Effects of Ferroptosis on Male Reproduction
Abstract
:1. Introduction
2. Ferroptosis and Its Associated Mechanisms
2.1. Overview of Ferroptosis
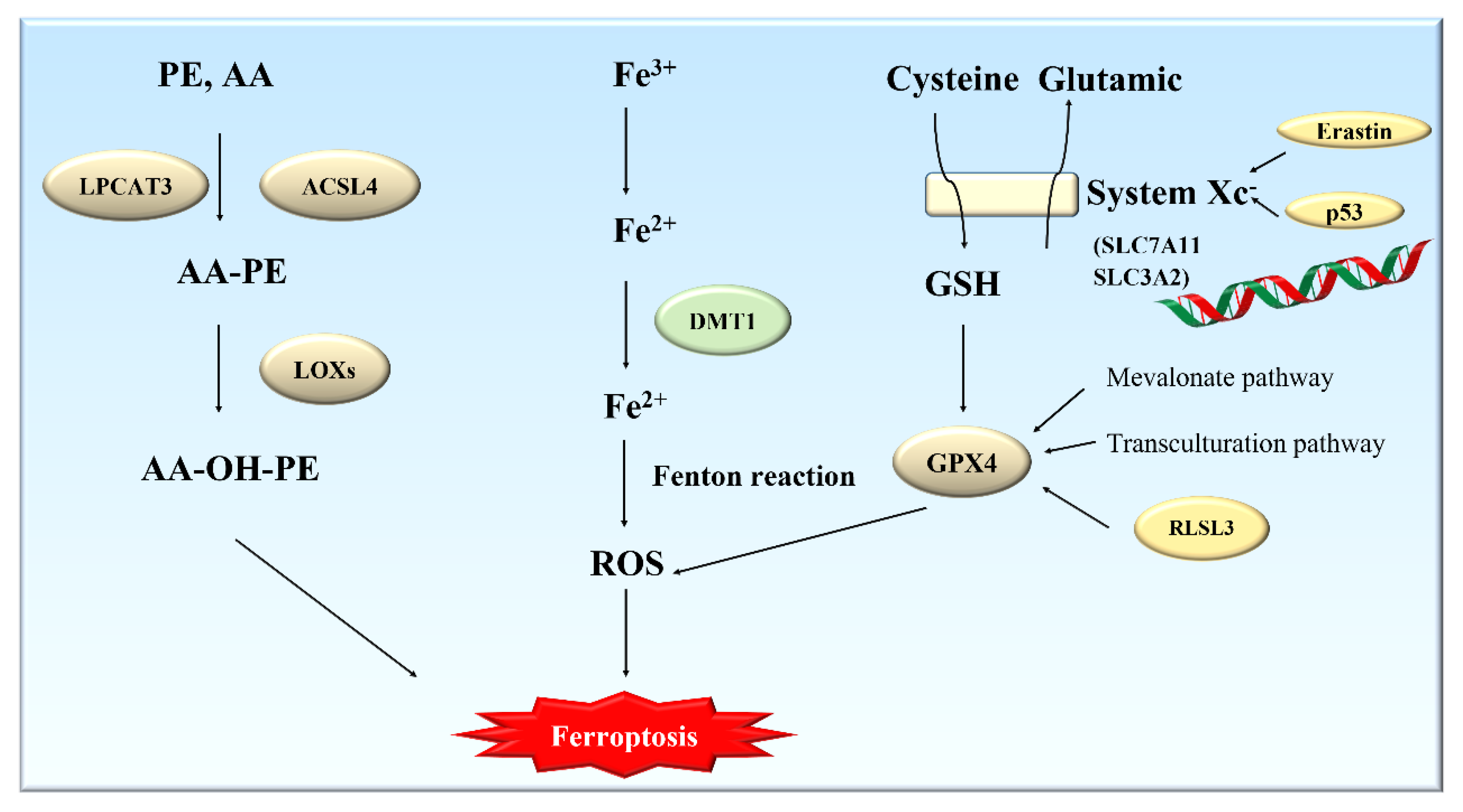
2.2. Mechanisms of Ferroptosis
2.2.1. Metabolism of Glutathione and Ferroptosis
2.2.2. Metabolism of Polyunsaturated Fatty Acids (PUFAs) and Ferroptosis
2.2.3. Metabolism of Iron and Ferroptosis
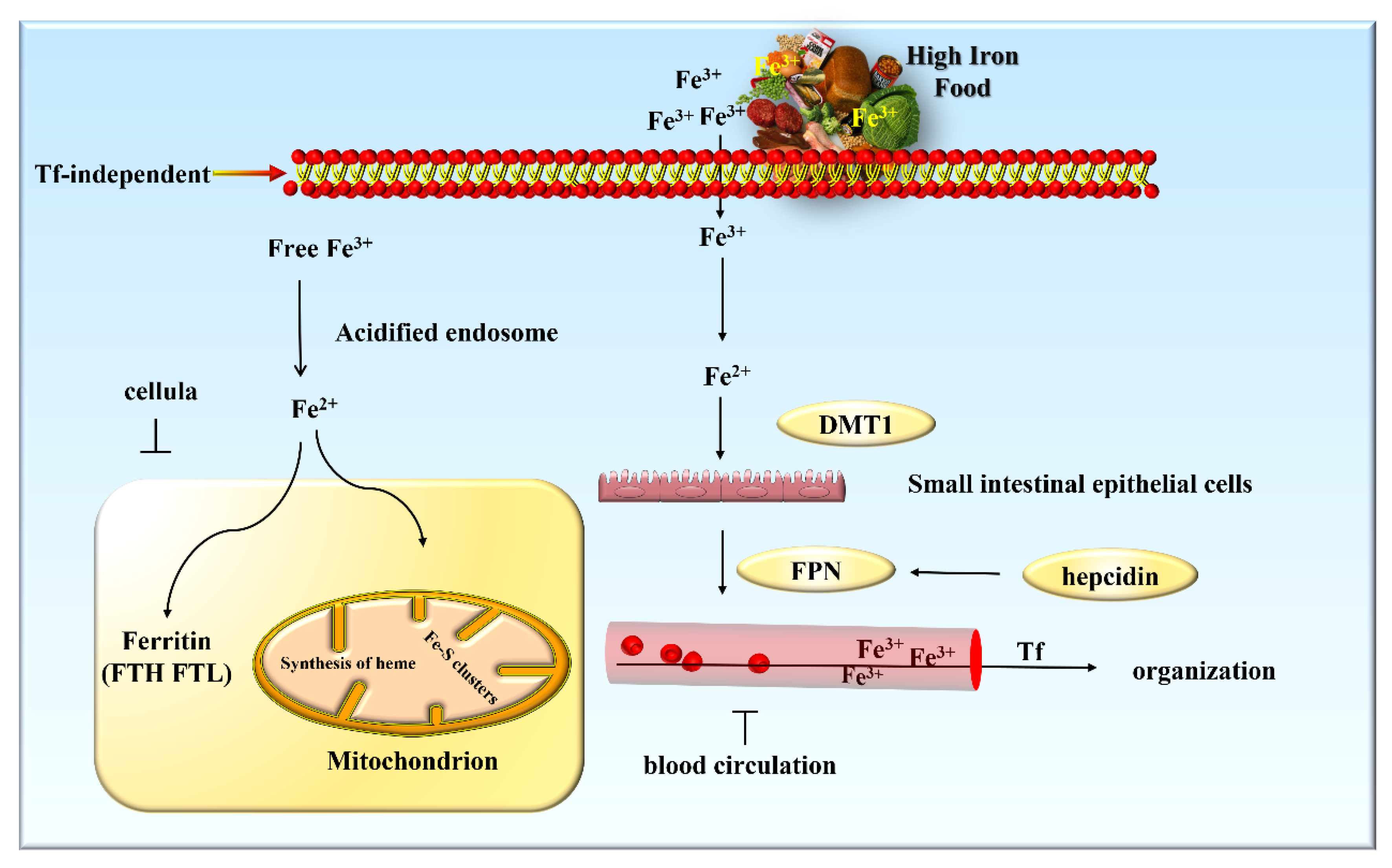
3. Iron Metabolism and Its Regulation
3.1. Iron Metabolism in the Body
3.2. Regulation of Iron Metabolism
3.3. Iron Plays an Important Role in Spermatogenesis
4. Relationship between Ferroptosis and Male Reproductive Disorders
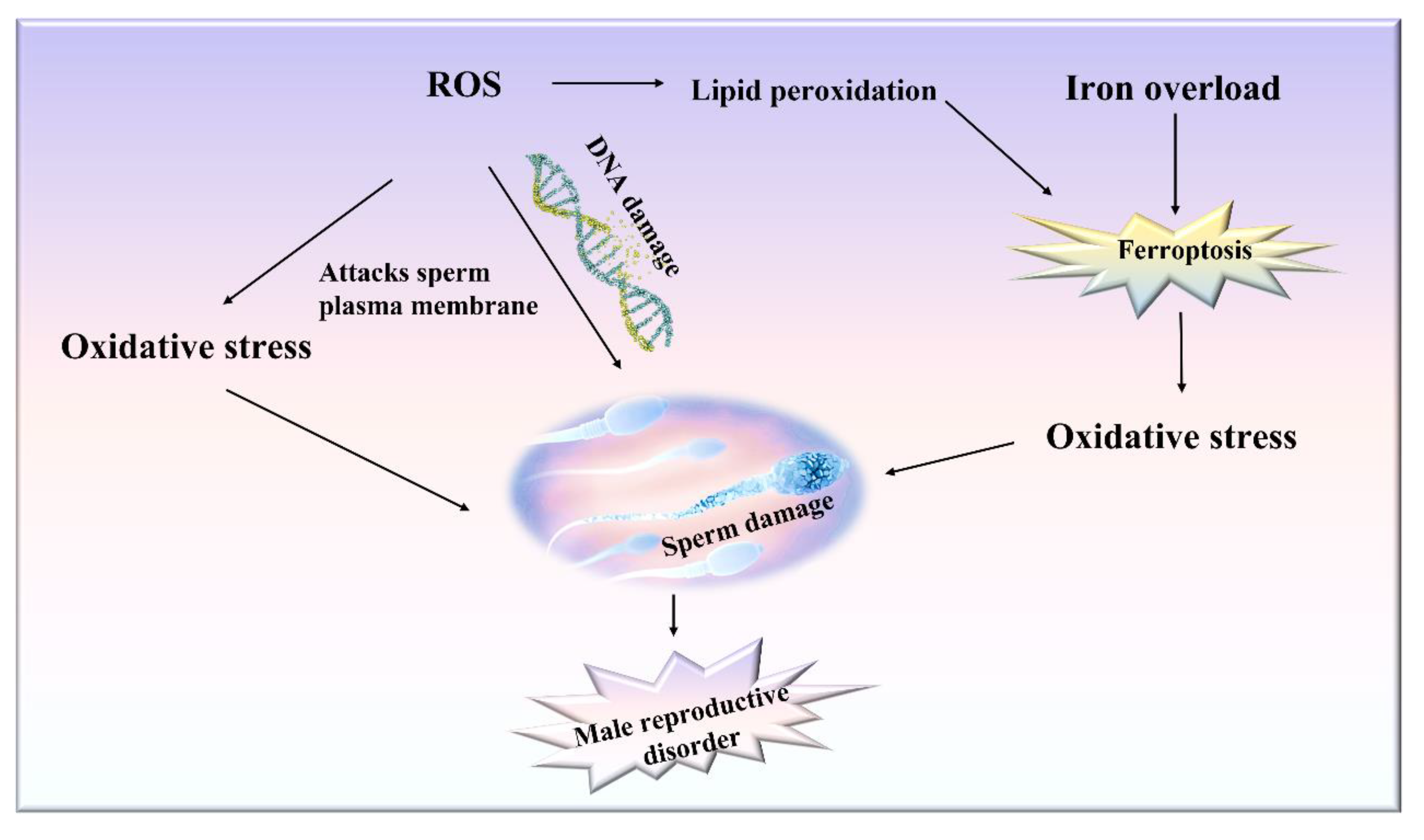
4.1. Effects of Ferroptosis on Cells in Testis
4.2. Testicular Lipid Peroxidation and ROS Accumulation Cause Male Reproductive Dysfunction
4.3. Reduced Testosterone Levels Caused by Iron Overload in the Testis
4.4. Enzymes and Genes Associated with Ferroptosis Play a Role in Spermatogenesis
4.5. Male Reproductive Diseases Should Be Treated by Inhibiting Ferroptosis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| RSL3 | RAS-selective lethal 3 |
| TF | Transferrin |
| GSH | Glutathione |
| GPX4 | Glutathione peroxidase 4 |
| Cys | Cysteine |
| PUFAs | Polyunsaturated fatty acids |
| IRE | Iron-responsive element |
| IRP1 and IRP2 | Iron-regulatory protein 1 and 2 |
| 5′-UTR | 5′ untranslated region |
| NCOA4 | Nuclear receptor coactivator 4 |
| SFT | Seminiferous tubule |
| SCs | Sertoli cells |
| BTB | Blood/testis barrier |
| Cp | Ceruloplasmin |
| Ferroportin | FPN |
| HIF2-α | Hypoxia-inducible factor 2-α |
| FTH | Ferritin heavy chain |
| FTL | Light chain |
| IRE | Iron-responsive element |
| MtF | Mitochondrial ferritin |
| ALOX15 | Arachidonate 15-lipoxygenase |
| β-TM | Beta-thalassemia major |
| NPs | Nanoparticles |
| IA | Idiopathic azoospermia |
| I/R | Ischemia/reperfusion |
| mGPx4 | Mitochondrial GPX4 |
| Nrf2 | Nuclear factor E2-related factor 2 |
| ARE | Antioxidant response elements |
| NAC | N-acetylcysteine |
| PD | Polydatin |
| LOXs | Lipoxygenases |
| DMT1 | Divalent metal transporter 1 |
References
- Camaschella, C. Iron deficiency: New insights into diagnosis and treatment. Hematol. Am. Soc. Hematol. Educ. Program 2015, 1, 8–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maneesh, M.; Jayalekshmi, H.J. Role of reactive oxygen species and antioxidants on pathophysiology of male reproduction. Indian J. Clin. Biochem. 2006, 21, 80–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merker, H.J.; Gunther, T.; Hollriegl, V.; Vormann, J.; Schumann, K. Lipid peroxidation and morphology of rat testis in magnesium deficiency. Andrologia 1996, 28, 43–51. [Google Scholar] [CrossRef]
