Abstract
Rhizosphere filamentous fungi of the genus Trichoderma, a dominant component of various soil ecosystem mycobiomes, are characterized by the ability to colonize plant roots. Detailed knowledge of the properties of Trichoderma, including metabolic activity and the type of interaction with plants and other microorganisms, can ensure its effective use in agriculture. The growing interest in the application of Trichoderma results from their direct and indirect biocontrol potential against a wide range of soil phytopathogens. They act through various complex mechanisms, such as mycoparasitism, the degradation of pathogen cell walls, competition for nutrients and space, and induction of plant resistance. With the constant exposure of plants to a variety of pathogens, especially filamentous fungi, and the increased resistance of pathogens to chemical pesticides, the main challenge is to develop biological protection alternatives. Among non-pathogenic microorganisms, Trichoderma seems to be the best candidate for use in green technologies due to its wide biofertilization and biostimulatory potential. Most of the species from the genus Trichoderma belong to the plant growth-promoting fungi that produce phytohormones and the 1-aminocyclopropane-1-carboxylate (ACC) deaminase enzyme. In the present review, the current status of Trichoderma is gathered, which is especially relevant in plant growth stimulation and the biocontrol of fungal phytopathogens.
1. Introduction
Plant diseases play a direct role in destroying natural resources in agriculture and are said to be a major cause of reducing the annual level of food production in the world, which, depending on the source, is estimated at the level of 10–40% [1,2,3,4]. The most serious losses, both for natural and production ecosystems, are attributed to soil-borne pathogens, among which fungi constitute the most numerous group of plant disease agents, destroying as much as one-third of all crops annually [5]. According to the Food and Agriculture Organization of the United Nations (FAO), plant mycoses most often affect the five most important world crops—rice, wheat, corn, potatoes, and soybean [5]. Recently, more than 19,000 species of fungi that cause diseases of crops are known worldwide [6]. Most of the fungal phytopathogens belong to the Ascomycota and Basidiomycota phyla, the most serious of which are representatives of the genera Cladosporium, Botrytis, Alternaria, Aspergillus, Verticillium, Pythium, Fusarium (Ascomycota), and Rhizoctonia (Basidiomycota) [7,8,9,10,11,12,13].
In recent decades, the agricultural environment has been under severe pressure from chemical pesticides, the most popular method of protecting plants against fungal pathogens [4]. Though the high effectiveness of chemical plant protection products, there are concerns about their safe use and impact on the environment, as well as human and animal health [4]. The result of the abuse of chemical pesticides is an increase in the resistance of pathogens to pesticides, and the contamination of soil and ground waters. Furthermore, pesticides have a detrimental effect on non-target organisms (e.g., beneficial insects, including pollinators), soil microbiomes, and the general condition of terrestrial and aquatic ecosystems [14,15].
To protect the environment from the negative effects of chemical fungicides, various actions and strategies of sustainable food production systems are taken, including Integrated Pest Management (IPM) and organic farming [16,17]. One of these strategies is the use of Biological Control Agents (BCAs), based on living microorganisms or their metabolites, and products of natural origin that control the population of plant pathogens [16,18]. Over the last several decades, the most effort has been made to examine the effectiveness and practicality of non-pathogenic bacteria and fungi in the hope of commercializing them as BCAs [19,20]. As a result of the conducted studies, a large number of bacterial and fungal strains have been employed as BCAs, including Pseudomonas spp., Bacillus spp., Streptomyces spp., Trichoderma spp., Glomus mosseae, Gliocladium virens, Pythium oligandrum, and Beauveria bassiana, which successfully control the soil-borne diseases of valuable crops caused by fungi, oomycetes, bacteria, and nematodes [19,20,21]. The introduction of microbiological technologies to plant cultivation, based on microorganisms with biostimulating properties, is a subsequent element of a sustainable agricultural tactic [22]. The above solutions are in line with the current trends included in the strategic programs of the EU and are consistent with the assumption of the European Green Deal (EGD) and the EU Biodiversity Strategy for 2030, which emphasize the importance and necessity of agroecology development, agricultural biologicalization, and area increase in the plants grown in ecological production systems [23].
Among filamentous fungi, most of the BCAs belong to the phylum Ascomycota and are mainly representatives of numerous species belonging to the genus Trichoderma [24]. Numerous studies documented the beneficial properties of avirulent Trichoderma strains which allow their use in plant protection, biostimulation, and biofertilization [4,25,26]. The effectiveness of using Trichoderma in agriculture depends on their metabolic activity and the type of interaction with plants and other microorganisms. These fungi effectively colonize the rhizoplane, rhizosphere, and plant roots, and produce several metabolites with anti-microbial (cell wall degrading enzymes, antibiotics, volatile, and non-volatile compounds) and biostimulating (phytohormones, phytoregulators) features. Moreover, Trichoderma is known for its intensive absorption of root schedules and interaction not only with pathogenic microorganisms, but also interactions with the entire soil microbiome [24,26]. The present review focuses on the properties of Trichoderma to select the strains with the best parameters and predispose them to commercialization as a protective agent against fungal phytopathogens and biostimulators in specific plant crops.
2. Characteristics of the Trichoderma Allowing Its Use in Agriculture
Trichoderma is a genus of mostly asexual (the teleomorphic forms are Hypocrea) filamentous fungi, widespread around the world, usually colonizing rotting wood and other forms of organic plant matter [27]. Trichoderma is a dominant component of the mycobiome of various soil ecosystems (such as farmland, prairie, forests, salt marshes, and deserts) in all climatic zones, including temperate and tropical regions, Antarctica, and the tundra [4,28]. The Trichoderma genus is classified as cosmopolitan, saprotrophic fungi, often living as endophytes of woody plants [28]. The systematics and taxonomy of these fungi have evolved since 1794 when Persoon first introduced the generic name Trichoderma [29]. However, Trichoderma taxonomy has undergone a remarkable transformation since 1969, when Rifai concluded that the genus encompassed more than a few species and divided the strains studied into nine “aggregate” species based on their morphological features [26,30]. It is worth emphasizing that the taxonomy of the genus Trichoderma is complicated and belongs to the most dynamically developing branches of mycology [26,31]. According to the current MycoBank classification, the Trichoderma genus belongs to the domain Eukaryota, kingdom Fungi, division Ascomycota, subdivision Pezizomycotina, class Sordariomycetes, order Hypocreales, and family Hypocreaceae. The genus Hypocrea/Trichoderma already includes more than 300 molecularly and morphologically characterized species, many of which have not yet been formally described [32,33,34].
The success of species belonging to the genus Trichoderma as biocontrol agents in the soil ecosystems results from their ability to rapid growth, the possibility of utilizing a variety of substrates, and resistance to many toxic chemicals, including fungicides (e.g., azoxystrobin, 3,4-dichloroaniline, and trifloxystrobin), herbicides, and other organic pollutants [28,35,36,37,38]. Other than that, Trichoderma was found to degrade some toxic contaminants through enzymes involved in cellulose/lignin degradation that have been shown to have xenobiotic-metabolizing enzyme potential [36,38]. For example, T. viride has been observed to degrade trinitrotoluene (TNT) [39], and T. inhamatum reduces hexavalent chromium [40]. Moreover, Trichoderma can adapt to changes in environmental conditions and abundantly produce conidia and chlamydospores [29,41,42]. The latest research indicates a much wider temperature range for the growth and development of Trichoderma than previously assumed and, above all, the optimal temperature for the production of metabolites important for interactions with the plant. Although the saprotrophic activity of Trichoderma strains is the highest in the range of 15 to 21 °C [43,44], it has been demonstrated for the DEMTkZ3A0 strain [26] that the temperature of 12 °C may be optimal for auxin and gibberellin synthesis and is suitable for high ACC deaminase activity. Trichoderma grows as a white or transparent (hyaline) mycelium on Potato Dextrose Agar (PDA), and the colony takes on various colorations (usually green-yellow or reddish) depending on sporulation color (Figure 1A) [41]. These fungi are characterized by multi-branched conidiophores with a pyramidal appearance and clusters of divergent, usually asymmetrical bent, flask-shaped/cylindrical phialides (Figure 1B), while the conidia are colorless or assume various shades of green, gray, or brown (Figure 1C) [29,35,41,45]. Moreover, some species produce a characteristic sweet or coconut odor [46]. Trichoderma is not only ubiquitous in the environment and is easy to be isolated, but can also be easily multiplied under controlled conditions on a variety of substrates and can be stored for many months without losing its viability and properties.
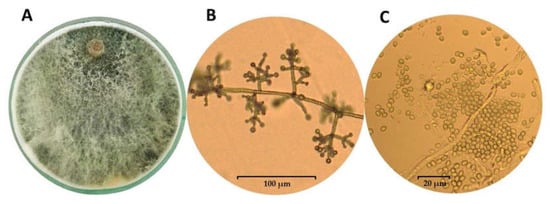
Figure 1.
Growth of the mycelium of Trichoderma koningiopsis TkZ3A0 strain (A) and the appearance of conidiophores (B) and conidia (C) observed in microcultures on PDA medium using an Olympus BX53 Upright Microscope equipped with a Olympus XC30 camera.
The potential of Trichoderma species as biological plant protection agents was first described in the early 1930s. Researcher Weindling [47] observed that the T. lignorum strain protects citrus seedlings against the Rhizoctonia solani pathogen through the mechanism of necrotrophic mycoparasitism. Since then, the biocontrol properties of Trichoderma have been extensively studied for the control of diseases caused by numerous soil phytopathogens [48]. The mechanisms by which Trichoderma reduces the occurrence of plant diseases include the competition for nutrients and space, synthesis of antifungal metabolites, mycoparasitism, production of lytic enzymes that degrade cell walls of fungal plant pathogens [4,49], as well as induction of plant resistance [50]. The most effective biocontrol properties are mainly attributed to the T. virens, T. harzianum, T. koningii, T. pseudokoningii, T. longibrachiatum, T. asperellum, T. polysporum, and T. viride, which have a significant impact on the development of plant diseases caused by R. solani, Sclerotium rolfsii, Pythium aphanidermatium, Gaeumannomyces graminis var. tritici, Verticillium dahliae, Fusarium oxysporum, and Fusarium culmorum, both under greenhouse and field conditions [7,24,26,51,52]. Furthermore, the application of Trichoderma strains to the soil increased the productivity and quality of crops of monocotyledons and dicotyledons, such as cucumbers, tomatoes, carrots, beans, corn, cotton, tobacco, millet, and ornamental grasses [53]. The stimulatory effect of Trichoderma on plants is probably related to their participation in the crosstalk between the growth hormones synthesized by these fungi and the defense hormones induced by them in the plant [54,55,56].
It should be noted that the Trichoderma fungi may also harm some areas of agriculture and human health. Their unfavorable effect in agriculture is mainly related to their ability of mycoparasitism and causes a disease entity known as green mold in mushroom (shiitake, oyster mushrooms, and champignons) cultivation [57]. The greatest losses are attributed to the T. aggressivum [58], T. pleurotum, and T. pleuroticola [59] genus. Several Trichoderma species are also included among the pathogens of cultivated plants. For instance, T. viride is the causative agent of onion green mold rot [41,42,60]. Recent reports indicate the occurrence of a new ear rot disease in maize in Europe caused by T. afroharzianum [61]. Some Trichoderma, especially T. brevicompactum, T. atroviride, and T. harzianum, can cause opportunistic infections in humans, including sinusitis, skin and liver infections, pneumonia, and stomatitis [62].
The biocontrol and biostimulation properties of Trichoderma directly translate into its wide application in agriculture. Due to the largest number of isolated anti-fungal bioactive compounds, Trichoderma is identified as the genus with the greatest biocontrol potential [18,63,64]. According to Rush et al. [64], Trichoderma species represent 50–60% of the fungal BCAs. Currently, at least 77 commercial Trichoderma-based biofungicides are available on the global market, including 7 approved by the European Commission for use in the Member States of the European Union (Supplementary Table S1) [18,64,65,66,67,68,69,70,71,72]. The above commercial preparations contain at least 36 different strains belonging to 13 species of the Trichoderma: T. asperellum (9), T. afroharzianum (formerly T. harzianum, 1), T. atroviride (8), T. atrobrunneum (1), T. asperelloides (1), T. fertile (1), T. gamsii (1), T. harzianum (7), T. hamatum (1), T. polysporum (1), T. stromaticum (1), T. virens (1), and T. viride (3), applied separately or in a consortium (Supplementary Table S1). It should be taken into account that due to the recent taxonomic changes within the Trichoderma, it is often difficult to precisely assign the strains constituting the active ingredient of biopreparations to the species [35,73,74].
3. Biocontrol Properties of Trichoderma against Fungal Phytopathogens
Trichoderma fungi use various complex direct or indirect mechanisms against fungal pathogens, which usually interact altogether in the biocontrol phenomenon (Figure 2) [4]. The direct impact on pathogens includes the production of cell wall degrading enzymes (CWDEs), synthesis of antibiotics, competition for space and nutrients (mainly carbon, nitrogen, and iron), and establishment of a direct parasitic relationship with the fungal pathogen [7,26,51,75]. On the other hand, Trichoderma indirectly induces local or systemic plant resistance through products (elicitors) released from the cell walls of the plant host (endoelicytors) and the infecting microorganism (exoelicytors) [50]. The type of mechanisms involved is often a strain characteristic and depends on the interaction type between the antagonist microorganism, pathogen, and the host plant [4,7].
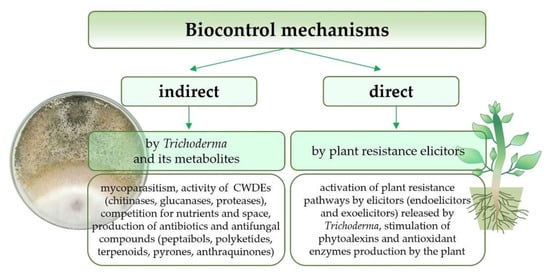
Figure 2.
Biocontrol mechanisms used by the Trichoderma genus against fungal pathogens.
3.1. Mycoparasitism as a Decisive Factor in Effective Biocontrol
Mycoparasitism is a phenomenon in which an antagonistic fungus (mycoparasite) can parasitize on another fungus (host) [4]. The fungi of the Trichoderma genus are mostly classified as necrotrophic mycoparasites [76]. Over 75 Trichoderma species with high mycoparasitic potential have already been described [75]. The mycoparasitic effect of Trichoderma necrotrophs on fungal pathogens includes prey sensing and chemotaxis, adhesion to the host, and physical attack through intense branching and coiling around the host’s hyphae. Moreover, Trichoderma can form appressoria-like penetration structures, homologs of pathogen appressoria (Figure 3) [77,78]. Chemical attack and degradation of the pathogen’s cell wall by hydrolytic enzymes and antifungal compounds produced by Trichoderma is the last stage of the mycoparasitic interaction, ultimately leading to the host death [76,77].
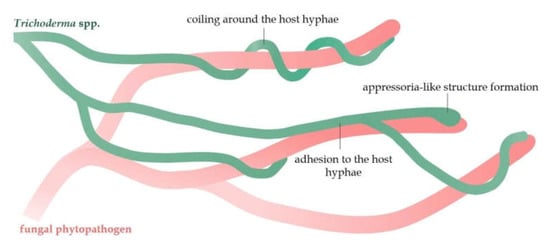
Figure 3.
A schematic mycoparasitic interaction of the Trichoderma hyphae with the hyphae of fungal pathogens.
