Flavonoids Seen through the Energy Perspective
Abstract
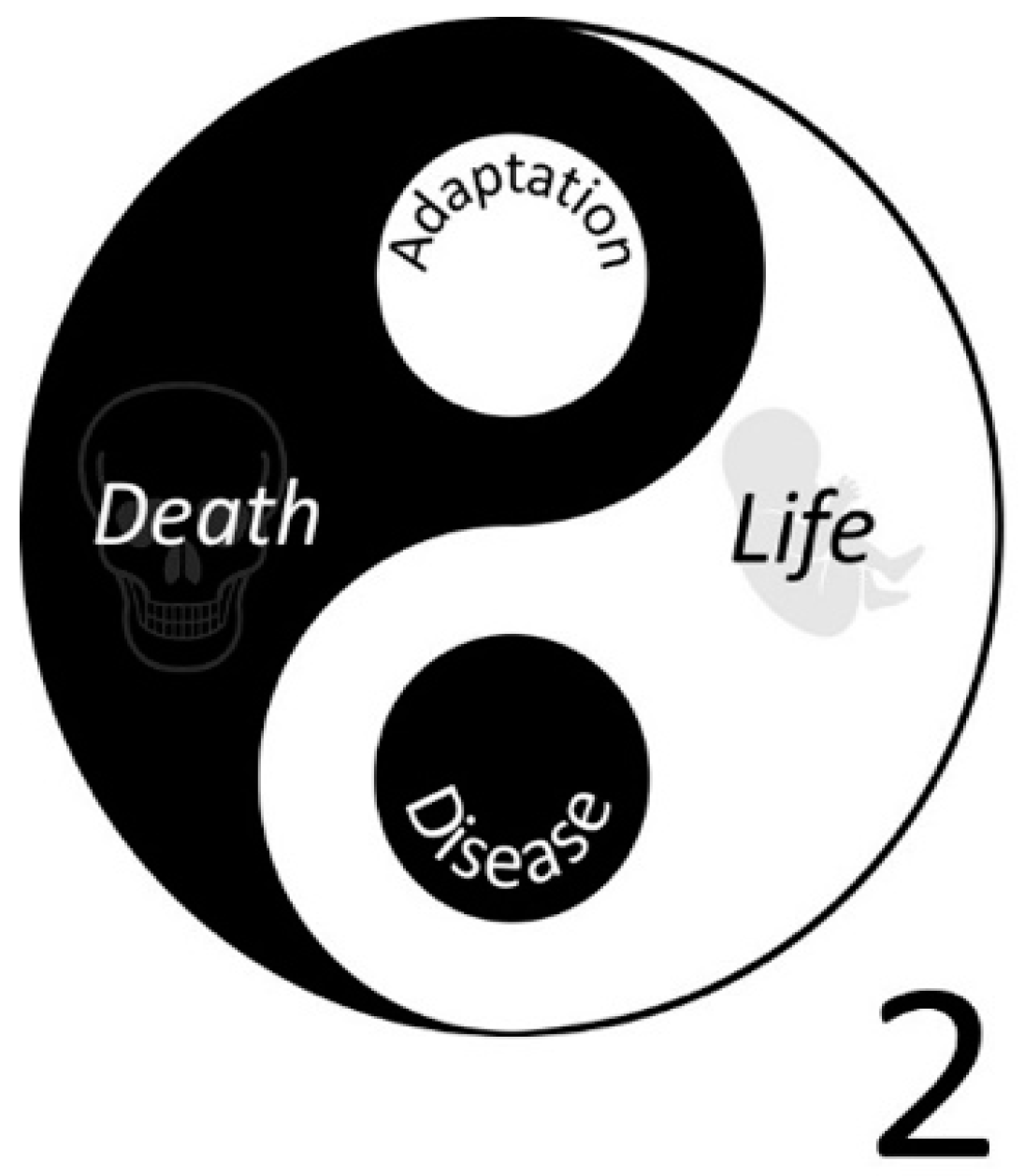
:1. The Dual Nature of Oxygen and Antioxidants
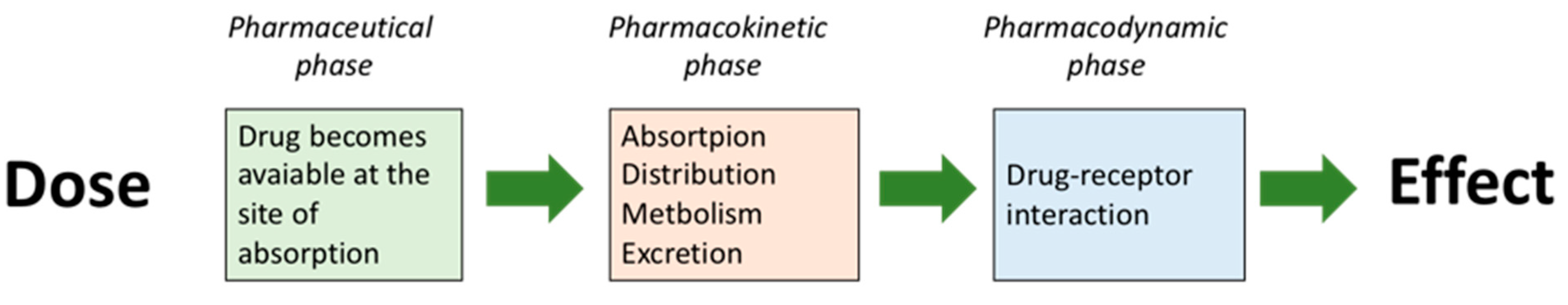
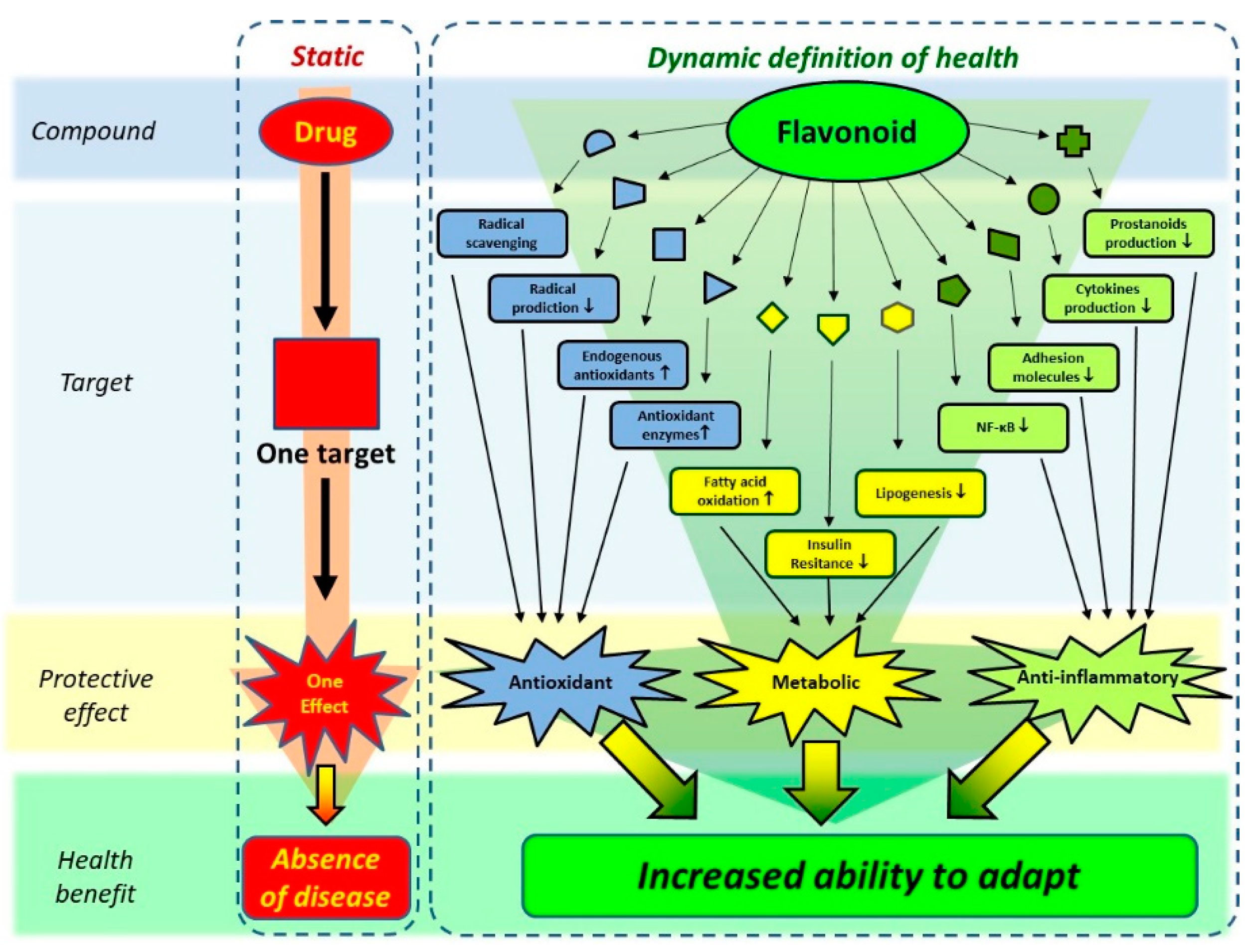
2. The Mode of Action of Flavonoids Seen through the Current Western Concept of Drug Action
2.1. Radical Scavenging
2.2. Capacity, Empowering the Endogenous Antioxidant Network
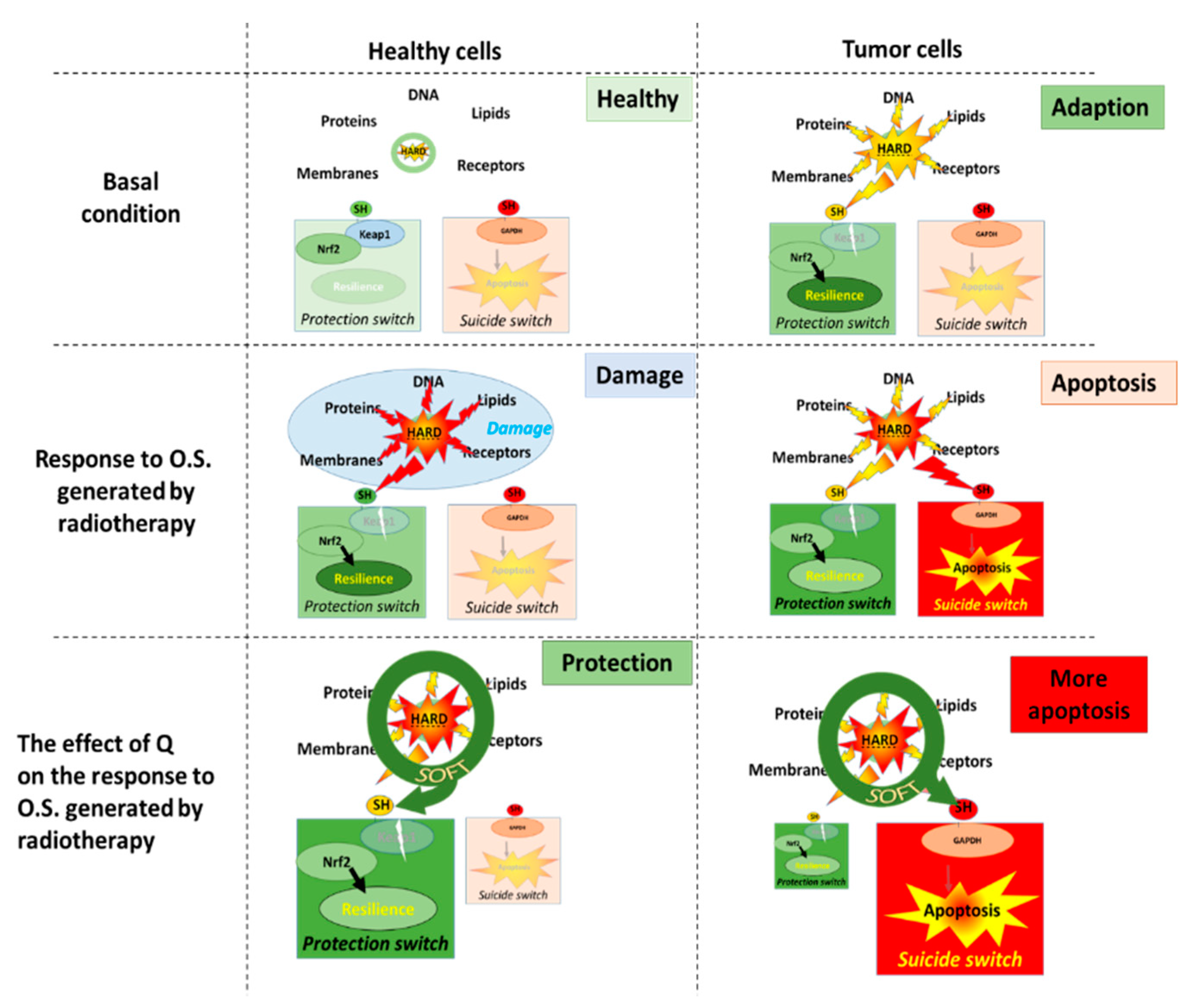
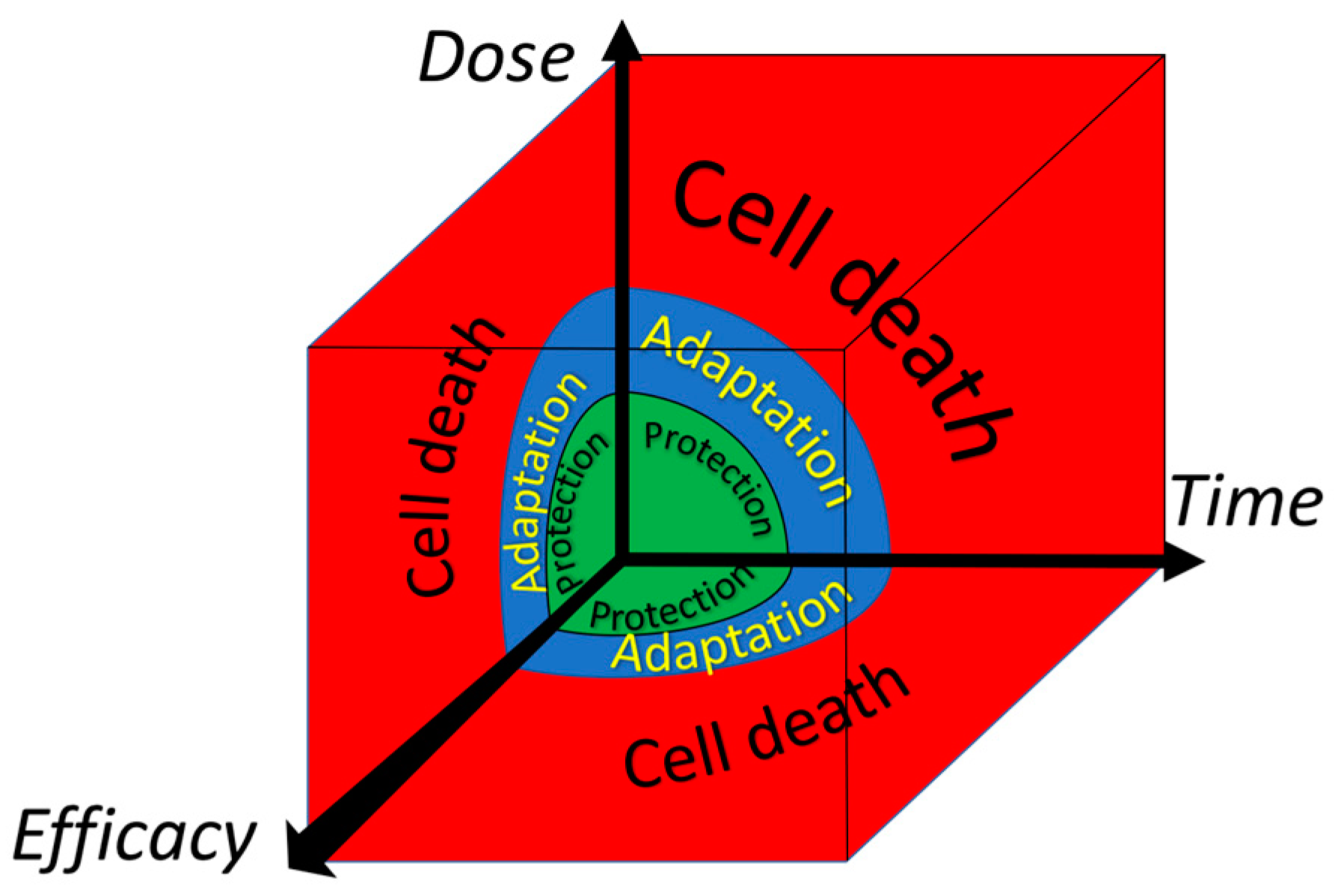
2.3. Empowering the Endogenous Adaptation
2.4. The Use of the Structure-Activity Relationship (SAR) to Elucidate the Mode of Action
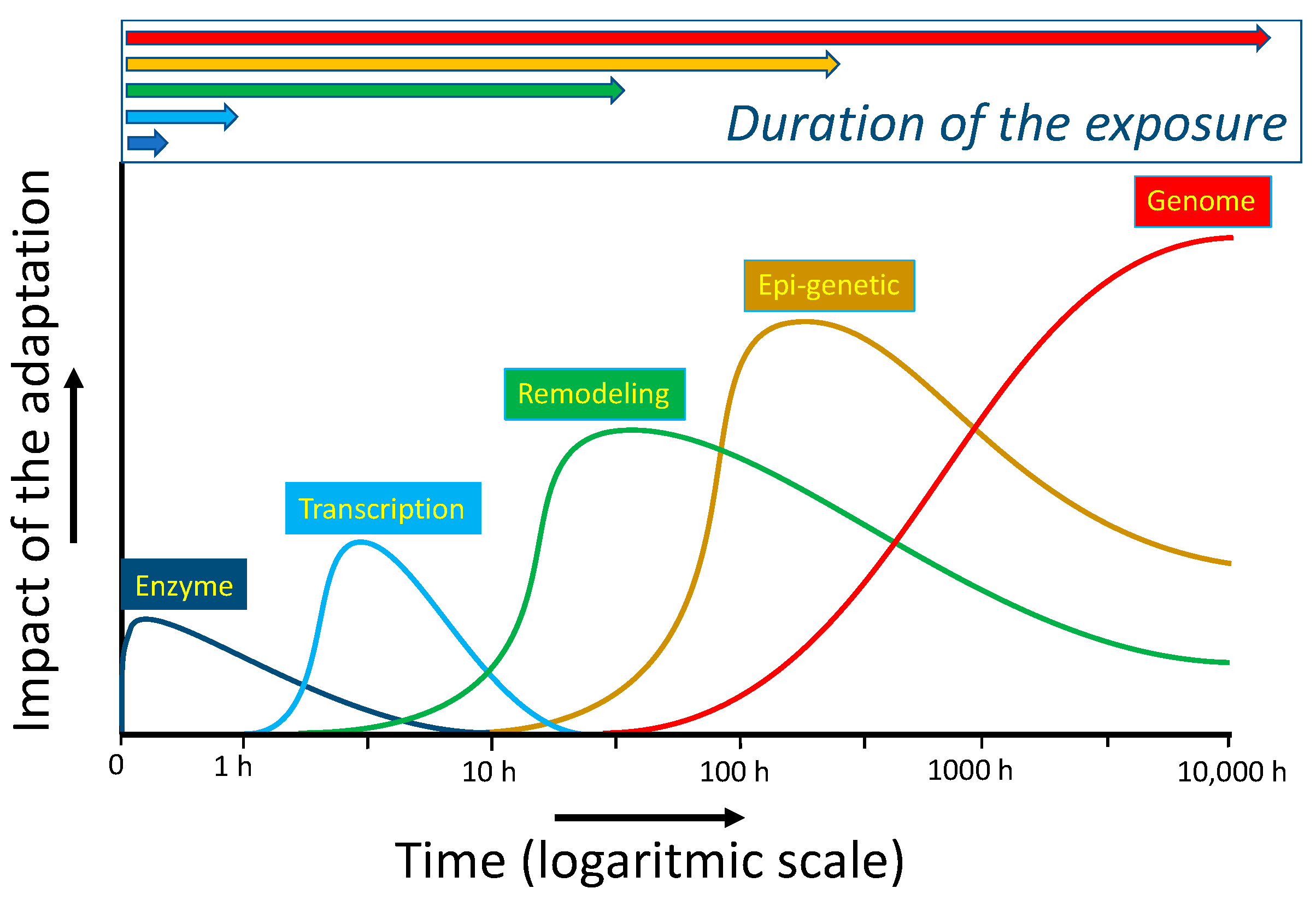
2.5. The Dimension of Time in the Mode of Action
2.6. Other Proposed Modes of Action of Flavonoids
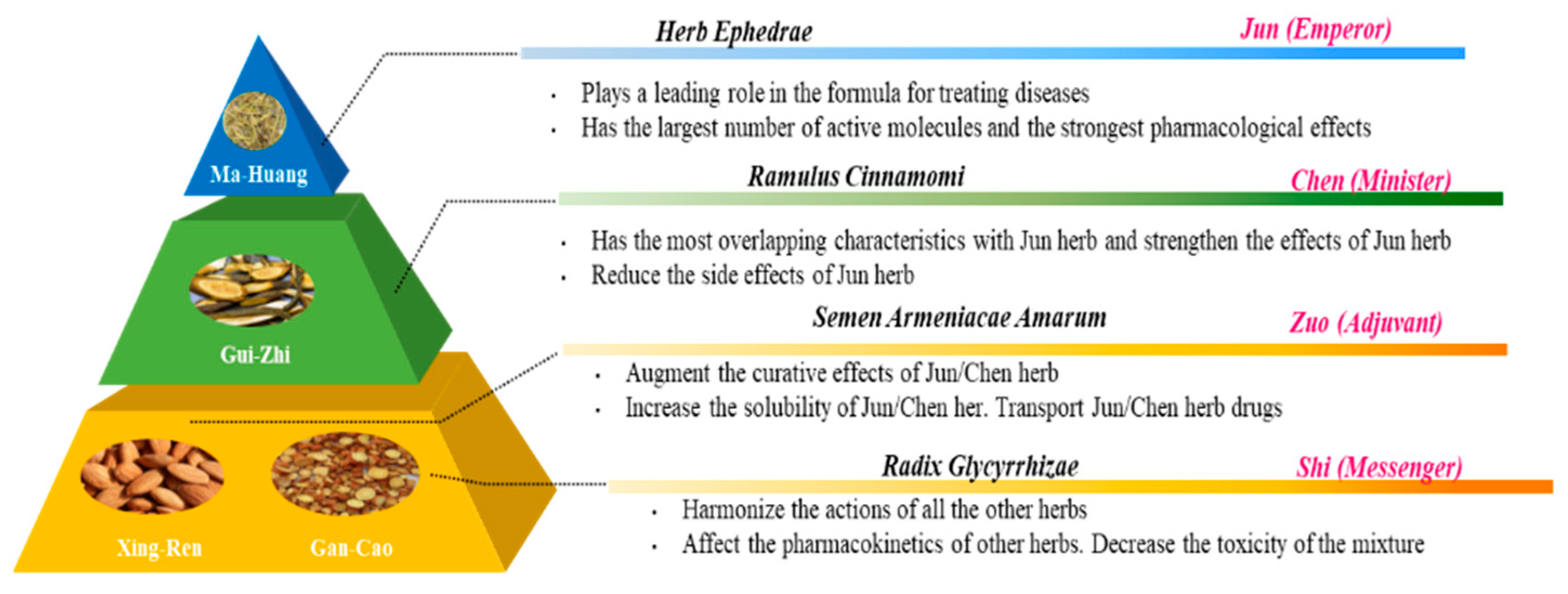
3. Back to the Future, Connection with Traditional Types of Medicine
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Yeo, J.; Lee, J.; Lee, S.; Kim, W.J. Polymeric Antioxidant Materials for Treatment of Inflammatory Disorders. Adv. Ther. 2021, 4, 2000270. [Google Scholar] [CrossRef]
- Haenen, G.R.M.M.; Veerman, M.; Bast, A. Reduction of β-adrenoceptor function by oxidative stress in the heart. Free Radic. Biol. Med. 1990, 9, 279–288. [Google Scholar] [CrossRef]
- Zhang, M.; Moalin, M.; Vervoort, L.; Li, Z.W.; Wu, W.B.; Haenen, G.R.M.M. Connecting Western and Eastern medicine from an energy perspective. Int. J. Mol. Sci. 2019, 20, 1512. [Google Scholar] [CrossRef] [Green Version]
- Milholland, B.; Dong, X.; Vijg, J. “Best-Guess” MRAD Provides Robust Evidence for a Limit to Human Lifespan: Reply to de Grey (Rejuvenation Res. 2017, 20, 261–262). Rejuvenation Res. 2017, 20, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, K.J.; Sthijns, M.M.; van der Vijgh, W.J.; Bast, A.; Haenen, G.R.M.M. The antioxidant flavonoid monoHER provides efficient protection and induces the innate Nrf2 mediated adaptation in endothelial cells subjected to oxidative stress. PharmaNutrition 2014, 2, 69–74. [Google Scholar] [CrossRef]
- Books, Z. What is health? The ability to adapt. Lancet 2009, 373, 781. [Google Scholar]
- Hunyadi, A. The mechanism(s) of action of antioxidants: From scavenging reactive oxygen/nitrogen species to redox signaling and the generation of bioactive secondary metabolites. Med. Res. Rev. 2019, 39, 2505–2533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bast, A.; Haenen, G.R.M.M. Ten misconceptions about antioxidants. Trends Pharmacol. Sci. 2013, 34, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Bast, A.; Haenen, G.R.M.M.; Doelman, C.J. Oxidants and antioxidants: State of the art. Am. J. Med. 1991, 91, 2–13. [Google Scholar] [CrossRef]