- Merker, H.J.; Gunther, T. Testis damage induced by zinc deficiency in rats. J. Trace Elem. Med. Biol. 1997, 11, 19–22. [Google Scholar] [CrossRef]
- Merker, H.J.; Vormann, J.; Gunther, T. Iron-induced injury of rat testis. Andrologia 1996, 28, 267–273. [Google Scholar] [CrossRef]
- Dolma, S.; Lessnick, S.L.; Hahn, W.C.; Stockwell, B.R. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 2003, 3, 285–296. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.S.; Stockwell, B.R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 2008, 15, 234–245. [Google Scholar] [CrossRef] [Green Version]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [Green Version]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.Y.; Song, J.B.; Yung, B.C.; Zhou, Z.J.; Wu, A.G.; Chen, X.Y. Emerging Strategies of Cancer Therapy Based on Ferroptosis. Adv. Mater. 2018, 30, 15. [Google Scholar] [CrossRef] [PubMed]
- Magtanong, L.; Dixon, S.J. Ferroptosis and Brain Injury. Dev. Neurosci. 2019, 40, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Suhara, T.; Baba, Y.; Kawasaki, N.K.; Higa, J.K.; Matsui, T. Pathological Roles of Iron in Cardiovascular Disease. Curr. Drug Targets 2018, 19, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Distefano, A.M.; Martin, M.V.; Cordoba, J.P.; Bellido, A.M.; D’Ippolito, S.; Colman, S.L.; Soto, D.; Roldan, J.A.; Bartoli, C.G.; Zabaleta, E.J.; et al. Heat stress induces ferroptosis-like cell death in plants. J. Cell Biol. 2017, 216, 463–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, T.; Ding, W.; Ji, X.; Ao, X.; Liu, Y.; Yu, W.; Wang, J. Molecular mechanisms of ferroptosis and its role in cancer therapy. J. Cell. Mol. Med. 2019, 23, 4900–4912. [Google Scholar] [CrossRef] [Green Version]
- Brigelius-Flohe, R.; Flohe, L. Regulatory Phenomena in the Glutathione Peroxidase Superfamily. Antioxid. Redox Signal. 2020, 33, 498–516. [Google Scholar] [CrossRef]
- Cao, J.Y.; Dixon, S.J. Mechanisms of ferroptosis. Cell. Mol. Life Sci. 2016, 73, 2195–2209. [Google Scholar] [CrossRef] [Green Version]
- Sato, H.; Tamba, M.; Ishii, T.; Bannai, S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J. Biol. Chem. 1999, 274, 11455–11458. [Google Scholar] [CrossRef] [Green Version]
- Imai, H.; Matsuoka, M.; Kumagai, T.; Sakamoto, T.; Koumura, T. Lipid Peroxidation-Dependent Cell Death Regulated by GPx4 and Ferroptosis. In Apoptotic and Non-Apoptotic Cell Death; Nagata, S., Nakano, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; Volume 403, pp. 143–170. [Google Scholar]
- Jiang, L.; Kon, N.; Li, T.Y.; Wang, S.J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015, 520, 57. [Google Scholar] [CrossRef] [Green Version]
