Engineered Zinc Finger Protein Targeting 2LTR Inhibits HIV Integration in Hematopoietic Stem and Progenitor Cell-Derived Macrophages: In Vitro Study
Abstract
:1. Introduction
2. Results
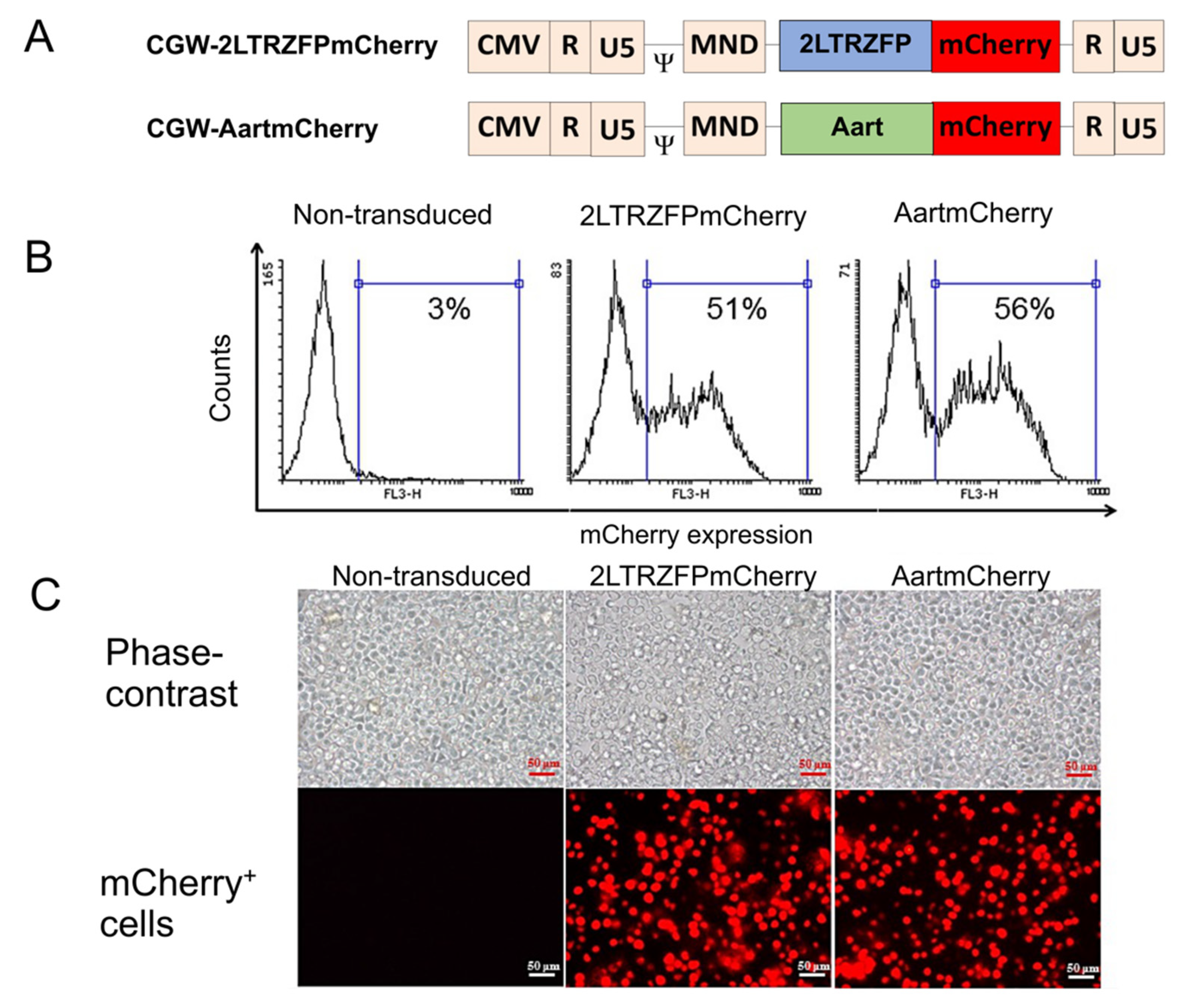
2.1. Generation of Human CD34+ HSPCs Expressing 2LTRZFPmCherry
2.2. The 2LTRZFPmCherry-Transduced Cells Maintained Good Viability and Showed No Difference in Levels of Pro-Apoptotic Proteins after the Lentiviral Transduction
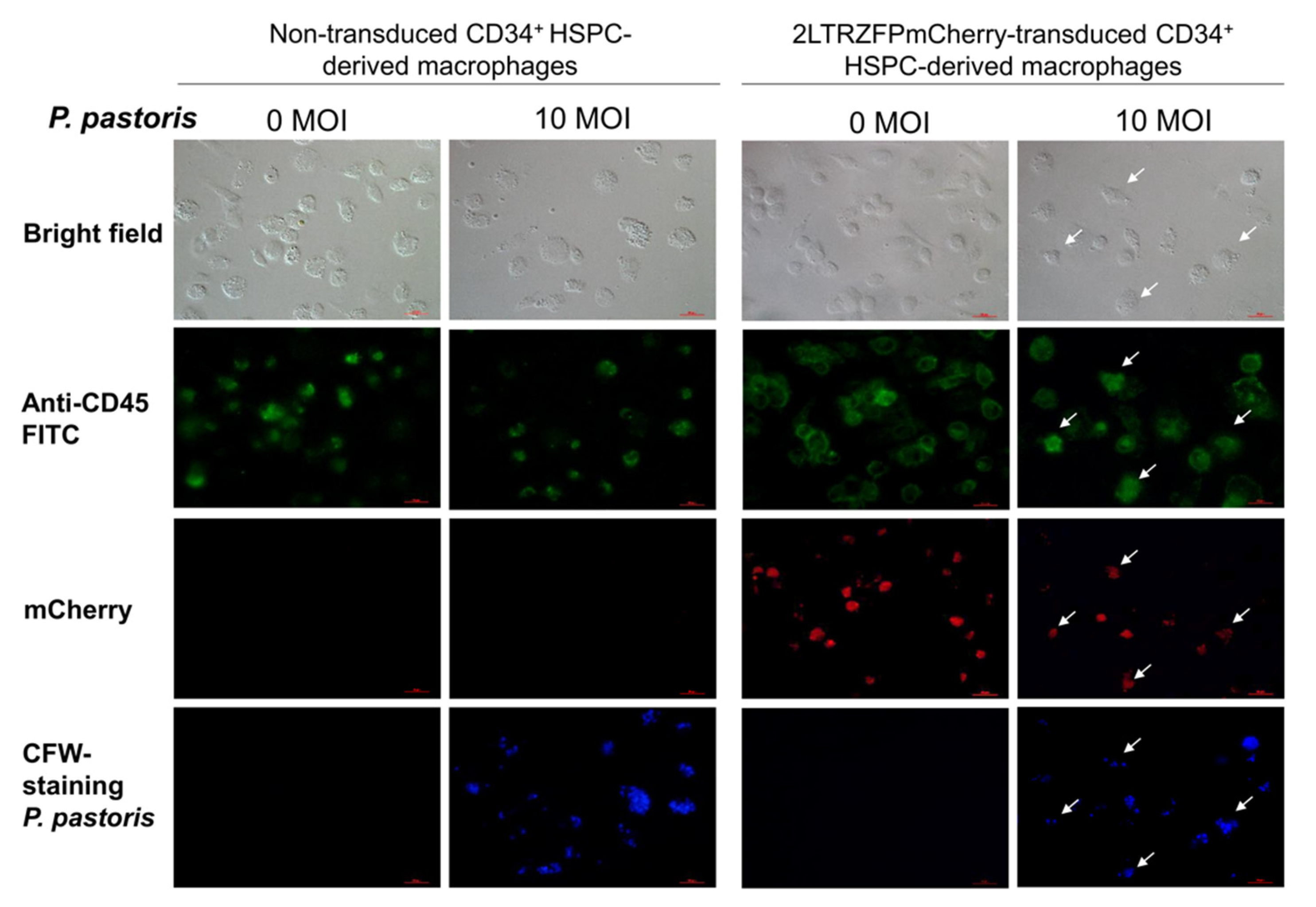
2.3. Functional Analyses of the CD34+ HSPC-Derived Macrophages Expressing 2LTRZFP
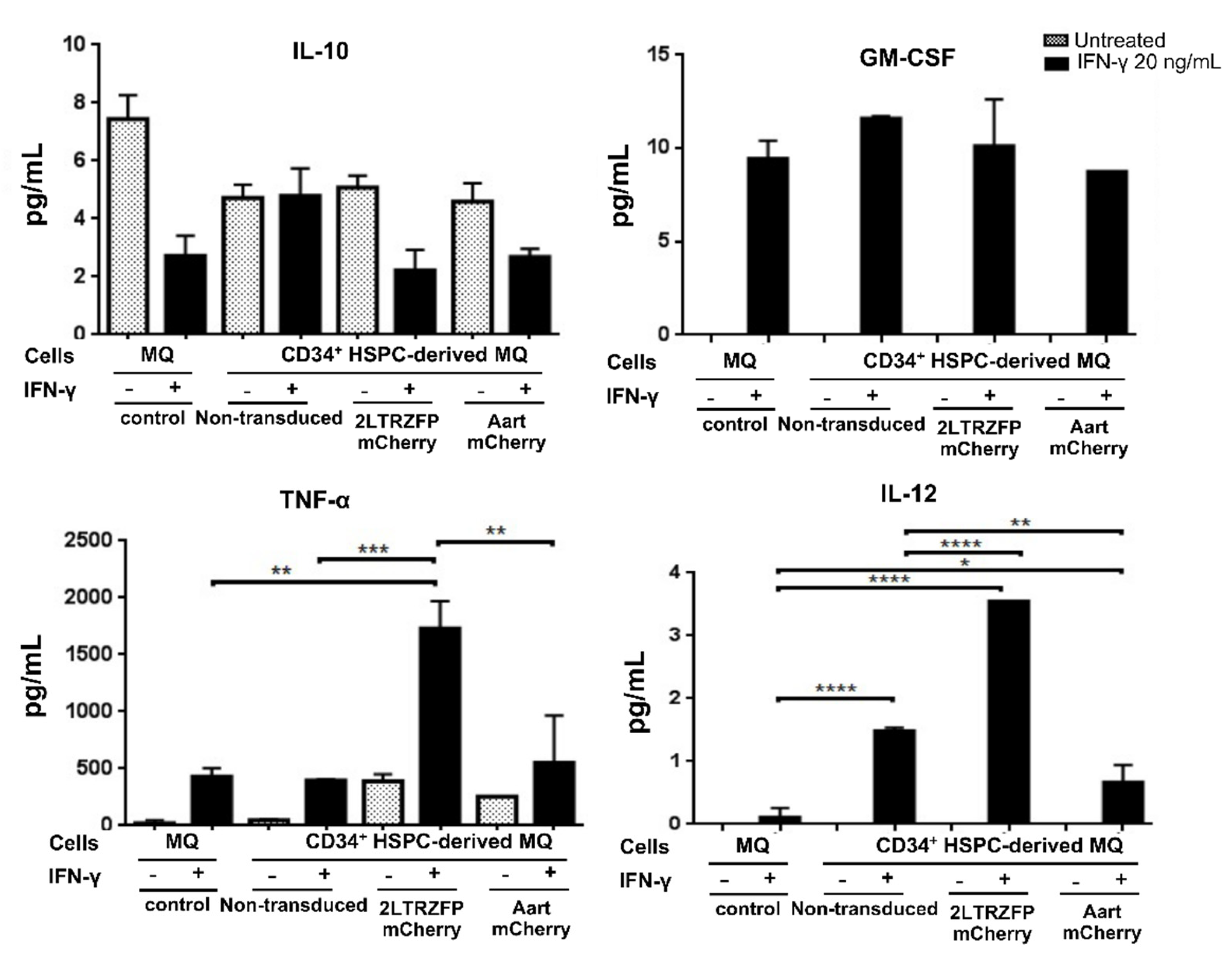
2.4. Cytokine Secretion Assay of the CD34+ HSPC-Derived Macrophages Expressing 2LTRZFP
2.5. 2LTRZFP Mediated Inhibition of HIV-1 Integration in CD34+ HSPCs and Their Mature Macrophages
3. Discussion
4. Materials and Methods
4.1. Ethical Approval and Consent to Participate
4.2. Isolation and Culture of Human CD34+ HSPCs
4.3. Generation of Human CD34+ HSPCs Stably Expressing 2LTRZFPmCherry or CGW-AartmCherry by Lentiviral Gene Transfer
4.4. Determination of Cell Viability by Trypan Blue Exclusion Assay
4.5. Apoptosis Assay
4.6. Differentiation of the 2LTRZFPmCherry-Transduced HSPCs into Mature Macrophages
4.7. Measurement of Phagocytosis of Human CD34+ HSPC-Derived Macrophages
4.8. Assessment of Cytokine Production in CD34+ HSPC-Derived Macrophages
4.9. VSV-G-Pseudotyped HIV-1NL4-3.Luc.R−.E− Infection
4.10. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arts, E.J.; Hazuda, D.J. HIV-1 antiretroviral drug therapy. Cold Spring Harb. Perspect. Med. 2012, 2, a007161. [Google Scholar] [CrossRef] [PubMed]
- Negredo, E.; Clotet, B. Efficacy of single-tablet darunavir, cobicistat, emtricitabine, and tenofovir alafenamide in the treatment of HIV-1. Expert Opin. Pharmacother. 2018, 19, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Chun, T.W.; Stuyver, L.; Mizell, S.B.; Ehler, L.A.; Mican, J.A.; Baseler, M.; Lloyd, A.L.; Nowak, M.A.; Fauci, A.S. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 1997, 94, 13193–13197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutter, G. Stem cell transplantation in strategies for curing HIV/AIDS. AIDS Res. Ther. 2016, 13, 31. [Google Scholar] [CrossRef] [Green Version]