- Bai, J.W.; Gao, Z.J.; Xiao, H.W.; Wang, X.T.; Zhang, Q. Polyphenol oxidase inactivation and vitamin C degradation kinetics of Fuji apple quarters by high humidity air impingement blanching. Int. J. Food Sci. Techonol. 2013, 48, 1135–1141. [Google Scholar] [CrossRef]
- Benzie, I.F. Evolution of antioxidant defence mechanisms. Eur. J. Nutr. 2000, 39, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Kohen, R.; Nyska, A. Invited review: Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derouiche, S. Oxidative stress associated with SARS-CoV-2 (COVID-19) increases the severity of the lung disease—A systematic review. J. Infect. Dis. Epidemiol. 2020, 6, 121. [Google Scholar]
- Wei, Z.; Li, X.; Li, X.; Liu, Q.; Cheng, Y. Oxidative stress in Parkinson’s disease: A systematic review and meta-analysis. Front. Mol. Neurosci. 2018, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Razavi, B.M.; Banach, M.; Hosseinzadeh, H. Quercetin and metabolic syndrome: A review. Phytother. Res. 2021, 35, 5352–5364. [Google Scholar] [CrossRef] [PubMed]
- Van der Greef, J.; van Wietmarschen, H.; Schroën, J.; Wang, M.; Hankemeier, T.; Xu, G. Systems biology-based diagnostic principles as pillars of the bridge between Chinese and Western medicine. Planta Med. 2010, 76, 2036–2047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franz, I. Paracelsus–Naturkundiger unter und über Tage. Mit einem Brückensch. zu Franz von Baader. In Geo.Alp; Universität Innsbruck: Innsbruck, Austria, 2007; Volume 1, pp. 33–43. [Google Scholar]
- Bast, A.; Haenen, G.R.M.M. The toxicity of antioxidants and their metabolites. Environ. Toxicol. Pharmacol. 2002, 11, 251–258. [Google Scholar] [CrossRef]
- Ariëns, E.J. Molecular Pharmacology V3: The Model of Action of Biology Active Compounds. In Medicinal Chemistry; Elsevier: Amsterdam, The Netherlands, 2012; p. 3. [Google Scholar]
- Petroz, G.C.; Sikich, N.; James, M.; van Dyk, H.; Shafer, S.L.; Schily, M.; Lerman, J. A phase I, two-center study of the pharmacokinetics and pharmacodynamics of dexmedetomidine in children. Anesthesiology 2006, 105, 1098–1110. [Google Scholar] [CrossRef]