- Stockwell, B.R. Ferroptosis: Death by lipid peroxidation. Free Radic. Biol. Med. 2018, 120, S7. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.T.; Guo, P.Y.; Xie, X.Z.; Wang, Y.; Chen, G. Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J. Cell. Mol. Med. 2017, 21, 648–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, S.Y.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Stockwell, B.R. Peroxidation Dixon, S.J.; Winter, G.E.; Musavi, L.S.; Lee, E.; Snijder, B.; et al. Human Haploid Cell Genetics Reveals Roles for Lipid Metabolism Genes in Nonapoptotic Cell Death. ACS Chem. Biol. 2015, 10, 1604–1609. [Google Scholar]
- Yang, W.S.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Shchepinov, M.S.; Stockwell, B.R. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, E4966. [Google Scholar]
- Noguchi, S.N.J.B.; Communications, B.R. Lipid peroxidation: Mechanisms, inhibition, and biological effects. Biochem. Biophys. Res. Commun. 2005, 338, 668–676. [Google Scholar]
- Lei, P.; Bai, T.; Sun, Y. Mechanisms of Ferroptosis and Relations With Regulated Cell Death: A Review. Front. Physiol. 2019, 10, 139. [Google Scholar] [CrossRef] [Green Version]
- Bogdan, A.R.; Miyazawa, M.; Hashimoto, K.; Tsuji, Y. Regulators of Iron Homeostasis: New Players in Metabolism, Cell Death, and Disease. Trends Biochem. Sci. 2016, 41, 274–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elstrott, B.; Khan, L.; Olson, S.; Raghunathan, V.; DeLoughery, T.; Shatzel, J.J. The role of iron repletion in adult iron deficiency anemia and other diseases. Eur. J. Haematol. 2020, 104, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to tango: Regulation of Mammalian iron metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef] [Green Version]
- Bonaccorsi di Patti, M.C.; Cutone, A.; Polticelli, F.; Rosa, L.; Lepanto, M.S.; Valenti, P.; Musci, G. The ferroportin-ceruloplasmin system and the mammalian iron homeostasis machine: Regulatory pathways and the role of lactoferrin. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2018, 31, 399–414. [Google Scholar] [CrossRef]
- Philpott, C.C.; Jadhav, S. The ins and outs of iron: Escorting iron through the mammalian cytosol. Free Radic. Biol. Med. 2019, 133, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.J.; Frazer, D.M. Current understanding of iron homeostasis. Am. J. Clin. Nutr. 2017, 106 (Suppl. S6), 1559s–1566s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arosio, P.; Elia, L.; Poli, M. Ferritin, cellular iron storage and regulation. IUBMB Life 2017, 69, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Hayano, M.; Yang, W.S.; Corn, C.K.; Pagano, N.C.; Stockwell, B.R. Loss of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death Differ. 2016, 23, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casey, J.L.; Hentze, M.W.; Koeller, D.M.; Caughman, S.W.; Rouault, T.A.; Klausner, R.D.; Harford, J.B. Iron-responsive elements: Regulatory RNA sequences that control mRNA levels and translation. Science 1988, 240, 924–928. [Google Scholar] [CrossRef]