Numerous Trichoderma genes encoding proteases (especially belonging to the subtilisin-like serine protease group) and oligopeptide transporters are expressed before and during the contact of various Trichoderma species with the host [79,80]. Moreover, it has been suggested that class IV G-protein coupled receptors (GPCRs), sensors for oligopeptides and compounds released from phytopathogen cell walls by the action of protease enzymes, are involved in the pathogenic host sensing [75]. Further signal transduction occurs through the conserved signaling cascade of G proteins, which contains three subunits: Gα, Gβ, and Gγ. T. atroviride mutants with a Gα subunit dysfunction completely lost their mycoparasitic capacity, decreased chitinolytic activity, and inhibited the production of the antifungal compound, 6-pentyl-α-pyrone [75,81,82]. In addition, there is biochemical evidence for the participation of Gα in the coiling of Trichoderma hyphae around the host hyphae. The presence of G-protein activators (mastoparan and fluoroaluminate) was found to increase the coiling of antagonist hyphae around nylon fibers [7]. Other than that, mitogen-activated protein kinases (MAPKs), especially pathogenicity MAPK (TmkA and Tmk1), are involved in signal transduction pathways in Trichoderma during mycoparasitism [75,83]. The removal of TmkA reduced the antagonistic interactions of T. virens towards S. rolfsii, R. solani, and Pythium ultimatum [75]. Moreover, the pathogenic fungi lectins and proteins containing cellulose-binding modules from Trichoderma hyphae may cooperate in the adhesion phase of mycoparasites to the victim’s hyphae [75].
The necrotrophic mycoparasitic effect was documented in the study by Błaszczyk et al. [49] during the interaction of T. atroviride AN240 and T. viride AN255 strains with F. avenaceum and F. graminearum, respectively. Similarly, the presence of the mycoparasites, T. harzianum [84] and T. virens [85], was found to be effective towards the hyphae of the R. solani. Moreover, effective mycoparasitic activity (coiling around the host hyphae and cell wall degradation) and changes in hyphal morphology were observed during the interaction of T. cerinum with the F. oxysporum [85]. Furthermore, the in vivo studies showed wilt disease suppression of chickpeas by the mycoparasitic T. cerinum Gur1 strain [86]. In their studies, Sánchez-Montesinos et al. [58] found the effective coiling of the T. aggressivum f. europaeum hyphae around the Botrytis cinerea, Sclerotinia sclerotiorum, Fusarium solani f. cucurbits, Pythium aphanidermatum, R. solani, and Mycosphaerella melonis.
Mycoparasitism by Trichoderma is not only limited to pathogenic hyphae of the host, and these fungi may use an additional biocontrol mechanism by acting on the pathogen’s conidia. The hyphae of the Trichoderma TkZ3A0 strain (with high similarity to the T. koningiopsis species) were able to parasitize on phialides with macroconidia of the F. culmorum phytopathogen (isolated from winter wheat with severe fusariosis symptoms) and showed clear chemotaxis and adhesion to their structures [26]. Changes in the morphology of macroconidia and inhibition of their germination (mycostasis) were observed, even in the early stages of the interaction [26].
3.2. The Production of Cell Wall Degrading Enzymes (CWDEs)
The production of CWDEs is an integral part of the final stage of mycoparasitism [4]. The fungal cell wall is mostly composed of 90% polysaccharides: chitin (a polymer of N-acetylglucosamine), β-(1,3)-, β-(1,4)- and β-(1,6)-glucans (composed of D-glucose units linked via β-glycosidic bonds), α-glucans, chitosan, mannan, and galactomannan, as well as proteins [87,88,89,90,91]. Trichoderma strains are mainly characterized by the ability to secrete a set of extracellular enzymes, including chitinases, β-(1,3)-, β-(1,6)-glucanases, and proteases, which hydrolyze the main components of the pathogen’s cell wall [4,15].
Chitinases are the most important group of lytic enzymes synthesized by Trichoderma fungi, hydrolyzing the β-glycosidic links between the C1 and C4 carbon of two adjacent N-acetylglucosamines in the chitin chain [92]. Most fungal chitinases belong to the glycoside hydrolase family 18 (GH18) with a molecular weight of 20 to 90 kDa [93,94]. The chitinolytic activity of Trichoderma results from the presence of genes (such as ech42, ech30, chit33, chit36, chit42, nag1, and chit18-13) encoding chitinases synthesis, the great variety of which is responsible for the effective biocontrol against a wide range of fungal phytopathogens [4,7,95]. Interestingly, the deletion of some chitinase genes of Trichoderma did not result in the loss of mycoparasitic and biocontrol capacity, probably due to the large reservoir of compensatory genes [75].
Glucanase enzymes degrade the cell walls of pathogenic fungi through two sets of enzymes, exo-β-glucanases, and endo-β-glucanases [91,96]. Presumably, the genes encoding this type of enzyme are over-represented in the genomes of Trichoderma compared to the genomes of other related fungi [75]. The description of the T. harzianum and Fusarium solani transcriptome interaction showed an overexpression of the bgn13.4 gene encoding β-(1,3)-glucanase during the later stages of the mycoparasitism [97]. Additionally, the deletion of the tvbgn3 gene encoding the enzyme β-(1,6)-glucanase, decreased the biocontrol potential of T. virens towards Pythium ultimum [98]. Increased chitinolytic and glucanolytic activity was demonstrated by the Trichoderma TkZ3A0 isolate in liquid cultures containing the lyophilized cell wall of the pathogenic F. culmorum strain, as the only carbon source [26]. Similarly, the Trichoderma ThJt1 isolate showed maximum enzymatic activity in the presence of chitin and S. rolfsii cell wall [99]. The addition of colloidal chitin and yeast extract as a carbon and nitrogen source, respectively, significantly increased the chitinolytic activity of T. asperellum strains [100]. This indicates an enhanced production of hydrolytic enzymes in the presence of a fungal pathogen.
In addition to chitinases and glucanases, a decisive role in the mycoparasitism process is played by proteolytic enzymes, catalyzing the hydrolysis of peptide bonds in proteins [77,101]. Prb1 protease synthesized by the T. harzianum was shown to play a crucial role in biological control. The prb1 transformants were shown to be up to five times more effective against R. solani [77,102]. In a further study, the overexpression of the T. virens extracellular serine protease gene tvsp1 increased the protection of cotton seedlings against R. solani by 15–32%, compared to the wild-type strain [103]. The aspartic protease P6281 secreted by the T. harzianum significantly inhibited spore germination and the growth of plant pathogenic fungi (especially B. cinerea and R. solani), as well as reduced the occurrence of cucumber, apple, and orange gray mold [104]. Furthermore, based on microscopic analyses, the effective degradation of the B. cinerea cell wall was found as a result of the P6281 protease action [104]. It is assumed that proteases produced by the Trichoderma species regulate the expression or action of other hydrolytic enzymes involved in mycoparasitism. For instance, transformants from T. harzianum that simultaneously overexpressed the β-1,6-glucanase gene bgn16.2 and papA (a gene that encodes an extracellular aspartyl protease) showed a 30% increase in β-1,6-glucanase activity compared to bgn16.2 single transformants [104,105].
3.3. The Production of Antibiotics and Other Antifungal Compounds
The Trichoderma species have been found to produce numerous secondary metabolites, over 370 of which belong to different classes of chemical compounds with strong antagonistic properties [4,29,106]. Most Trichoderma strains produce non-volatile and volatile organic compounds (VOCs), the most important of which are peptaibols and polyketides [91].
Trichoderma is considered to be one of the richest sources of peptaibols, and over 80% of entries in the “Peptaibiotics Database” belong to different species of this genus [107]. Peptaibols are defined as polypeptide antibiotics with a molecular weight of 500 to 2200 Da, rich in non-proteinogenic amino acids, especially alpha-aminoisobutyric acid (Aib) and isovaline (Iva). These compounds are also characterized by the presence of an acylated N-terminus and amino alcohols (e.g., phenylalaninol or leucinol) at the C-terminal residue [34,108,109]. Peptaibols are produced by non-ribosomal peptide synthetases (NRPSs) [91], and three main gene-encoding NPRSs, tex1, tex2, and tex3, were identified in the Trichoderma genomes [75]. Trichoderma species synthesize various antibiotics of this group. For instance, 17 biotechnologically and agriculturally important species from the Longibrachiatum Clade, (e.g., T. citrinoviride, T. longibrachiatum, T. pseudokoningii, and T. reesei) produce several new peptaibols, mainly related to trichobrachins, suzukacillins, trichoaureocins, trichocellins, longibrachins, trichokonins, trichosporins, alamethicins, and brevicelsins [33]. Moreover, T. harzianum has been found to synthesize trichorzins (HA, MA, and PA), harzianins, trichotoxin, and trichokindins [110]. Other than that, T. atroviride releases peptaibols, such as atroviridins A–C and neoatroviridins A–D, while T. viride produces trichotoxins A and B, trichodecenins, trichorovins, and trichocellins [91,110]. A study conducted by Tamandegani et al. [34] found that T. asperellum and T. longibrachiatum strains increased peptaibol production (trichotoxins and longibrachins) during in vitro interactions with fungal plant pathogens F. moniliforme, F. culmorum, F. graminearum, F. oxysporum, Alternaria solani, and R. solani. This study also indicated a significant increase in the peptaibol synthetase tex1 expression during the interaction of T. asperellum with R. solani. Moreover, three analogs of peptaibol trichogin completely inhibited the growth of phytopathogens, B. cinerea, F. graminearum, and Penicillium expansum. After foliar application, these analogs effectively (even by 95%) reduced the symptoms of a bean, grapevine, and ripe grape disease caused by B. cinerea [111].
Additionally, the Trichoderma species show the ability to produce polyketides, a structurally diverse group of biologically active compounds derived from bacteria, fungi, and plants [112]. The polyketides include antibiotics (e.g., tetracyclines and macrolides), mycotoxins, and pigments [105]. Polyketides are synthesized from simple units such as acetyl-CoA and malonyl-CoA by polyketide synthases (PKSs) [113]. Although Trichoderma genomes are abundant in PKS-encoding genes, only a few researchers have focused on the genetics and polyketide biosynthesis by these fungi. Detailed phylogenomic analysis of the PKS-encoding genes of T. reesei, T. virens, and T. atroviride showed that most of the polyketide synthases belong to the lovastatin/citrinin or fumonisin clades, and are present as orthologs in all three species [114]. Further, the PKS gene pks4 from T. reesei is responsible for green pigmentation and the stability of the conidial cell wall, as well as the antagonistic capacity against other fungi [115]. Moreover, two PKS-encoding genes, pksT-1 and pksT-2, from T. harzianum were differentially expressed during contact with R. solani and F. oxysporum phytopathogens [116].
Numerous Trichoderma species produce secondary metabolites belonging to the group of terpenoids, pyrones, anthraquinones, and epipolythiodioxopiperazines (ETPs) [109,117]. The terpenoids identified in Trichoderma include tetracyclic diterpenes (e.g., harziandion), sesquiterpenes, such as trichothecenes (trichodermin and harzianum A), as well as triterpene viridin [109]. Furthermore, T. viride, T. harzianum, and T. koningii species produce the volatile antibiotic 6-phenyl-α-pyrone (6PAP), which is responsible for the characteristic coconut smell and the biological control against F. oxysporum [29]. Importantly, T. harzianum, T. viride, and T. aureoviride produce numerous anthraquinone pigments, such as pachybasin, chrysophanol, and emodin, which have strong antagonistic properties against pathogenic fungi [118], and mediate in mycoparasitic coiling [119]. The best-known ETP is gliotoxin, named after the fungus Gliocladium fimbriatum (later identified as T. virens) from which gliotoxin was originally isolated [109]. Gliotoxin exhibits immunosuppressive activity and is a virulence factor of the human pathogen Aspergillus fumigatus, but also plays a crucial role in biological control due to its strong fungitoxic activity [120]. T. virens mutants with a deletion of the gliP gene encoding gliotoxin synthesis showed an impaired mycoparasitic capacity against P. ultimum and S. sclerotiorum [121]. Moreover, Trichoderma fungi, especially T. harzianum, produce harzianic acid (belonging to the subgroup of tetramic acids) with greatly interesting properties due to its antifungal activity, stimulation of plant growth, and chelating abilities [122].
3.4. Competition for Nutrients and Space
The antagonistic fungi can deprive pathogens of space and nutrients by colonizing a common habitat, i.e., plant tissues, rhizospheres, or phyllospheres [4]. This depends on their properties, the degree of colonization of the host plant, and adaptation to the environmental conditions in which they live [4,91]. As for the successful competition with pathogens for niches and nutrients, Trichoderma should have effective plant colonization strategies and be abundant in a niche where competition with other fungi occurs [4]. Species belonging to this genus are widely known for their rapid growth and are considered to be aggressive competitors. They quickly colonize various substrates and eliminate slower-growing pathogens [123]. Trichoderma fungi are characterized by a particularly high growth rate on glucose and sucrose [26]. At the same time, it is worth emphasizing that there is a wide range of substrates that these fungi can utilize as the only source of C and energy, which allows them to effectively use a variety of rhizodeposites, both simple sugars and polymeric carbohydrates that are components of cell walls [26,91]. Trichoderma species have a much better ability to mobilize and uptake nutrients from the soil compared to other microorganisms [24]. This process is related to the biosynthesis of gluconic, citric, and fumaric organic acids, which lower the soil pH and increase the solubilization of phosphates and microelements (iron, manganese, or magnesium) [124].
The greatest importance in a relation to the competition phenomenon in Trichoderma is attributed to siderophores, a low molecular weight (less than 10 KDa) chelator compound with a high affinity for iron (Fe), produced under iron-deficiency stress [125,126]. The Fe ions are cofactors for many enzymes and are an important factor for the proper growth and development of plants, but also many microorganisms [127]. The microbial siderophores are usually divided into three groups, hydroxamate, catecholate, and carboxylate, based on the chemical nature and coordination sites with iron [128]. Generally, fungi synthesize hydroxamate-type siderophores, such as coprogens, ferrichromes, and fusarinines, which share a common structural unit, N5-acyl-N5-hydroxyornithine [109,129]. Under neutral pH conditions and in the presence of oxygen, iron is present in the soil mainly in the form of Fe3+. In the aerobic environment, Fe tends to form insoluble iron oxides, which makes it unavailable for plants [127]. Siderophores can bind with insoluble iron (Fe3+) and then transform it into a soluble form of Fe2+, easily assimilated by plants and microorganisms (Figure 4) [130].
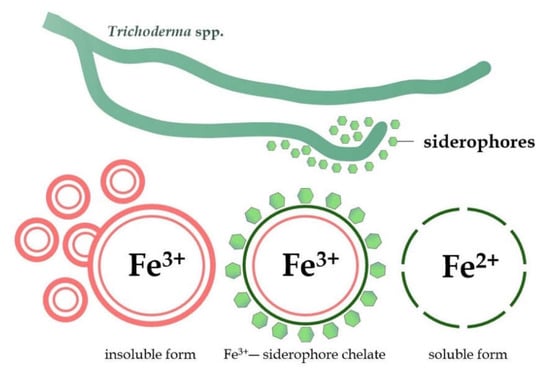
Figure 4.
Transformation of the insoluble form of iron (Fe3+) into a soluble and easily assimilate form (Fe2+) by siderophores produced by Trichoderma fungi.
Trichoderma can inhibit the growth and activity of target soil pathogens by depriving them of iron sources from a common niche [131]. In vitro studies of the interaction between T. harzianum and Fusarium acuminatum, Alternaria alternata, and Alternaria infectoria pathogens showed that nutrient starvation caused the death of the studied fungi [132]. Moreover, the iron competition was described as one of the key factors in the antagonism of T. asperellum against F. oxysporum f. sp. lycopersici and the protection of tomato plants against wilt disease [133]. It was also reported that Trichoderma can effectively compete for simple and complex carbon substrates with phytopathogens of the genera Colletotrichum sp., Botrytis sp., Verticillium sp., and Phytophthora sp. [123]. Further, the enzymes that mediated competition for nutrients and space were responsible for the potent inhibitory effect of Trichoderma on B. cinerea, F. graminearum, and Macrophomina phaseolina pathogens [134].