- Lees, P.; Cunningham, F.; Elliott, J. Principles of pharmacodynamics and their applications in veterinary pharmacology. J. Vet. Pharmacol. Ther. 2004, 27, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Kenakin, T. Principles: Receptor theory in pharmacology. Trends Pharmacol. Sci. 2004, 25, 186–192. [Google Scholar] [CrossRef]
- Stephenson, R. A modification of receptor theory. Br. J. Pharmacol. 1956, 11, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Ezechiáš, M. Combinations of a full and partial agonist: Experimental evidence of curved isoboles. Toxicol. Lett. 2021, 350, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Kenakin, T. Agonist-receptor efficacy II: Agonist trafficking of receptor signals. Trends Pharmacol. Sci. 1995, 16, 232–238. [Google Scholar] [CrossRef] [Green Version]
- Morrison, C.F.; Stephenson, J.A. Nicotine injections as the conditioned stimulus in discrimination learning. Psychopharmacologia 1969, 15, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Moalin, M.; Van Strijdonck, G.P.; Beckers, M.; Hagemen, G.J.; Borm, P.J.; Bast, A.; Haenen, G.R.M.M. A planar conformation and the hydroxyl groups in the B and C rings play a pivotal role in the antioxidant capacity of quercetin and quercetin derivatives. Molecules 2011, 16, 9636–9650. [Google Scholar] [CrossRef] [Green Version]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Sepahpour, S.; Selamat, J.; Abdul Manap, M.Y.; Khatib, A.; Abdull Razis, A.F. Comparative analysis of chemical composition, antioxidant activity and quantitative characterization of some phenolic compounds in selected herbs and spices in different solvent extraction systems. Molecules 2018, 23, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galano, A.; Raúl Alvarez-Idaboy, J. Computational strategies for predicting free radical scavengers’ protection against oxidative stress: Where are we and what might follow? Int. J. Quantum Chem. 2019, 119, 25665. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Liu, Y.; Zhang, L.; An, L.; Chen, R.; Liu, Y.; Luo, Q.; Li, Y.; Wang, H.; Xue, Y. Computational study on the antioxidant property of coumarin-fused coumarins. Food Chem. 2020, 304, 125446. [Google Scholar] [CrossRef] [PubMed]