- Iwai, K. Regulation of cellular iron metabolism: Iron-dependent degradation of IRP by SCF(FBXL5) ubiquitin ligase. Free Radic. Biol. Med. 2019, 133, 64–68. [Google Scholar] [CrossRef]
- Munro, H.N. Iron regulation of ferritin gene expression. J. Cell Biochem. 1990, 44, 107–115. [Google Scholar] [CrossRef]
- Hirota, K. An intimate crosstalk between iron homeostasis and oxygen metabolism regulated by the hypoxia-inducible factors (HIFs). Free Radic. Biol. Med. 2019, 133, 118–129. [Google Scholar] [CrossRef]
- Dowdle, W.E.; Nyfeler, B.; Nagel, J.; Elling, R.A.; Liu, S.; Triantafellow, E.; Menon, S.; Wang, Z.; Honda, A.; Pardee, G.; et al. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat. Cell Biol. 2014, 16, 1069–1079. [Google Scholar] [CrossRef]
- Tvrda, E.; Peer, R.; Sikka, S.C.; Agarwal, A. Iron and copper in male reproduction: A double-edged sword. J. Assist. Reprod. Genet. 2015, 32, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Wise, T.; Lunstra, D.D.; Rohrer, G.A.; Ford, J.J. Relationships of testicular iron and ferritin concentrations with testicular weight and sperm production in boars. J. Anim. Sci. 2003, 81, 503–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griswold, M.D. Protein secretions of Sertoli cells. Int. Rev. Cytol. 1988, 110, 133–156. [Google Scholar] [PubMed]
- Toebosch, A.; Kroos, M.J.; Grootegoed, J.A. Transport of transferrin-bound iron into rat Sertoli cells and spermatids. Int. J. Androl. 2010, 10, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Morales, C.R.J.B. Receptor-mediated endocytosis of transferrin by Sertoli cells of the rat. Biol. Reprod. 2019, 35, 393–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, C.; Sylvester, S.R.; Griswold, M.D. Transport of iron and transferrin synthesis by the seminiferous epithelium of the rat in vivo. Biol. Reprod. 1987, 37, 995–1005. [Google Scholar] [CrossRef]
- Leichtmann-Bardoogo, Y.; Cohen, L.A.; Weiss, A.; Marohn, B.; Schubert, S.; Meinhardt, A.; Meyron-Holtz, E.G. Compartmentalization and regulation of iron metabolism proteins protect male germ cells from iron overload. Am. J. Physiology. Endocrinol. Metab. 2012, 302, E1519–E1530. [Google Scholar] [CrossRef] [Green Version]
- Carlsen, C.U.; Mller, J.K.S.; Skibsted, L.H. Heme-iron in lipid oxidation. Coord. Chem. Rev. 2005, 249, 485–498. [Google Scholar] [CrossRef]
- Nikolaev, A.A.; Lutskiĭ, D.; Nikolaeva, N.N.; Lozhkina, L.V.J.U.N. Iron and nonheme iron protein metabolism in ejaculates with varying degrees of fertility. Urol. Nefrol. 1998, 5, 27–31. [Google Scholar]
- Tvrda, E.; Knazicka, Z.; Lukacova, J.; Schneidgenova, M.; Goc, Z.J. Relationships between iron and copper content, motility characteristics and antioxidant status in bovine seminal plasma. J. Microbiol. Biotechnol. Food Sci. 2012, 2, 536–547. [Google Scholar]