- Chun, T.W.; Davey, R.T., Jr.; Engel, D.; Lane, H.C.; Fauci, A.S. Re-emergence of HIV after stopping therapy. Nature 1999, 401, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y. Recent update in HIV vaccine development. Clin. Exp. Vaccine Res. 2016, 5, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Caskey, M.; Klein, F.; Lorenzi, J.C.; Seaman, M.S.; West, A.P., Jr.; Buckley, N.; Kremer, G.; Nogueira, L.; Braunschweig, M.; Scheid, J.F.; et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 2015, 522, 487–491, Corrigendum in Nature 2016, 535, 580. [Google Scholar] [CrossRef] [Green Version]
- Sung, J.A.; Lam, S.; Garrido, C.; Archin, N.; Rooney, C.M.; Bollard, C.M.; Margolis, D.M. Expanded cytotoxic T-cell lymphocytes target the latent HIV reservoir. J. Infect. Dis. 2015, 212, 258–263. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Kitchen, S.G.; Chen, I.S.Y.; Ng, H.L.; Zack, J.A.; Yang, O.O. HIV-1-Specific Chimeric Antigen Receptors Based on Broadly Neutralizing Antibodies. J. Virol. 2016, 90, 6999–7006. [Google Scholar] [CrossRef] [Green Version]
- Kitchen, S.G.; Zack, J.A. Engineering HIV-Specific Immunity with Chimeric Antigen Receptors. AIDS Patient Care STDs 2016, 30, 556–561. [Google Scholar] [CrossRef] [Green Version]
- Brown, T.R. I am the Berlin patient: A personal reflection. AIDS Res. Hum. Retrovir. 2015, 31, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Hutter, G.; Nowak, D.; Mossner, M.; Ganepola, S.; Mussig, A.; Allers, K.; Schneider, T.; Hofmann, J.; Kucherer, C.; Blau, O.; et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N. Engl. J. Med. 2009, 360, 692–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allers, K.; Hutter, G.; Hofmann, J.; Loddenkemper, C.; Rieger, K.; Thiel, E.; Schneider, T. Evidence for the cure of HIV infection by CCR5Delta32/Delta32 stem cell transplantation. Blood 2011, 117, 2791–2799. [Google Scholar] [CrossRef] [PubMed]
- Symons, J.; Vandekerckhove, L.; Hutter, G.; Wensing, A.M.; van Ham, P.M.; Deeks, S.G.; Nijhuis, M. Dependence on the CCR5 coreceptor for viral replication explains the lack of rebound of CXCR4-predicted HIV variants in the Berlin patient. Clin. Infect. Dis. 2014, 59, 596–600. [Google Scholar] [CrossRef] [Green Version]