- Alisi, I.O.; Uzairu, A.; Abechi, S.E. In silico design of hydrazone antioxidants and analysis of their free radical-scavenging mechanism by thermodynamic studies. Beni-Suef Univ. J. Basic Appl. Sci. 2019, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Amorati, R.; Baschieri, A.; Cowden, A.; Valgimigli, L. The antioxidant activity of quercetin in water solution. Biomimetics 2017, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Moalin, M.; van Strijdonck, G.P.; Bast, A.; Haenen, G.R.M.M. Competition between ascorbate and glutathione for the oxidized form of methylated quercetin metabolites and analogues: Tamarixetin, 4′ o-methylquercetin, has the lowest thiol reactivity. J. Agric. Food Chem. 2012, 60, 9292–9297. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Moalin, M.; Zhang, M.; Vervoort, L.; Hursel, E.; Mommers, A.; Haenen, G.R.M.M. The Flow of the Redox Energy in Quercetin during Its Antioxidant Activity in Water. Int. J. Mol. Sci. 2020, 21, 6015. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Moalin, M.; Zhang, M.; Vervoort, L.; Mommers, A.; Haenen, G.R.M.M. Delocalization of the Unpaired Electron in the Quercetin Radical: Comparison of Experimental ESR Data with DFT Calculations. Int. J. Mol. Sci. 2020, 21, 2033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niki, E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: In vitro and in vivo evidence. Free Radic. Biol. Med. 2014, 66, 3–12. [Google Scholar] [CrossRef]
- Arts, M.J.; Dallinga, J.S.; Voss, H.-P.; Haenen, G.R.; Bast, A. A new approach to assess the total antioxidant capacity using the TEAC assay. Food Chem. 2004, 88, 567–570. [Google Scholar] [CrossRef]
- Boots, A.W.; Kubben, N.; Haenen, G.R.M.M.; Bast, A. Oxidized quercetin reacts with thiols rather than with ascorbate: Implication for quercetin supplementation. Biochem. Biophys. Res. Commun. 2003, 308, 560–565. [Google Scholar] [CrossRef]
- Vrolijk, M.F.; Opperhuizen, A.; Jansen, E.H.; Godschalk, R.W.; Van Schooten, F.J.; Bast, A.; Haenen, G.R.M.M. The shifting perception on antioxidants: The case of vitamin E and β-carotene. Redox Biol. 2015, 4, 272–278. [Google Scholar] [CrossRef] [Green Version]
- Heijnen, C.G.M.; Haenen, G.R.M.M.; Vekemans, J.A.; Bast, A. Peroxynitrite scavenging of flavonoids: Structure activity relationship. Environ. Toxicol. Pharmacol. 2001, 10, 199–206. [Google Scholar] [CrossRef]
- Heijnen, C.G.M.; Haenen, G.R.M.M.; Van Acker, F.; Van der Vijgh, W.; Bast, A. Flavonoids as peroxynitrite scavengers: The role of the hydroxyl groups. Toxicol. In Vitro 2001, 15, 3–6. [Google Scholar] [CrossRef]
