Kinase-Inhibitors in Iodine-Refractory Differentiated Thyroid Cancer—Focus on Occurrence, Mechanisms, and Management of Treatment-Related Hypertension
Abstract
:1. Introduction
Multikinase Inhibitors
2. Methods
2.1. Eligibility Criteria
2.2. Search
2.3. Study Selection
3. Results
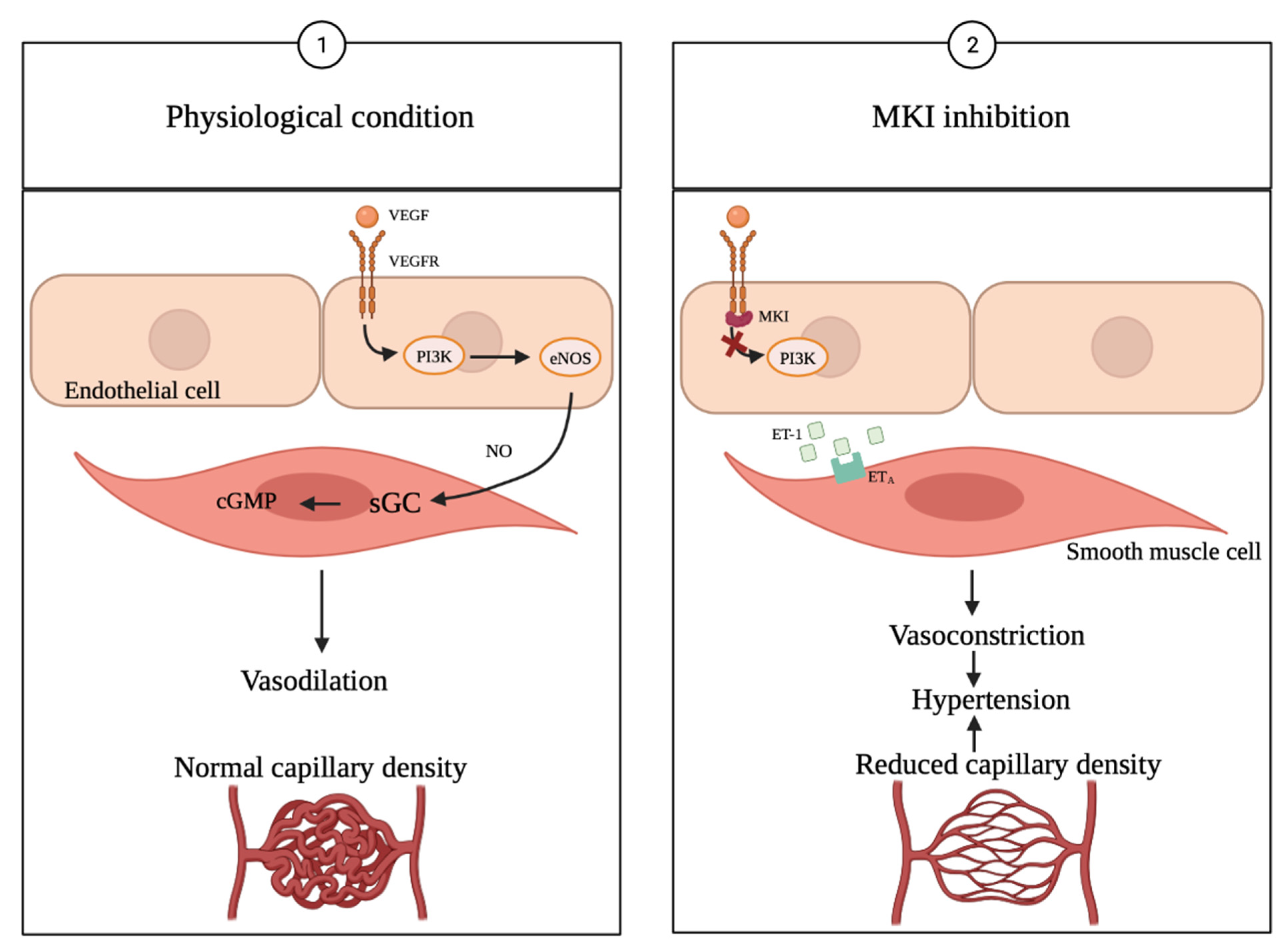
3.1. Multikinase Inhibitor Treatment Causes Hypertension
3.2. Clinical Study Findings
3.3. Management of MKI-Induced Hypertension
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AE(s) | adverse effect(s) |
| ATC | anaplastic thyroid cancer |
| BP | blood pressure |
| CV | cardiovascular |
| DBP | diastolic blood pressure |
| DTC | differentiated thyroid cancer |
| EGF | epidermal growth factor |
| EGFR | epidermal growth factor receptor |
| FGFR | fibroblast growth factor receptor |
| FTC | follicular thyroid cancer |
| HCC | hepatocellular carcinoma |
| HTC | Hürthle cell thyroid cancer |
| HGF | hepatocyte growth factor |
| HGFR | hepatocyte growth factor receptor |
| MTC | medullary thyroid cancer |
| MKI | multikinase inhibitor |
| ORR | overall response rate |
| OS | overall survival |
| PTC | papillary thyroid cancer |
| PDGF | platelet-derived growth factor |
| PDGFR | platelet-derived growth factor receptor |
| PDTC | poorly differentiated thyroid cancer |
| PFS | progression-free survival |
| RAI | radioactive iodine |
| RCC | Renal clear cell carcinoma |
| RR-DTC | RAI refractory DTC |
| RTK | receptor tyrosine kinases |
| SBP | systolic blood pressure |
| TEAE | treatment-emerged adverse effect |
| TE-HTN | treatment-emerged hypertension |
| TC | thyroid cancer |
| TK(s) | tyrosine kinase(s) |
| VEGFR | vascular endothelial growth factor receptor |
| VEGF | vascular endothelial growth factor |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974–2013. JAMA 2017, 317, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Kiyota, N.; Yamazaki, T.; Chayahara, N.; Nakano, K.; Inagaki, L.; Toda, K.; Enokida, T.; Minami, H.; Imamura, Y.; et al. A Phase II study of the safety and efficacy of lenvatinib in patients with advanced thyroid cancer. Future Oncol. 2019, 15, 717–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carling, T.; Udelsman, R. Thyroid cancer. Annu. Rev. Med. 2014, 65, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef]
- Tirrò, E.; Martorana, F.; Romano, C.; Vitale, S.R.; Motta, G.; Di Gregorio, S.; Massimino, M.; Pennisi, M.S.; Stella, S.; Puma, A.; et al. Molecular Alterations in Thyroid Cancer: From Bench to Clinical Practice. Genes 2019, 10, 709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- John, W.; Baynes, M.H.D. Medical Biochemistry; Elsevier Health Sciences: Edinburgh, UK, 2019; Chapter 28. [Google Scholar]
- Zschäbitz, S.; Grüllich, C. Lenvantinib: A Tyrosine Kinase Inhibitor of VEGFR 1-3, FGFR 1-4, PDGFRα, KIT and RET. Recent Results Cancer Res. 2018, 211, 187–198. [Google Scholar] [CrossRef]
- Stjepanovic, N.; Capdevila, J. Multikinase inhibitors in the treatment of thyroid cancer: Specific role of lenvatinib. Biologics 2014, 8, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Prete, A.; Borges de Souza, P.; Censi, S.; Muzza, M.; Nucci, N.; Sponziello, M. Update on Fundamental Mechanisms of Thyroid Cancer. Front. Endocrinol. 2020, 11, 102. [Google Scholar] [CrossRef]
- Marotta, V.; Sciammarella, C.; Colao, A.; Faggiano, A. Application of molecular biology of differentiated thyroid cancer for clinical prognostication. Endocr. Relat. Cancer 2016, 23, R499–R515. [Google Scholar] [CrossRef] [Green Version]
- Lorusso, L.; Cappagli, V.; Valerio, L.; Giani, C.; Viola, D.; Puleo, L.; Gambale, C.; Minaldi, E.; Campopiano, M.C.; Matrone, A.; et al. Thyroid Cancers: From Surgery to Current and Future Systemic Therapies through Their Molecular Identities. Int. J. Mol. Sci. 2021, 22, 3117. [Google Scholar] [CrossRef] [PubMed]
- Marotta, V.; Chiofalo, M.G.; Di Gennaro, F.; Daponte, A.; Sandomenico, F.; Vallone, P.; Costigliola, L.; Botti, G.; Ionna, F.; Pezzullo, L. Kinase-inhibitors for iodine-refractory differentiated thyroid cancer: Still far from a structured therapeutic algorithm. Crit. Rev. Oncol. Hematol. 2021, 162, 103353. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Wang, P. Lenvatinib in Management of Solid Tumors. Oncologist 2020, 25, e302–e310. [Google Scholar] [CrossRef] [Green Version]