- Kiem, H.P.; Jerome, K.R.; Deeks, S.G.; McCune, J.M. Hematopoietic-stem-cell-based gene therapy for HIV disease. Cell Stem Cell 2012, 10, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Kitchen, S.G.; Shimizu, S.; An, D.S. Stem cell-based anti-HIV gene therapy. Virology 2011, 411, 260–272. [Google Scholar] [CrossRef] [Green Version]
- Li, M.J.; Kim, J.; Li, S.; Zaia, J.; Yee, J.K.; Anderson, J.; Akkina, R.; Rossi, J.J. Long-term inhibition of HIV-1 infection in primary hematopoietic cells by lentiviral vector delivery of a triple combination of anti-HIV shRNA, anti-CCR5 ribozyme, and a nucleolar-localizing TAR decoy. Mol. Ther. 2005, 12, 900–909. [Google Scholar] [CrossRef]
- Mitsuyasu, R.T.; Merigan, T.C.; Carr, A.; Zack, J.A.; Winters, M.A.; Workman, C.; Bloch, M.; Lalezari, J.; Becker, S.; Thornton, L.; et al. Phase 2 gene therapy trial of an anti-HIV ribozyme in autologous CD34+ cells. Nat. Med. 2009, 15, 285–292. [Google Scholar] [CrossRef] [Green Version]
- Wolstein, O.; Boyd, M.; Millington, M.; Impey, H.; Boyer, J.; Howe, A.; Delebecque, F.; Cornetta, K.; Rothe, M.; Baum, C.; et al. Preclinical safety and efficacy of an anti-HIV-1 lentiviral vector containing a short hairpin RNA to CCR5 and the C46 fusion inhibitor. Mol. Ther. Methods Clin. Dev. 2014, 1, 11. [Google Scholar] [CrossRef]
- Podsakoff, G.M.; Engel, B.C.; Carbonaro, D.A.; Choi, C.; Smogorzewska, E.M.; Bauer, G.; Selander, D.; Csik, S.; Wilson, K.; Betts, M.R.; et al. Selective survival of peripheral blood lymphocytes in children with HIV-1 following delivery of an anti-HIV gene to bone marrow CD34+ cells. Mol. Ther. 2005, 12, 77–86. [Google Scholar] [CrossRef]
- Holt, N.; Wang, J.; Kim, K.; Friedman, G.; Wang, X.; Taupin, V.; Crooks, G.M.; Kohn, D.B.; Gregory, P.D.; Holmes, M.C.; et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat. Biotechnol. 2010, 28, 839–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saydaminova, K.; Ye, X.; Wang, H.; Richter, M.; Ho, M.; Chen, H.; Xu, N.; Kim, J.S.; Papapetrou, E.; Holmes, M.C.; et al. Efficient genome editing in hematopoietic stem cells with helper-dependent Ad5/35 vectors expressing site-specific endonucleases under microRNA regulation. Mol. Ther. Methods Clin. Dev. 2015, 1, 14057. [Google Scholar] [CrossRef] [PubMed]