3.5. The Induction of Plant Resistance in Response to Biotic Stress
The ability of Trichoderma strains to colonize plant roots and establish a robust and stable relationship with them is particularly crucial in biological plant protection. Fungi belonging to this genus can indirectly affect pathogenic microorganisms via plants by inducing their local or systemic defense mechanisms [91,109]. The induction of plant resistance is a consequence of the action of various elicitors (inducers of the defense response) released from the cells of the microorganisms (exoelicitors) and plant tissues (endoelicitors). The elicitors are classified into two groups: (1) race specific elicitors that trigger gene-to-gene type defense only in specific host cultivars, and (2) released from pathogenic and non-pathogenic strains general elicitors that trigger non-race specific defense both in host and non-host plant [135,136]. Different classes of elicitors were characterized, including oligosaccharides (e.g., glucans and chitins, and oligogalacturonides), proteins and peptides (e.g., endoxylanase and elicitins), glycopeptides and glycoproteins (e.g., glycopeptide fragments of invertase), glycolipids (e.g., lipopolysaccharides), and lipophilic compounds (e.g., fatty acids) [135,136]. The activation of signal transduction pathways by elicitors lead to the physical, biochemical, and molecular changes in plants, such as ion flow across the membrane, the production of reactive oxygen species (ROS), creating a physical barrier to prevent the spread of phytopathogens (callose deposition, reinforcement of plant cell wall), and the synthesis of different defense compounds (for instance, phytoalexins, volatile organic compounds, enzymes, and phytohormones) [136,137,138,139].
Various plant species, both monocotyledonous and dicotyledonous, show an increased activity of the immune response in the presence of non-pathogenic fungi of the Trichoderma [131]. The plant defense response is primarily based on the recognition of conserved domains, such as the microbe-associated molecular pattern (MAMP) or pathogen-associated molecular pattern (PAMP) [140]. These domains induce two types of innate immunity in plants: (1) MAMP-triggered immunity (MTI)/PAMP-triggered immunity (PTI) and (2) effector-triggered immunity (ETI) (Figure 5) [26].
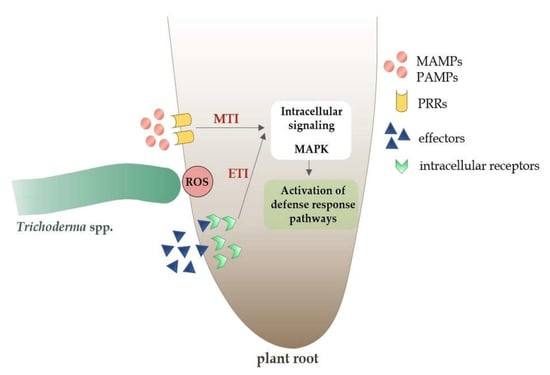
Figure 5.
Plant defense response pathways to microbe-associated molecular pattern (MAMPs) molecules and effectors from non-pathogenic Trichoderma spp.
Both transmembrane pattern recognition receptors (PRRs) and intracellular receptors recognize particular molecular patterns of MAMP/PAMP and ETI molecules, initiating local MTI/PTI or ETI immunity [91,141]. In the case of the ETI-type immune response, it is usually stronger than MTI/PTI and leads to programmed cell death as a result of hypersensitive response (HR) activation [142]. Mitogen-activated protein kinases (MAPKs) transmit information from receptors to plant cells and initiate the systemic cascade of the immune response (Figure 5) [143].
Three types of induced resistance in plants are activated as a result of the MTI and ETI immune pathways induction by Trichoderma: (1) systemic acquired resistance (SAR) effective against biotrophic pathogens, (2) induced systemic resistance (ISR) effective against necrotrophic pathogens, and (3) induced resistance (IR) effective in defense against biotrophic and necrotrophic pathogens and some abiotic stress factors [144,145,146,147]. The SAR is characterized by the expression of pathogenesis-related (PR) protein genes and the production of salicylic acid (SA) as a signaling molecule [145,147]. In turn, jasmonic acid (JA) and ethylene (ET) are crucial signaling molecules in ISR-type immunity [146,147]. The IR immunity is activated by β-aminobutyric acid (BABA) and involves abscisic acid (ABA) as a signaling molecule [144] (Figure 6).
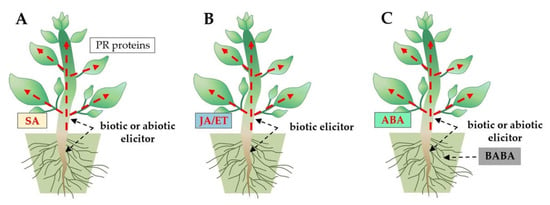
Figure 6.
Three types of induced resistance in plants: (A) systemic acquired resistance (SAR), (B) induced systemic resistance (ISR), and (C) induced resistance (IR), trigged by Trichoderma species (biotic elicitor). Signaling molecules, such as phytohormones salicylic acid (SA), jasmonic acid (JA)/ethylene (ET), and abscisic acid (ABA), are involved in SAR, ISR, and IR immunity, respectively.
The increase in the plant resistance marker’s (enzymes and metabolites) activity, as a result of the action of Trichoderma elicitors, indicates the induction of signaling pathways of the plant’s defense response. The inoculation of wheat seeds with Trichoderma TkZ3A0 strain conidia significantly increased the activity of the phenylalanine (PAL) and tyrosine lyase (TAL), catalase (CAT), guaiacol peroxidase (GPX), as well as glucanase and chitinase (PR proteins) in the plant stems and roots [26]. The PAL, a principal enzyme of the phenylpropanoid pathway, plays a crucial role in phytoalexins production and lignin biosynthesis [26,148]. On the other hand, peroxidase, catalase, and superoxide dismutase are produced by plant cells to protect against damage and oxidative stress [136]. The characteristics of the induced resistance against fungal pathogen Colletotrichum truncatum were demonstrated by chili pepper (Capsicum annum L.) treated with T. harzianum (as a foliar spray) and T. asperellum (as seed treatment) strains [149]. Moreover, the application of T. harzianum reduced disease development and enhanced the resistance of the grapevine against Plasmopara viticola [150]. In another report, T. longibrachiatum mediated plant systemic resistance to B. cinerea challenge through the activation of signaling pathways associated with the phytohormones JA/ET and SA in cucumber [151].
Priming adaptive strategy is particularly promising for the effectiveness of the resistance induction method against pathogens. The characteristic feature of this strategy is that a plant pre-inoculated with an elicitor can activate one or several defense pathways more rapidly and robustly after infection by pathogens than non-induced plants [152,153]. The plant colonization by Trichoderma species can prime defense response, enabling robust plant reactions to further pathogen attack [154].
4. The Effect of Trichoderma in Enhancing Plant Tolerance to Abiotic Stress
The presence of Trichoderma in the rhizosphere and plant tissues leads to increased plant tolerance to both biotic and abiotic stresses [155]. The most harmful environmental stresses that menace crop yield are drought, salinity, heavy metal accumulation, and extreme temperatures [156,157,158]. Adverse environmental conditions can disrupt the photosynthesis process, generate high ROS content, affect the translocation of nutrients, and modify plant hormonal balance, causing cell damage and plant necrosis [141]. However, the inoculation of tomato seedlings with T. harzianum under salinity and drought stress conditions resulted in the maintenance of photosynthetic efficiency and effectively reduced ROS accumulation [159]. Moreover, inoculation of wheat seedlings with T. longibrachiatum strain conidia enhanced the tolerance of wheat to salt stress and significantly increased the concentration of antioxidant enzymes [160]. It was found that the rapeseed (Brassica napus L.) plants inoculated with T. parareesei had a high salt and oxidative stress tolerance. Upon the root inoculation of this strain, rapeseed plants showed a significantly greater yield compared to non-inoculated plants. In addition, T. parareesei enhanced the tolerance to salinity and drought stress in rapeseed by increasing the expression of ACCO1, NCED3, and PYL4 genes related to the ABA and ET hormonal pathways [157]. Durum wheat inoculated with T. harzianum showed a higher tolerance to moderate drought stress by 52% under optimal nitrogen fertilization; however, nutrient availability in the soil and environmental conditions significantly influenced this response [161].
Furthermore, Trichoderma was found to increase the plant’s tolerance to low-temperature stress. The inoculation of tomato (Solanum lycopersicum L.) plants with T. harzianum strain efficiently alleviated the adverse effects of cold stress leading to an increased fresh and dry weight of shoots and roots, as well as improved photosynthesis, growth rate, and leaf water content. In addition, TAS14 and P5CS gene-encoding enzymes and metabolic proteins that mediate plant tolerance to low-temperature stress were up-regulated with T. harzianum inoculation [162]. On the other hand, T. koningii proved to be beneficial in imparting heat stress tolerance to the tomato plants through the enhanced modulation of antioxidants. The application of this strain reduced the ROS accumulation and protected the plant cells from oxidative damage under high-temperature stress [163].
5. Trichoderma as a Biocontrol Agent against Other Plant Pathogenic Organisms
Numerous strains of Trichoderma have been developed as biocontrol agents against plant diseases caused by bacteria, nematodes, and insects [158,164]. There are more than 200 species of plant pathogenic bacteria, among which Agrobacterium, Pseudomonas, Erwinia, Ralstonia, and Xanthomonas cause the most severe crop losses worldwide [165]. The antibacterial activity of Trichoderma is most often attributed to the secretion of secondary metabolites, the most important of which are peptaibols, gliotoxin, polyketides, gliovirin, and pyrones [165,166]. T. pseudoharzianum and T. asperelloides showed a strong inhibitory effect on the growth of the serious bacterial pathogen of tomato plants, Ralstonia solanacearum. Changes in the morphology of R. solanacearum cells (the rupturing of cell walls, the disintegration of the cell membrane, and leakage of cell contents) were observed as a result of the action of various secondary compounds from Trichoderma [165]. Moreover, T. hamatum synthesized the bioactive volatile secondary metabolite, 6PAP, which effectively inhibited the growth of Acidovorax avenae, Erutimacarafavora, and Xanthomonas campestris [167]. Sarsaiya et al. [168] demonstrated for the first time that the endophytic strain of T. longibrachiatum, isolated from the medicine plant Dendrobium nobile, produced the dendrobine compound responsible for enhancing human immunity, preventing metastatic cancer, and therapeutic effects on Alzheimer’s disease. Other than that, dendrobine exhibited strong toxic activity against bacterial plant pathogens, such as Bacillus subtilis, Bacillus mycoides, and Staphylococcus sp. [168]. This indicates the potential applications of Trichoderma metabolites in both agricultural and medical fields.
Plant-parasitic nematodes (PPNs) represent a vital threat to agricultural production. Worldwide crop damage caused by plant nematodes has been estimated at around 12% [169]. Trichoderma was found to have strong nematicidal activity against PPNs. The biocontrol action of Trichoderma against phytoparasitic nematodes occurs through the direct parasitism of eggs and larvae as well as the production of hydrolytic enzymes (chitinases and proteases) and secondary metabolites (volatile and non-volatile) that destroy the cuticle of nematodes. Furthermore, Trichoderma hyphae may form a physical and chemical protective barrier over the plant roots [170]. The application of T. harzianum to tomato seeds greatly reduced the severity of disease caused by the root-knot nematode Meloidogyne javanica. In this study, T. harzianum efficiently penetrated the nematode mass matrix and decreased egg hatching. In tomato plants, defense enzymes activity (peroxidase, polyphenol oxidase, and PAL) was significantly increased [171]. Moreover, T. asperellum and T. harzianum have been reported to inhibit the penetration of Pratylenchus brachyurus in soybeans by producing non-volatile compounds with nematicidal activity [170].
Recent studies demonstrated that Trichoderma can enhance plant protection against insect pests, such as aphids, thrips, moths, and caterpillars [172]. Trichoderma directly affects pests through some antagonistic mechanisms, the most important of which are parasitism and the production of insecticidal secondary metabolites, antifeedant compounds, and repellents. Moreover, Trichoderma has been reported to directly reduce the detrimental effect of these pathogens by the activation of plant defense mechanisms [173]. T. longibrachiatum showed an entomopathogenic effect on the development of Leucinodes orbonalis, one of the major pests of Solanum melongena (eggplant) [174]. Further examination indicated that there was an intense parasitic growth of the T. gamsii hyphae across the lower abdomen and respiratory ostiole of the Delia radicum larva [175]. Other than that, T. harzianum, T. asperellum, and T. hamatum showed pathogenic potential against Cetarovacuna lanigera, a destructive insect pest of sugarcane that is responsible for reducing the quality, yield, and sugar content [176].
6. Plant Growth-Promoting Properties of Trichoderma
Trichoderma is often associated with the root ecosystems of the host plants. Therefore, Trichoderma is usually defined as the genus of symbiotic, opportunistic, and avirulent microorganisms that colonize the roots and stimulate plant growth through mechanisms similar to those used by mycorrhizal fungi [7]. The beneficial effects of Trichoderma species on plants include the promotion of their growth, improvement to root structure and condition, enhancement of seed germination and viability, as well as increased photosynthesis efficiency, flowering, and yield quality [177]. The most important stimulating factor at almost all stages of plant growth and development is the synthesis of phytohormones and phytoregulators [26,178].
6.1. Plant Root Colonization
Numerous rhizosphere Trichoderma species can colonize the root surfaces of both monocotyledonous and dicotyledonous plants, leading to significant changes in plant metabolism [179]. Colonization by the Trichoderma species involves the recognition of host plant, attachment and penetration of plant roots, and resistance of Trichoderma to metabolites produced by the plants in response to invasion by foreign organisms (pathogenic or non-pathogenic) [7]. Due to the colonization of a wide range of host plants, Trichoderma presumably developed effective strategies to overcome plant defense mechanisms [179].
Numerous studies confirmed the ability of Trichoderma not only to colonize the rhizosphere soil, but also to colonize the root surface [180,181]. The root colonization by Trichoderma was previously observed mainly in the root hair and elongation zone. However, recent studies confirmed the colonization of the root cap border cells (RBCs) zone by these fungi [26]. The RBCs play a crucial role in the interaction between plants and soil microorganisms [182,183]. The RBCs are released into the soil environment from the outer layer of the cap cells during root elongation (Figure 7A). It is assumed that these cells and the associated root exudates constitute a valuable source of nutrients and biologically active compounds for microorganisms [182]. Moreover, RBCs influence the composition and structure of the microbial rhizosphere community and act as attractants for microorganisms, facilitating the interaction with the plants and root colonization [182,184]. Simultaneously, RBCs are considered a crucial agent in plant protection against various biotic and abiotic stresses [185]. The ability of Trichoderma to colonize RBCs, as demonstrated for the wheat roots inoculated with DEMTkZ3A0 strain conidia (Figure 7B) [26], indicates an important role of these fungi in the indirect defense mechanism against phytopathogens through the formation of a mantle-like structure.
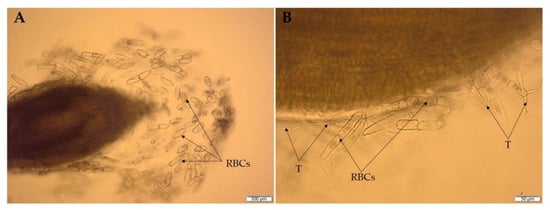
Figure 7.
Wheat root border cells (RBCs) (A) and RBC colonization (B) by Trichoderma DEMTkZ3A0 hyphae (T) observed in microcultures on glass slides using an Olympus BX53 Upright Microscope equipped with a Olympus XC30 camera.
It was found that the recognition and adhesion of Trichoderma to the root surface of the host plants is mediated by hydrophobins, which play a fundamental role in cell communication, fungal morphogenesis (including infectious structures), and the adhesion of hyphae to hydrophobic surfaces [186,187]. For instance, the T. harzianum gene, qid74, encodes a cysteine-rich protein, involved in the adhesion of fungal hyphae to the tomato root system [188]. On the other hand, the deletion of the TasHyd1 hydrophobin gene from T. asperellum resulted in a change in the hyphae wettability and serious impairment in root attachment and colonization [187]. Trichoderma also produces proteins with a high affinity for cellulose, among which cerato-platanins and swollenins are crucial for the effective root colonization of plants [180,189]. T. atroviride strains that overexpressed the Taswo1 swollenin gene showed improved colonization of pepper (Capsicum annuum L.) roots and a stronger plant growth-promoting effect [189]. Furthermore, the root colonization by Trichoderma is closely related to the production of cellulolytic, proteolytic, pectinolytic, and xylanolytic plant cell wall degrading enzymes (PCWDEs), enabling the colonization of the root epidermis and cortex area [91,179,190]. Moreover, PCWDEs may release fragments of the plant cell wall, acting as plant resistance endoelicitors [26].
