Function of Chloroplasts in Plant Stress Responses
Abstract
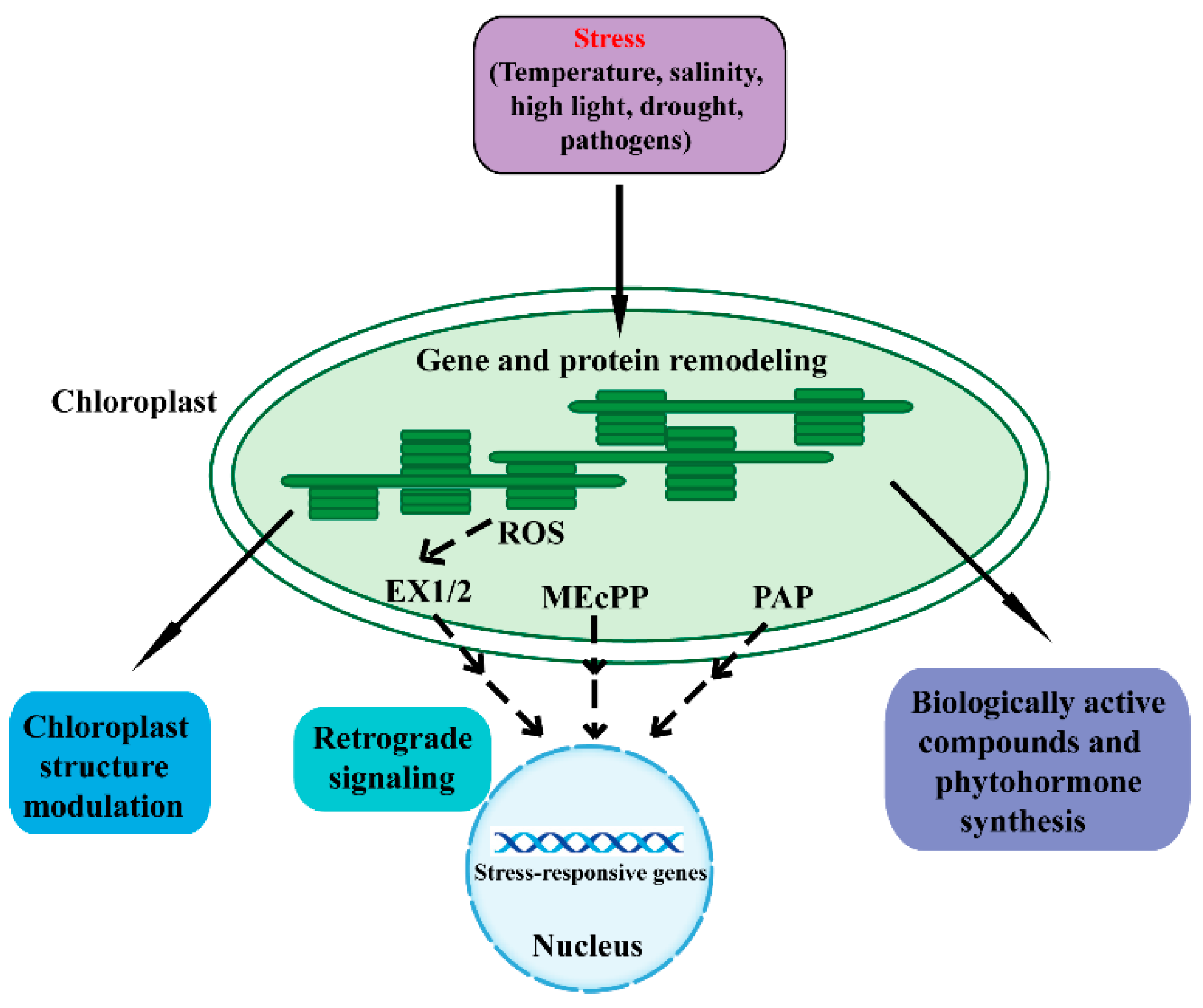
:1. Introduction
2. Chloroplast Response to Abiotic Stress
2.1. Response to Heat Stress
2.2. Response to Low-Temperature Stress
2.3. Response to Salt Stress
2.4. Response to High-Light Stress
2.5. Response to Drought Stress
3. Chloroplast Response to Biotic Stress
| Components | Species | Process/Stimulus | Molecular Function | Reference |
|---|---|---|---|---|
| Heat stress | ||||
| SGR | Musa acuminata | Chlorophyll degradation | Stay-green protein | [56] |
| Rca1 | Triticum aestivum | Modulate the activity of Rubisco | A catalytic chaperone | [57] |
| CDJ2 | Solanum lycopersicum, Lycopersicon esculentum | Protect Rubisco activity | Chloroplast-targeted DnaJ protein | [58,59] |
| Ub2 | Triticum aestivum | Improve antioxidant capacity | Ubiquitin/26S proteasome system | [61] |
| Hsp21 | Arabidopsis thaliana | Associate with the thylakoid membranes | Small heat-shock protein chaperone | [65] |
| Chilling stress | ||||
| FBPase, SBPase | Zea mays L. | Compensate for decreases in photosynthetic capacity | Enzymes involved in the Calvin cycle | [71] |
| DUA1 | Oryza sativa | Regulate chloroplast development | RNA-binding protein | [72] |
| WHY1 | Solanum lycopersicum | Upregulate the PSII key D1 reaction center protein encoding gene psbA; maintain high Rubisco content | WHIRLY proteins—the plant-specific DNA-binding proteins | [73,74] |
| CDJ1 | Lycopersicon esculentum | Maintain PSII activity | Chloroplast-targeted DnaJ protein | [75] |
| RBD1 | Arabidopsis thaliana | Regulate chloroplast protein translation by influencing 23S rRNA processing | Chloroplast RNA-binding protein | [80] |
| Salt stress | ||||
| SP1 | Arabidopsis thaliana | Induce the degradation of translocon at the outer envelope membrane of chloroplasts (TOC) | Uiquitin E3 ligase suppressor of PPI1 locus 1 | [83,84] |
| Hsp70, Clp protease | Arabidopsis thaliana | Protect plants from salinity-triggered oxidative stress | Hsp70—chaperone; Clp—a protease system | [85] |
| RH22 | Brassica rapa | Affect chloroplast gene translation | Cloroplast-targeted DEAD-box RNA helicase | [86] |
| CspA, CspB | Oryza sativa | Impart SGR phenotype | RNA-binding bacterial chaperones | [87] |
| High-light stress | ||||
| SAL1-PAP | Arabidopsis thaliana | Regulate stress-inducible nuclear genes | Components of retrograde pathway | [16] |
| FtSH | Chlamydomonas reinhardtii | Control the quality of thylakoid membrane proteins | Thylakoid membrane protease | [91] |
| Drought stress | ||||
| SAL1-PAP | Arabidopsis thaliana | Regulate the stress-inducible nuclear genes | Components of retrograde pathway | [16] |
| RH22 | Brassica rapa | Affect chloroplast genes’ translation | Chloroplast-targeted DEAD-box RNA helicase | [86] |
| CTR1 | Oryza sativa | Interact with two chloroplast-localized proteins, OsCP12 and OsRP1 | RING Ub E3 ligase | [93] |
| PhyB | Oryza sativa | Repress the activity of ascorbate peroxidase | Phytochrome B | [94] |
| LOX6 | Zea mays | Additional storage of nitrogen | Mesophyll lipoxygenase in chloroplast | [95] |
| NADP-ME4 | Salsola laricifolia | Alleviate chlorophyll content decrease and PSII photochemical efficiency | NADP-malic enzyme | [96] |
| BBX21 | Solanum tuberosum | Reduce chloroplast electron transport capacity | B-box (BBX) protein | [97] |
| Biotic stress | ||||
| PP2C62, PP2C26 | Arabidopsis thaliana | Catalyze the dephosphorylation of the photosynthesis-related protein, chaperonin-60 | Components of the serine/threonine-specific protein phosphatase family | [107] |
| XopL | Nicotiana benthamiana | Eliminate stromules formation and chloroplast relocation | E3 ubiquitin ligase | [108] |
| WKS1 | Triticum aestivum | Phosphorylate thylakoid-associated ascorbate peroxidase (tAPX) and detoxify peroxides; phosphorylate an extrinsic member of photosystem II (PSII) PsbO | A serine/threonine kinase | [109,110] |
| THF1 | Nicotiana benthamiana | Maintain chloroplast homeostasis | Chloroplastic protein thylakoid formation1 | [111] |
| APX8 | Oryza sativa | Regulate H2O2 accumulation | Thylakoid membrane-bound ascorbate peroxidase | [112] |
| LHCB5 | Oryza sativa | Regulate ROS accumulation | Light-harvesting complex II protein | [113] |
| NTRC | Arabidopsis thaliana | Modulate chloroplast-generated ROS | Redox detoxification system NADPH-dependent thioredoxin reductase C | [114] |
| MPK3, MPK6 | Arabidopsis thaliana | Manipulate plant photosynthetic activities and promote ROS accumulation | Mitogen-activated protein kinase | [115] |
| PsbQ | Nicotiana benthamiana, Arabidopsis thaliana | A target for pathogen suppression and contributes to plant immunity responses | The oxygen evolving complex of photosystem II | [118] |
| ALC | Solanum lycopersicum, Arabidopsis thaliana | Regulate disease-associated necrotic cell death | Chloroplast genes with altered responses to coronatine | [119] |