- Resteghini, C.; Cavalieri, S.; Galbiati, D.; Granata, R.; Alfieri, S.; Bergamini, C.; Bossi, P.; Licitra, L.; Locati, L.D. Management of tyrosine kinase inhibitors (TKI) side effects in differentiated and medullary thyroid cancer patients. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Marotta, V.; Sciammarella, C.; Vitale, M.; Colao, A.; Faggiano, A. The evolving field of kinase inhibitors in thyroid cancer. Crit. Rev. Oncol. Hematol. 2015, 93, 60–73. [Google Scholar] [CrossRef] [Green Version]
- Wirth, L.J.; Tahara, M.; Robinson, B.; Francis, S.; Brose, M.S.; Habra, M.A.; Newbold, K.; Kiyota, N.; Dutcus, C.E.; Mathias, E.; et al. Treatment-emergent hypertension and efficacy in the phase 3 Study of (E7080) lenvatinib in differentiated cancer of the thyroid (SELECT). Cancer 2018, 124, 2365–2372. [Google Scholar] [CrossRef]
- Ancker, O.V.; Wehland, M.; Bauer, J.; Infanger, M.; Grimm, D. The Adverse Effect of Hypertension in the Treatment of Thyroid Cancer with Multi-Kinase Inhibitors. Int. J. Mol. Sci. 2017, 18, 625. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, Y.; Matsui, J.; Matsushima, T.; Obaishi, H.; Miyazaki, K.; Nakamura, K.; Tohyama, O.; Semba, T.; Yamaguchi, A.; Hoshi, S.S.; et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc. Cell 2014, 6, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Facemire, C.S.; Nixon, A.B.; Griffiths, R.; Hurwitz, H.; Coffman, T.M. Vascular endothelial growth factor receptor 2 controls blood pressure by regulating nitric oxide synthase expression. Hypertension 2009, 54, 652–658. [Google Scholar] [CrossRef] [Green Version]
- Costache, M.I.; Ioana, M.; Iordache, S.; Ene, D.; Costache, C.A.; Săftoiu, A. VEGF Expression in Pancreatic Cancer and Other Malignancies: A Review of the Literature. Rom. J. Intern. Med. 2015, 53, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Shibuya, M.; Claesson-Welsh, L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp. Cell Res. 2006, 312, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Goel, H.L.; Mercurio, A.M. VEGF targets the tumour cell. Nat. Rev. Cancer 2013, 13, 871–882. [Google Scholar] [CrossRef]
- Mohamad Pakarul Razy, N.H.; Wan Abdul Rahman, W.F.; Win, T.T. Expression of Vascular Endothelial Growth Factor and Its Receptors in Thyroid Nodular Hyperplasia and Papillary Thyroid Carcinoma: A Tertiary Health Care Centre Based Study. Asian Pac. J. Cancer Prev. 2019, 20, 277–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laursen, R.; Wehland, M.; Kopp, S.; Pietsch, J.; Infanger, M.; Grosse, J.; Grimm, D. Effects and Role of Multikinase Inhibitors in Thyroid Cancer. Curr. Pharm. Des. 2016, 22, 5915–5926. [Google Scholar] [CrossRef]
- Marotta, V.; Sciammarella, C.; Capasso, M.; Testori, A.; Pivonello, C.; Chiofalo, M.G.; Gambardella, C.; Grasso, M.; Antonino, A.; Annunziata, A.; et al. Germline Polymorphisms of the VEGF Pathway Predict Recurrence in Nonadvanced Differentiated Thyroid Cancer. J. Clin. Endocrinol. Metab. 2017, 102, 661–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marotta, V.; Sciammarella, C.; Capasso, M.; Testori, A.; Pivonello, C.; Chiofalo, M.G.; Pivonello, R.; Pezzullo, L.; Botti, G.; Colao, A.; et al. Preliminary data of VEGF-A and VEGFR-2 polymorphisms as predictive factors of radiological response and clinical outcome in iodine-refractory differentiated thyroid cancer treated with sorafenib. Endocrine 2017, 57, 539–543. [Google Scholar] [CrossRef] [Green Version]
- Kish, J.K.; Chatterjee, D.; Wan, Y.; Yu, H.T.; Liassou, D.; Feinberg, B.A. Lenvatinib and Subsequent Therapy for Radioactive Iodine-Refractory Differentiated Thyroid Cancer: A Real-World Study of Clinical Effectiveness in the United States. Adv. Ther. 2020, 37, 2841–2852. [Google Scholar] [CrossRef] [PubMed]
- Eder, J.P.; Vande Woude, G.F.; Boerner, S.A.; LoRusso, P.M. Novel therapeutic inhibitors of the c-Met signaling pathway in cancer. Clin. Cancer Res. 2009, 15, 2207–2214. [Google Scholar] [CrossRef] [Green Version]