The plant-derived sucrose, a predominant resource supplied to Trichoderma cells, is associated with the rapid growth of fungi and facilitated root colonization [75,191]. A similar effect is shown by carbon substrates (mono- and disaccharides) secreted by the mucigel layer of the roots during the mycorrhiza process [75]. In the presence of plant sucrose, the intracellular invertase from T. virens (TvInv) was shown to play an important role in the mechanisms controlling the symbiotic association with maize (Zea mays L.) roots [191]. Some important solute transporters, such as di/tripeptide transporter and the permease or intracellular invertase system involved in the obtaining and transport of root exudates, were described in the Trichoderma genomes [192].
6.2. The Synthesis of Phytohormones and Metabolites Influencing the Phytohormonal Balance
The microbial production of phytohormones and phytoregulators is one of the direct mechanisms contributing to the rapid and stable colonization of soil by microorganisms and their promoting effect on plant growth [193,194]. Furthermore, the colonization of plant tissues by phytohormone-synthesizing soil microorganisms affects the hormonal balance of plants and their interaction with microorganisms [195,196]. Microbial phytohormones play crucial roles in agriculture with a growing interest in their industrial production, especially with the use of fungal cultures [197]. Phytohormones participate in the regulation of complex and interrelated plant immune signaling pathways, ensuring a rapid defense response and adaptation to various environmental conditions [198]. Several representatives of the genus Trichoderma are characterized by the ability to produce phytohormones (auxin and gibberellin) and phytoregulators, including the 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase enzyme, which regulates the ethylene biosynthetic pathway [26,198,199]. The synthesis of the indole-3-acetic acid (IAA) hormone by Trichoderma results in the enhancement of the colonization capacity of the rhizosphere, rhizoplane, and roots of monocotyledons and dicotyledons, which was observed during the interaction of the T. harzianum mutant with a cucumber [200]. Moreover, the growth stimulation of cucumber roots was enhanced by the T. asperellum Q1 strain, capable of producing a complex of growth hormones (IAA, gibberellin, and ABA) [201]. Furthermore, the reinforcement growth of wheat seedlings was observed upon Trichoderma strain inoculation, capable of producing IAA, gibberellin, and ACC deaminase [26].
6.2.1. The Production of Auxin Phytohormone Indole-3-Acetic Acid (IAA)
Several species of Trichoderma produce auxin phytohormones, especially IAA, which is crucial for most of the processes responsible for the proper growth and development of plants. It is assumed that IAA is the main factor determining competition between fungal species inhabiting the same niche [202,203]. The production of microbial IAA usually depends on the presence of its precursor, L-tryptophan. However, some microorganisms synthesize IAA in amino acid independent pathways [199]. The in vitro production of IAA by Trichoderma strains has been demonstrated in the presence of tryptophan [26,204]. A similar dependence was also found in the case of endophytic bacteria strains [205] and Mortierella fungi [199,206] with biostimulatory potential. The production of IAA by Trichoderma also depends on abiotic factors, such as temperature and pH [26,207].
IAA at low concentrations stimulates root elongation, while the high concentration of IAA is responsible for the proper formation of lateral and adventitious roots [26]. Upon the inoculation of T. virens and T. atroviride, the biomass production and stimulation of lateral root growth of the wild-type Arabidopsis thaliana seedlings were enhanced [208]. In the presence of T. atroviride closely related strains, the number of A. thaliana secondary roots increased by 64 to 90% compared to non-inoculated plants [203]. Moreover, mutations in the aux1, big, eir1, and axr1 genes involved in auxin signaling and transport were found to reduce the stimulating effect of T. virens on plant root growth and development [208]. The synthesis of IAA by the T. harzianum mutant may affect the composition of the rhizosphere and rhizoplane soil and facilitate the colonization of cucumber roots and stems [200]. Other than that, the improvement of plant growth due to the action of IAA derived from the Trichoderma species was also reported in many other agricultural plants, including tomato [209], sorghum [204], bean [53], wheat [210], and pepper [211].
6.2.2. The Production of Gibberellin Phytohormones
Gibberellins (GAs) are one of the main phytohormones, the low concentration of which affects the proper germination of seeds, the growth of plant roots and shoots, leaf expansion, and flower development [199]. These properties are responsible for the extensive application of GAs in agriculture to improve the quality of agricultural and horticultural crops [212]. Almost 136 different GA compounds are already known, while only GA1, GA3, GA4, and GA7 from the C19-GA group are biologically active [213,214].
Several scientific reports indicate the ability of numerous species from the Trichoderma to synthesize GAs [26,215,216]. After the application of T. koningiopsis isolates, tomato growth was significantly improved, possibly through GA action [215]. Moreover, the T. asperellum Q1 strain synthesizing both IAA, GA, and ABA, increased the concentration of these hormones in leaves of cucumber seedlings [201]. The accumulation of GA3 produced by T. harzianum in a combination with IAA was found to increase the plant growth-promoting effect [216]. Furthermore, the production of GA by the Trichoderma strain was positively correlated with the synthesis of phytohormone IAA and phytoregulator ACC-deaminase [26].
6.2.3. The Production of the ACC-Deaminase Enzyme
Plant growth stimulation also occurs in the presence of Trichoderma with the ability to produce the ACC-deaminase enzyme (ACCD), which lowers the ethylene (ET) levels in plants by cleaving the ET precursor ACC into α-ketobutyrate and ammonia [217]. The ET is produced by plants in a response to numerous environmental biotic and abiotic stresses [218]. This phytohormone (ET) is also involved in the regulation of various physiological processes of the plant, in part through complex interactions with other phytohormones [219]. However, high levels of ET can inhibit root elongation and growth, often leading to plant death [220,221].
Several species of Trichoderma fungi have the considerable ability to produce ACCD involved in promoting plant growth [26,222,223]. Rauf et al. [218] proved an increased tolerance of wheat to waterlogging stress as a result of the activity of ACCD produced by T. asperellum. Moreover, ACCD from T. harzianum positively influenced the germination and growth of maize seedlings under greenhouse conditions [223]. The genes encoding the ACCD enzyme were identified in the genomes of Trichoderma. The silencing of the Tas-acdS gene from T. asperellum showed a decreased ability to stimulate the root elongation of canola plants [224].
The release of ACC by plant roots into the rhizosphere soil to attract and interact with plant growth-promoting microorganisms (PGPMs) suggests an ethylene-independent function of ACC [225]. A study conducted by Viterbo et al. [224] showed that the addition of ACC to the medium as the sole nitrogen source increased the ACCD activity of T. asperellum T203 strain. Moreover, tomato plants pretreated with ACC proved less serious disease symptoms caused by the V. dahliae pathogen, compared to untreated controls. This confirms that ACC may act as a signal molecule for control defense and enhance plant resistance to diseases [225].
6.3. Nutrient Solubilization and Enhancement Bioavailability of Essential Elements
Trichoderma plays a crucial role in enhancing plant growth by the production of vitamins, increasing the solubility of nutrients contained in the rhizosphere (phosphates, Fe3+, Cu2+, Mn4+, ZnO), and supplementing the plant with the necessary elements (mainly nitrogen, phosphorus, potassium, and microelements) for their proper growth and yield [26,91,179,226].
Amongst all the plant nutrients, phosphorus (P) is probably present in the soil in the forms with the most limited bioavailability to plants [227]. The soil application of Trichoderma strains was demonstrated experimentally to increase inorganic phosphate solubilization due to extra-cellular phytase activity [228] and acidification of the soil environment by acetic, butyric, citric, and fumaric acids production [229]. The ability of Trichoderma to solubilize phosphates was correlated with improved beans, wheat [230], rice [231], soybean [232], and mangrove [228] growth.
A study conducted by Li et al. [233] showed an increased nutrient (P, K, Mg, and Zn) uptake by tomato plants after pre-inoculation with T. asperellum CHF 78 strain. Moreover, the T. harzianum strain produced in liquid cultures diffusible metabolites capable of reducing Fe(III) and Cu(II) [234]. On the other hand, Singh et al. [235] proved that T. asperellum T42 mediated the enhancement in host biomass, total nitrogen content, production of nitric oxide (NO), and the accumulation of cytosolic Ca2+ in tobacco.
7. Conclusions and Future Prospects
The Trichoderma─plant─pathogen interaction is a very dynamic and multi-dependent system. Detailed knowledge of the Trichoderma mechanisms towards plants and pathogens can significantly increase the effectiveness of their action. Trichoderma uses several complex direct and indirect biocontrol mechanisms, both against biotic stresses, such as wide spectrum of pathogenic microorganisms (fungi, bacteria, insects, and nematodes), and abiotic stresses—unfavorable environmental conditions.
The knowledge about the extraordinary abilities of Trichoderma gained in recent years contributes to the creation of biopreparations based on strains with a comprehensive and beneficial effect on plants. These Trichoderma preparations will find wide application in organic farming to combat plant diseases of various etiologies, where they have a chance to provide complete protection without the use of chemical pesticides. In turn, the resistance of Trichoderma to chemical pesticides will make it possible to combine these fungi in preparations with low concentrations of various, especially newly introduced and modified, chemical pesticides. Furthermore, Trichoderma has the potential to become the basis of new phytoremediation technologies due to its resistance to a variety of toxic chemicals, both organic and inorganic, and increase plant tolerance to stress factors under conditions of xenobiotic contamination. Importantly, these solutions are in line with the idea of sustainable agriculture.
In the coming years, the methods of enhancing the effectiveness and reliability of Trichoderma preparations will be improved through treatments increasing the competitiveness of Trichoderma in the rhizosphere and rhizoplane. This can be achieved through strong and stable colonization of these zones and the combination with microorganisms supporting the positive effects of Trichoderma, the so-called supporting strains [236,237]. For instance, the volatile compounds (VCs) from some bacteria have been shown to significantly affect the secretion of antimicrobial and antifungal compounds by T. virens and T. harzianum [238].
Fungi belonging to the genus Trichoderma have become an excellent model for the study of substances determining the nature of the interaction of microorganisms with the plants, i.e., effector-like molecules [239]. Research on Trichoderma identified several plant interaction effectors, including proteins (cerato-platanins, glycoside hydrolases, hydrophobins, and small secreted cysteine-rich proteins), secondary metabolites (lactones, peptaibols, polyketides, terpenes, trichothecenes, VOCs, and phytohormones), and small (20−30 nucleotide long) non-coding RNAs. Direct evidence for the role of beneficial microorganisms RNAs on the suppression of plant immunity to establish a symbiotic relationship is sought. Trichoderma, through the production of RNAs, establishes symbiotic and non-pathogenic associations with plants [240]. Since most symbiotic effectors are members of the same families present in phytopathogens, further intensive studies of the effector molecules produced by Trichoderma will explain not only the course of interaction with microorganisms that favorably affect plants, but also those that may lead to a negative impact on agriculture.
The great importance of Trichoderma in signaling and influencing the virulence of pathogens is associated with the ability to release an unusual universal signaling substance, N-acetylglucosamine (GlcNAc), as a result of chitinolytic activity [241]. A crucial step in a successful attack on fungal hosts is the production of hydrolytic enzymes that permeabilize and degrade the fungal cell wall [242].
The main challenge is to develop and use in Trichoderma research the latest, comprehensive, advanced, and at the same time cheap, fast, and effective methods of detecting and testing antagonists, combining various modes of action and causing a cascade of reactions [243,244,245,246]. The research will also be aimed at the accurate and quick estimation of the risk of using BCAs based on Trichoderma, their toxicity and ecotoxicity not only in in vitro conditions, but above all in natural growing conditions in vitro and in situ, before being introduced to the market as biocontrols, biostimulations or bioremediation preparations. Social fears of introducing new microorganisms with antifungal and antibacterial properties into the environment should be eliminated.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23042329/s1.