- Abram, M.E.; Ferris, A.L.; Shao, W.; Alvord, W.G.; Hughes, S.H. Nature, position, and frequency of mutations made in a single cycle of HIV-1 replication. J. Virol. 2010, 84, 9864–9878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuevas, J.M.; Geller, R.; Garijo, R.; Lopez-Aldeguer, J.; Sanjuan, R. Extremely High Mutation Rate of HIV-1 In Vivo. PLoS Biol. 2015, 13, e1002251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contento, R.L.; Molon, B.; Boularan, C.; Pozzan, T.; Manes, S.; Marullo, S.; Viola, A. CXCR4-CCR5: A couple modulating T cell functions. Proc. Natl. Acad. Sci. USA 2008, 105, 10101–10106. [Google Scholar] [CrossRef] [Green Version]
- Molon, B.; Gri, G.; Bettella, M.; Gomez-Mouton, C.; Lanzavecchia, A.; Martinez, A.C.; Manes, S.; Viola, A. T cell costimulation by chemokine receptors. Nat. Immunol. 2005, 6, 465–471. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, Q.; Gendron, P.; Zhu, W.; Guo, F.; Cen, S.; Wainberg, M.A.; Liang, C. CRISPR/Cas9-Derived Mutations Both Inhibit HIV-1 Replication and Accelerate Viral Escape. Cell Rep. 2016, 15, 481–489. [Google Scholar] [CrossRef] [Green Version]
- Sakkhachornphop, S.; Jiranusornkul, S.; Kodchakorn, K.; Nangola, S.; Sirisanthana, T.; Tayapiwatana, C. Designed zinc finger protein interacting with the HIV-1 integrase recognition sequence at 2-LTR-circle junctions. Protein Sci. 2009, 18, 2219–2230. [Google Scholar] [CrossRef] [Green Version]
- Sakkhachornphop, S.; Barbas, C.F., 3rd; Keawvichit, R.; Wongworapat, K.; Tayapiwatana, C. Zinc finger protein designed to target 2-long terminal repeat junctions interferes with human immunodeficiency virus integration. Hum. Gene Ther. 2012, 23, 932–942. [Google Scholar] [CrossRef]
- Khamaikawin, W.; Saoin, S.; Nangola, S.; Chupradit, K.; Sakkhachornphop, S.; Hadpech, S.; Onlamoon, N.; Ansari, A.A.; Byrareddy, S.N.; Boulanger, P.; et al. Combined Antiviral Therapy Using Designed Molecular Scaffolds Targeting Two Distinct Viral Functions, HIV-1 Genome Integration and Capsid Assembly. Mol. Ther. Nucleic Acids 2015, 4, e249. [Google Scholar] [CrossRef]
