) | 98.0 (45.0–150.0) | 102.0 (45.0–150.0) | 17 (17.7%) | |
| AP (U/L) | 84.0 (30.0–707.0) | 81.0 (51.0–707.0) | 87.0 (30.0–254.0) | 16 (16.7%) | |
| GGT (U/L) | 52.0 (7.0–1576.0) | 53.0 (18.0–1576.0) | 49.0 (7.0–710.0) | 16 (16.7%) | |
| AST (U/L) | 30.5 (17.0–144.0) | 34.0 (14.0–224.0) | 32.0 (14.0–224.0) | 33 (34.4%) | |
| ALT (U/L) | 30.0 (7.0–143.0) | 33.5 (12.0–129.0) | 29.0 (7.0–143.0) | 16 (16.7%) | |
| Albumin (g/L) | 43.3 (33.0–243.0) | 41.3 (33.0–47.3) | 44.4 (33.7–243.0) | 37 (38.5%) | |
| Morbidity | |||||
| No morbidity | 50 (52.1%) | 5 (22.7%) | 45 (59.2%) | ||
| Grade I | 8 (8.3%) | 2 (9.1%) | 6 (7.9%) | ||
| Grade II | 21 (21.9%) | 6 (27.3%) | 15 (19.7%) | ||
| Grade III | 10 (10.4%) | 4 (18.2%) | 6 (7.9%) | ||
| Grade IV | 3 (3.1%) | 2 (9.1%) | 1 (1.3%) | ||
| Grade V | 4 (4.2%) | 3 (13.6%) | 1 (1.3%) | ||
| Postoperative stay | |||||
| ICU (days) | 2.0 (0.0–18.0) | 3.0 (0.0–15.0) | 1.0 (0.0–18.0) | ||
| Total hospitalization (days) | 14.0 (3.0–117.0) | 22.0 (5.0–117.0) | 11.0 (3.0–44.0) | ||
| PHLF ISGLS | |||||
| no PHLF | 76 (77.1%) | 76 (100.0%) | |||
| Grade A | 7 (7.3%) | 7 (31.8%) | |||
| Grade B | 6 (6.3%) | 6 (27.3%) | |||
| Grade C | 9 (9.4%) | 9 (40.1%) |
| Parameter | Subcohort (n = 24) | PHLF Cohort (n = 10) | no PHLF Cohort (n = 14) | Missing Values | |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 9 (37.5%) | 4 (40.0%) | 5 (35.7%) | ||
| Female | 15 (62.5%) | 6 (60.0%) | 9 (64.3%) | ||
| Age (years) | 59.1 (35.0–81.3) | 60.2 (35.0–76.9) | 58.3 (38.4–81.3) | ||
| Hepatic resection | |||||
| Minor (<3 segments) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| Major (≥3 segments) | 24 (100.0%) | 10 (100.0%) | 14 (100.0%) | ||
| Tumor type | |||||
| CRLM | 4 (16.7%) | 0 (0.0%) | 4 (28.6%) | ||
| HCC | 2 (8.3%) | 1 (10.0%) | 1 (7.1%) | ||
| CCA | 13 (54.2%) | 8 (80.0%) | 5 (35.7%) | ||
| Pancreas related | 2 (8.3%) | 1 (10.0%) | 1 (7.1%) | ||
| Others | 3 (12.5%) | 0 (0.0%) | 3 (21.4%) | ||
| Hepatic comorbidities | |||||
| Steatosis (%) | 5.0 (0.0–25.0%) | 2.0 (0.0–10.0%) | 8.0 (0.0–25.0) | ||
| Steatohepatitis | 7 (30.4%) | 2 (20.0%) | 5 (38.5%) | 1 (4.2%) | |
| Fibrosis | 10 (43.5%) | 5 (50.0%) | 5 (38.5%) | 1 (4.2%) | |
| CASH | 4 (17.4%) | 1 (10.0%) | 3 (23.1%) | 1 (4.2%) | |
| Treatment | |||||
| Neoadjuvant CTx | 6 (35.3%) | 3 (42.7%) | 3 (30.0%) | 7 (29.2%) | |
| Intraoperative RBCs | 3 (12.5%) | 2 (20.0%) | 1 (7.1%) | ||
| Preoperative parameters | |||||
| PDR (%) | 25.0 (15.0–32.0) | 26.0 (22.0–32.0) | 24.5 (15.0–31.0 | 7 (29.2%) | |
| Platelets (×103/µL) | 283.0 (144.0–1082.0) | 242.2 (172.0–333.0) | 324.0 (144.0–1082.0) | 4 (16.7%) | |