Author Contributions
Conceptualization, R.T.; writing—original draft preparation, R.T.; writing—review and editing, R.T., A.N., E.O. and J.J.-Ś.; visualization, R.T. and A.N.; supervision, R.T., E.O. and J.J.-Ś. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 6PAP | 6-phenyl-α-pyrone |
| ABA | Abscisic acid |
| ACC | 1-aminocyclopropane-1-carboxylate |
| ACCD | ACC-deaminase enzyme |
| Aib | Alpha-aminoisobutyric acid |
| BABA | β-aminobutyric acid |
| BCAs | Biological control agents |
| CAT | Catalase |
| CWDEs | Cell wall degrading enzymes |
| EGD | European Green Deal |
| ET | Ethylene |
| ETI | Effector-triggered immunity |
| ETPs | Epipolythiodioxopiperazines |
| EU | European Union |
| FAO | Food and Agriculture Organization of the United Nations |
| GAs | Gibberellins |
| GH18 | Glycoside hydrolase family 18 |
| GPCRs | G-protein coupled receptors |
| GPX | Guaiacol peroxidase |
| HR | Hypersensitive response |
| IAA | Indole-3-acetic acid |
| IPM | Integrated pest management |
| IR | Induced resistance |
| ISR | Induced systemic resistance |
| Iva | Isovaline |
| JA | Jasmonic acid |
| MAMP | Microbe-associated molecular pattern |
| MAPKs | Mitogen-activated protein kinases |
| MTI | MAMP-triggered immunity |
| NO | Nitric oxide |
| NRPSs | Non-ribosomal peptide synthetases |
| P | Phosphorus |
| PAL | Phenylalanine lyase |
| PAMP | Pathogen-associated molecular pattern |
| PCWDEs | Plant cell wall degrading enzymes |
| PDA | Potato dextrose agar |
| PKSs | Polyketide synthases |
| PPNs | Plant-parasitic nematodes |
| PR | Pathogenesis-related |
| PRRs | Pattern recognition receptors |
| PTI | PAMP-triggered immunity |
| RBCs | Root cap border cells |
| ROS | Reactive oxygen species |
| SA | Salicylic acid |
| SAR | Systemic acquired resistance |
| TAL | Tyrosine lyase |
| VOCs | Volatile organic compounds |
References
- Sharma, R.R.; Singh, D.; Singh, R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biol. Control. 2009, 50, 205–221. [Google Scholar] [CrossRef]
- Savary, S.; Ficke, A.; Aubertot, N.J.; Hollier, C. Crop losses due to diseases and their implications for global food production losses and food security. Food Secur. 2012, 4, 519–537. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Wisniewski, M.; Droby, S.; Liu, Y. Review: Utilization of antagonistic yeast to manage postharvest fungal diseases of fruits. Int. J. Food Microbiol. 2013, 167, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanpour, M.; Omidvari, M.; Abbaszadeh-Dahaji, P.; Omidvar, R.; Kariman, K. Mechanisms underlying the protective effects of beneficial fungi against plant diseases. Biol. Control 2018, 117, 147–157. [Google Scholar] [CrossRef]
- Almeida, F.B.; Rodrigues, L.M.; Coelho, C. The still underestimated problem of fungal diseases worldwide. Front. Microbiol. 2019, 10, 214. [Google Scholar] [CrossRef]
- Jain, A.; Sarsaiya, S.; Wu, Q.; Lu, Y.; Shi, J. A review of plant leaf fungal diseases and its environment speciation. Bioengineered 2019, 10, 409–424. [Google Scholar] [CrossRef]
- Benítez, T.; Rincón, M.A.; Limón, C.M.; Codón, C.A. Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. 2004, 7, 249–260. [Google Scholar]
- Escrivá, L.; Front, G.; Manyes, L. In vivo toxicity studies of fusarium mycotoxins in the last decade: A review. Food Chem. Toxicol. 2015, 78, 185–206. [Google Scholar] [CrossRef]
- Zhang, D.D.; Wang, Y.X.; Chen, Y.J.; Kong, Q.Z.; Gui, J.Y.; Li, Y.N.; Bao, M.Y.; Dai, X. Identification and characterization of a pathogenicity-related gene VdCYP1 from Verticillium dahliae. Sci. Rep. 2016, 6, 27979. [Google Scholar] [CrossRef]
- Doehlemann, G.; Ökmen, B.; Zhu, W.; Sharon, A. Plant pathogenic fungi. Microbiol. Spectr. 2017, 5, 1–23. [Google Scholar] [CrossRef]
- Krylov, B.V.; Petruk, I.M.; Glushko, I.N.; Khaldeeva, V.E.; Mokeeva, L.V.; Bilanenko, N.E.; Lebedin, S.Y.; Eremin, A.S.; Nifantiev, E.N. Carbohydrate specificity of antibodies against phytopathogenic fungi of the Aspergillus genus. Appl. Biochem. Microbiol. 2018, 54, 522–527. [Google Scholar] [CrossRef]
- Li, H.; Bian, R.; Liu, Q.; Yang, L.; Pang, T.; Salaipeth, L.; Andika, B.I.; Kondo, H.; Sun, L. Identification of a novel hypovirulence-inducing Hypovirus from Alternaria alternaria. Front. Microbiol. 2019, 10, 1076. [Google Scholar] [CrossRef]
- Abbas, A.; Khan, U.S.; Khan, U.W.; Saleh, A.T.; Khan, U.H.M.; Ullah, S.; Ali, A.; Ikram, M. Antagonist effects of strains of Bacillus spp. against Rhizoctonia solani for their protection against several plant diseases: Alternatives to chemical pesticides. C. R. Biol. 2019, 342, 124–135. [Google Scholar] [CrossRef]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef]
- Alizadeh, M.; Vasebi, Y.; Safaie, N. Microbial antagonists against plant pathogens in Iran: A review. Open Agric. 2020, 5, 404–440. [Google Scholar] [CrossRef]
- Rahman, S.F.; Singh, E.; Pieterse, C.M.J.; Schenk, P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef]
- Grasswitz, T.R. Integrated pest management (IPM) for small-scale farms in developed economies: Challenges and opportunities. Insects 2019, 10, 179. [Google Scholar] [CrossRef]
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.L.; Kannangara, S.D.; Promputtha, I. Fungi vs. fungi in biocontrol: An overview of fungal antagonists applied against fungal plant pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 718. [Google Scholar] [CrossRef]
- Subedi, P.; Gattoni, K.; Liu, W.; Lawrence, K.S.; Park, S.W. Current utility of plant growth-promoting rhizobacteria as biological control agents towards plant-parasitic nematodes. Plants 2020, 9, 1167. [Google Scholar] [CrossRef]
- Niu, B.; Wang, W.; Yuan, Z.; Sederoff, R.R.; Sederoff, H.; Chiang, V.L.; Borriss, R. Microbial interactions within multiple-strain Biological Control Agents impact soil-borne plant disease. Front Microbiol. 2020, 11, 585404. [Google Scholar] [CrossRef]
- Savita, S.A. Fungi as biological control agents. In Biofertilizers for Sustainable Agriculture and Environment: Soil Biology; Giri, B., Prasad, R., Wu, Q.S., Varma, A., Eds.; Springer: Cham, Switzerland, 2019; Volume 55. [Google Scholar] [CrossRef]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; De Rosa, A.; Gioia, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. Trichoderma-based biostimulants modulate rhizosphere microbial populations and improve N uptake efficiency, yield, and nutritional quality of leafy vegetables. Front. Plant Sci. 2018, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Montanarella, L.; Panagos, P. The relevance of sustainable soil management within the European Green Deal. Land Use Policy 2021, 100, 104950. [Google Scholar] [CrossRef]
- Gajera, H.; Domadiya, R.; Patel, S.; Kapopara, M.; Golakiya, B. Molecular mechanism of Trichoderma as bio-control agents against phytopathogen system—A review. Curr. Res. Microbiol. Biotechnol. 2013, 1, 133–142. [Google Scholar]
- Martínez-Medina, A.; Alguacil, M.D.M.; Pascual, J.A.; Van Wees, S.C.M. Phytohormone profiles induced by Trichoderma isolates correspond with their biocontrol and plant growth-promoting activity on melon plants. J. Chem. Ecol. 2014, 40, 804–815. [Google Scholar] [CrossRef]
- Jaroszuk-Ściseł, J.; Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Majewska, M.; Hanaka, A.; Tyśkiewicz, K.; Pawlik, A.; Janusz, G. Phytohormones (auxin, gibberellin) and ACC deaminase in vitro synthesized by the mycoparasitic Trichoderma DEMTkZ3A0 strain and changes in the level of auxin and plant resistance markers in wheat seedlings inoculated with this strain conidia. Int. J. Mol. Sci. 2019, 20, 4923. [Google Scholar] [CrossRef]
- Howell, C.R. Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Plant Dis. 2003, 87, 1–10. [Google Scholar] [CrossRef]
- Kamala, T.; Devi, S.I.; Sharma, K.C.; Kennedy, K. Phylogeny and taxonomical investigation of Trichoderma spp. from Indian region of Indo-Burma biodiversity hot spot region with special reference to Manipur. BioMed Res. Int. 2015, 285261. [Google Scholar] [CrossRef]
- Błaszczyk, L.; Siwulski, M.; Sobieralski, K.; Lisiecka, J.; Jędryczka, M. Trichoderma spp.—Application and prospects for use in organic farming and industry. J. Plant Prot. Res. 2014, 54, 309–317. [Google Scholar] [CrossRef]
- Samuels, G.J.; Dodd, S.L.; Lu, B.S.; Petrini, O.; Schroers, H.J.; Druzhinina, I.S. The Trichoderma koningii aggregate species. Stud. Mycol. 2006, 56, 67–133. [Google Scholar] [CrossRef]
- Hassan, M.M.; Farid, M.A.; Gaber, A. Rapid identification of Trichoderma koningiopsis and Trichoderma longibrachiatum using sequence-characterized amplified region markers. Egypt J. Biol. Pest. Control 2019, 29, 13. [Google Scholar] [CrossRef]
- Bissett, J.; Gams, W.; Jaklitsch, W.; Samuels, G.J. Accepted Trichoderma names in the year 2015. IMA Fungus 2015, 6, 263–295. [Google Scholar] [CrossRef]
- Marik, T.; Tyagi, C.; Balázs, D.; Urbán, P.; Szepesi, Á.; Bakacsy, L.; Endre, G.; Rakk, D.; Szekeres, A.; Andersson, M.A.; et al. Structural diversity and bioactivities of peptaibol compounds from the longibrachiatum clade of the filamentous fungal genus Trichoderma. Front. Microbiol. 2019, 10, 1434. [Google Scholar] [CrossRef]
- Tamandegani, P.R.; Marik, T.; Zafari, D.; Balázs, D.; Vágvölgyi, C.; Szekeres, A.; Kredics, L. Changes in peptaibol production of Trichoderma species during in vitro antagonistic interactions with fungal plant pathogens. Biomolecules 2020, 10, 730. [Google Scholar] [CrossRef]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Cocaign, A.; Bui, L.C.; Silar, P.; Chan Ho Tong, L.; Busi, F.; Lamouri, A.; Mougin, C.; Rodrigues-Lima, F.; Dupret, J.M.; Dairou, J. Biotransformation of Trichoderma spp. and their tolerance to aromatic amines, a major class of pollutants. Appl. Environ. Microbiol. 2013, 79, 4719–4726. [Google Scholar] [CrossRef]
- De Padua, J.C.; dela Cruz, T.E.E. Isolation and characterization of nickel-tolerant Trichoderma strains from marine and terrestrial environments. J. Fungi 2021, 7, 591. [Google Scholar] [CrossRef]
- Escudero-Leyva, E.; Alfaro-Vargas, P.; Muñoz-Arrieta, R.; Charpentier-Alfaro, C.; Granados-Montero, M.M.; Valverde-Madrigal, K.S.; Pérez-Villanueva, M.; Méndez-Rivera, M.; Rodríguez-Rodríguez, C.E.; Chaverri, P.; et al. Tolerance and biological removal of fungicides by Trichoderma species isolated from the endosphere of wild Rubiaceae plants. Front. Agron. 2022, 3, 772170. [Google Scholar] [CrossRef]
- Alothman, Z.; Bahkali, A.; Elgorban, A.; Al-Otaibi, M.; Ghfar, A.; Gabr, S.; Wabaidur, S.; Habila, M.; Ahmed, A. Bioremediation of explosive TNT by Trichoderma viride. Molecules 2020, 25, 1393. [Google Scholar] [CrossRef]
- Morales-Barrera, L.; Cristiani-Urbina, E. Hexavalent chromium removal by a Trichoderma inhamatum fungal strain isolated from tannery effluent. Water Air Soil Pollut. 2008, 187, 327–336. [Google Scholar] [CrossRef]
- Sandle, T. Trichoderma. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 644–646. [Google Scholar] [CrossRef]
- Qiao, M.; Du, X.; Zhang, Z.; Xu, J.P.; Yu, Z.F. Three new species of soil-inhabiting Trichoderma from southwest China. MycoKeys 2018, 44, 63–80. [Google Scholar] [CrossRef]
- Eastburn, D.M.; Butler, E.E. Effect of soil moisture and temperature on the saprophytic ability of Trichoderma harzianum. Mycologia 1991, 83, 257–263. [Google Scholar] [CrossRef]
- Poosapati, S.; Ravulapalli, P.D.; Tippirishetty, N.; Vishwanathaswamy, D.K.; Chunduri, S. Selection of high temperature and salinity tolerant Trichoderma isolates with antagonistic activity against Sclerotium rolfsii. SpringerPlus 2014, 3, 1–11. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Kumar, D. Trichoderma: A beneficial antifungal agent and insights into its mechanisms of biocontrol potential. Egypt. J. Biol. Pest Control 2020, 30, 133. [Google Scholar] [CrossRef]
- Siddiquee, S. Morphology-based characterization of Trichoderma species. In Practical Handbook of the Biology and Molecular Diversity of Trichoderma Species from Tropical Regions; Springer: Cham, Switzerland, 2017; pp. 41–73. [Google Scholar] [CrossRef]
- Weindling, R. Trichoderma lignorum as a parasite of other soil fungi. Phytopathology 1932, 22, 837–845. [Google Scholar]
- Kumar, M.A.; Sharma, P. Morphological characterization of biocontrol isolates of Trichoderma to study the correlation between morphological characters and biocontrol efficacy. Int. Lett. Nat. Sci. 2016, 55, 57–67. [Google Scholar] [CrossRef]
- Błaszczyk, L.; Basińska-Barczak, A.; Ćwiek-Kupczyńska, H.; Gromadzka, K.; Popiel, D.; Stępień, Ł. Suppressive effect of Trichoderma spp. on toxigenic Fusarium species. Pol. J. Microbiol. 2017, 66, 85–100. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Yu, C.; Dou, K.; Wang, M.; Li, Y.; Chen, J. Synergistic effect of Trichoderma-derived antifungal metabolites and cell wall degrading enzymes on enhanced biocontrol of Fusarium oxysporum f. sp. cucumerinum. Biol. Control 2016, 94, 37–46. [Google Scholar] [CrossRef]
- Rai, S.; Kashyap, P.L.; Kumar, S.; Srivastava, A.K.; Ramteke, P.W. Identification, characterization and phylogenetic analysis of antifungal Trichoderma from tomato rhizosphere. SpringerPlus 2016, 5, 1939. [Google Scholar] [CrossRef]
- Srivastava, M.; Kumar, V.; Shahid, M.; Pandey, S.; Singh, A. Trichoderma—A potential and effective bio fungicide and alternative source against notable phytopathogens: A review. Afr. J. Agric. Res. 2016, 11, 310–316. [Google Scholar] [CrossRef]
- Hoyos-Carvajal, L.; Orduz, S.; Bissett, J. Growth stimulation in bean (Phaseolus vulgaris L.) by Trichoderma. Biol. Control 2009, 51, 409–416. [Google Scholar] [CrossRef]
- Grant, M.R.; Jones, J.D. Hormone (dis)harmony moulds plant health and disease. Science 2009, 324, 750–752. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Leon-Reyes, A.; Van der Ent, S.; Van Wees, S.C.M. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Čarná, M.; Repka, V.; Skůpa, P.; Šturdík, E. Auxins in defense strategies. Biologia 2014, 69, 1255–1263. [Google Scholar] [CrossRef]
- Sánchez-Montesinos, B.; Santos, M.; Moreno-Gavíra, A.; Marín-Rodulfo, T.; Gea, F.J.; Diánez, F. Biological control of fungal diseases by Trichoderma aggressivum f. europaeum and its compatibility with fungicides. J. Fungi 2021, 7, 598. [Google Scholar] [CrossRef] [PubMed]
- Gea, F.J.; Navarro, M.J.; Santos, M.; Diánez, F.; Carrasco, J. Control of fungal diseases in mushroom crops while dealing with fungicide resistance: A review. Microorganisms 2021, 9, 585. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Bae, K.S.; Yu, S.H. Two new species of Trichoderma associated with green mold of oyster mushroom cultivation in Korea. Mycobiology 2006, 34, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Szczech, M.; Staniaszek, M.; Habdas, H.; Uliński, Z.; Szymański, J. Trichoderma spp.—The cause of green mold on polish mushroom farms. J. Fruit Ornam. Plant Res. 2008, 69, 105–114. [Google Scholar] [CrossRef]
- Pfordt, A.; Schiwek, S.; Karlovsky, P.; von Tiedemann, A. Trichoderma afroharzianum ear rot–a new disease on maize in Europe. Front. Agron. 2020, 2, 547758. [Google Scholar] [CrossRef]
- Ram, R.M.; Singh, H.B. Trichoderma spp.: An opportunistic pathogen. Biotech Today 2018, 8, 16–24. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Kumar, N.; Shandilya, C.; Mohapatra, S.; Bhayana, S.; Varma, A. Revisiting plant–microbe interactions and microbial consortia application for enhancing sustainable agriculture: A review. Front. Microbiol. 2020, 11, 560406. [Google Scholar] [CrossRef]
- Rush, T.A.; Shrestha, H.K.; Gopalakrishnan Meena, M.; Spangler, M.K.; Ellis, J.C.; Labbé, J.L.; Abraham, P.E. Bioprospecting Trichoderma: A systematic roadmap to screen genomes and natural products for biocontrol applications. Front. Fungal Biol. 2021, 2, 716511. [Google Scholar] [CrossRef]
- Kovács, C.; Csótó, A.; Pál, K.; Nagy, A.; Fekete, E.; Karaffa, L.; Kubicek, C.P.; Sándor, E. The biocontrol potential of endophytic Trichoderma fungi isolated from Hungarian grapevines. Part I. Isolation, identification and in vitro studies. Pathogens 2021, 10, 1612. [Google Scholar] [CrossRef]
- EU Pesticides Database. Available online: https://ec.europa.eu/food/plants/pesticides/eu-pesticides-database_pl (accessed on 24 January 2022).
- BPDB: Bio-Pesticides Database. Available online: https://sitem.herts.ac.uk/aeru/bpdb/atoz.htm (accessed on 24 January 2022).
- Biopesticide Products and Active Ingredients Registered for Use in USA by the United States Environmental Protection Agency (EPA). Available online: https://www.epa.gov/ingredients-used-pesticide-products/biopesticide-active-ingredients (accessed on 24 January 2022).
- List of Trichoderma-Based Biopesticides Registered in Brazil. Available online: https://www.agrolink.com.br/agrolinkfito/busca-direta-produto (accessed on 24 January 2022).
- Registered Biocontrol and Biopesticide Products around the World. Available online: https://bioprotectionportal.com/ (accessed on 24 January 2022).
- Registered Pesticide Products in Canada. Available online: https://pr-rp.hc-sc.gc.ca/pi-ip/result-eng.php?1=0&2=501&3=pr&4=n&5=1&6=ASC&7=B&8=E (accessed on 24 January 2022).
- Registered Bio-Fungicides with Trichoderma as the Active Ingredient Use in New Zealand and Australia. Available online: https://agrimm.co.nz/ (accessed on 24 January 2022).