- Lemmens, K.J.; van de Wier, B.; Vaes, N.; Ghosh, M.; van Zandvoort, M.A.; van der Vijgh, W.J.; Bast, A.; Haenen, G.R. The flavonoid 7-mono-O-(β-hydroxyethyl)-rutoside is able to protect endothelial cells by a direct antioxidant effect. Toxicol. In Vitro 2014, 28, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Vervoort, L.; Moalin, M.; Mommers, A.; Douny, C.; den Hartog, G.J.; Haenen, G.R.M.M. The chemical reactivity of (−)-epicatechin quinone mainly resides in its B-ring. Free Radic. Biol. Med. 2018, 124, 31–39. [Google Scholar] [CrossRef]
- Benhar, M. Oxidants, Antioxidants and Thiol Redox Switches in the Control of Regulated Cell Death Pathways. Antioxidants 2020, 9, 309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, S.C.; Li, X.; Henzl, M.T.; Beamer, L.J.; Hannink, M. Structure of the Keap1:Nrf2 interface provides mechanistic insight into Nrf2 signaling. EMBO J. 2006, 25, 3605–3617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sthijns, M.M.J.P.E.; Schiffers, P.M.; Janssen, G.M.; Lemmens, K.J.A.; Ides, B.; Vangrieken, P.; Bouwman, F.G.; Mariman, E.C.; Pader, I.; Arner, E.S.J.; et al. Rutin protects against H2O2-triggered impaired relaxation of placental arterioles and induces Nrf2-mediated adaptation in Human Umbilical Vein Endothelial Cells exposed to oxidative stress. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1177–1189. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhang, F.; Hong, C.Q.; Giuliano, A.E.; Cui, X.J.; Zhou, G.J.; Zhang, G.J.; Cui, Y.K. Critical protein GAPDH and its regulatory mechanisms in cancer cells. Cancer Biol. Med. 2015, 12, 10. [Google Scholar] [PubMed]
- Hara, M.R.; Agrawal, N.; Kim, S.F.; Cascio, M.B.; Fujimuro, M.; Ozeki, Y.; Takahashi, M.; Cheah, J.H.; Tankou, S.K.; Hester, L.D.; et al. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat. Cell Biol. 2005, 7, 665–674. [Google Scholar] [CrossRef]
- Giorgio, M. Oxidative stress and the unfulfilled promises of antioxidant agents. Ecancermedicalscience 2015, 9, 556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marengo, B.; Nitti, M.; Furfaro, A.L.; Colla, R.; De Ciucis, C.; Marinari, U.M.; Pronzato, M.A.; Traverso, N.; Domenicotti, C. Redox Homeostasis and Cellular Antioxidant Systems: Crucial Players in Cancer Growth and Therapy. Oxidative Med. Cell. Longev. 2016, 2016, 6235641. [Google Scholar] [CrossRef] [Green Version]