| RipAL | Ralstonia solanacearum | Localized to chloroplasts and targeted chloroplast lipids | Type III effector proteins | [121] |
| Tsn1 | Triticum aestivum | Interact with effector protein ToxA | A unique wheat disease resistance-like gene, regulated by the circadian clock and light | [122] |
| Rpi-vnt1.1 | Nicotiana benthamiana | Recognize the effector protein AVRvnt1, and mediate a light-dependent immune response | A nucleotide-binding leucine-rich repeat (NLR) protein | [123] |
| CAS | Arabidopsis thaliana | Recognize the effector protein, and interfere with the salicylic acid (SA) signaling pathway | A chloroplast-localized calcium-sensing receptor | [124] |
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Fedoroff, N.V.; Battisti, D.S.; Beachy, R.N.; Cooper, P.J.; Fischhoff, D.A.; Hodges, C.N.; Knauf, V.C.; Lobell, D.; Mazur, B.J.; Molden, D.; et al. Radically Rethinking Agriculture for the 21st Century. Science 2010, 327, 833–834. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, A.; Li, X.; Lu, C. The Role of Chloroplast Gene Expression in Plant Responses to Environmental Stress. Int. J. Mol. Sci. 2020, 21, 6082. [Google Scholar] [CrossRef]
- Littlejohn, G.R.; Breen, S.; Smirnoff, N.; Grant, M. Chloroplast Immunity Illuminated. New Phytol. 2021, 229, 3088–3107. [Google Scholar] [CrossRef]
- Serrano, I.; Audran, C.; Rivas, S. Chloroplasts at Work During Plant Innate Immunity. J. Exp. Bot. 2016, 67, 3845–3854. [Google Scholar] [CrossRef]
- Mamaeva, A.; Taliansky, M.; Filippova, A.; Love, A.J.; Golub, N.; Fesenko, I. The Role of Chloroplast Protein Remodeling in Stress Responses and Shaping of The Plant Peptidome. New Phytol. 2020, 227, 1326–1334. [Google Scholar] [CrossRef]
- Watson, S.J.; Sowden, R.G.; Jarvis, P. Abiotic Stress-induced Chloroplast Proteome Remodelling: A Mechanistic Overview. J. Exp. Bot. 2018, 69, 2773–2781. [Google Scholar] [CrossRef]
- Bose, J.; Munns, R.; Shabala, S.; Gilliham, M.; Pogson, B.; Tyerman, S.D. Chloroplast Function and Ion Regulation in Plants Growing on Saline Soils: Lessons from Halophytes. J. Exp. Bot. 2017, 68, 3129–3143. [Google Scholar] [CrossRef]
- Daniell, H.; Lin, C.S.; Yu, M.; Chang, W.J. Chloroplast Genomes: Diversity, Evolution, and Applications in Genetic Engineering. Genome Biol. 2016, 17, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huo, Y.; Gao, L.; Liu, B.; Yang, Y.; Kong, S.; Sun, Y.; Yang, Y.; Wu, X. Complete Chloroplast Genome Sequences of Four Allium Species: Comparative and Phylogenetic Analyses. Sci. Rep. 2019, 9, 12250. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.X.; Phua, S.Y.; Crisp, P.; McQuinn, R.; Pogson, B.J. Learning the Languages of the Chloroplast: Retrograde Signaling and Beyond. Annu. Rev. Plant Biol. 2016, 67, 25–53. [Google Scholar] [CrossRef]
- Crawford, T.; Lehotai, N.; Strand, Å. The Role of Retrograde Signals during Plant Stress Responses. J. Exp. Bot. 2018, 69, 2783–2795. [Google Scholar] [CrossRef]
- de Souza, A.; Wang, J.Z.; Dehesh, K. Retrograde Signals: Integrators of Interorganellar Communication and Orchestrators of Plant Development. Annu. Rev. Plant Biol. 2017, 6, 85–108. [Google Scholar] [CrossRef]
- Wu, G.Z.; Meyer, E.H.; Wu, S.; Bock, R. Extensive Posttranscriptional Regulation of Nuclear Gene Expression by Plastid Retrograde Signals. Plant Physiol. 2019, 180, 2034–2048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, J.C.; Burch-Smith, T.M. Chloroplasts as Mediators of Plant Biotic Interactions over Short and Long Distances. Curr. Opin. Plant Biol. 2019, 50, 148–155. [Google Scholar] [CrossRef]
- Estavillo, G.M.; Crisp, P.A.; Pornsiriwong, W.; Wirtz, M.; Collinge, D.; Carrie, C.; Giraud, E.; Whelan, J.; David, P.; Javot, H.; et al. Evidence for A SAL1-PAP Chloroplast Retrograde Pathway that Functions in Drought and High Light Signaling in Arabidopsis. Plant Cell 2011, 23, 3992–4012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Häusler, R.E.; Heinrichs, L.; Schmitz, J.; Flügge, U.I. How Sugars might Coordinate Chloroplast and Nuclear Gene Expression during Acclimation to High Light Intensities. Mol. Plant 2014, 7, 1121–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramel, F.; Birtic, S.; Ginies, C.; Soubigou-Taconnat, L.; Triantaphylidès, C.; Havaux, M. Carotenoid Oxidation Products are Stress Signals that Mediate Gene Responses to Singlet Oxygen in Plants. Proc. Natl. Acad. Sci. USA 2012, 109, 5535–5540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shumbe, L.; Bott, R.; Havaux, M. Dihydroactinidiolide, a High Light-Induced β-carotene Derivative that Can Regulate Gene Expression and Photoacclimation in Arabidopsis. Mol. Plant 2014, 7, 1248–1251. [Google Scholar] [CrossRef] [Green Version]