- Chaverri, P.; Branco-Rocha, F.; Jaklitsch, W.; Gazis, R.; Degenkolb, T.; Samuels, G.J. Systematics of the Trichoderma harzianum species complex and the re-identification of commercial biocontrol strains. Mycologia 2015, 107, 558–590. [Google Scholar] [CrossRef]
- Fanelli, F.; Liuzzi, V.C.; Logrieco, A.F.; Altomare, C. Genomic characterization of Trichoderma atrobrunneum (T. harzianum species complex) ITEM 908: Insight into the genetic endowment of a multi-target biocontrol strain. BMC Genom. 2018, 19, 662. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Seidl-Seiboth, V.; Herrera-Estrella, A.; Horwitz, B.A.; Kenerley, C.M.; Monte, E.; Mukherjee, P.K.; Zeilinger, S.; Grigoriev, I.V.; Kubicek, C.P. Trichoderma: The genomics of opportunistic success. Nat. Rev. Microbiol. 2011, 9, 749–759. [Google Scholar] [CrossRef]
- Seidl-Seiboth, V.; Ihrmark, K.; Druzhinina, I.S.; Karlsson, M. Molecular evolution of Trichoderma chitinases. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M.G., Eds.; Elsevier: Oxford, UK, 2014; pp. 67–78. [Google Scholar] [CrossRef]
- Mukherjee, M.; Mukherjee, P.K.; Horwitz, B.A.; Zachow, C.; Berg, G.; Zeilinger, S. Trichoderma-plant-pathogen interactions: Advances in genetics of biological control. Indian J. Microbiol. 2012, 53, 522–529. [Google Scholar] [CrossRef]
- Moreno-Ruiz, D.; Lichius, A.; Turra, D.; Di Pietro, A.; Zeilinger, S. Chemotropism assays for plant symbiosis and mycoparasitism related compound screening in Trichoderma atroviride. Front. Microbiol. 2020, 11, 601251. [Google Scholar] [CrossRef]
- Suarez, M.B.; Vizcaino, J.A.; Llobell, A.; Monte, E. Characterization of genes encoding novel peptidases in the biological fungus Trichoderma harzianum CECT 2413 using the TrichoEST functional genomics approach. Curr. Genet. 2007, 51, 331–342. [Google Scholar] [CrossRef]
- Seidl, V.; Song, L.; Lindquist, E.; Gruber, S.; Koptchinskiy, A.; Zeilinger, S.; Schmoll, M.; Martínez, P.; Sun, J.; Grigoriev, I.; et al. Transcriptomic response of the mycoparasitic fungus Trichoderma atroviride to the presence of a fungal prey. BMC Genom. 2009, 10, 567. [Google Scholar] [CrossRef]
- Rocha-Ramirez, V.; Omero, C.; Chet, I.; Horwitz, B.A.; Herrera-Estrella, A. Trichoderma atroviride G-protein alpha-subunit gene tga1 is involved in mycoparasitic coiling and conidiation. Eukaryot. Cell 2002, 1, 594–605. [Google Scholar] [CrossRef]
- Reithner, B.; Brunner, K.; Schuhmacher, R.; Peissl, I.; Seidl, V.; Krska, R.; Zeilinger, S. The G protein α subunit Tga1 of Trichoderma atroviride is involved in chitinase formation and differential production of antifungal metabolites. Fungal Genet. Biol. 2005, 42, 749–760. [Google Scholar] [CrossRef]
- Schmoll, M. The information highways of a biotechnological workhorse—Signal transduction in Hypocrea jecorina. BMC Genom. 2008, 9, 430. [Google Scholar] [CrossRef]
- Almeida, F.B.; Cerqueira, F.M.; Silva Rdo, N.; Ulhoa, C.J.; Lima, A.L. Mycoparasitism studies of Trichoderma harzianum strains against Rhizoctonia solani: Evaluation of coiling and hydrolytic enzyme production. Biotechnol. Lett. 2007, 29, 1189–1193. [Google Scholar] [CrossRef]
- Mukherjee, P.K. Genomics of biological control—Whole genome sequencing of two mycoparasitic Trichoderma spp. Curr. Sci. 2011, 101, 268. [Google Scholar]
- Khare, E.; Kumar, S.; Kim, K. Role of peptaibols and lytic enzymes of Trichoderma cerinum Gur1 in biocontrol of Fusarium oxysporum and chickpea wilt. Environ. Sustain. 2018, 1, 39–47. [Google Scholar] [CrossRef]
- Martin, K.; McDougall, B.M.; McIlroy, S.; Chen, J.; Seviour, R.J. Biochemistry and molecular biology of exocellular fungal β-(1,3)- and β-(1,6)-glucanases. FEMS Microbiol. Rev. 2007, 31, 168–192. [Google Scholar] [CrossRef]
- Matroudi, S.; Zamani, M.R.; Motallebi, M. Molecular cloning of chitinase 33 (chit33) gene from Trichoderma atroviride. Braz. J. Microbiol. 2008, 39, 433–437. [Google Scholar] [CrossRef]
- Kang, X.; Kirui, A.; Muszyński, A.; Dickwella Widanage, M.C.; Chen, A.; Azadi, P.; Wang, P.; Mentink-Vigier, F.; Wang, T. Molecular architecture of fungal cell wall revealed by solid-state NMR. Nat. Commun. 2018, 9, 2747. [Google Scholar] [CrossRef]
- Ribeiro, M.S.; de Paula, R.G.; Voltan, A.R.; de Castro, R.G.; Carraro, C.B.; de Assis, L.J.; Steindorff, A.S.; Goldman, G.H.; Silva, R.N.; Ulhoa, C.J.; et al. Endo β 1,3 glucanase (GH16 family) from Trichoderma harzianum participates in cell wall biogenesis but is not essential for antagonism against plant pathogens. Biomolecules 2019, 9, 781. [Google Scholar] [CrossRef]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “secrets” of a multitalented biocontrol agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- Loc, N.H.; Huy, N.D.; Quang, H.T.; Lan, T.T.; Ha, T.T.T. Characterization and antifungal activity of extracellular chitinase from a biocontrol fungus, Trichoderma asperellum PQ34. Mycology 2020, 11, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Hartl, L.; Zach, S.; Seidl-Seiboth, V. Fungal chitinases: Diversity, mechanistic properties and biotechnological potential. Appl. Microbiol. Biotechnol. 2012, 93, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Hamid, R.; Khan, M.A.; Ahmad, M.; Ahmad, M.M.; Abdin, M.Z.; Musarrat, J.; Javed, S. Chitinases: An update. J. Pharm. Bioallied Sci. 2013, 5, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Duo-Chuan, L. Review of fungal chitinases. Mycopathologia 2006, 161, 345–360. [Google Scholar] [CrossRef] [PubMed]
- De Marco, J.L.; Felix, C.R. Purification and characterization of a β-glucanase produced by Trichoderma harzianum showing biocontrol potential. Braz. Arch. Biol. Technol. 2007, 50, 21–29. [Google Scholar] [CrossRef]
- Vieira, P.M.; Coelho, A.S.G.; Steindorff, A.S.; de Siqueira, S.J.L.; do Nascimento Silva, R.; Ulhoa, C.J. Identification of differentially expressed genes from Trichoderma harzianum during growth on cell wall of Fusarium solani as a tool for biotechnological application. BMC Genom. 2013, 14, 177. [Google Scholar] [CrossRef]
- Djonović, S.; Pozo, M.J.; Kenerley, C.M. Tvbgn3, a beta-1,6-glucanase from the biocontrol fungus Trichoderma virens, is involved in mycoparasitism and control of Pythium ultimum. Appl. Environ. Microbiol. 2006, 72, 7661–7670. [Google Scholar] [CrossRef]
- Mallikharjuna Rao, K.L.N.; Raju, K.S.; Ravisankar, H. Cultural condition on the production of extracellular enzymes by Trichoderma isolates from tobacco rhizosphere. Braz. J. Microbiol. 2016, 47, 25–32. [Google Scholar] [CrossRef]
- Gueye, N.; Kumar, G.K.; Ndiaye, M.; Sall, S.Y.D.; Ndiaye, M.A.F.; Diop, T.A.; Ram, M.R. Factors affecting the chitinase activity of Trichoderma asperellum isolated from agriculture field soils. J. Appl. Biol. Biotechnol. 2020, 8, 41–44. [Google Scholar] [CrossRef]
- De Souza, P.M.; De Assis Bittencourt, M.L.; Caprara, C.C.; De Freitas, M.; De Almeida, R.P.C.; Silveira, D.; Fonseca, Y.M.; Ferreira Filho, E.X.; Pessoa Junior, A.; Megalhães, P.O. A biotechnology perspective of fungal proteases. Braz. J. Microbiol. 2015, 46, 337–346. [Google Scholar] [CrossRef]
- De Marco, J.L.; Felix, C.R. Characterization of a protease produced by a Trichoderma harzianum isolate which controls cocoa plant witches’ broom disease. BMC Biochem. 2002, 3, 3. [Google Scholar] [CrossRef]
- Pozo, M.J.; Baek, J.M.; García, J.M.; Kenerley, C.M. Functional analysis of tvsp1, a serine protease-encoding gene in the biological agent Trichoderma virens. Fungal Genet. Biol. 2004, 41, 336–348. [Google Scholar] [CrossRef]
- Deng, J.-J.; Huang, W.-Q.; Li, Z.-W.; Lu, D.-L.; Zhang, Y.; Luo, X.-C. Biocontrol activity of recombinant aspartic protease from Trichoderma harzianum against pathogenic fungi. Enzyme Microb. Technol. 2018, 112, 35–42. [Google Scholar] [CrossRef]
- Delgado-Jarana, J.; Rincón, A.M.; Benítez, T. Aspartyl protease from Trichoderma harzianum CECT 2413: Cloning and characterization. Microbiology 2002, 148, 1305–1315. [Google Scholar] [CrossRef]
- Eslahi, N.; Kowsari, M.; Zamani, M.R.; Motallebi, M. Correlation study between biochemical and molecular pathways of Trichoderma harzianum recombinant strains on plant growth and health. J. Plant Growth Regul. 2021, 1–17. [Google Scholar] [CrossRef]
- Neumann, N.K.; Stoppacher, N.; Zeilinger, S.; Degenkolb, T.; Bruckner, H.; Schuhmacher, R. The peptaibiotics database—A comprehensive online source. Chem. Biodivers. 2015, 12, 743–751. [Google Scholar] [CrossRef]
- Daniel, J.F.; Filho, E.R. Peptaibols of Trichoderma. Nat. Prod. Rep. 2007, 24, 1128–1141. [Google Scholar] [CrossRef]
- Zeilinger, S.; Gruber, S.; Bansal, R.; Mukherjee, P.K. Secondary metabolism in Trichoderma—Chemistry meets genomics. Fungal Biol. Rev. 2016, 30, 74–90. [Google Scholar] [CrossRef]
- Siddiquee, S. Fungal volatile organic compounds: Emphasis on their plant growth-promoting. In Volatiles and Food Security; Choudhary, D., Sharma, A., Agarwal, P., Varma, A., Tuteja, N., Eds.; Springer: Singapore, 2017; pp. 313–333. [Google Scholar] [CrossRef]
- De Zotti, M.; Sella, L.; Bolzonello, A.; Gabbatore, L.; Peggion, C.; Bortolotto, A.; Elmaghraby, I.; Tundo, S.; Favaron, F. Targeted amino acid substitutions in a Trichoderma peptaibol confer activity against fungal plant pathogens and protect host tissues from Botrytis cinerea infection. Int. J. Mol. Sci. 2020, 21, 7521. [Google Scholar] [CrossRef]
- Zeilinger, S.; García-Estrada, C.; Martín, J.-F. Fungal secondary metabolites in the “OMICS” era. In Biosynthesis and Molecular Genetics of Fungal Secondary Metabolites, 2nd ed.; Zeilinger, S., Martín, J.-F., García-Estrada, C., Eds.; Springer: New York, NY, USA, 2015; pp. 1–12. [Google Scholar] [CrossRef]
- Keller, N.P.; Turner, G.; Bennett, J.W. Fungal secondary metabolism—From biochemistry to genomics. Nat. Rev. Microbiol. 2005, 3, 937–947. [Google Scholar] [CrossRef]
- Baker, S.E.; Perrone, G.; Richardson, A.; Gallo, A.; Kubicek, C.P. Phylogenomic analysis of polyketide synthase-encoding genes in Trichoderma. Microbiology 2012, 158, 147–154. [Google Scholar] [CrossRef]
- Atanasova, L.; Knox, B.P.; Kubicek, C.P.; Druzhinina, I.S.; Baker, S.E. The polyketide synthase gene pks4 of Trichoderma reesei provides pigmentation and stress resistance. Eukaryot. Cell 2013, 12, 1499–1508. [Google Scholar] [CrossRef]
- Yao, L.; Tan, C.; Song, J.; Yang, Q.; Yu, L.; Li, X. Isolation and expression of two polyketide synthase genes from Trichoderma harzianum 88 during mycoparasitism. Braz. J. Microbiol. 2016, 47, 468–479. [Google Scholar] [CrossRef][Green Version]
- Siddiquee, S. Recent advancements on the role and analysis of volatile compounds (VOCs) from Trichoderma. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 139–175. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Lo, C.-T.; Shibu, M.A.; Leu, Y.-L.; Jen, B.-Y.; Peng, K.-C. Study on the anthraquinones separated from the cultivation of Trichoderma harzianum strain Th-R16 and their biological activity. J. Agric. Food Chem. 2009, 57, 7288–7292. [Google Scholar] [CrossRef]
- Lin, Y.R.; Lo, C.T.; Li, S.Y.; Peng, K.C. Involvement of pachybasin and emodin in self-regulation of Trichoderma harzianum mycoparasitic coiling. J. Agric. Food Chem. 2012, 60, 2123–2128. [Google Scholar] [CrossRef]
- Scharf, D.H.; Brakhage, A.A.; Mukherjee, P.K. Gliotoxin—Bane or boon? Environ. Microbiol. 2016, 18, 1096–1109. [Google Scholar] [CrossRef]
- Vargas, W.A.; Mukherjee, P.K.; Laughlin, D.; Wiest, A.; Moran-Diez, M.E.; Kenerley, C.M. Role of gliotoxin in the symbiotic and pathogenic interactions of Trichoderma virens. Microbiology 2014, 160, 2319–2330. [Google Scholar] [CrossRef]
- Vinale, F.; Flematti, G.; Sivasithamparam, K.; Lorito, M.; Marra, M.; Skelton, B.W.; Ghisalberti, E.L. Harzianic acid, an antifungal and plant growth promoting metabolite from Trichoderma harzianum. J. Nat. Prod. 2009, 72, 2032–2035. [Google Scholar] [CrossRef]
- Oszust, K.; Cybulska, J.; Frąc, M. How do Trichoderma genus fungi win a nutritional competition battle against soft fruit pathogens? A report on niche overlap nutritional potentiates. Int. J. Mol. Sci. 2020, 21, 4235. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma-plant pathogen interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Khan, A.; Singh, P.; Srivastava, A. Synthesis, nature and utility of universal iron chelator—Siderophore: A review. Microbiol. Res. 2018, 212–213, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, Y.; Kong, S. Effects of Trichoderma asperellum and its siderophores on endogenous auxin in Arabidopsis thaliana under iron-deficiency stress. Int. Microbiol. 2020, 23, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Miethke, M. Molecular strategies of microbial iron assimilation: From high-affinity complexes to cofactor assembly systems. Metallomics 2013, 5, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Banerjee, S.; Sengupta, C. Siderophore production by antagonistic fungi. J. Biopestic. 2017, 10, 105–112. [Google Scholar]
- Chowdappa, S.; Jagannath, S.; Konappa, N.; Udayashankar, A.C.; Jogaiah, S. Detection and characterization of antibacterial siderophores secreted by endophytic fungi from Cymbidium aloifolium. Biomolecules 2020, 10, 1412. [Google Scholar] [CrossRef]
- Srivastava, M.P.; Gupta, S.; Sharm, Y.K. Detection of siderophore production from different cultural variables by CAS-agar plate assay. Asian J. Pharm. Pharmacol. 2018, 4, 66–69. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Mokhtar, H.; Aid, D. Contribution in isolation and identification of some pathogenic fungi from wheat seeds, and evaluation of antagonistic capability of Trichoderma harzianum against those isolated fungi in vitro. Agri. Biol. J. North Am. 2013, 4, 145–154. [Google Scholar] [CrossRef]