- Levi, S.; Corsi, B.; Bosisio, M.; Invernizzi, R.; Volz, A.; Sanford, D.; Arosio, P.; Drysdale, J. A human mitochondrial ferritin encoded by an intronless gene. J. Biol. Chem. 2001, 276, 24437–24440. [Google Scholar] [CrossRef] [Green Version]
- Metzendorf, C.; Lind, M.I. Drosophila mitoferrin is essential for male fertility: Evidence for a role of mitochondrial iron metabolism during spermatogenesis. BMC Dev. Biol. 2010, 10, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levi, S.; Arosio, P. Mitochondrial ferritin. Int. J. Biochem. Cell Biol. 2004, 36, 1887–1889. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Clarkson, J.S.; Fishel, S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol. Reprod. 1989, 41, 183–197. [Google Scholar] [CrossRef]
- Kerr, J.R.; Barah, F.; Chiswick, M.L.; McDonnell, G.V.; Smith, J.; Chapman, M.D.; Bingham, J.B.; Kelleher, P.; Sheppard, M.N. Evidence for the role of demyelination, HLA-DR alleles, and cytokines in the pathogenesis of parvovirus B19 meningoencephalitis and its sequelae. J. Neurol. Neurosurg. Psychiatry 2002, 73, 739–746. [Google Scholar] [CrossRef] [Green Version]
- Kurniawan, A.L.; Lee, Y.C.; Shih, C.K.; Hsieh, R.H.; Chen, S.H.; Chang, J.S. Alteration in iron efflux affects male sex hormone testosterone biosynthesis in a diet-induced obese rat model. Food Funct. 2019, 10, 4113–4123. [Google Scholar] [CrossRef] [PubMed]
- Abarikwu, S.O.; Wokoma, A.F.S.; Mgbudom-Okah, C.J.; Omeodu, S.I.; Ohanador, R. Effect of Fe and Cd Co-Exposure on Testicular Steroid Metabolism, Morphometry, and Spermatogenesis in Mice. Biol. Trace Elem. Res. 2019, 190, 109–123. [Google Scholar] [CrossRef]
- Li, L.; Hao, Y.; Zhao, Y.; Wang, H.; Zhao, X.; Jiang, Y.; Gao, F. Ferroptosis is associated with oxygen-glucose deprivation/reoxygenation-induced Sertoli cell death. Int. J. Mol. Med. 2018, 41, 3051–3062. [Google Scholar] [CrossRef] [Green Version]
- Bromfield, E.G.; Walters, J.L.H.; Cafe, S.L.; Bernstein, I.R.; Stanger, S.J.; Anderson, A.L.; Aitken, R.J.; McLaughlin, E.A.; Dun, M.D.; Gadella, B.M.; et al. Differential cell death decisions in the testis: Evidence for an exclusive window of ferroptosis in round spermatids. Mol. Hum. Reprod. 2019, 25, 241–256. [Google Scholar] [CrossRef]
- Aitken, R.J.; Clarkson, J.S.; Hargreave, T.B.; Irvine, D.S.; Wu, F.C. Analysis of the relationship between defective sperm function and the generation of reactive oxygen species in cases of oligozoospermia. Int. J. Androl. 1989, 10, 214–220. [Google Scholar] [CrossRef]
- Sharma, R.K.; Agarwal, A. Role of reactive oxygen species in male infertility. Urology 1996, 48, 835–850. [Google Scholar] [CrossRef]