- Sen, N.; Hara, M.R.; Kornberg, M.D.; Cascio, M.B.; Bae, B.-I.; Shahani, N.; Thomas, B.; Dawson, T.M.; Dawson, V.L.; Snyder, S.H.; et al. Nitric oxide-induced nuclear GAPDH activates p300/CBP and mediates apoptosis. Nat. Cell Biol. 2008, 10, 866–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirover, M.A. Pleiotropic effects of moonlighting glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in cancer progression, invasiveness, and metastases. Cancer Metastasis Rev. 2018, 37, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Russell, G.; Veal, D.; Hancock, J.T. Is glyceraldehyde-3-phosphate dehydrogenase a central redox mediator? React. Oxyg. Species 2020, 9, 48–69. [Google Scholar] [CrossRef]
- Brown, A.C.; Fraser, T.R. On the Connection between Chemical Constitution and Physiological Action; with special reference to the Physiological Action of the Salts of the Ammonium Bases derived from Strychnia, Brucia, Thebaia, Codeia, Morphia, and Nicotia. J. Anat. Physiol. 1868, 2, 224–242. [Google Scholar]
- Wen, C.; Zhang, J.; Zhang, H.; Duan, Y.; Ma, H. Study on the structure-activity relationship of watermelon seed antioxidant peptides by using molecular simulations. Food Chem. 2021, 364, 130432. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.M.; Wang, P.; Ge, G.B.; Dai, Z.R.; Wu, D.C.; Zou, L.W.; Dou, T.Y.; Zhang, T.Y.; Yang, L.; Hou, J. Structure-activity relationships of flavonoids as natural inhibitors against E. coli beta-glucuronidase. Food Chem. Toxicol. 2017, 109, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.C.; Fortes, G.A.C.; Camargo, L.T.F.M.; Camargo, A.J.; Ferri, P.H. Antioxidant effects of polyphenolic compounds and structure-activity relationship predicted by multivariate regression tree. LWT Food Sci. Technol. 2021, 137, 110366. [Google Scholar] [CrossRef]
- Yang, J.; Chen, J.; Hao, Y.; Liu, Y. Identification of the DPPH radical scavenging reaction adducts of ferulic acid and sinapic acid and their structure-antioxidant activity relationship. LWT Food Sci. Technol. 2021, 146, 111411. [Google Scholar] [CrossRef]
- Ochiai, K.; Fujii, S. Structure-property and structure-activity relationships of phenylferrocene derivatives as androgen receptor antagonists. Bioorg. Med. Chem. Lett. 2021, 46, 128141. [Google Scholar] [CrossRef]
- Arora, A.; Nair, M.G.; Strasburg, G.M. Structure-activity relationships for antioxidant activities of a series of flavonoids in a liposomal system. Free Radic. Biol. Med. 1998, 24, 1355–1363. [Google Scholar] [CrossRef]
- Musialik, M.; Kuzmicz, R.; Pawlowski, T.S.; Litwinienko, G. Acidity of Hydroxyl Groups: An Overlooked Influence on Antiradical Properties of Flavonoids. J. Org. Chem. 2009, 74, 2699–2709. [Google Scholar] [CrossRef]