| SB (mg/dL) | 1.3 (0.2–6.1) | 2.0 (0.3–6.1) | 0.6 (0.2–2.5) | 4 (16.7%) | |
| PT (%) | 104.0 (73.0–126.2) | 95.3 (73.0–124.0) | 113.0 (97.0–126.2) | 6 | |
| AP (U/L) | 238.0 (42.0–1432.0) | 468.3 (65.0–1432.0) | 98.0 (42.0- 158.0) | 4 (16.7%) | |
| GGT (U/L) | 228.0 (7.0–2576.0) | 335.4 (27.0–2576.0) | 121.0 (7.0–382.0) | 8 (33.3%) | |
| AST (U/L) | 40.0 (14.0–64.0) | 39.0 (14.0–65.0) | 40.0 (19.0–64.0) | 8 (33.3%) | |
| ALT (U/L) | 49.0 (10.0–257.0) | 61.0 (12.0–257.0) | 39.0 (10.0–127.0) | 6 (25.0%) | |
| Albumin (g/L) | 43.1 (32.1–74.2) | 37.9 (34.4–44.5) | 46.6 (32.1–74.2) | 7 (29.2%) | |
| Morbidity | |||||
| No morbidity | 8 (33.3%) | 1 (10.0%) | 7 (50.0%) | ||
| Grade I | 4 (16.7%) | 2 (20.0%) | 2 (14.3%) | ||
| Grade II | 5 (20.8%) | 2 (20.0%) | 3 (21.4%) | ||
| Grade III | 3 (12.5%) | 1 (10.0%) | 2 (14.3%) | ||
| Grade IV | 2 (8.3%) | 2 (20.0%) | 0 (0.0%) | ||
| Grade V | 2 (8.3%) | 2 (20.0%) | 0 (0.0%) | ||
| Postoperative stay | |||||
| ICU (days) | 3.0 (0.0–15.0) | 4.0 (0.0–15.0) | 2.0 (0.0–9.0) | ||
| Total hospitalization (days) | 11.0 (3.0–24.0) | 12.0 (3.0–23.0) | 11.0 (4.0–24.0) | ||
| PHLF ISGLS | |||||
| no PHLF | 14 (58.3%) | 14 (100%) | |||
| Grade A | 3 (12.5%) | 3 (30.0%) | |||
| Grade B | 4 (16.7%) | 4 (40.0%) | |||
| Grade C | 3 (12.5%) | 3 (30.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunnthaler, L.; Hammond, T.G.; Pereyra, D.; Santol, J.; Probst, J.; Laferl, V.; Resch, U.; Aiad, M.; Janoschek, A.S.; Gruenberger, T.; et al. HMGB1-Mediated Cell Death—A Crucial Element in Post-Hepatectomy Liver Failure. Int. J. Mol. Sci. 2024, 25, 7150. https://doi.org/10.3390/ijms25137150
Brunnthaler L, Hammond TG, Pereyra D, Santol J, Probst J, Laferl V, Resch U, Aiad M, Janoschek AS, Gruenberger T, et al. HMGB1-Mediated Cell Death—A Crucial Element in Post-Hepatectomy Liver Failure. International Journal of Molecular Sciences. 2024; 25(13):7150. https://doi.org/10.3390/ijms25137150
Chicago/Turabian StyleBrunnthaler, Laura, Thomas G. Hammond, David Pereyra, Jonas Santol, Joel Probst, Valerie Laferl, Ulrike Resch, Monika Aiad, Anna Sofie Janoschek, Thomas Gruenberger, and et al. 2024. "HMGB1-Mediated Cell Death—A Crucial Element in Post-Hepatectomy Liver Failure" International Journal of Molecular Sciences 25, no. 13: 7150. https://doi.org/10.3390/ijms25137150
APA StyleBrunnthaler, L., Hammond, T. G., Pereyra, D., Santol, J., Probst, J., Laferl, V., Resch, U., Aiad, M., Janoschek, A. S., Gruenberger, T., Hackl, H., Starlinger, P., & Assinger, A. (2024). HMGB1-Mediated Cell Death—A Crucial Element in Post-Hepatectomy Liver Failure. International Journal of Molecular Sciences, 25(13), 7150. https://doi.org/10.3390/ijms25137150