- Jones, R.; Mann, T.; Sherins, R. Peroxidative breakdown of phospholipids in human spermatozoa, spermicidal properties of fatty acid peroxides, and protective action of seminal plasma. Fertil. Steril. 1979, 31, 531–537. [Google Scholar] [CrossRef]
- Storey, B.T. Biochemistry of the induction and prevention of lipoperoxidative damage in human spermatozoa. Mol. Hum. Reprod. 1997, 3, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.G.; Storey, B.T. Differential incorporation of fatty acids into and peroxidative loss of fatty acids from phospholipids of human spermatozoa. Mol. Reprod. Dev. 1995, 42, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.M.; Lewis, S.E.; McKelvey-Martin, V.J.; Thompson, W. A comparison of baseline and induced DNA damage in human spermatozoa from fertile and infertile men, using a modified comet assay. Mol. Hum. Reprod. 1996, 2, 613–619. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.G.; Jurisicova, A.; Casper, R.F. Detection of Deoxyribonucleic Acid Fragmentation in Human Sperm: Correlation with Fertilization in Vitro. Biol. Reprod. 1997, 56, 3. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Gordon, E.; Harkiss, D.; Twigg, J.P.; Milne, P.; Jennings, Z.; Irvine, D.S. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol. Reprod. 1998, 59, 1037–1046. [Google Scholar] [CrossRef]
- Chen, M.J.; Peng, S.S.; Lu, M.Y.; Yang, Y.L.; Jou, S.T.; Chang, H.H.; Chen, S.U.; Lin, D.T.; Lin, K.H. Effect of iron overload on impaired fertility in male patients with transfusion-dependent beta-thalassemia. Pediatr. Res. 2018, 83, 655–661. [Google Scholar] [CrossRef]
- De Sanctis, V.; Soliman, A.T.; Yassin, M.A.; Di Maio, S.; Daar, S.; Elsedfy, H.; Soliman, N.; Kattamis, C. Hypogonadism in male thalassemia major patients: Pathophysiology, diagnosis and treatment. Acta Biomater. 2018, 89, 6–15. [Google Scholar]
- Lucesoli, F.; Fraga, C.G. Oxidative damage to lipids and DNA concurrent with decrease of antioxidants in rat testes after acute iron intoxication. Arch. Biochem. Biophys. 1995, 316, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Sundarraj, K.; Manickam, V.; Raghunath, A.; Periyasamy, M.; Viswanathan, M.P.; Perumal, E. Repeated exposure to iron oxide nanoparticles causes testicular toxicity in mice. Environ. Toxicol. 2017, 32, 594–608. [Google Scholar] [CrossRef]
- Jing, T.; Wang, P.; Liu, Y.; Zhao, J.; Niu, X.; Wang, X. Pathological changes in Sertoli cells and dysregulation of divalent metal transporter 1 with iron responsive element in the testes of idiopathic azoospermia patients. Andrologia 2018, 50, 2. [Google Scholar] [CrossRef] [PubMed]