- Lemanska, K.; Szymusiak, H.; Tyrakowska, B.; Zielinski, R.; Soffers, A.E.; Rietjens, I.M. The influence of pH on antioxidant properties and the mechanism of antioxidant action of hydroxyflavones. Free Radic. Biol. Med. 2001, 31, 869–881. [Google Scholar] [CrossRef]
- Pomatto, L.C.D.; Davies, K.J.A. The role of declining adaptive homeostasis in ageing. J. Physiol. 2017, 595, 7275–7309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, K.J.A. Age and sex determine the effectiveness of redox adaptive homeostasis. Free Radic. Biol. Med. 2021, 165, 3. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.M.M.; den Hartog, G.J.M.; Bast, A. Oxidative damage shifts from lipid peroxidation to thiol arylation by catechol-containing antioxidants. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2002, 1583, 279–284. [Google Scholar] [CrossRef]
- Sthijns, M.M.J.P.E.; Weseler, A.R.; Bast, A.; Haenen, G.R.M.M. Time in Redox Adaptation Processes: From Evolution to Hormesis. Int. J. Mol. Sci. 2016, 17, 1649. [Google Scholar] [CrossRef] [Green Version]
- Van De Wier, B.; Koek, G.H.; Bast, A.; Haenen, G.R.M.M. The potential of flavonoids in the treatment of non-alcoholic fatty liver disease. Crit. Rev. Food Sci. Nutr. 2017, 57, 834–855. [Google Scholar] [CrossRef] [PubMed]
- Sarrica, A.; Kirika, N.; Romeo, M.; Salmona, M.; Diomede, L. Safety and toxicology of magnolol and honokiol. Planta Med. 2018, 84, 1151–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Packer, J.E.; Slater, T.F.; Willson, R.L. Direct observation of a free radical interaction between vitamin E and vitamin C. Nature 1979, 278, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Rietjens, S.J.; Bast, A.; Haenen, G.R.M.M. New insights into controversies on the antioxidant potential of the olive oil antioxidant hydroxytyrosol. J. Agric. Food Chem. 2007, 55, 7609–7614. [Google Scholar] [CrossRef]
- Tao, W.; Xu, X.; Wang, X.; Li, B.; Wang, Y.; Li, Y.; Yang, L. Network pharmacology-based prediction of the active ingredients and potential targets of Chinese herbal Radix Curcumae formula for application to cardiovascular disease. J. Ethnopharmacol. 2013, 145, 1–10. [Google Scholar] [CrossRef]