- Le Douce, V.; Herbein, G.; Rohr, O.; Schwartz, C. Molecular mechanisms of HIV-1 persistence in the monocyte-macrophage lineage. Retrovirology 2010, 7, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bracq, L.; Xie, M.; Lambele, M.; Vu, L.T.; Matz, J.; Schmitt, A.; Delon, J.; Zhou, P.; Randriamampita, C.; Bouchet, J.; et al. T cell-macrophage fusion triggers multinucleated giant cell formation for HIV-1 spreading. J. Virol. 2017, 91, e01237-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.Z.; Au, P.; Chen, T.; Shao, Y.; Daheron, L.M.; Bai, H.; Arzigian, M.; Fukumura, D.; Jain, R.K.; Scadden, D.T. Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat. Biotechnol. 2007, 25, 317–318. [Google Scholar] [CrossRef] [PubMed]
- Dreier, B.; Beerli, R.R.; Segal, D.J.; Flippin, J.D.; Barbas, C.F., 3rd. Development of zinc finger domains for recognition of the 5′-ANN-3′ family of DNA sequences and their use in the construction of artificial transcription factors. J. Biol. Chem. 2001, 276, 29466–29478. [Google Scholar] [CrossRef] [Green Version]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [Green Version]
- Aiken, C. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 1997, 71, 5871–5877. [Google Scholar] [CrossRef] [Green Version]
- Zhen, A.; Kitchen, S. Stem-cell-based gene therapy for HIV infection. Viruses 2013, 6, 1–12. [Google Scholar] [CrossRef]
- van Griensven, J.; De Clercq, E.; Debyser, Z. Hematopoietic stem cell-based gene therapy against HIV infection: Promises and caveats. AIDS Rev. 2005, 7, 44–55. [Google Scholar]
- Pernet, O.; Yadav, S.S.; An, D.S. Stem cell-based therapies for HIV/AIDS. Adv. Drug Deliv. Rev. 2016, 103, 187–201. [Google Scholar] [CrossRef] [Green Version]
- Johnston, R. Gene Therapy to Cure HIV: Where to from Here? AIDS Patient Care STDS 2016, 30, 531–533. [Google Scholar] [CrossRef]
- Werkmeister, J.A.; Ramshaw, J.A. Recombinant protein scaffolds for tissue engineering. Biomed. Mater. 2012, 7, 012002. [Google Scholar] [CrossRef] [PubMed]
- Biffi, A.; Bartolomae, C.C.; Cesana, D.; Cartier, N.; Aubourg, P.; Ranzani, M.; Cesani, M.; Benedicenti, F.; Plati, T.; Rubagotti, E.; et al. Lentiviral vector common integration sites in preclinical models and a clinical trial reflect a benign integration bias and not oncogenic selection. Blood 2011, 117, 5332–5339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kambal, A.; Mitchell, G.; Cary, W.; Gruenloh, W.; Jung, Y.; Kalomoiris, S.; Nacey, C.; McGee, J.; Lindsey, M.; Fury, B.; et al. Generation of HIV-1 resistant and functional macrophages from hematopoietic stem cell-derived induced pluripotent stem cells. Mol. Ther. 2011, 19, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Kuritzkes, D.R. Hematopoietic stem cell transplantation for HIV cure. J. Clin. Investig. 