- Jayarangaiah, A.; Sidhu, G.; Brown, J.; Barrett-Campbell, O.; Bahtiyar, G.; Youssef, I.; Arora, S.; Skwiersky, S.; McFarlane, S.I. Therapeutic options for advanced thyroid cancer. Int. J. Clin. Endocrinol. Metab. 2019, 5, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Cabanillas, M.E.; de Souza, J.A.; Geyer, S.; Wirth, L.J.; Menefee, M.E.; Liu, S.V.; Shah, K.; Wright, J.; Shah, M.H. Cabozantinib as Salvage Therapy for Patients with Tyrosine Kinase Inhibitor-Refractory Differentiated Thyroid Cancer: Results of a Multicenter Phase II International Thyroid Oncology Group Trial. J. Clin. Oncol. 2017, 35, 3315–3321. [Google Scholar] [CrossRef]
- Takahashi, S.; Tahara, M.; Ito, K.; Tori, M.; Kiyota, N.; Yoshida, K.; Sakata, Y.; Yoshida, A. Safety and Effectiveness of Lenvatinib in 594 Patients with Unresectable Thyroid Cancer in an All-Case Post-Marketing Observational Study in Japan. Adv. Ther. 2020, 37, 3850–3862. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Sugino, K.; Matsuzu, K.; Masaki, C.; Akaishi, J.; Hames, K.; Tomoda, C.; Suzuki, A.; Uruno, T.; Ohkuwa, K.; et al. Rapid disease progression after discontinuation of lenvatinib in thyroid cancer. Medicine 2020, 99, e19408. [Google Scholar] [CrossRef] [PubMed]
- Aydemirli, M.D.; Kapiteijn, E.; Ferrier, K.R.M.; Ottevanger, P.B.; Links, T.P.; van der Horst-Schrivers, A.N.A.; Broekman, K.E.; Groenwold, R.H.H.; Zwaveling, J. Effectiveness and toxicity of lenvatinib in refractory thyroid cancer: Dutch real-life data. Eur. J. Endocrinol. 2020, 182, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Kiyota, N.; Robinson, B.; Shah, M.; Hoff, A.O.; Taylor, M.H.; Li, D.; Dutcus, C.E.; Lee, E.K.; Kim, S.B.; Tahara, M. Defining Radioiodine-Refractory Differentiated Thyroid Cancer: Efficacy and Safety of Lenvatinib by Radioiodine-Refractory Criteria in the SELECT Trial. Thyroid 2017, 27, 1135–1141. [Google Scholar] [CrossRef] [Green Version]
- Brose, M.S.; Smit, J.; Lin, C.C.; Pitoia, F.; Fellous, M.; DeSanctis, Y.; Schlumberger, M.; Tori, M.; Sugitani, I. Timing of multikinase inhibitor initiation in differentiated thyroid cancer. Endocr. Relat. Cancer 2017, 24, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Tahara, M.; Schlumberger, M.; Elisei, R.; Habra, M.A.; Kiyota, N.; Paschke, R.; Dutcus, C.E.; Hihara, T.; McGrath, S.; Matijevic, M.; et al. Exploratory analysis of biomarkers associated with clinical outcomes from the study of lenvatinib in differentiated cancer of the thyroid. Eur. J. Cancer 2017, 75, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Sugino, K.; Nagahama, M.; Kitagawa, W.; Ohkuwa, K.; Uruno, T.; Matsuzu, K.; Suzuki, A.; Masaki, C.; Akaishi, J.; Hames, K.Y.; et al. Clinical factors related to the efficacy of tyrosine kinase inhibitor therapy in radioactive iodine refractory recurrent differentiated thyroid cancer patients. Endocr. J. 2018, 65, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Molina-Vega, M.; García-Alemán, J.; Sebastián-Ochoa, A.; Mancha-Doblas, I.; Trigo-Pérez, J.M.; Tinahones-Madueño, F. Tyrosine kinase inhibitors in iodine-refractory differentiated thyroid cancer: Experience in clinical practice. Endocrine 2018, 59, 395–401. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; Habra, M.A. Lenvatinib: Role in thyroid cancer and other solid tumors. Cancer Treat. Rev. 2016, 42, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Hasskarl, J. Sorafenib. Recent Results Cancer Res. 2010, 184, 61–70. [Google Scholar] [CrossRef]
- Zimmerman, E.I.; Roberts, J.L.; Li, L.; Finkelstein, D.; Gibson, A.; Chaudhry, A.S.; Schuetz, E.G.; Rubnitz, J.E.; Inaba, H.; Baker, S.D. Ontogeny and sorafenib metabolism. Clin. Cancer Res. 2012, 18, 5788–5795. [Google Scholar] [CrossRef] [Green Version]
- White, P.T.; Cohen, M.S. The discovery and development of sorafenib for the treatment of thyroid cancer. Expert Opin. Drug Discov. 2015, 10, 427–439. [Google Scholar] [CrossRef] [Green Version]
- Lenvima, Lenvatinib. Available online: https://pro.medicin.dk/Medicin/Praeparater/7785 (accessed on 25 September 2021).