- Lu, W.I.; Lu, D.P. Impact of Chinese Herbal Medicine on American Society and Health Care System: Perspective and Concern. Evid.-Based Complement. Altern. Med. 2014, 2014, 251891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.; Zhang, X.; Wang, Z.; Zheng, C.; Li, P.; Huang, C.; Tao, W.; Xiao, W.; Wang, Y.; Huang, L.; et al. Deciphering the combination principles of Traditional Chinese Medicine from a systems pharmacology perspective based on Ma-huang Decoction. J. Ethnopharmacol. 2013, 150, 619–638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Schiffers, P.; Janssen, G.; Vrolijk, M.; Vangrieken, P.; Haenen, G.R.M.M. The cardiovascular side effects of Ma Huang due to its use in isolation in the Western world. Eur. J. Integr. Med. 2018, 18, 18–22. [Google Scholar] [CrossRef]
- Zhang, M.; Moalin, M.; Haenen, G.R.M.M. Connecting West and East. Int. J. Mol. Sci. 2019, 20, 2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Vrolijk, M.; Haenen, G.R.M.M. The Screening of Anticholinergic Accumulation by Traditional Chinese Medicine. Int. J. Mol. Sci. 2018, 19, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooper, L.; Kay, C.; Abdelhamid, A.; Kroon, P.A.; Cohn, J.S.; Rimm, E.B.; Cassidy, A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: A systematic review and meta-analysis of randomized trials. Am. J. Clin. Nutr. 2012, 95, 740–751. [Google Scholar] [CrossRef]
- Rosch, P.J. Bioelectromagnetic and Subtle Energy Medicine the Interface between Mind and Matter. Ann. N. Y. Acad. Sci. 2009, 1172, 297–311. [Google Scholar] [CrossRef]
- Zapata, F.; Pastor-Ruiz, V.; Ortega-Ojeda, F.; Montalvo, G.; Victoria Ruiz-Zolle, A.; Garcia-Ruiz, C. Human ultra-weak photon emission as non-invasive spectroscopic tool for diagnosis of internal states—A review. J. Photochem. Photobiol. B. Biol. 2021, 216, 112141. [Google Scholar] [CrossRef]
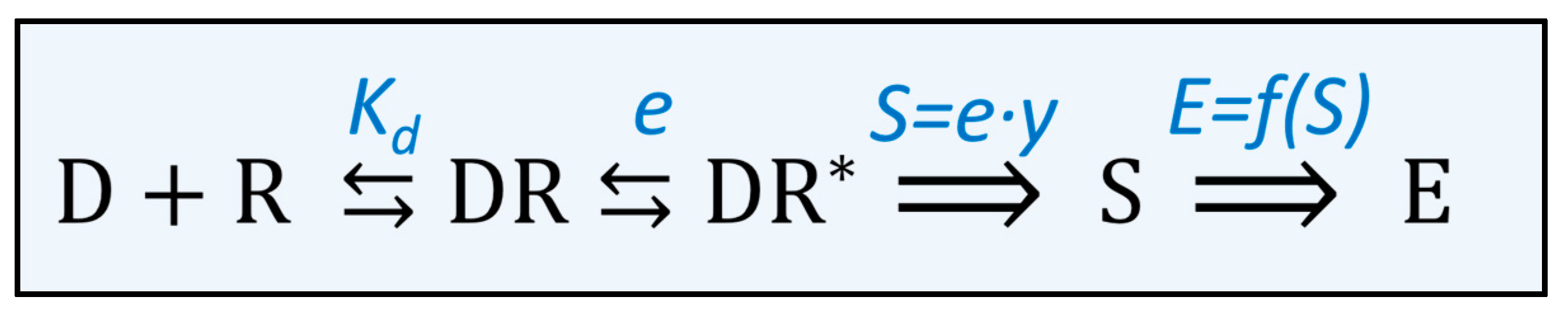


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhang, M.; Haenen, G.R.M.M.; Vervoort, L.; Moalin, M. Flavonoids Seen through the Energy Perspective. Int. J. Mol. Sci. 2022, 23, 187. https://doi.org/10.3390/ijms23010187
Li Z, Zhang M, Haenen GRMM, Vervoort L, Moalin M. Flavonoids Seen through the Energy Perspective. International Journal of Molecular Sciences. 2022; 23(1):187. https://doi.org/10.3390/ijms23010187
Chicago/Turabian StyleLi, Zhengwen, Ming Zhang, Guido R. M. M. Haenen, Lily Vervoort, and Mohamed Moalin. 2022. "Flavonoids Seen through the Energy Perspective" International Journal of Molecular Sciences 23, no. 1: 187. https://doi.org/10.3390/ijms23010187