2016, 126, 432–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellano, P.; Prevedel, L.; Eugenin, E.A. HIV-infected macrophages and microglia that survive acute infection become viral reservoirs by a mechanism involving Bim. Sci. Rep. 2017, 7, 12866. [Google Scholar] [CrossRef] [Green Version]
- Kang, E.M.; De Witte, M.; Malech, H.; Morgan, R.A.; Carter, C.; Leitman, S.F.; Childs, R.; Barrett, A.J.; Little, R.; Tisdale, J.F. Gene therapy-based treatment for HIV-positive patients with malignancies. J. Hematother. Stem Cell Res. 2002, 11, 809–816. [Google Scholar] [CrossRef]
- Amado, R.G.; Mitsuyasu, R.T.; Rosenblatt, J.D.; Ngok, F.K.; Bakker, A.; Cole, S.; Chorn, N.; Lin, L.S.; Bristol, G.; Boyd, M.P.; et al. Anti-human immunodeficiency virus hematopoietic progenitor cell-delivered ribozyme in a phase I study: Myeloid and lymphoid reconstitution in human immunodeficiency virus type-1-infected patients. Hum. Gene Ther. 2004, 15, 251–262. [Google Scholar] [CrossRef] [Green Version]
- Kurian, K.M.; Watson, C.J.; Wyllie, A.H. Retroviral vectors. Mol. Pathol. 2000, 53, 173–176. [Google Scholar] [CrossRef] [Green Version]
- DiGiusto, D.L.; Krishnan, A.; Li, L.; Li, H.; Li, S.; Rao, A.; Mi, S.; Yam, P.; Stinson, S.; Kalos, M.; et al. RNA-based gene therapy for HIV with lentiviral vector-modified CD34+ cells in patients undergoing transplantation for AIDS-related lymphoma. Sci. Transl. Med. 2010, 2, 36ra43. [Google Scholar] [CrossRef] [Green Version]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef] [Green Version]
- Martinez, F.O.; Gordon, S.; Locati, M.; Mantovani, A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. J. Immunol. 2006, 177, 7303–7311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, P.; Budnick, I.; Singh, M.; Thiruppathi, M.; Alharshawi, K.; Elshabrawy, H.; Holterman, M.J.; Prabhakar, B.S. Dual Role of GM-CSF as a Pro-Inflammatory and a Regulatory Cytokine: Implications for Immune Therapy. J. Interferon Cytokine Res. 2015, 35, 