- Ancker, O.V.; Krüger, M.; Wehland, M.; Infanger, M.; Grimm, D. Multikinase Inhibitor Treatment in Thyroid Cancer. Int. J. Mol. Sci. 2019, 21, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabometyx, Cabozantinib. Available online: https://pro.medicin.dk/Medicin/Praeparater/8195 (accessed on 25 September 2021).
- Tucker, N. Cabozantinib Lands Breakthrough Threapy Designation in RAI-Refractory Diffenrentiated Thyroid Cancer. Available online: https://www.targetedonc.com/view/cabozantinib-lands-breakthrough-therapy-designation-in-rai-refractory-differentiated-thyroid-cancer (accessed on 23 March 2021).
- Locati, L.D.; Piovesan, A.; Durante, C.; Bregni, M.; Castagna, M.G.; Zovato, S.; Giusti, M.; Ibrahim, T.; Puxeddu, E.; Fedele, G.; et al. Real-world efficacy and safety of lenvatinib: Data from a compassionate use in the treatment of radioactive iodine-refractory differentiated thyroid cancer patients in Italy. Eur. J. Cancer 2019, 118, 35–40. [Google Scholar] [CrossRef]
- Bair, S.M.; Choueiri, T.K.; Moslehi, J. Cardiovascular complications associated with novel angiogenesis inhibitors: Emerging evidence and evolving perspectives. Trends Cardiovasc. Med. 2013, 23, 104–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicenas, J.; Cicenas, E. Multi-kinase inhibitors, AURKs and cancer. Med. Oncol. 2016, 33, 43. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Yazici, Y.D.; Calzada, G.; Wang, Z.Y.; Younes, M.N.; Jasser, S.A.; El-Naggar, A.K.; Myers, J.N. Sorafenib inhibits the angiogenesis and growth of orthotopic anaplastic thyroid carcinoma xenografts in nude mice. Mol. Cancer Ther. 2007, 6, 1785–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gollob, J.A.; Wilhelm, S.; Carter, C.; Kelley, S.L. Role of Raf kinase in cancer: Therapeutic potential of targeting the Raf/MEK/ERK signal transduction pathway. Semin. Oncol. 2006, 33, 392–406. [Google Scholar] [CrossRef]
- Bentzien, F.; Zuzow, M.; Heald, N.; Gibson, A.; Shi, Y.; Goon, L.; Yu, P.; Engst, S.; Zhang, W.; Huang, D.; et al. In vitro and in vivo activity of cabozantinib (XL184), an inhibitor of RET, MET, and VEGFR2, in a model of medullary thyroid cancer. Thyroid 2013, 23, 1569–1577. [Google Scholar] [CrossRef] [Green Version]
- Brose, M.S.; Robinson, B.; Sherman, S.I.; Krajewska, J.; Lin, C.C.; Vaisman, F.; Hoff, A.O.; Hitre, E.; Bowles, D.W.; Hernando, J.; et al. Cabozantinib for radioiodine-refractory differentiated thyroid cancer (COSMIC-311): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1126–1138. [Google Scholar] [CrossRef]
- Worden, F.; Fassnacht, M.; Shi, Y.; Hadjieva, T.; Bonichon, F.; Gao, M.; Fugazzola, L.; Ando, Y.; Hasegawa, Y.; Park, D.J.; et al. Safety and tolerability of sorafenib in patients with radioiodine-refractory thyroid cancer. Endocr. Relat. Cancer 2015, 22, 877–887. [Google Scholar] [CrossRef] [Green Version]
- Brose, M.S.; Nutting, C.M.; Jarzab, B.; Elisei, R.; Siena, S.; Bastholt, L.; de la Fouchardiere, C.; Pacini, F.; Paschke, R.; Shong, Y.K.; et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: A randomised, double-blind, phase 3 trial. Lancet 2014, 384, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Brose, M.S.; Nutting, C.M.; Sherman, S.I.; Shong, Y.K.; Smit, J.W.; Reike, G.; Chung, J.; Kalmus, J.; Kappeler, C.; Schlumberger, M. Rationale and design of decision: A double-blind, randomized, placebo-controlled phase III trial evaluating the efficacy and safety of sorafenib in patients with locally advanced or metastatic radioactive iodine (RAI)-refractory, differentiated thyroid cancer. BMC Cancer 2011, 11, 349. [Google Scholar] [CrossRef] [Green Version]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef] [Green Version]
- Taylor, M.H.; Takahashi, S.; Capdevila, J.; Tahara, M.; Leboulleux, S.; Kiyota, N.; Dutcus, C.E.; Xie, R.; Robinson, B.; Sherman, S.; et al. Correlation of Performance Status and Neutrophil-Lymphocyte Ratio with Efficacy in Radioiodine-Refractory Differentiated Thyroid Cancer Treated with Lenvatinib. Thyroid 2021, 31, 1226–1234. [Google Scholar] [CrossRef]
- Tahara, M.; Kiyota, N.; Hoff, A.O.; Badiu, C.; Owonikoko, T.K.; Dutcus, C.E.; Suzuki, T.; Ren, M.; Wirth, L.J. Impact of lung metastases on overall survival in the phase 3 SELECT study of lenvatinib in patients with radioiodine-refractory differentiated thyroid cancer. Eur. J. Cancer 2021, 147, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Tahara, M.; Brose, M.S.; Wirth, L.J.; Suzuki, T.; Miyagishi, H.; Fujino, K.; Dutcus, C.E.; Gianoukakis, A. Impact of dose interruption on the efficacy of lenvatinib in a phase 3 study in patients with radioiodine-refractory differentiated thyroid cancer. Eur. J. Cancer 2019, 106, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, B.; Schlumberger, M.; Wirth, L.J.; Dutcus, C.E.; Song, J.; Taylor, M.H.; Kim, S.B.; Krzyzanowska, M.K.; Capdevila, J.; Sherman, S.I.; et al. Characterization of Tumor Size Changes Over Time From the Phase 3 Study of Lenvatinib in Thyroid Cancer. J. Clin. Endocrinol. Metab. 2016, 101, 4103–4109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiyota, N.; Schlumberger, M.; Muro, K.; Ando, Y.; Takahashi, S.; Kawai, Y.; Wirth, L.; Robinson, B.; Sherman, S.; Suzuki, T.; et al. Subgroup analysis of Japanese patients in a phase 3 study of lenvatinib in radioiodine-refractory differentiated thyroid cancer. Cancer Sci. 2015, 106, 1714–1721. [Google Scholar] [CrossRef] [Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- PubMed.gov. Available online: https://pubmed.ncbi.nlm.nih.gov (accessed on 2 November 2021).