- Macchi, C.; Steffani, L.; Oleari, R.; Lettieri, A.; Valenti, L.; Dongiovanni, P.; Romero-Ruiz, A.; Tena-Sempere, M.; Cariboni, A.; Magni, P.; et al. Iron overload induces hypogonadism in male mice via extrahypothalamic mechanisms. Mol. Cell. Endocrinol. 2017, 454, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Holladay, S.D.; Wolf, D.C.; Ahmed, S.A.; Robertson, J.L. Reproductive and developmental toxicity of arsenic in rodents: A review. Int. J. Toxicol. 2006, 25, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Chung, J.Y.; Lee, S.G.; Kim, J.Y.; Park, J.E.; Kim, W.R.; Joo, B.S.; Han, S.H.; Yoo, K.S.; Yoo, Y.H.; et al. Arsenic trioxide-induced apoptosis in TM4 Sertoli cells: The potential involvement of p21 expression and p53 phosphorylation. Toxicology 2011, 285, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Meng, P.; Zhang, S.; Jiang, X.; Cheng, S.; Zhang, J.; Cao, X.; Qin, X.; Zou, Z.; Chen, C. Arsenite induces testicular oxidative stress in vivo and in vitro leading to ferroptosis. Ecotoxicol. Environ. Saf. 2020, 194, 110360. [Google Scholar] [CrossRef]
- Abarikwu, S.O.; Oruitemeka, S.; Uwadileke, I.A.; Omeodu, S.I.; Okoye, N.F.; Mgbudom-Okah, C.J.; Ohanador, R. Oral administration of cadmium depletes intratesticular and epididymal iron levels and inhibits lipid peroxidation in the testis and epididymis of adult rats. J. Trace Elem. Med. Biol. 2018, 48, 213–223. [Google Scholar] [CrossRef]
- Shi, F.; Zhang, Z.; Cui, H.; Wang, J.; Wang, Y.; Tang, Y.; Yang, W.; Zou, P.; Ling, X.; Han, F.; et al. Analysis by transcriptomics and metabolomics for the proliferation inhibition and dysfunction through redox imbalance-mediated DNA damage response and ferroptosis in male reproduction of mice and TM4 Sertoli cells exposed to PM(2.5). Ecotoxicol. Environ. Saf. 2022, 238, 113569. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Zhao, T.; Chen, J.; Kang, L.; Wei, Y.; Han, L.; Shen, L.; Long, C.; Wu, S.; et al. Di-(2-ethylhexyl) phthalate exposure leads to ferroptosis via the HIF-1α/HO-1 signaling pathway in mouse testes. J. Hazard. Mater. 2022, 426, 127807. [Google Scholar] [CrossRef]
- Kocpinar, E.F.; Gonul Baltaci, N.; Ceylan, H.; Kalin, S.N.; Erdogan, O.; Budak, H. Effect of a Prolonged Dietary Iron Intake on the Gene Expression and Activity of the Testicular Antioxidant Defense System in Rats. Biol. Trace Elem. Res. 2020, 195, 135–141. [Google Scholar] [CrossRef]
- Alvarez, J.G.; Storey, B.T. Role of glutathione peroxidase in protecting mammalian spermatozoa from loss of motility caused by spontaneous lipid peroxidation. Gamete Res. 1989, 23, 77–90. [Google Scholar] [CrossRef]
- Chen, K.; Mai, Z.; Zhou, Y.; Gao, X.; Yu, B. Low NRF2 mRNA expression in spermatozoa from men with low sperm motility. Tohoku J. Exp. Med. 2012, 228, 259–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Liu, Z.; Gao, J.; Li, H.; Wang, X.; Li, Y.; Sun, F. Inhibition of ferroptosis attenuates busulfan-induced oligospermia in mice. Toxicology 2020, 440, 152489. [Google Scholar]