- Strand, A.; Asami, T.; Alonso, J.; Ecker, J.R.; Chory, J. Chloroplast to Nucleus Communication Triggered by Accumulation of Mg-protoporphyrinIX. Nature 2003, 421, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Vogel, M.O.; Moore, M.; König, K.; Pecher, P.; Alsharafa, K.; Lee, J.; Dietz, K.J. Fast Retrograde Signaling in Response to High Light Involves Metabolite Export, MITOGEN-ACTIVATED PROTEIN KINASE6, and AP2/ERF Transcription Factors in Arabidopsis. Plant Cell 2014, 26, 1151–1165. [Google Scholar] [CrossRef] [Green Version]
- Woodson, J.D.; Perez-Ruiz, J.M.; Schmitz, R.J.; Ecker, J.R.; Chory, J. Sigma Factor-Mediated Plastid Retrograde Signals Control Nuclear Gene Expression. Plant J. 2013, 73, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Savchenko, T.; Baidoo, E.E.; Chehab, W.E.; Hayden, D.M.; Tolstikov, V.; Corwin, J.A.; Kliebenstein, D.J.; Keasling, J.D.; Dehesh, K. Retrograde Signaling by the Plastidial Metabolite MEcPP Regulates Expression of Nuclear Stress-response Genes. Cell 2012, 149, 1525–1535. [Google Scholar] [CrossRef] [Green Version]
- Mignolet-Spruyt, L.; Xu, E.; Idänheimo, N.; Hoeberichts, F.A.; Mühlenbock, P.; Brosché, M.; Van Breusegem, F.; Kangasjärvi, J. Spreading the News: Subcellular and Organellar Reactive Oxygen Species Production and Signalling. J. Exp. Bot. 2016, 67, 3831–3844. [Google Scholar] [CrossRef] [Green Version]
- Tripathy, B.C.; Oelmüller, R. Reactive Oxygen Species Generation and Signaling in Plants. Plant Signal Behav. 2012, 7, 1621–1633. [Google Scholar] [CrossRef]
- Chan, K.X.; Crisp, P.A.; Estavillo, G.M.; Pogson, B.J. Chloroplast-to-Nucleus Communication: Current Knowledge, Experimental Strategies and Relationship to Drought Stress Signaling. Plant Signal Behav. 2010, 5, 1575–1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, P.; Liu, F.; Li, R.; Wang, S.; Luo, J. Chloroplasts- Beyond Energy Capture and Carbon Fixation: Tuning of Photosynthesis in Response to Chilling Stress. Int. J. Mol. Sci. 2019, 20, 5046. [Google Scholar] [CrossRef] [Green Version]
- Triantaphylidès, C.; Havaux, M. Singlet Oxygen in Plants: Production, Detoxification and Signaling. Trends Plant Sci. 2009, 14, 219–228. [Google Scholar] [CrossRef]
- González-Pérez, S.; Gutiérrez, J.; García-García, F.; Osuna, D.; Dopazo, J.; Lorenzo, Ó.; Revuelta, J.L.; Arellano, J.B. Early Transcriptional Defense Responses in Arabidopsis Cell Suspension Culture under High-Light Conditions. Plant Physiol. 2011, 156, 1439–1456. [Google Scholar] [CrossRef] [Green Version]
- Meskauskiene, R.; Nater, M.; Goslings, D.; Kessler, F.; Op den Camp, R.; Apel, K.F.L.U. A Negative Regulator of Chlorophyll Biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2001, 98, 12826–12831. [Google Scholar] [CrossRef] [Green Version]
- Wagner, D.; Przybyla, D.; Op den Camp, R.; Kim, C.; Landgraf, F.; Lee, K.P.; Würsch, M.; Laloi, C.; Nater, M.; Hideg, E.; et al. The Genetic Basis of Singlet Oxygen-Induced Stress Responses of Arabidopsis thaliana. Science 2004, 306, 1183–1185. [Google Scholar] [CrossRef] [PubMed]
- Op den Camp, R.G.; Przybyla, D.; Ochsenbein, C.; Laloi, C.; Kim, C.; Danon, A.; Wagner, D.; Hideg, E.; Göbel, C.; Feussner, I.; et al. Rapid Induction of Distinct Stress Responses After the Release of Singlet Oxygen in Arabidopsis. Plant Cell 2003, 15, 2320–2332. [Google Scholar] [CrossRef] [Green Version]
- Page, M.T.; McCormac, A.C.; Smith, A.G.; Terry, M.J. Singlet Oxygen Initiates a Plastid Signal Controlling Photosynthetic Gene Expression. New Phytol. 2017, 213, 1168–1180. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.; Kim, C.; Landgraf, F.; Apel, K. EXECUTER1- and EXECUTER2-Dependent Transfer of Stress-Related Signals from the Plastid to the Nucleus of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2007, 104, 10270–10275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.; Apel, K. Singlet Oxygen-Mediated Signaling in Plants: Moving from Flu to Wild Type Reveals an Increasing Complexity. Photosynth. Res. 2013, 116, 455–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uberegui, E.; Hall, M.; Lorenzo, Ó.; Schröder, W.P.; Balsera, M. An Arabidopsis Soluble Chloroplast Proteomic Analysis Reveals the Participation of the Executer Pathway in Response to Increased Light Conditions. J. Exp. Bot. 2015, 66, 2067–2077. [Google Scholar] [CrossRef] [Green Version]
- Carmody, M.; Crisp, P.A.; d’Alessandro, S.; Ganguly, D.; Gordon, M.; Havaux, M.; Albrecht-Borth, V.; Pogson, B.J. Uncoupling High Light Responses from Singlet Oxygen Retrograde Signaling and Spatial-Temporal Systemic Acquired Acclimation. Plant Physiol. 2016, 171, 1734–1749. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Kim, C.; Xu, X.; Piskurewicz, U.; Dogra, V.; Singh, S.; Mahler, H.; Apel, K. Singlet Oxygen- and EXECUTER1-Mediated Signaling Is Initiated in Grana Margins and Depends on the Protease FtsH2. Proc. Natl. Acad. Sci. USA 2016, 113, E3792–E3800. [Google Scholar] [CrossRef] [Green Version]