- Segarra, G.; Casanova, E.; Aviles, M.; Trillas, I. Trichoderma asperellum strain T34 controls Fusarium wilt disease in tomato plants in soilless culture through competition for iron. Microb. Ecol. 2010, 59, 141–149. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Wang, M.-H. Isolation and molecular identification of Trichoderma species from wetland soil and their antagonistic activity against phytopathogens. Physiol. Mol. Plant Pathol. 2020, 109, 101458. [Google Scholar] [CrossRef]
- Montesano, M.; Brader, G.; Palva, E.T. Pathogen derived elicitors: Searching for receptors in plants. Mol. Plant Pathol. 2003, 4, 73–79. [Google Scholar] [CrossRef]
- Thakur, M.; Sohal, B.S. Role of elicitors in inducing resistance in plants against pathogen infection: A review. ISRN Biochem. 2013, 762412. [Google Scholar] [CrossRef]
- Harman, G.E.; Herrera-Estrella, A.H.; Horwitz, B.A.; Lorito, M. Special issue: Trichoderma—From basic biology to biotechnology. Microbiology 2012, 158, 1–2. [Google Scholar] [CrossRef]
- Aljbory, Z.; Chen, M.-S. Indirect plant defense against insect herbivores: A review. Insect Sci. 2018, 25, 2–23. [Google Scholar] [CrossRef]
- Bhaskar, R.; Xavier, L.S.E.; Udayakumaran, G.; Kumar, D.S.; Venkatesh, R.; Nagella, P. Biotic elicitors: A boon for the in-vitro production of plant secondary metabolites. Plant Cell Tiss. Organ. Cult. 2021, 147, 1–18. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Hanaka, A.; Ozimek, E.; Reszczyńska, E.; Jaroszuk-Ściseł, J.; Stolarz, M. Plant tolerance to drought stress in the presence of supporting bacteria and fungi: An efficient strategy in horticulture. Horticulturae 2021, 7, 390. [Google Scholar] [CrossRef]
- De Schutter, K.; Van Damme, E.J.M. Protein-carbohydrate interactions as part of plant defense and animal immunity. Molecules 2015, 20, 9029–9053. [Google Scholar] [CrossRef]
- Jagodzik, P.; Tajdel-Zielińska, M.; Cieśla, A.; Marczak, M.; Ludwikow, A. Mitogen-activated protein kinase cascades in plant hormone signalling. Front. Plant Sci. 2018, 9, 1387. [Google Scholar] [CrossRef]
- Ton, J.; Jakab, G.; Toquin, V.; Flors, V.; Iavicoli, A.; Maeder, M.N.; Métraux, J.-P.; Mauch-Mani, B. Dissecting the β-aminobutyric acid-induced priming phenomenon in Arabidopsis. Plant Cell 2005, 17, 987–999. [Google Scholar] [CrossRef]
- Shoresh, M.; Harman, G.E.; Mastoury, F. Induced systemic resistance and plant response to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Hartman, G.L.; Pawlowski, M.L.; Chang, H.-X.; Hill, C.B. Successful technologies and approaches used to develop and manage resistance against crop diseases and pests. In Woodhead Publishing Series in Food Science, Technology and Nutrition. Emerging Technologies for Promoting Food Security; Madramootoo, C., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 43–66. [Google Scholar] [CrossRef]
- Boccardo, N.A.; Segretin, M.E.; Hernandez, I.; Mirkin, F.G.; Chaćon, O.; Lopez, Y.; Borrás-Hidalgo, O.; Bravo-Almonacid, F.F. Expression of pathogenesis-related proteins in transplastomic tobacco plants confers resistance to filamentous pathogens under field trials. Sci. Rep. 2019, 9, 2791. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.-Y.; Sattler, S.A.; Cortez, G.S.; Vermerris, W.; Sattler, S.E.; Kang, C. Biochemical and structural analysis of substrate specificity of a phenylalanine ammonia-lyase. Plant Physiol. 2018, 176, 1452–1468. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Mishra, S.; Ray, S.; Raghuwanshi, R.; Singh, H.B. Differential reprogramming of defense network in Capsicum annum L. plants against Colletotrichum truncatum infection by phyllospheric and rhizospheric Trichoderma strains. J. Plant Growth Regul. 2020, 39, 751–763. [Google Scholar] [CrossRef]
- Perazzolli, M.; Dagostin, S.; Ferrari, A.; Elad, Y.; Pertot, I. Induction of systemic resistance against Plasmopara viticolain grapevine by Trichoderma harzianum T39 and benzothiadiazole. Biol. Control 2008, 47, 228–234. [Google Scholar] [CrossRef]
- Yuan, M.; Huang, Y.; Ge, W.; Jia, Z.; Song, S.; Zhang, L.; Huang, Y. Involvement of jasmonic acid, ethylene and salicylic acid signaling pathways behind the systemic resistance induced by Trichoderma longibrachiatum H9 in cucumber. BMC Genom. 2019, 20, 144. [Google Scholar] [CrossRef]
- Ton, J.; van der Ent, S.; van Hulten, M.; Pozo, M.; van Oosten, V.; van Loon, L.C.; Mauch-Mani, B.; Turlings, T.C.J.; Pieterse, C.M.J. Priming as a mechanism behind induced resistance against pathogens, insects and abiotic stress. IOBC/wprs Bull. 2009, 44, 3–13. [Google Scholar]
- Aranega-Bou, P.; de la O Leyva, M.; Finiti, I.; García-Agustín, P.; González-Bosch, C. Priming of plant resistance by natural compounds. Hexanoic acid as a model. Front. Plant Sci. 2014, 5, 488. [Google Scholar] [CrossRef]
- Gupta, R.; Bar, M. Plant immunity, priming, and systemic resistance as mechanisms for Trichoderma spp. biocontrol. In Trichoderma. Rhizosphere Biology; Sharma, A., Sharma, P., Eds.; Springer: Singapore, 2020; pp. 81–110. [Google Scholar] [CrossRef]
- Verma, H.; Kumar, D.; Kumar, V.; Kumari, M.; Singh, S.K.; Sharma, V.K.; Droby, S.; Santoyo, G.; White, J.F.; Kumar, A. The potential application of endophytes in management of stress from drought and salinity in crop plants. Microorganisms 2021, 9, 1729. [Google Scholar] [CrossRef]
- Hidangmayum, A.; Dwivedi, P. Plant responses to Trichoderma spp. and their tolerance to abiotic stresses: A review. J. Pharmacogn. Phytochem. 2018, 7, 758–766. [Google Scholar]
- Poveda, J. Trichoderma parareesei favors the tolerance of rapeseed (Brassica napus L.) to salinity and drought due to a chorismate mutase. Agronomy 2020, 10, 118. [Google Scholar] [CrossRef]
- Abdullah, N.S.; Doni, F.; Mispan, M.S.; Saiman, M.Z.; Yusuf, Y.M.; Oke, M.A.; Suhaimi, N.S.M. Harnessing Trichoderma in agriculture for productivity and sustainability. Agronomy 2021, 11, 2559. [Google Scholar] [CrossRef]
- Azad, K.; Kaminskyj, S. A fungal endophyte strategy for mitigating the effect of salt and drought stress on plant growth. Symbiosis 2016, 68, 73–78. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, Y.; Xu, B. Application of plant-growth-promoting fungi Trichoderma longibrachiatum T6 enhances tolerance of wheat to salt stress through improvement of antioxidative defense system and gene expression. Front. Plant Sci. 2016, 7, 1405. [Google Scholar] [CrossRef]
- Silletti, S.; Di Stasio, E.; Van Oosten, M.J.; Ventorino, V.; Pepe, O.; Napolitano, M.; Marra, R.; Woo, S.L.; Cirillo, V.; Maggio, A. Biostimulant activity of Azotobacter chroococcum and Trichoderma harzianum in durum wheat under water and nitrogen deficiency. Agronomy 2021, 11, 380. [Google Scholar] [CrossRef]
- Ghorbanpour, A.; Salimi, A.; Ghanbary, M.A.T.; Pirdashti, H.; Dehestani, A. The effect of Trichoderma harzianum in mitigating low temperature stress in tomato (Solanum lycopersicum L.) plants. Sci. Hortic. 2018, 230, 134–141. [Google Scholar] [CrossRef]
- Tripathi, R.; Keswani, C.; Tewari, R. Trichoderma koningii enhances tolerance against thermal stress by regulating ROS metabolism in tomato (Solanum lycopersicum L.) plants. J. Plant Interact. 2021, 16, 116–126. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Musumeci, M.A. Trichoderma as biological control agent: Scope and prospects to improve efficacy. World J. Microbiol. Biotechnol. 2021, 37, 90. [Google Scholar] [CrossRef]
- Khan, R.A.A.; Najeeb, S.; Mao, Z.; Ling, J.; Yang, Y.; Li, Y.; Xie, B. Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic bacteria and root-knot nematode. Microorganisms 2020, 8, 401. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Woo, S.L.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Ruocco, M.; Lanzuise, S.; et al. Trichoderma secondary metabolites active on plants and fungal pathogens. Open Mycol. J. 2014, 8, 127–139. [Google Scholar] [CrossRef]
- Baazeem, A.; Almanea, A.; Manikandan, P.; Alorabi, M.; Vijayaraghavan, P.; Abdel-Hadi, A. In vitro antibacterial, antifungal, nematocidal and growth promoting activities of Trichoderma hamatum FB10 and its secondary metabolites. J. Fungi 2021, 7, 331. [Google Scholar] [CrossRef] [PubMed]
- Sarsaiya, S.; Jain, A.; Fan, X.; Jia, Q.; Xu, Q.; Shu, F.; Zhou, Q.; Shi, J.; Chen, J. New insights into detection of a dendrobine compound from a novel endophytic Trichoderma longibrachiatum strain and its toxicity against phytopathogenic bacteria. Front. Microbiol. 2020, 11, 337. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.; Abril-Urias, P.; Escobar, C. Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, mycorrhizal and endophytic fungi. Front. Microbiol. 2020, 11, 992. [Google Scholar] [CrossRef]
- De Oliveira, C.M.; Almeida, N.O.; Côrtes, M.V.D.C.B.; Lobo, M., Jr.; da Rocha, M.R.; Ulhoa, C.J. Biological control of Pratylenchus brachyurus with isolates of Trichoderma spp. on soybean. Biol. Control 2021, 152, 104425. [Google Scholar] [CrossRef]
- Sahebani, N.; Hadavi, N. Biological control of the root-knot nematode Meloidogyne javanica by Trichoderma harzianum. Soil Biol. Biochem. 2008, 40, 2016–2020. [Google Scholar] [CrossRef]
- Coppola, M.; Cascone, P.; Di Lelio, I.; Woo, S.L.; Lorito, M.; Rao, R.; Pennacchio, F.; Guerrieri, E.; Digilio, M.C. Trichoderma atroviride P1 colonization of tomato plants enhances both direct and indirect defense barriers against insects. Front. Physiol. 2019, 10, 813. [Google Scholar] [CrossRef]
- Poveda, J. Trichoderma as biocontrol agent against pests: New uses for a mycoparasite. Biol. Control. 2021, 159, 104634. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Pal, S. Entomopathogenic potential of Trichoderma longibrachiatum and its comparative evaluation with malathion against the insect pest Leucinodes orbonalis. Environ. Monit. Assess. 2016, 188, 37. [Google Scholar] [CrossRef]
- Razinger, J.; Lutz, M.; Schroers, H.J.; Urek, G.; Grunder, J. Evaluation of insect associated and plant growth promoting fungi in the control of cabbage root flies. J. Econ. Entomol. 2014, 107, 1348–1354. [Google Scholar] [CrossRef]
- Islam, M.S.; Subbiah, V.K.; Siddiquee, S. Efficacy of entomopathogenic Trichoderma isolates against sugarcane woolly aphid, Ceratovacuna lanigera Zehntner (Hemiptera: Aphididae). Horticulturae 2022, 8, 2. [Google Scholar] [CrossRef]
- Halifu, S.; Deng, X.; Song, X.; Song, R. Effects of two Trichoderma strains on plant growth, rhizosphere soil nutrients and fungal community of Pinus sylvestris var. mongolica annual seedlings. Forests 2019, 10, 758. [Google Scholar] [CrossRef]
- Cai, W.-J.; Ye, T.-T.; Wang, Q.; Cai, B.-D.; Feng, Y.-Q. A rapid approach to investigate spatiotemporal distribution of phytohormones in rice. Plant Methods 2016, 12, 47. [Google Scholar] [CrossRef]
- Contreras-Cornejo, X.A.; Macías-Rodríguez, L.; del-Val, E.; Larsen, J. Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: Interactions with plants. FEMS Microbiol. Ecol. 2016, 92, 1–17. [Google Scholar] [CrossRef]
- Brotman, Y.; Kapuganti, J.G.; Viterbo, A. Trichoderma. Curr. Biol. 2010, 20, R390–R391. [Google Scholar] [CrossRef]
- Nogueira-Lopez, G.; Greenwood, D.R.; Middleditch, M.; Winefield, C.; Eaton, C.; Steyaert, J.M.; Mendoza-Mendoza, A. The apoplastic secretome of Trichoderma virens during interaction with maize roots shows an inhibition of plant defense and scavenging oxidative stress secreted proteins. Front. Plant Sci. 2018, 9, 409. [Google Scholar] [CrossRef]
- Jaroszuk-Ściseł, J.; Kurek, E.; Rodzik, B.; Winiarczyk, K. Interactions between rye (Secale cereale) root border cells (RBCs) and pathogenic and nonpathogenic rhizosphere strains of Fusarium culmorum. Mycol. Res. 2009, 113, 1053–1061. [Google Scholar] [CrossRef]
- Hawes, M.; Allen, C.; Turgeon, G.; Curlango-Rivera, G.; Tran, T.M.; Huskey, D.A.; Xiong, Z. Root border cells and their role in plant defense. Annu. Rev. Phytopathol. 2016, 54, 143–161. [Google Scholar] [CrossRef]
- Jaroszuk-Ściseł, J.; Kurek, E. Komórki graniczne korzenia i ich rola w interakcjach roślina-mikroorganizmy glebowe. Postęp. Nauk Rol. 2008, 1, 43–56. [Google Scholar]
- Liu, Q.; Li, K.; Guo, X.; Ma, L.; Guo, Y.; Liu, Z. Developmental characteristics of grapevine seedlings root border cells and their response to ρ-hydroxybenzoic acid. Plant Soil 2019, 443, 199–218. [Google Scholar] [CrossRef]
- Kershaw, M.J.; Talbot, N.J. Hydrophobins and repellents: Proteins with fundamental roles in fungal morphogenesis. Fungal Genet. Biol. 1998, 23, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Viterbo, A.; Chet, I. TasHyd1, a new hydrophobin gene from the biocontrol agent Trichoderma asperellum, is involved in plant root colonization. Mol. Plant Pathol. 2006, 7, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Samolski, I.; Rincón, A.M.; Pinzón, L.M.; Viterbo, A.; Monte, E. The qid74 gene from Trichoderma harzianum has a role in root architecture and plant biofertilization. Microbiology 2012, 158, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cruz, R.; Mehta, R.; Atriztán-Hernández, K.; Martínez-Villamil, O.; del Rayo Sánchez-Carbente, M.; Sánchez-Reyes, A.; Lira-Ruan, V.; González-Chávez, C.A.; Tabche-Barrera, M.L.; Bárcenas-Rodríguez, R.C.; et al. Effects on Capsicum annuum plants colonized with Trichoderma atroviride P. Karst strains genetically modified in Taswo1, a gene coding for a protein with expansin-like activity. Plants 2021, 10, 1919. [Google Scholar] [CrossRef]