585–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, J.P.; Zhang, X.; Frauwirth, K.A.; Mosser, D.M. Biochemical and functional characterization of three activated macrophage populations. J. Leukoc. Biol. 2006, 80, 1298–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moonmuang, S.; Saoin, S.; Chupradit, K.; Sakkhachornphop, S.; Israsena, N.; Rungsiwiwut, R.; Tayapiwatana, C. Modulated expression of the HIV-1 2LTR zinc finger efficiently interferes with the HIV integration process. Biosci. Rep. 2018, 38, BSR20181109. [Google Scholar] [CrossRef] [Green Version]
- Berges, B.K.; Rowan, M.R. The utility of the new generation of humanized mice to study HIV-1 infection: Transmission, prevention, pathogenesis, and treatment. Retrovirology 2011, 8, 65. [Google Scholar] [CrossRef] [Green Version]
- Barclay, S.L.; Yang, Y.; Zhang, S.; Fong, R.; Barraza, A.; Nolta, J.A.; Torbett, B.E.; Abedi, M.; Bauer, G.; Anderson, J.S. Safety and efficacy of a tCD25 preselective combination anti-HIV lentiviral vector in human hematopoietic stem and progenitor cells. Stem Cells 2015, 33, 870–879. [Google Scholar] [CrossRef]
- Heredia, A.; Le, N.; Gartenhaus, R.B.; Sausville, E.; Medina-Moreno, S.; Zapata, J.C.; Davis, C.; Gallo, R.C.; Redfield, R.R. Targeting of mTOR catalytic site inhibits multiple steps of the HIV-1 lifecycle and suppresses HIV-1 viremia in humanized mice. Proc. Natl. Acad. Sci. USA 2015, 112, 9412–9417. [Google Scholar] [CrossRef] [Green Version]
- Scott, C.T.; DeFrancesco, L. Gene therapy’s out-of-body experience. Nat. Biotechnol. 2016, 34, 600–607. [Google Scholar] [CrossRef]
- Peterson, C.W.; Haworth, K.G.; Burke, B.P.; Polacino, P.; Norman, K.K.; Adair, J.E.; Hu, S.L.; Bartlett, J.S.; Symonds, G.P.; Kiem, H.P. Multilineage polyclonal engraftment of Cal-1 gene-modified cells and in vivo selection after SHIV infection in a nonhuman primate model of AIDS. Mol. Ther. Methods Clin. Dev. 2016, 3, 16007. [Google Scholar] [CrossRef] [Green Version]