- Dogra, V.; Duan, J.; Lee, K.P.; Lv, S.; Liu, R.; Kim, C. FtsH2-Dependent Proteolysis of EXECUTER1 Is Essential in Mediating Singlet Oxygen-Triggered Retrograde Signaling in Arabidopsis thaliana. Front Plant Sci. 2017, 8, 1145. [Google Scholar] [CrossRef] [Green Version]
- Shumbe, L.; D’Alessandro, S.; Shao, N.; Chevalier, A.; Ksas, B.; Bock, R.; Havaux, M. METHYLENE BLUE SENSITIVITY 1 (MBS1) is Required for Acclimation of Arabidopsis to Singlet Oxygen and Acts Downstream of β-cyclocitral. Plant Cell Environ. 2017, 40, 216–226. [Google Scholar] [CrossRef] [Green Version]
- Maruta, T.; Noshi, M.; Tanouchi, A.; Tamoi, M.; Yabuta, Y.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. H2O2-Triggered Retrograde Signalingfrom Chloroplasts to Nucleus Plays Specific Role in Response to Stress. J. Biol. Chem. 2012, 287, 11717–11729. [Google Scholar] [CrossRef] [Green Version]
- Petrov, V.D.; Van Breusegem, F. Hydrogen Peroxide-A Central Hub for Information Flow in Plant Cells. AoB Plants 2012, 2012, pls014. [Google Scholar] [CrossRef] [Green Version]
- Benn, G.; Bjornson, M.; Ke, H.; De Souza, A.; Balmond, E.I.; Shaw, J.T.; Dehesh, K. Plastidial Metabolite MEcPP Induces a Transcriptionally Centered Stress-response Hub Via the Transcription Factor CAMTA3. Proc. Natl. Acad. Sci. USA 2016, 113, 8855–8860. [Google Scholar] [CrossRef] [Green Version]
- Walley, J.; Xiao, Y.; Wang, J.Z.; Baidoo, E.E.; Keasling, J.D.; Shen, Z.; Briggs, S.P.; Dehesh, K. Plastid-Produced Interorgannellar Stress Signal MEcPP Potentiates Induction of the Unfolded Protein Response in Endoplasmic Reticulum. Proc. Natl. Acad. Sci. USA 2015, 112, 6212–6217. [Google Scholar] [CrossRef] [Green Version]
- Lemos, M.; Xiao, Y.; Bjornson, M.; Wang, J.Z.; Hicks, D.; Souza, A.; Wang, C.Q.; Yang, P.; Ma, S.; Dinesh-Kumar, S.; et al. The Plastidial Retrograde Signal Methyl Erythritol Cyclopyrophosphate Is a Regulator of Salicylic Acid and Jasmonic Acid Crosstalk. J. Exp. Bot. 2016, 67, 1557–1566. [Google Scholar] [CrossRef] [Green Version]
- Pornsiriwong, W.; Estavillo, G.M.; Chan, K.X.; Tee, E.E.; Ganguly, D.; Crisp, P.A.; Phua, S.Y.; Zhao, C.; Qiu, J.; Park, J.; et al. A Chloroplast Retrograde Signal, 3’-Phosphoadenosine 5’-Phosphate, Acts as A Secondary Messenger in Abscisic Acid Signaling in Stomatal Closure and Germination. eLife 2017, 6, e23361. [Google Scholar] [CrossRef]
- Chan, K.X.; Mabbitt, P.D.; Phua, S.Y.; Mueller, J.W.; Nisar, N.; Gigolashvili, T.; Stroeher, E.; Grassl, J.; Arlt, W.; Estavillo, G.M.; et al. Sensing and Signaling of Oxidative Stress in Chloroplasts by Inactivation of the SAL1 Phosphoadenosine Phosphatase. Proc. Natl. Acad. Sci. USA 2016, 113, E4567–E4576. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.L.; Chen, J.H.; He, N.Y.; Guo, F.Q. Metabolic Reprogramming in Chloroplasts under Heat Stress in Plants. Int. J. Mol. Sci. 2018, 19, 849. [Google Scholar] [CrossRef] [Green Version]
- Yoo, Y.H.; Hong, W.J.; Jung, K.H. A Systematic View Exploring the Role of Chloroplasts in Plant Abiotic Stress Responses. Biomed. Res Int. 2019, 2019, 6534745. [Google Scholar] [CrossRef]
- Rossi, S.; Burgess, P.; Jespersen, D.; Huang, B. Heat-Induced Leaf Senescence Associated with Chlorophyll Metabolism in Bentgrass Lines Differing in Heat Tolerance. Crop Sci. 2017, 57, S169–S178. [Google Scholar] [CrossRef]
- Jespersen, D.; Zhang, J.; Huang, B. Chlorophyll Loss Associated with Heat-induced Senescence in Bentgrass. Plant Sci. 2016, 249, 1–12. [Google Scholar] [CrossRef]
- Li, Z.; Tang, M.; Hassan, M.J.; Zhang, Y.; Han, L.; Peng, Y. Adaptability to High Temperature and Stay-Green Genotypes Associated with Variations in Antioxidant, Chlorophyll Metabolism, and γ-Aminobutyric Acid Accumulation in Creeping Bentgrass Species. Front. Plant Sci. 2021, 12, 750728. [Google Scholar] [CrossRef]
- Jiao, B.; Meng, Q.; Lv, W. Roles of Stay-green (SGR) Homologs during Chlorophyll Degradation in Green Plants. Bot. Stud. 2020, 61, 25. [Google Scholar] [CrossRef]
- Ono, K.; Kimura, M.; Matsuura, H.; Tanaka, A.; Ito, H. Jasmonate Production through Chlorophyll A Degradation by Stay-Green in Arabidopsis thaliana. J. Plant Physiol. 2019, 238, 53–62. [Google Scholar] [CrossRef]
- Shimoda, Y.; Ito, H.; Tanaka, A. Arabidopsis STAY-GREEN, Mendel’s Green Cotyledon Gene, Encodes Magnesium-Dechelatase. Plant Cell 2016, 28, 2147–2160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Pang, X.; Xu, L.; Fang, R.; Huang, X.; Guan, P.; Lu, W.; Zhang, Z. Accumulation of Soluble Sugars in Peel at High Temperature Leads to Stay-green Ripe Banana Fruit. J. Exp. Bot. 2009, 60, 4051–4062. [Google Scholar] [CrossRef]