- Clinicaltrial.gov. Available online: https://clinicaltrials.gov (accessed on 2 November 2021).
- Pandey, A.K.; Singhi, E.K.; Arroyo, J.P.; Ikizler, T.A.; Gould, E.R.; Brown, J.; Beckman, J.A.; Harrison, D.G.; Moslehi, J. Mechanisms of VEGF (Vascular Endothelial Growth Factor) Inhibitor-Associated Hypertension and Vascular Disease. Hypertension 2018, 71, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Wasserstrum, Y.; Kornowski, R.; Raanani, P.; Leader, A.; Pasvolsky, O.; Iakobishvili, Z. Hypertension in cancer patients treated with anti-angiogenic based regimens. Cardiooncology 2015, 1, 6. [Google Scholar] [CrossRef] [Green Version]
- Cabanillas, M.E.; Ryder, M.; Jimenez, C. Targeted Therapy for Advanced Thyroid Cancer: Kinase Inhibitors and Beyond. Endocr. Rev. 2019, 40, 1573–1604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakes, F.M.; Chen, J.; Tan, J.; Yamaguchi, K.; Shi, Y.; Yu, P.; Qian, F.; Chu, F.; Bentzien, F.; Cancilla, B.; et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol. Cancer Ther. 2011, 10, 2298–2308. [Google Scholar] [CrossRef] [Green Version]
- Sueta, D.; Suyama, K.; Sueta, A.; Tabata, N.; Yamashita, T.; Tomiguchi, M.; Takeshita, T.; Yamamoto-Ibusuki, M.; Yamamoto, E.; Izumiya, Y.; et al. Lenvatinib, an oral multi-kinases inhibitor, -associated hypertension: Potential role of vascular endothelial dysfunction. Atherosclerosis 2017, 260, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Reed, N.; Glen, H.; Gerrard, G.; Good, J.; Lei, M.; Lyon, A.R.; Strachan, M.; Wadsley, J.; Newbold, K. Expert Consensus on the Management of Adverse Events During Treatment with Lenvatinib for Thyroid Cancer. Clin. Oncol. 2020, 32, e145–e153. [Google Scholar] [CrossRef] [PubMed]
- Dienstmann, R.; Braña, I.; Rodon, J.; Tabernero, J. Toxicity as a biomarker of efficacy of molecular targeted therapies: Focus on EGFR and VEGF inhibiting anticancer drugs. Oncologist 2011, 16, 1729–1740. [Google Scholar] [CrossRef]
- Small, H.Y.; Montezano, A.C.; Rios, F.J.; Savoia, C.; Touyz, R.M. Hypertension due to antiangiogenic cancer therapy with vascular endothelial growth factor inhibitors: Understanding and managing a new syndrome. Can. J. Cardiol. 2014, 30, 534–543. [Google Scholar] [CrossRef]
- de Jesus-Gonzalez, N.; Robinson, E.; Moslehi, J.; Humphreys, B.D. Management of antiangiogenic therapy-induced hypertension. Hypertension 2012, 60, 607–615. [Google Scholar] [CrossRef] [Green Version]
- Bæk Møller, N.; Budolfsen, C.; Grimm, D.; Krüger, M.; Infanger, M.; Wehland, M.; Magnusson, N.E. Drug-Induced Hypertension Caused by Multikinase Inhibitors (Sorafenib, Sunitinib, Lenvatinib and Axitinib) in Renal Cell Carcinoma Treatment. Int. J. Mol. Sci. 2019, 20, 4712. [Google Scholar] [CrossRef] [Green Version]
- Institute, N.c. Common Terminology Criteria for Adverse Events (CTCAE). Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed on 12 August 2021).
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the Management of Arterial Hypertension. J. Hypertens. 2018, 36, 2284–2309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, W.J. Systemic hypertension. Curr. Probl. Cardiol. 2007, 32, 201–259. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, J.; Newbold, K.; Licitra, L.; Popovtzer, A.; Moreso, F.; Zamorano, J.; Kreissl, M.; Aller, J.; Grande, E. Optimisation of treatment with lenvatinib in radioactive iodine-refractory differentiated thyroid cancer. Cancer Treat. Rev. 2018, 69, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Nervo, A.; Gallo, M.; Samà, M.T.; Felicetti, F.; Alfano, M.; Migliore, E.; Marchisio, F.; Berardelli, R.; Arvat, E.; Piovesan, A. Lenvatinib in Advanced Radioiodine-refractory Thyroid Cancer: A Snapshot of Real-life Clinical Practice. Anticancer. Res. 2018, 38, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Kiyota, N.; Tahara, M. Optimal use of lenvatinib in the treatment of advanced thyroid cancer. Cancers Head Neck 2017, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Cabanillas, M.E.; Schlumberger, M.; Jarzab, B.; Martins, R.G.; Pacini, F.; Robinson, B.; McCaffrey, J.C.; Shah, M.H.; Bodenner, D.L.; Topliss, D.; et al. A phase 2 trial of lenvatinib (E7080) in advanced, progressive, radioiodine-refractory, differentiated thyroid cancer: A clinical outcomes and biomarker assessment. Cancer 2015, 121, 2749–2756. [Google Scholar] [CrossRef]
- Fierro-Maya, L.F.; González, G.G.; Melo, L.J.R.; Cuéllar, A.A.C.; Carreño, A.; Córdoba, C. Safety and efficacy of sorafenib in patients with advanced thyroid carcinoma: A phase II study (NCT02084732). Arch. Endocrinol. Metab. 2021, 65, 404–410. [Google Scholar] [CrossRef]
- Hoftijzer, H.; Heemstra, K.A.; Morreau, H.; Stokkel, M.P.; Corssmit, E.P.; Gelderblom, H.; Weijers, K.; Pereira, A.M.; Huijberts, M.; Kapiteijn, E.; et al. Beneficial effects of sorafenib on tumor progression, but not on radioiodine uptake, in patients with differentiated thyroid carcinoma. Eur. J. Endocrinol. 2009, 161, 923–931. [Google Scholar] [CrossRef] [Green Version]