- Omara, F.O.; Blakley, B.R. Vitamin E is protective against iron toxicity and iron-induced hepatic vitamin E depletion in mice. J. Nutr. 1993, 123, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.K.; Xu, J.; Mehra, M.R. Antioxidant N-acetyl cysteine reverses cigarette smoke-induced myocardial infarction by inhibiting inflammation and oxidative stress in a rat model. Lab. Investig. 2012, 92, 224–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ince, S.; Avdatek, F.; Demirel, H.H.; Arslan-Acaroz, D.; Goksel, E.; Kucukkurt, I. Ameliorative effect of polydatin on oxidative stress-mediated testicular damage by chronic arsenic exposure in rats. Andrologia 2016, 48, 518–524. [Google Scholar] [CrossRef]
- Seidel, U.; Huebbe, P.; Rimbach, G. Taurine: A Regulator of Cellular Redox Homeostasis and Skeletal Muscle Function. Mol. Nutr. Food Res. 2019, 63, e1800569. [Google Scholar] [CrossRef]
- Poggiali, E.; Cassinerio, E.; Zanaboni, L.; Cappellini, M.D. An update on iron chelation therapy. Blood Transfus. 2012, 10, 411–422. [Google Scholar]
- Zeng, L.; Zhou, J.; Wang, X.; Zhang, Y.; Wang, M.; Su, P. Cadmium attenuates testosterone synthesis by promoting ferroptosis and blocking autophagosome-lysosome fusion. Free. Radic. Biol. Med. 2021, 176, 176–188. [Google Scholar] [CrossRef]
- Ou, Z.; Wen, Q.; Deng, Y.; Yu, Y.; Chen, Z.; Sun, L. Cigarette smoking is associated with high level of ferroptosis in seminal plasma and affects semen quality. Reprod. Biol. Endocrinol. 2020, 18, 55. [Google Scholar] [CrossRef]
- Kalpravidh, R.W.; Siritanaratkul, N.; Insain, P.; Charoensakdi, R.; Panichkul, N.; Hatairaktham, S.; Srichairatanakool, S.; Phisalaphong, C.; Rachmilewitz, E.; Fucharoen, S. Improvement in oxidative stress and antioxidant parameters in beta-thalassemia/Hb E patients treated with curcuminoids. Clin. Biochem. 2010, 43, 424–429. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Cao, X.; He, C.; Guo, X.; Cai, H.; Aierken, A.; Hua, J.; Peng, S. Effects of Ferroptosis on Male Reproduction. Int. J. Mol. Sci. 2022, 23, 7139. https://doi.org/10.3390/ijms23137139
Liu Y, Cao X, He C, Guo X, Cai H, Aierken A, Hua J, Peng S. Effects of Ferroptosis on Male Reproduction. International Journal of Molecular Sciences. 2022; 23(13):7139. https://doi.org/10.3390/ijms23137139
Chicago/Turabian StyleLiu, Yang, Xuanhong Cao, Chen He, Xinrui Guo, Hui Cai, Aili Aierken, Jinlian Hua, and Sha Peng. 2022. "Effects of Ferroptosis on Male Reproduction" International Journal of Molecular Sciences 23, no. 13: 7139. https://doi.org/10.3390/ijms23137139
APA StyleLiu, Y., Cao, X., He, C., Guo, X., Cai, H., Aierken, A., Hua, J., & Peng, S. (2022). Effects of Ferroptosis on Male Reproduction. International Journal of Molecular Sciences, 23(13), 7139. https://doi.org/10.3390/ijms23137139