- Kumar, R.R.; Goswami, S.; Singh, K.; Dubey, K.; Singh, S.; Sharma, R.; Verma, N.; Kala, Y.K.; Rai, G.K.; Grover, M.; et al. Identification of Putative RuBisCo Activase (TaRca1)-The Catalytic Chaperone Regulating Carbon Assimilatory Pathway in Wheat (Triticum aestivum) under the Heat Stress. Front. Plant Sci. 2016, 7, 986. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Kong, F.; Zhang, S.; Meng, X.; Wang, Y.; Meng, Q. A Tomato Chloroplast-Targeted DnaJ Protein Protects Rubisco Activity under Heat Stress. J. Exp. Bot. 2015, 66, 3027–3040. [Google Scholar] [CrossRef] [Green Version]
- Kong, F.; Deng, Y.; Wang, G.; Wang, J.; Liang, X.; Meng, Q. LeCDJ1, A Chloroplast DnaJ Protein, Facilitates Heat Tolerance in Transgenic Tomatoes. J. Integr. Plant Biol. 2014, 56, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Danilova, M.N.; Kudryakova, N.V.; Andreeva, A.A.; Doroshenko, A.S.; Pojidaeva, E.S.; Kusnetsov, V.V. Differential Impact of Heat Stress on the Expression of Chloroplast-Encoded Genes. Plant Physiol. Biochem. 2018, 129, 90–100. [Google Scholar] [CrossRef]
- Tian, F.; Gong, J.; Zhang, J.; Feng, Y.; Wang, G.; Guo, Q.; Wang, W. Overexpression of Monoubiquitin Improves Photosynthesis in Transgenic Tobacco Plants Following High Temperature Stress. Plant Sci. 2014, 226, 92–100. [Google Scholar] [CrossRef]
- Chovancek, E.; Zivcak, M.; Botyanszka, L.; Hauptvogel, P.; Yang, X.; Misheva, S.; Hussain, S.; Brestic, M. Transient Heat Waves May Affect the Photosynthetic Capacity of Susceptible Wheat Genotypes Due to Insufficient Photosystem I Photoprotection. Plants 2019, 8, 282. [Google Scholar] [CrossRef] [Green Version]
- Vacca, R.A.; Valenti, D.; Bobba, A.; Merafina, R.S.; Passarella, S.; Marra, E. Cytochrome C is Released in a Reactive Oxygen Species-Dependent Manner and is Degraded Via Caspase-like Proteases in Tobacco Bright-Yellow 2 Cells En Route to Heat Shock-Induced Cell Death. Plant Physiol. 2006, 141, 208–219. [Google Scholar] [CrossRef] [Green Version]
- Martins, M.Q.; Rodrigues, W.P.; Fortunato, A.S.; Leitão, A.E.; Rodrigues, A.P.; Pais, I.P.; Martins, L.D.; Silva, M.J.; Reboredo, F.H.; Partelli, F.L.; et al. Protective Response Mechanisms to Heat Stress in Interaction with High [CO2] Conditions in Coffea spp. Front Plant Sci. 2016, 7, 947. [Google Scholar] [CrossRef] [Green Version]
- Bernfur, K.; Rutsdottir, G.; Emanuelsson, C. The Chloroplast-Localized Small Heat Shock Protein Hsp21 Associates with the Thylakoid Membranes in Heat-Stressed Plants. Protein Sci. 2017, 26, 1773–1784. [Google Scholar] [CrossRef] [PubMed]
- Cen, W.; Liu, J.; Lu, S.; Jia, P.; Yu, K.; Han, Y.; Li, R.; Luo, J. Comparative Proteomic Analysis of QTL CTS-12 Derived from Wild Rice (Oryza rufipogon Griff.), in the Regulation of Cold Acclimation and De-Acclimation of Rice (Oryza sativa L.) in Response to Severe Chilling Stress. BMC Plant Biol. 2018, 18, 163. [Google Scholar] [CrossRef]
- Hong, W.J.; Jiang, X.; Ahn, H.R.; Choi, J.; Kim, S.R.; Jung, K.H. Systematic Analysis of Cold Stress Response and Diurnal Rhythm Using Transcriptome Data in Rice Reveals the Molecular Networks Related to Various Biological Processes. Int. J. Mol. Sci. 2020, 21, 6872. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Xiao, J.; Bao, F. Effects of Chilling on the Structure, Function and Development of Chloroplasts. Front. Plant Sci. 2018, 9, 1715. [Google Scholar] [CrossRef] [Green Version]
- Skupień, J.; Wójtowicz, J.; Kowalewska, Ł.; Mazur, R.; Garstka, M.; Gieczewska, K.; Mostowska, A. Dark-Chilling Induces Substantial Structural Changes and Modifies Galactolipid and Carotenoid Composition During Chloroplast Biogenesis in Cucumber (Cucumis sativus L.) Cotyledons. Plant Physiol. Biochem. 2017, 111, 107–118. [Google Scholar] [CrossRef]
- Xue, M.; Guo, T.; Ren, M.; Wang, Z.; Tang, K.; Zhang, W.; Wang, M. Constitutive Expression of Chloroplast Glycerol-3-Phosphate Acyltransferase from Ammopiptanthus Mongolicus Enhances Unsaturation of Chloroplast Lipids and Tolerance to Chilling, Freezing and Oxidative Stress in Transgenic Arabidopsis. Plant Physiol. Biochem. 2019, 143, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Kingston-Smith, A.H.; Harbinson, J.; Williams, J.; Foyer, C.H. Effect of Chilling on Carbon Assimilation, Enzyme Activation, and Photosynthetic Electron Transport in the Absence of Photoinhibition in Maize Leaves. Plant Physiol. 1997, 114, 1039–1046. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Wang, Y.; Wu, J.; Han, X.; Gu, X.; Lu, T.; Zhang, Z. The RNA Editing Factor DUA1 is Crucial to Chloroplast Development at Low Temperature in Rice. New Phytol. 2019, 221, 834–849. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, K.; Kong, F.; Zhang, S.; Meng, C.; Yang, M.; Liu, Z.; Wang, Y.; Ma, N.; Meng, Q. Whirly1 Enhances Tolerance to Chilling Stress in Tomato via Protection of Photosystem II and Regulation of Starch Degradation. New Phytol. 2019, 221, 1998–2012. [Google Scholar] [CrossRef]
- Zhuang, K.; Wang, J.; Jiao, B.; Chen, C.; Zhang, J.; Ma, N.; Meng, Q. WHIRLY1 Maintains Leaf Photosynthetic Capacity in Tomato by Regulating the Expression of RbcS1 Under Chilling Stress. J. Exp. Bot. 2020, 71, 3653–3663. [Google Scholar] [CrossRef]