- Brose, M.S.; Schlumberger, M.; Peña, C.; Kappeler, C. Sorafenib for patients with differentiated thyroid cancer—authors’ reply. Lancet 2015, 385, 228–229. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; Takahashi, S. Managing the adverse events associated with lenvatinib therapy in radioiodine-refractory differentiated thyroid cancer. Semin. Oncol. 2019, 46, 57–64. [Google Scholar] [CrossRef]
- Ptinopoulou, A.G.; Sprangers, B. Tyrosine kinase inhibitor-induced hypertension-marker of anti-tumour treatment efficacy or cardiovascular risk factor? Clin. Kidney J. 2020, 14, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Budolfsen, C.; Faber, J.; Grimm, D.; Krüger, M.; Bauer, J.; Wehland, M.; Infanger, M.; Magnusson, N.E. Tyrosine Kinase Inhibitor-Induced Hypertension: Role of Hypertension as a Biomarker in Cancer Treatment. Curr. Vasc. Pharmacol. 2019, 17, 618–634. [Google Scholar] [CrossRef] [PubMed]
- Izzedine, H.; Ederhy, S.; Goldwasser, F.; Soria, J.C.; Milano, G.; Cohen, A.; Khayat, D.; Spano, J.P. Management of hypertension in angiogenesis inhibitor-treated patients. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2009, 20, 807–815. [Google Scholar] [CrossRef]
- Laffin, L.J.; Bakris, G.L. Endothelin antagonism and hypertension: An evolving target. Semin. Nephrol. 2015, 35, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Lang, N.N.; Herrmann, J.; van den Meiracker, A.H.; Danser, A.H.J. Recent Advances in Hypertension and Cardiovascular Toxicities with Vascular Endothelial Growth Factor Inhibition. Hypertension 2017, 70, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Iglesias, L.; Klain, M.; Pitoia, F.; Schlumberger, M.J. Radioactive iodine-refractory differentiated thyroid cancer: An uncommon but challenging situation. Arch. Endocrinol. Metab. 2017, 61, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Krajewska, J.; Kukulska, A.; Jarzab, B. Efficacy of lenvatinib in treating thyroid cancer. Expert Opin. Pharmacother. 2016, 17, 1683–1691. [Google Scholar] [CrossRef]
- Kreissl, M.C.; Janssen, M.J.R.; Nagarajah, J. Current Treatment Strategies in Metastasized Differentiated Thyroid Cancer. J. Nucl. Med. 2019, 60, 9–15. [Google Scholar] [CrossRef] [Green Version]
- De Leo, S.; Di Stefano, M.; Persani, L.; Fugazzola, L.; Colombo, C. Lenvatinib as first-line treatment for advanced thyroid cancer: Long progression-free survival. Endocrine 2021, 72, 462–469. [Google Scholar] [CrossRef]
- Berdelou, A.; Borget, I.; Godbert, Y.; Nguyen, T.; Garcia, M.E.; Chougnet, C.N.; Ferru, A.; Buffet, C.; Chabre, O.; Huillard, O.; et al. Lenvatinib for the Treatment of Radioiodine-Refractory Thyroid Cancer in Real-Life Practice. Thyroid 2018, 28, 72–78. [Google Scholar] [CrossRef]
- de la Fouchardiere, C.; Alghuzlan, A.; Bardet, S.; Borget, I.; Borson Chazot, F.; Do Cao, C.; Godbert, Y.; Leenhardt, L.; Zerdoud, S.; Leboulleux, S. The medical treatment of radioiodine-refractory differentiated thyroid cancers in 2019. A TUTHYREF(®) network review. Bull Cancer 2019, 106, 812–819. [Google Scholar] [CrossRef]
- Song, E.; Kim, M.; Kim, E.Y.; Kim, B.H.; Shin, D.Y.; Kang, H.C.; Ahn, B.C.; Kim, W.B.; Shong, Y.K.; Jeon, M.J.; et al. Lenvatinib for Radioactive Iodine-Refractory Differentiated Thyroid Carcinoma and Candidate Biomarkers Associated with Survival: A Multicenter Study in Korea. Thyroid 2020, 30, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.K.; Kim, S.M.; Kim, B.H.; Kim, M.J.; Lim, D.J.; Kim, M.H.; Shin, D.Y.; Kang, H.C.; Ahn, B.C.; Kim, S.W.; et al. Lesion-Based Evaluation Predicts Treatment Response to Lenvatinib for Radioactive Iodine-Refractory Differentiated Thyroid Cancer: A Korean Multicenter Retrospective Study. Thyroid 2019, 29, 1811–1819. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, N.; Wang, X.; Ohmoto, A.; Urasaki, T.; Sato, Y.; Nakano, K.; Nishizawa, M.; Yunokawa, M.; Ono, M.; Tomomatsu, J.; et al. Sequential Analysis of Neutrophil-to-lymphocyte Ratio for Differentiated Thyroid Cancer Patients Treated with Lenvatinib. In Vivo 2020, 34, 709–714. [Google Scholar] [CrossRef] [Green Version]
- Ito, Y.; Onoda, N.; Kudo, T.; Masuoka, H.; Higashiyama, T.; Kihara, M.; Miya, A.; Miyauchi, A. Sorafenib and Lenvatinib Treatment for Metastasis/Recurrence of Radioactive Iodine-refractory Differentiated Thyroid Carcinoma. In Vivo 2021, 35, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Lückerath, K.; Schmid, J.S.; Higuchi, T.; Kreissl, M.C.; Grelle, I.; Reiners, C.; Buck, A.K.; Lapa, C. Thyroglobulin fluctuations in patients with iodine-refractory differentiated thyroid carcinoma on lenvatinib treatment—Initial experience. Sci. Rep. 2016, 6, 28081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brose, M.S.; Worden, F.P.; Newbold, K.L.; Guo, M.; Hurria, A. Effect of Age on the Efficacy and Safety of Lenvatinib in Radioiodine-Refractory Differentiated Thyroid Cancer in the Phase III SELECT Trial. J. Clin. Oncol. 2017, 35, 2692–2699. [Google Scholar] [CrossRef]