- Mitsuyasu, R.T.; Zack, J.A.; Macpherson, J.L.; Symonds, G.P. Phase I/II Clinical Trials Using Gene-Modified Adult Hematopoietic Stem Cells for HIV: Lessons Learnt. Stem Cells Int. 2011, 2011, 393698. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.X.; Cannon, P.M. The clinical applications of genome editing in HIV. Blood 2016, 127, 2546–2552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kronenwett, R.; Martin, S.; Haas, R. The role of cytokines and adhesion molecules for mobilization of peripheral blood stem cells. Stem Cells 2000, 18, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Tschan, M.P.; Fischer, K.M.; Fung, V.S.; Pirnia, F.; Borner, M.M.; Fey, M.F.; Tobler, A.; Torbett, B.E. Alternative splicing of the human cyclin D-binding Myb-like protein (hDMP1) yields a truncated protein isoform that alters macrophage differentiation patterns. J. Biol. Chem. 2003, 278, 42750–42760. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chupradit, K.; Khamaikawin, W.; Sakkhachornphop, S.; Puaninta, C.; Torbett, B.E.; Borwornpinyo, S.; Hongeng, S.; Wattanapanitch, M.; Tayapiwatana, C. Engineered Zinc Finger Protein Targeting 2LTR Inhibits HIV Integration in Hematopoietic Stem and Progenitor Cell-Derived Macrophages: In Vitro Study. Int. J. Mol. Sci. 2022, 23, 2331. https://doi.org/10.3390/ijms23042331
Chupradit K, Khamaikawin W, Sakkhachornphop S, Puaninta C, Torbett BE, Borwornpinyo S, Hongeng S, Wattanapanitch M, Tayapiwatana C. Engineered Zinc Finger Protein Targeting 2LTR Inhibits HIV Integration in Hematopoietic Stem and Progenitor Cell-Derived Macrophages: In Vitro Study. International Journal of Molecular Sciences. 2022; 23(4):2331. https://doi.org/10.3390/ijms23042331
Chicago/Turabian StyleChupradit, Koollawat, Wannisa Khamaikawin, Supachai Sakkhachornphop, Chaniporn Puaninta, Bruce E. Torbett, Suparerk Borwornpinyo, Suradej Hongeng, Methichit Wattanapanitch, and Chatchai Tayapiwatana. 2022. "Engineered Zinc Finger Protein Targeting 2LTR Inhibits HIV Integration in Hematopoietic Stem and Progenitor Cell-Derived Macrophages: In Vitro Study" International Journal of Molecular Sciences 23, no. 4: 2331. https://doi.org/10.3390/ijms23042331
APA StyleChupradit, K., Khamaikawin, W., Sakkhachornphop, S., Puaninta, C., Torbett, B. E., Borwornpinyo, S., Hongeng, S., Wattanapanitch, M., & Tayapiwatana, C. (2022). Engineered Zinc Finger Protein Targeting 2LTR Inhibits HIV Integration in Hematopoietic Stem and Progenitor Cell-Derived Macrophages: In Vitro Study. International Journal of Molecular Sciences, 23(4), 2331. https://doi.org/10.3390/ijms23042331