- Kong, F.; Deng, Y.; Zhou, B.; Wang, G.; Wang, Y.; Meng, Q. A Chloroplast-Targeted DnaJ Protein Contributes to Maintenance of Photosystem II under Chilling Stress. J. Exp. Bot. 2014, 65, 143–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Ye, T.; Zhong, B.; Liu, X.; Chan, Z. Comparative Proteomic and Metabolomic Analyses Reveal Mechanisms of Improved Cold Stress Tolerance in Bermudagrass (Cynodon dactylon (L.) Pers.) by Exogenous Calcium. J. Integr. Plant Biol. 2014, 56, 1064–1079. [Google Scholar] [CrossRef]
- van Buer, J.; Cvetkovic, J.; Baier, M. Cold Regulation of Plastid Ascorbate Peroxidases Serves as A Priming Hub Controlling ROS Signaling in Arabidopsis thaliana. BMC Plant Biol. 2016, 16, 163. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Ye, L.; Wang, Y.; Zhou, X.; Yang, J.; Wang, J.; Cao, K.; Zou, Z. Melatonin Increases the Chilling Tolerance of Chloroplast in Cucumber Seedlings by Regulating Photosynthetic Electron Flux and the Ascorbate-Glutathione Cycle. Front. Plant Sci. 2016, 7, 1814. [Google Scholar] [CrossRef] [Green Version]
- Soliman, M.H.; Alayafi, A.A.M.; El Kelish, A.A.; Abu-Elsaoud, A.M. Acetylsalicylic Acid Enhance Tolerance of Phaseolus vulgaris L. to Chilling Stress, Improving Photosynthesis, Antioxidants and Expression of Cold Stress Responsive Genes. Bot. Stud. 2018, 59, 6. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Bai, G.; Wang, S.; Yang, L.; Yang, F.; Wang, Y.; Zhu, J.K.; Hua, J. Chloroplast RNA-Binding Protein RBD1 Promotes Chilling Tolerance through 23S rRNA Processing in Arabidopsis. PLoS Genet. 2016, 12, e1006027. [Google Scholar] [CrossRef]
- He, Y.; Yu, C.; Zhou, L.; Chen, Y.; Liu, A.; Jin, J.; Hong, J.; Qi, Y.; Jiang, D. Rubisco Decrease is Involved in Chloroplast Protrusion and Rubisco-containing Body Formation in Soybean (Glycine max.) Under Salt Stress. Plant Physiol. Biochem. 2014, 74, 118–124. [Google Scholar] [CrossRef]
- Hossain, M.S.; ElSayed, A.I.; Moore, M.; Dietz, K.J. Redox and Reactive Oxygen Species Network in Acclimation for Salinity Tolerance in Sugar Beet. J. Exp. Bot. 2017, 68, 1283–1298. [Google Scholar] [CrossRef] [Green Version]
- Ling, Q.; Jarvis, P. Regulation of Chloroplast Protein Import by the Ubiquitin E3 Ligase SP1 Is Important for Stress Tolerance in Plants. Curr. Biol. 2015, 25, 2527–2534. [Google Scholar] [CrossRef] [Green Version]
- Ling, Q.; Jarvis, P. Plant Signaling: Ubiquitin Pulls the Trigger on Chloroplast Degradation. Curr. Biol. 2016, 26, R38–R40. [Google Scholar] [CrossRef] [Green Version]
- Pulido, P.; Llamas, E.; Rodriguez-Concepcion, M. Both Hsp70 Chaperone and Clp Protease Plastidial Systems are Required for Protection Against Oxidative Stress. Plant Signal Behav. 2017, 12, e1290039. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, G.; Lee, K.; Park, S.J.; Kim, Y.O.; Kang, H. A Chloroplast-Targeted Cabbage DEAD-box RNA Helicase BrRH22 Confers Abiotic Stress Tolerance to Transgenic Arabidopsis Plants by Affecting Translation of Chloroplast Transcripts. Plant Physiol. Biochem. 2018, 127, 336–342. [Google Scholar] [CrossRef]
- Guddimalli, R.; Somanaboina, A.K.; Palle, S.R.; Edupuganti, S.; Kummari, D.; Palakolanu, S.R.; Naravula, J.; Gandra, J.; Qureshi, I.A.; Marka, N.; et al. Overexpression of RNA-binding Bacterial Chaperones in Rice Leads to Stay-green Phenotype, Improved Yield and Tolerance to Salt and Drought Stresses. Physiol. Plant. 2021, 173, 1351–1368. [Google Scholar] [CrossRef]
- Schuster, M.; Gao, Y.; Schöttler, M.A.; Bock, R.; Zoschke, R. Limited Responsiveness of Chloroplast Gene Expression during Acclimation to High Light in Tobacco. Plant Physiol. 2020, 182, 424–435. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Gao, Y.; Wang, H.; Yang, X.; Zhai, H.; Du, Y. Stimulation of Cyclic Electron Flow around PSI As a Response to the Combined Stress of High Light and High Temperature in Grape Leaves. Funct. Plant Biol. 2018, 45, 1038–1045. [Google Scholar] [CrossRef]
- Exposito-Rodriguez, M.; Laissue, P.P.; Yvon-Durocher, G.; Smirnoff, N.; Mullineaux, P.M. Photosynthesis-Dependent H2O2 Transfer from Chloroplasts to Nuclei Provides a High-light Signalling Mechanism. Nat. Commun. 2017, 8, 49. [Google Scholar] [CrossRef] [Green Version]
- Malnoë, A.; Wang, F.; Girard-Bascou, J.; Wollman, F.A.; de Vitry, C. Thylakoid FtsH Protease Contributes to Photosystem II and Cytochrome B6f Remodeling in Chlamydomonas reinhardtii under Stress Conditions. Plant Cell 2014, 26, 373–390. [Google Scholar] [CrossRef] [Green Version]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive Oxygen Species Homeostasis and Signalling During Drought and Salinity Stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Lim, S.D.; Lee, C.; Jang, C.S. The Rice RING E3 ligase, OsCTR1, Inhibits Trafficking to The Chloroplasts of OsCP12 and OsRP1, and Its Overexpression Confers Drought Tolerance in Arabidopsis. Plant Cell Environ. 2014, 37, 1097–1113. [Google Scholar] [CrossRef]