- Suzuki, C.; Kiyota, N.; Imamura, Y.; Goto, H.; Suto, H.; Chayahara, N.; Toyoda, M.; Ito, Y.; Miya, A.; Miyauchi, A.; et al. Exploratory analysis of prognostic factors for lenvatinib in radioiodine-refractory differentiated thyroid cancer. Head Neck 2019, 41, 3023–3032. [Google Scholar] [CrossRef]
- Jerkovich, F.; García Falcone, M.G.; Pitoia, F. The experience of an Endocrinology Division on the use of tyrosine multikinase inhibitor therapy in patients with radioiodine-resistant differentiated thyroid cancer. Endocrine 2019, 64, 632–638. [Google Scholar] [CrossRef]
- Balmelli, C.; Railic, N.; Siano, M.; Feuerlein, K.; Cathomas, R.; Cristina, V.; Güthner, C.; Zimmermann, S.; Weidner, S.; Pless, M.; et al. Lenvatinib in Advanced Radioiodine-Refractory Thyroid Cancer—A Retrospective Analysis of the Swiss Lenvatinib Named Patient Program. J. Cancer 2018, 9, 250–255. [Google Scholar] [CrossRef] [Green Version]
- Masaki, C.; Sugino, K.; Saito, N.; Akaishi, J.; Hames, K.Y.; Tomoda, C.; Suzuki, A.; Matsuzu, K.; Uruno, T.; Ohkuwa, K.; et al. Efficacy and Limitations of Lenvatinib Therapy for Radioiodine-Refractory Differentiated Thyroid Cancer: Real-World Experiences. Thyroid 2020, 30, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Jerkovich, F.; Califano, I.; Bueno, F.; Carrera, J.M.; Giglio, R.; Abelleira, E.; Pitoia, F. Real-life use of lenvatinib in patients with differentiated thyroid cancer: Experience from Argentina. Endocrine 2020, 69, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Gianoukakis, A.G.; Dutcus, C.E.; Batty, N.; Guo, M.; Baig, M. Prolonged duration of response in lenvatinib responders with thyroid cancer. Endocr. Relat. Cancer 2018, 25, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.S.; Khankin, E.V.; Karumanchi, S.A.; Humphreys, B.D. Hypertension induced by vascular endothelial growth factor signaling pathway inhibition: Mechanisms and potential use as a biomarker. Semin. Nephrol. 2010, 30, 591–601. [Google Scholar] [CrossRef] [Green Version]
- Tahara, M.; Takami, H.; Ito, Y.; Sugino, K.; Takahashi, S.; Takeyama, H.; Tsutsui, H.; Hara, H.; Mitsuma, A.; Yamashita, H.; et al. Cohort study exploring the effect of lenvatinib on differentiated thyroid cancer. Endocr. J. 2018, 65, 1071–1074. [Google Scholar] [CrossRef] [Green Version]
- Jasim, S.; Iniguez-Ariza, N.M.; Hilger, C.R.; Chintakuntlawar, A.V.; Ryder, M.M.; Morris, J.C., 3rd; Bible, K.C. Optimizing lenvatinib therapy in patients with metastatic radioactive iodine-resistant differentiated thyroid cancers. Endocr. Pract. 2017, 23, 1254–1261. [Google Scholar] [CrossRef]
- Suyama, K.; Tomiguchi, M.; Takeshita, T.; Sueta, A.; Yamamoto-Ibusuki, M.; Shimokawa, M.; Yamamoto, Y.; Iwase, H. Factors involved in early lenvatinib dose reduction: A retrospective analysis. Med. Oncol. 2018, 35, 19. [Google Scholar] [CrossRef]
- Haddad, R.I.; Schlumberger, M.; Wirth, L.J.; Sherman, E.J.; Shah, M.H.; Robinson, B.; Dutcus, C.E.; Teng, A.; Gianoukakis, A.G.; Sherman, S.I. Incidence and timing of common adverse events in Lenvatinib-treated patients from the SELECT trial and their association with survival outcomes. Endocrine 2017, 56, 121–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porcelli, T.; Luongo, C.; Sessa, F.; Klain, M.; Masone, S.; Troncone, G.; Bellevicine, C.; Schlumberger, M.; Salvatore, D. Long-term management of lenvatinib-treated thyroid cancer patients: A real-life experience at a single institution. Endocrine 2021, 73, 358–366. [Google Scholar] [CrossRef]
- Agarwal, M.; Thareja, N.; Benjamin, M.; Akhondi, A.; Mitchell, G.D. Tyrosine Kinase Inhibitor-Induced Hypertension. Curr. Oncol. Rep. 2018, 20, 65. [Google Scholar] [CrossRef]
- Oh, H.S.; Shin, D.Y.; Kim, M.; Park, S.Y.; Kim, T.H.; Kim, B.H.; Kim, E.Y.; Kim, W.B.; Chung, J.H.; Shong, Y.K.; et al. Extended Real-World Observation of Patients Treated with Sorafenib for Radioactive Iodine-Refractory Differentiated Thyroid Carcinoma and Impact of Lenvatinib Salvage Treatment: A Korean Multicenter Study. Thyroid 2019, 29, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Iwasaki, H.; Takasaki, H.; Suganuma, N.; Sakai, R.; Masudo, K.; Nakayama, H.; Rino, Y.; Masuda, M. Efficacy and tolerability of initial low-dose lenvatinib to treat differentiated thyroid cancer. Medicine 2019, 98, e14774. [Google Scholar] [CrossRef] [PubMed]