- Chen, J.; Karuppiah, V.; Dou, K. Multiplayer interaction of Trichoderma and plant in the induced plant resistance. In New and Future Developments in Microbial Biotechnology and Bioengineering; Gupta, V.K., Zeilinger, S., Singh, H.B., Druzhinina, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 141–155. [Google Scholar] [CrossRef]
- Vargas, W.A.; Mandawe, J.C.; Kenerley, C.M. Plant-derived sucrose is a key element in the symbiotic association between Trichoderma virens and maize plants. Plant Physiol. 2009, 151, 792–808. [Google Scholar] [CrossRef]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef]
- Tamayo-Velez, A.; Osorio, N.W. Co-inoculation with an arbuscular mycorrhizal fungus and a phosphate-solubilizing fungus promotes the plant growth and phosphate uptake of avocado plantlets in a nursery. Botany 2017, 95, 539–545. [Google Scholar] [CrossRef]
- Zhou, L.S.; Tang, K.; Guo, S.X. The plant growth-promoting fungus (PGPF) Alternaria sp. A13 markedly enhances Salvia miltiorrhiza root growth and active ingredient accumulation under greenhouse and field conditions. Int. J. Mol. Sci. 2018, 19, 270. [Google Scholar] [CrossRef]
- Jaroszuk-Ściseł, J.; Kurek, E.; Trytek, M. Efficiency of indoleacetic acid, gibberellic acid and ethylene synthesized in vitro by Fusarium culmorum strains with different effects on cereal growth. Biologia 2014, 69, 281–292. [Google Scholar] [CrossRef]
- Mefteh, F.B.; Daoud, A.; Bouket, A.C.; Alenezi, F.N.; Luptakova, L.; Rateb, M.E.; Kadri, A.; Gharsallah, N.; Belbahri, L. Fungal root microbiome from healthy and brittle leaf diseased date palm trees (Phoenix dactylifera L.) reveals a hidden untapped arsenal of antibacterial and broad spectrum antifungal secondary metabolites. Front. Biol. 2017, 8, 307. [Google Scholar] [CrossRef]
- Shi, W.L.; Chen, X.L.; Wang, L.X.; Gong, Z.T.; Li, S.; Li, C.L.; Xie, B.B.; Zhang, W.; Shi, M.; Li, C.; et al. Cellular and molecular insight into the inhibition of primary root growth of Arabidopsis induced by peptaibols, a class of linear peptide antibiotics mainly produced by Trichoderma spp. J. Exp. Bot. 2016, 67, 2191–2205. [Google Scholar] [CrossRef]
- Alfiky, A.; Weisskopf, L. Deciphering Trichoderma-plant-pathogen interactions for better development of biological applications. J. Fungi 2021, 7, 61. [Google Scholar] [CrossRef]
- Ozimek, E.; Jaroszuk-Ściseł, J.; Bohacz, J.; Korniłłowicz-Kowalska, T.; Tyśkiewicz, R.; Słomka, A.; Nowak, A.; Hanaka, A. Synthesis of indoleacetic acid, gibberellic acid and ACC-deaminase by Mortierella strains promote winter wheat seedlings growth under different conditions. Int. J. Mol. Sci. 2018, 19, 3218. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, Z.; Yang, X.; Shen, Q. Trichoderma harzianum T-E5 significantly affects cucumber root exudates and fungal community in the cucumber rhizosphere. Appl. Soil Ecol. 2013, 72, 41–48. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y. Effects of phosphate solubilization and phytohormone production of Trichoderma asperellum Q1 on promoting cucumber growth under salt stress. J. Integr. Agr. 2015, 14, 1588–1597. [Google Scholar] [CrossRef]
- Fu, S.F.; Wei, J.Y.; Chen, H.W.; Liu, Y.Y.; Lu, H.Y.; Chou, J.Y. Indole-3-acetic acid: A widespread physiological code in interactions of fungi with other organisms. Plant Signal. Behav. 2015, 10, 1–9. [Google Scholar] [CrossRef]
- Nieto-Jacobo, M.F.; Steyaert, J.M.; Salazar-Badillo, F.B.; Nguyen, D.V.; Rostás, M.; Braithwaite, M.; De Souza, J.T.; Jimenez-Bremont, J.F.; Ohkura, M.; Stewart, A.; et al. Environmental growth conditions of Trichoderma spp. affects indole acetic acid derivatives, volatile organic compounds, and plant growth promotion. Front. Plant Sci. 2017, 8, 102. [Google Scholar] [CrossRef]
- Saber, W.I.A.; Ghoneem, K.M.; Rashad, Y.M.; Al-Askar, A.A. Trichoderma harzianum WKY1: An indole acetic acid producer for growth improvement and anthracnose disease control in sorghum. Biocontrol Sci. Technol. 2017, 27, 654–676. [Google Scholar] [CrossRef]
- Woźniak, M.; Gałązka, A.; Tyśkiewicz, R.; Jaroszuk-Ściseł, J. Endophytic bacteria potentially promote plant growth by synthesizing different metabolites and their phenotypic/physiological profiles in the Biolog GEN III MicroPlateTM test. Int. J. Mol. Sci. 2019, 20, 5283. [Google Scholar] [CrossRef] [PubMed]
- Ozimek, E.; Hanaka, A. Mortierella Species as the plant growth-promoting fungi present in the agricultural soils. Agriculture 2021, 11, 7. [Google Scholar] [CrossRef]
- Napitupulu, T.P.; Kanti, A.; Sudiana, I.M. Evaluation of the environmental factors modulating indole-3-acetic acid (IAA) production by Trichoderma harzianum InaCC F88. IOP Conf. Ser. Earth Environ. Sci. 2019, 308, 012060. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef]
- Bader, A.N.; Salerno, G.L.; Covacevich, F.; Consolo, V.F. Native Trichoderma harzianum strains from Argentina produce indole-3 acetic acid and phosphorus solubilization, promote growth and control wilt disease on tomato (Solanum lycopersicum L.). J. King Saud Univ. Sci. 2020, 32, 867–873. [Google Scholar] [CrossRef]
- Illescas, M.; Pedrero-Méndez, A.; Pitorini-Bovolini, M.; Hermosa, R.; Monte, E. Phytohormone production profiles in Trichoderma species and their relationship to wheat plant responses to water stress. Pathogens 2021, 10, 991. [Google Scholar] [CrossRef]
- Sánchez-Montesinos, B.; Diánez, F.; Moreno-Gavíra, A.; Gea, F.J.; Santos, M. Role of Trichoderma aggressivum f. europaeum as plant-growth promoter in horticulture. Agronomy 2020, 10, 1004. [Google Scholar] [CrossRef]
- De Oliveira, J.; Rodrigues, C.; Vandenberghe, L.P.S.; Câmara, M.C.; Libardi, N.; Soccol, C.R. Gibberellic acid production by different fermentation systems using citric pulp as substrate/support. BioMed Res. Int. 2017, 5191046. [Google Scholar] [CrossRef]
- Salazar-Cerezo, S.; Martínez-Montiel, N.; García-Sánchez, J.; Pérez-Y-Terrón, R.; Martínez-Contreras, R.D. Gibberellin biosynthesis and metabolism: A convergent route for plants, fungi and bacteria. Microbiol. Res. 2018, 208, 85–98. [Google Scholar] [CrossRef]
- Cen, Y.K.; Lin, J.G.; Wang, Y.L.; Wang, J.Y.; Liu, Z.Q.; Zheng, Y.G. The gibberellin producer Fusarium fujikuroi: Methods and technologies in the current toolkit. Front. Bioeng. Biotechnol. 2020, 8, 232. [Google Scholar] [CrossRef]
- You, J.; Zhang, J.; Wu, M.; Yang, L.; Chen, W.; Li, G. Multiple criteria-based screening of Trichoderma isolates for biological control of Botrytis cinerea on tomato. Biol. Control 2016, 101, 31–38. [Google Scholar] [CrossRef]
- Kamalov, L.S.; Turgunov, K.K.; Aripova, S.F.; Abdilalimov, O. Gibberillin A-3 from the microscopic fungus Trichoderma harzianum. Chem. Nat. Compd. 2018, 54, 421–422. [Google Scholar] [CrossRef]
- Todorovic, B.; Glick, B.R. The interconversion of ACC deaminase and D-cysteine desulfhydrase by directed mutagenesis. Planta 2008, 229, 193–205. [Google Scholar] [CrossRef]
- Rauf, M.; Awais, M.; Ud-Din, A.; Ali, K.; Gul, H.; Rahman, M.M.; Hamayun, M.; Arif, M. Molecular mechanisms of the 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase producing Trichoderma asperellum MAP1 in enhancing wheat tolerance to waterlogging stress. Front. Plant Sci. 2021, 11, 614971. [Google Scholar] [CrossRef]
- Dubois, M.; Van den Broeck, L.; Inzé, D. The pivotal role of ethylene in plant growth. Trends Plant Sci. 2018, 23, 1–14. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Rossi, M.J.; Soares, C.R.F.S.; McConkey, B.J.; Glick, B.R. New insights into 1-aminocyclopropane-1-carboxylate (ACC) deaminase phylogeny, evolution and ecological significance. PLoS ONE 2014, 9, e99168. [Google Scholar] [CrossRef]
- Saravanakumar, K.; MubarakAli, D.; Kathiresan, K.; Wang, M.-H. An evidence of fungal derived 1-aminocyclopropane-1-carboxylate deaminase promoting the growth of mangroves. Beni-Suef Univ. J. Appl. 2018, 7, 446–451. [Google Scholar] [CrossRef]
- Zhang, F.; Dou, K.; Liu, C.; Chen, F.; Wu, W.; Yang, T.; Li, L.; Liu, T.; Yu, L. The application potential of Trichoderma T-soybean containing 1-aminocyclopropane-1-carboxylate for maize production. Physiol. Mol. Plant Pathol. 2020, 110, 101475. [Google Scholar] [CrossRef]
- Viterbo, A.; Landau, U.; Kim, S.; Chernin, L.; Chet, I. Characterization of ACC deaminase from the biocontrol and plant growth-promoting agent Trichoderma asperellum T203. FEMS Microbiol. Lett. 2010, 305, 42–48. [Google Scholar] [CrossRef]
- Tsolakidou, M.-D.; Pantelides, L.S.; Tzima, A.K.; Kang, S.; Paplomatas, E.J.; Tsaltas, D. Disruption and overexpression of the gene encoding ACC (1-aminocyclopropane-1-carboxylic acid) deaminase in soil-borne fungal pathogen Verticillium dahliae revealed the role of ACC as a potential regulator of virulence and plant defense. Mol. Plant Microbe Interact. 2019, 32, 639–653. [Google Scholar] [CrossRef]
- Stewart, A.; Hill, R. Applications of Trichoderma in plant growth promotion. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 415–428. [Google Scholar] [CrossRef]
- Menezes-Blackburn, D.; Giles, C.; Darch, T.; George, T.S.; Blackwell, M.; Stutter, M.; Shand, C.; Lumsdon, D.; Cooper, P.; Wendler, R.; et al. Opportunities for mobilizing recalcitrant phosphorus from agricultural soils: A review. Plant Soil 2018, 427, 5–16. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Shanmuga Arasu, V.; Kathiresan, K. Effect of Trichoderma on soil phosphate solubilization and growth improvement of Avicennia marina. Aquat. Bot. 2013, 104, 101–105. [Google Scholar] [CrossRef]
- Scervino, J.M.; Mesa, M.P.; Monica, I.D.; Recchi, M.; Moreno, N.S.; Godeas, A. Soil fungal isolates produce different organic acid patterns involved in phosphate salt solubilization. Biol. Fertil. Soils 2010, 46, 755–763. [Google Scholar] [CrossRef]
- Öğüt, M.; Akdağ, C.; Düzdemir, O.; Sakin, M.A. Single and double inoculation with Azospirillum/Trichoderma: The effects on dry bean and wheat. Biol. Fertil. Soils 2005, 41, 262–272. [Google Scholar] [CrossRef]
- Borges Chagas, L.F.; Chagas Junior, A.F.; Rodrigues de Carvalho, M.; de Oliveira Miller, L.; Orozco Colonia, B.S. Evaluation of the phosphate solubilization potential of Trichoderma strains (Trichoplus JCO) and effects on rice biomass. J. Soil Sci. Plant Nutr. 2015, 15, 794–804. [Google Scholar] [CrossRef]
- Paul, S.; Rakshit, A. Effect of seed bio-priming with Trichoderma viride strain BHU-2953 for enhancing soil phosphorus solubilization and uptake in soybean (Glycine max). J. Soil Sci. Plant Nutr. 2021, 21, 1041–1052. [Google Scholar] [CrossRef]
- Li, Y.T.; Hwang, S.G.; Huang, Y.M.; Huang, C.H. Effects of Trichoderma asperellum on nutrient uptake and Fusarium wilt of tomato. Crop Protect. 2018, 110, 275–282. [Google Scholar] [CrossRef]
- Altomare, C.; Norvell, W.A.; Björkman, T.; Harman, G.E. Solubilization of phosphates and micronutrients by the plant-growth-promoting and biocontrol fungus Trichoderma harzianum Rifai 1295-22. Appl. Environ. Microbiol. 1999, 65, 2926–2933. [Google Scholar] [CrossRef]
- Singh, B.N.; Dwivedi, P.; Sarma, B.K.; Singh, G.S.; Singh, H.B. Trichoderma asperellum T42 reprograms tobacco for enhanced nitrogen utilization efficiency and plant growth when fed with N nutrients. Front. Plant Sci. 2018, 9, 163. [Google Scholar] [CrossRef]
- Massart, S.; Martinez-Medina, M.; Jijakli, M.H. Biological control in the microbiome era: Challenges and opportunities. Biol. Control 2015, 89, 98–108. [Google Scholar] [CrossRef]
- Massart, S.; Perazzolli, M.; Höfte, M.; Pertot, I.; Jijakli, M.H. Impact of the omic technologies for understanding the modes of action of biological control agents against plant pathogens. Biol. Control 2015, 60, 725–746. [Google Scholar] [CrossRef]
- Li, N.; Islam, M.T.; Kang, S. Secreted metabolite-mediated interactions between rhizosphere bacteria and Trichoderma biocontrol agents. PLoS ONE 2019, 14, e0227228. [Google Scholar] [CrossRef]
- Ramírez-Valdespino, C.A.; Casas-Flores, S.; Olmedo-Monfil, V. Trichoderma as a model to study effector-like molecules. Front. Microbiol. 2019, 10, 1030. [Google Scholar] [CrossRef]
- Estrada-Rivera, M.; Rebolledo-Prudencio, O.G.; Pérez-Robles, D.A.; Rocha Medina, M.C.; González-López, M.C.; Casas-Flores, S. Histone deacetylase HDA-2 is essential in Trichoderma to modulate multiple responses in Arabidopsis. Plant Physiol. 2019, 179, 1343–1361. [Google Scholar] [CrossRef]
- Ansari, S.; Kumar, V.; Bhatt, D.N.; Irfan, M.; Datta, A. N-acetylglucosamine sensing and metabolic engineering for attenuating human and plant pathogens. Bioengineering 2022, 9, 64. [Google Scholar] [CrossRef]
- Karlsson, M.; Atanasova, L.; Jensen, D.F.; Zeilinger, S. Necrotrophic mycoparasites and their genomes. Microbiol. Spectrum 2017, 5, 2–5. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef]
- Anonymous. Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. Off. J. Eur. Union L 2009, 309, 1–50. [Google Scholar]
- Anonymous. Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorization of plant protection products. Off. J. Eur. Union L 2011, 155, 127–175. [Google Scholar]
- Anonymous. Commission Regulation (EU) No 284/2013 of 1 March 2013 setting out the data requirements for plant protection products, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. Off. J. Eur. Union L 2013, 93, 85–152. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).