- Yoo, Y.H.; Nalini Chandran, A.K.; Park, J.C.; Gho, Y.S.; Lee, S.W.; An, G.; Jung, K.H. OsPhyB-Mediating Novel Regulatory Pathway for Drought Tolerance in Rice Root Identified by a Global RNA-Seq Transcriptome Analysis of Rice Genes in Response to Water Deficiencies. Front. Plant Sci. 2017, 8, 580. [Google Scholar] [CrossRef]
- Abbaraju, H.K.R.; Gupta, R.; Appenzeller, L.M.; Fallis, L.P.; Hazebroek, J.; Zhu, G.; Bourett, T.M.; Howard, R.J.; Weers, B.; Lafitte, R.H.; et al. A vegetative storage protein improves drought tolerance in maize. Plant Biotechnol. J. 2021. [Google Scholar] [CrossRef]
- Wen, Z.; Wang, Y.; Xia, C.; Zhang, Y.; Zhang, H. Chloroplastic SaNADP-ME4 of C(3)-C(4) Woody Desert Species Salsola laricifolia Confers Drought and Salt Stress Resistance to Arabidopsis. Plants 2021, 10, 1827. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ocampo, G.; Ploschuk, E.L.; Mantese, A.; Crocco, C.D.; Botto, J.F. BBX21 Reduces Abscisic Acid Sensitivity, Mesophyll Conductance and Chloroplast Electron Transport Capacity to Increase Photosynthesis and Water Use Efficiency in Potato Plants Cultivated Under Moderated Drought. Plant J. 2021, 108, 1131–1144. [Google Scholar] [CrossRef]
- Faye, J.M.; Akata, E.A.; Sine, B.; Diatta, C.; Cisse, N.; Fonceka, D.; Morris, G.P. Quantitative and Population Genomics Suggest a Broad Role of Stay-Green Loci In the Drought Adaptation of Sorghum. Plant Genome 2021, e20176. [Google Scholar] [CrossRef]
- Park, E.; Nedo, A.; Caplan, J.L.; Dinesh-Kumar, S.P. Plant-Microbe Interactions: Organelles and the Cytoskeleton in Action. New Phytol. 2018, 217, 1012–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomura, H.; Komori, T.; Uemura, S.; Kanda, Y.; Shimotani, K.; Nakai, K.; Furuichi, T.; Takebayashi, K.; Sugimoto, T.; Sano, S.; et al. Chloroplast-Mediated Activation of Plant Immune Signalling in Arabidopsis. Nat. Commun. 2012, 3, 926. [Google Scholar] [CrossRef] [Green Version]
- de Torres Zabala, M.; Littlejohn, G.; Jayaraman, S.; Studholme, D.; Bailey, T.; Lawson, T.; Tillich, M.; Licht, D.; Bölter, B.; Delfino, L.; et al. Chloroplasts Play a Central Role in Plant Defence and Are Targeted by Pathogen Effectors. Nat. Plants 2015, 1, 15074. [Google Scholar] [CrossRef]
- Bilgin, D.D.; Zavala, J.A.; Zhu, J.; Clough, S.J.; Ort, D.R.; DeLucia, E.H. Biotic Stress Globally Downregulates Photosynthesis Genes. Plant Cell Environ. 2010, 33, 1597–1613. [Google Scholar] [CrossRef] [Green Version]
- Göhre, V.; Jones, A.M.; Sklenář, J.; Robatzek, S.; Weber, A.P. Molecular Crosstalk between PAMP-triggered Immunity and Photosynthesis. Mol. Plant Microbe Interact. 2012, 25, 1083–1092. [Google Scholar] [CrossRef]
- Caplan, J.L.; Kumar, A.S.; Park, E.; Padmanabhan, M.S.; Hoban, K.; Modla, S.; Czymmek, K.; Dinesh-Kumar, S.P. Chloroplast Stromules Function during Innate Immunity. Dev. Cell 2015, 34, 45–57. [Google Scholar] [CrossRef] [Green Version]
- Park, E.; Caplan, J.L.; Dinesh-Kumar, S.P. Dynamic Coordination of Plastid Morphological Change by Cytoskeleton for Chloroplast-Nucleus Communication during Plant Immune Responses. Plant Signal Behav. 2018, 13, e1500064. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Jimenez-Gongora, T.; Krenz, B.; Lozano-Duran, R. Chloroplast Clustering around the Nucleus is A General Response to Pathogen Perception in Nicotiana benthamiana. Mol. Plant Pathol. 2019, 20, 1298–1306. [Google Scholar] [CrossRef] [Green Version]
- Akimoto-Tomiyama, C.; Tanabe, S.; Kajiwara, H.; Minami, E.; Ochiai, H. Loss of Chloroplast-Localized Protein Phosphatase 2Cs in Arabidopsis thaliana Leads to Enhancement of Plant Immunity and Resistance to Xanthomonas campestris pv. campestris Infection. Mol. Plant Pathol. 2018, 19, 1184–1195. [Google Scholar] [CrossRef] [Green Version]
- Erickson, J.L.; Adlung, N.; Lampe, C.; Bonas, U.; Schattat, M.H. The Xanthomonas effector XopL Uncovers the Role of Microtubules in Stromule Extension and Dynamics in Nicotiana benthamiana. Plant J. 2018, 93, 856–870. [Google Scholar] [CrossRef] [Green Version]
- Gou, J.Y.; Li, K.; Wu, K.; Wang, X.; Lin, H.; Cantu, D.; Uauy, C.; Dobon-Alonso, A.; Midorikawa, T.; Inoue, K.; et al. Wheat Stripe Rust Resistance Protein WKS1 Reduces the Ability of the Thylakoid-Associated Ascorbate Peroxidase to Detoxify Reactive Oxygen Species. Plant Cell 2015, 27, 1755–1770. [Google Scholar] [CrossRef]
- Wang, S.; Li, Q.P.; Wang, J.; Yan, Y.; Zhang, G.L.; Yan, Y.; Zhang, H.; Wu, J.; Chen, F.; Wang, X.; et al. YR36/WKS1-Mediated Phosphorylation of PsbO, an Extrinsic Member of Photosystem II, Inhibits Photosynthesis and Confers Stripe Rust Resistance in Wheat. Mol. Plant 2019, 12, 1639–1650. [Google Scholar] [CrossRef] [Green Version]
- Hamel, L.P.; Sekine, K.T.; Wallon, T.; Sugiwaka, Y.; Kobayashi, K.; Moffett, P. The Chloroplastic Protein THF1 Interacts with the Coiled-Coil Domain of the Disease Resistance Protein N’ and Regulates Light-Dependent Cell Death. Plant Physiol. 2016, 171, 658–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, G.; Yin, D.; Zhao, J.; Chen, H.; Guo, L.; Zhu, L.; Zhai, W. The Rice Thylakoid Membrane-Bound Ascorbate Peroxidase OsAPX8 Functions in Tolerance to Bacterial Blight. Sci. Rep. 2016, 6, 26104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Zhang, S.; Hu, J.; Sun, W.; Padilla, J.; He, Y.; Li, Y.; Yin, Z.; Liu, X.; Wang, W.; et al. Phosphorylation-Guarded Light-Harvesting complex II Contributes to Broad-Spectrum Blast Resistance in Rice. Proc. Natl. Acad. Sci. USA 2019, 116, 17572–17577. [Google Scholar] [CrossRef] [Green Version]
- Ishiga, Y.; Ishiga, T.; Ikeda, Y.; Matsuura, T.; Mysore, K.S. NADPH-Dependent Thioredoxin Reductase C Plays a Role in Nonhost Disease Resistance Against Pseudomonas syringae Pathogens by Regulating Chloroplast-Generated Reactive Oxygen Species. Peer. J. 2016, 4, e1938. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Yang, L.; Zhu, Q.; Wu, H.; He, Y.; Liu, Y.; Xu, J.; Jiang, D.; Zhang, S. Active Photosynthetic Inhibition Mediated by MPK3/MPK6 is Critical to Effector-Triggered Immunity. PLoS Biol. 2018, 16, e2004122. [Google Scholar] [CrossRef]
- Lewis, L.A.; Polanski, K.; de Torres-Zabala, M.; Jayaraman, S.; Bowden, L.; Moore, J.; Penfold, C.A.; Jenkins, D.J.; Hill, C.; Baxter, L.; et al. Transcriptional Dynamics Driving MAMP-Triggered Immunity and Pathogen Effector-Mediated Immunosuppression in Arabidopsis Leaves Following Infection with Pseudomonas syringae pv tomato DC3000. Plant Cell 2015, 27, 3038–3064. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Froehlich, J.E.; Elowsky, C.; Msanne, J.; Ostosh, A.C.; Zhang, C.; Awada, T.; Alfano, J.R. Distinct Pseudomonas Type-III Effectors Use a Cleavable Transit Peptide to Target Chloroplasts. Plant J. 2014, 77, 310–321. [Google Scholar] [CrossRef]
- Rodríguez-Herva, J.J.; González-Melendi, P.; Cuartas-Lanza, R.; Antúnez-Lamas, M.; Río-Alvarez, I.; Li, Z.; López-Torrejón, G.; Díaz, I.; Del Pozo, J.C.; Chakravarthy, S.; et al. A Bacterial Cysteine Protease Effector Protein Interferes with Photosynthesis to Suppress Plant Innate Immune Responses. Cell Microbiol. 2012, 14, 669–681. [Google Scholar] [CrossRef] [Green Version]
- Wangdi, T.; Uppalapati, S.R.; Nagaraj, S.; Ryu, C.M.; Bender, C.L.; Mysore, K.S. A Virus-Induced Gene Silencing Screen Identifies a Role for Thylakoid Formation1 in Pseudomonas syringae pv Tomato Symptom Development in Tomato and Arabidopsis. Plant Physiol. 2010, 152, 281–292. [Google Scholar] [CrossRef] [Green Version]
- Rossi, F.R.; Krapp, A.R.; Bisaro, F.; Maiale, S.J.; Pieckenstain, F.L.; Carrillo, N. Reactive Oxygen Species Generated in Chloroplasts Contribute to Tobacco Leaf Infection by the Necrotrophic Fungus Botrytis cinerea. Plant J. 2017, 92, 761–773. [Google Scholar] [CrossRef] [Green Version]
- Nakano, M.; Mukaihara, T. Ralstonia solanacearum Type III Effector RipAL Targets Chloroplasts and Induces Jasmonic Acid Production to Suppress Salicylic Acid-Mediated Defense Responses in Plants. Plant Cell Physiol. 2018, 59, 2576–2589. [Google Scholar] [CrossRef] [PubMed]
- Faris, J.D.; Zhang, Z.; Lu, H.; Lu, S.; Reddy, L.; Cloutier, S.; Fellers, J.P.; Meinhardt, S.W.; Rasmussen, J.B.; Xu, S.S.; et al. A Unique Wheat Disease Resistance-like Gene Governs Effector-triggered Susceptibility to Necrotrophic Pathogens. Proc. Natl. Acad. Sci. USA 2010, 107, 13544–13549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, C.; Xu, H.; Huang, J.; Sun, B.; Zhang, F.; Savage, Z.; Duggan, C.; Yan, T.; Wu, C.H.; Wang, Y.; et al. Pathogen Manipulation of Chloroplast Function Triggers a Light-dependent Immune Recognition. Proc. Natl. Acad. Sci. USA 2020, 117, 9613–9620. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Yang, G.; Ma, M.; Liu, X.; Li, B.; Xie, J.; Fu, Y.; Chen, T.; Yu, Y.; Chen, W.; et al. An Effector of a Mecrotrophic Fungal Pathogen Targets the Calcium-sensing Receptor in Chloroplasts to Inhibit Host Resistance. Mol. Plant Pathol. 2020, 21, 686–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Feng, L.; Alyafei, M.A.M.; Jaleel, A.; Ren, M. Function of Chloroplasts in Plant Stress Responses. Int. J. Mol. Sci. 2021, 22, 13464. https://doi.org/10.3390/ijms222413464
Song Y, Feng L, Alyafei MAM, Jaleel A, Ren M. Function of Chloroplasts in Plant Stress Responses. International Journal of Molecular Sciences. 2021; 22(24):13464. https://doi.org/10.3390/ijms222413464
Chicago/Turabian StyleSong, Yun, Li Feng, Mohammed Abdul Muhsen Alyafei, Abdul Jaleel, and Maozhi Ren. 2021. "Function of Chloroplasts in Plant Stress Responses" International Journal of Molecular Sciences 22, no. 24: 13464. https://doi.org/10.3390/ijms222413464