- Keizer, R.J.; Gupta, A.; Mac Gillavry, M.R.; Jansen, M.; Wanders, J.; Beijnen, J.H.; Schellens, J.H.; Karlsson, M.O.; Huitema, A.D. A model of hypertension and proteinuria in cancer patients treated with the anti-angiogenic drug E7080. J. Pharmacokinet. Pharmacodyn. 2010, 37, 347–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masaki, C.; Sugino, K.; Saito, N.; Saito, Y.; Tanaka, T.; Ogimi, Y.; Maeda, T.; Osaku, T.; Akaishi, J.; Hames, K.Y.; et al. Lenvatinib induces early tumor shrinkage in patients with advanced thyroid carcinoma. Endocr. J. 2017, 64, 819–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markowitz, J.N.; Fancher, K.M. Cabozantinib: A Multitargeted Oral Tyrosine Kinase Inhibitor. Pharmacotherapy 2018, 38, 357–369. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, Z.; Ji, Q.; Ge, M.; Shi, F.; Qin, J.; Wang, F.; Chen, G.; Zhang, Y.; Huang, R.; et al. A Randomized, Phase III Study of Lenvatinib in Chinese Patients with Radioiodine-Refractory Differentiated Thyroid Cancer. Clin. Cancer Res. 2021, 27, 5502. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, S.M.; Chang, H.; Kim, B.W.; Lee, Y.S.; Chang, H.S.; Park, C.S. Safety of Tyrosine Kinase Inhibitors in Patients with Differentiated Thyroid Cancer: Real-World Use of Lenvatinib and Sorafenib in Korea. Front. Endocrinol. 2019, 10, 384. [Google Scholar] [CrossRef]
- Schlumberger, M.; Elisei, R.; Müller, S.; Schöffski, P.; Brose, M.; Shah, M.; Licitra, L.; Krajewska, J.; Kreissl, M.C.; Niederle, B.; et al. Overall survival analysis of EXAM, a phase III trial of cabozantinib in patients with radiographically progressive medullary thyroid carcinoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 2813–2819. [Google Scholar] [CrossRef]
- Elisei, R.; Schlumberger, M.J.; Müller, S.P.; Schöffski, P.; Brose, M.S.; Shah, M.H.; Licitra, L.; Jarzab, B.; Medvedev, V.; Kreissl, M.C.; et al. Cabozantinib in progressive medullary thyroid cancer. J. Clin. Oncol. 2013, 31, 3639–3646. [Google Scholar] [CrossRef] [Green Version]
- Verburg, F.A.; Amthauer, H.; Binse, I.; Brink, I.; Buck, A.; Darr, A.; Dierks, C.; Koch, C.; König, U.; Kreissl, M.C.; et al. Questions and Controversies in the Clinical Application of Tyrosine Kinase Inhibitors to Treat Patients with Radioiodine-Refractory Differentiated Thyroid Carcinoma: Expert Perspectives. Horm. Metab. Res. Horm. Stoffwechs. Horm. Metab. 2021, 53, 149–160. [Google Scholar] [CrossRef]

| Drug | Target | Half-Life in Plasma | Metabolism | Approval for DTC | Structure |
|---|---|---|---|---|---|
| Sorafenib | VEGFR-2 and -3 PDGFR, c-Kit, RET/PTC, RAF [41] | 36 h [41] | Hepatic CYP3A4 and UGT1A9 [42] | 2013 [43] | 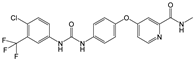 |
| Lenvatinib | , RET, c-KIT, FGFR 1-4 [3] | 28 h [44] | Hepatic CYP3A4 [45] | 2015 [28] | 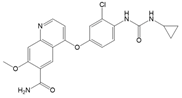 |
| Cabozantinib | VEGFR-2, c-MET, RET [31] | 100–120 h [46] | Hepatic CYP3A4 [46] | 2021 [47] | 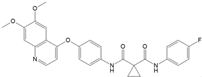 |
| Title | Design | Number of Participants | Doses and Cycles | Results | Status |
|---|---|---|---|---|---|
| A Study of Cabozantinib Compared with Placebo in Subjects with Radioiodine-refractory Differentiated Thyroid Cancer Who Have Progressed After Prior VEGFR-targeted Therapy (COSMIC-311 Trial) NCT03690388 [54] | Interventional, randomized, double blind | Planned 300 Enrolled187 | 60 mg or 20 mg cabozantinib or placebo equivalent once daily | PFS vs. placebo: median not reached (96% CI 5.7–not estimable) versus 1.9 months (1.8–3.6); hazard ratio 0.22 (96% CI 0.13–0.36; p < 0.0001) | Active, not recruiting |
| A Double-Blind Randomized Phase III Study Evaluating the Efficacy and Safety of Sorafenib Compared to Placebo in Locally Advanced/Metastatic RAI-Refractory Differentiated Thyroid Cancer (DECISISON) NCT0098428 [55,56,57] | Interventional, double blind, randomized | 417 | Sorafenib 800 mg/day (400 mg every 12 h) Placebo twice daily (approximately every 12 h). | PFS vs. placebo: hazard ratio, 0.59 (95% CI 0.45–0.76); p < 0.0001; median 10.8 vs. 5.8 months | Completed |
| A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial of Lenvatinib (E7080) in 131I-Refractory Differentiated Thyroid Cancer (SELECT) NCT01321554 [37,58,59,60,61,62,63] | Interventional, double blind, randomized | 392 | Randomization phase: Starting dose—24 mg Lenvatinib orally (two 10 mg tablets and one 4 mg tablet) once daily, continuously, or placebo matching the lenvatinib treatment. | PFS Treatment: 18.3 months Placebo: 3.6 months HR: 0.21 (99% CI: 0.14 to 0.31) ORR Treatment: 64.8% Placebo: 1.5%. | Completed |
| Condition | Systolic Blood Pressure (mmHg) | Diastolic Blood Pressure (mmHg) | |
|---|---|---|---|
| Optimal | <120 | and | <80 |
| Normal | 120–129 | and/or | 80–84 |
| High normal | 130–139 | and/or | 85–89 |
| Grade 1 hypertension | 140–159 | and/or | 90–99 |
| Grade 2 hypertension | 160–179 | and/or | 100–109 |
| Grade 3 hypertension | ≥180 | and/or | ≥110 |
| Isolated systolic hypertension | ≥140 | and | <90 |
| Title | Daily Dose | Most Frequent AEs (Not Including Serious) | Most Frequent Serious AEs |
|---|---|---|---|
| Evaluating the Safety and Efficacy of Oral Lenvatinib in Medullary and Iodine-131 Refractory, Unresectable Differentiated Thyroid Cancers, Stratified by Histology NCT00784303 [83] | 24 mg lenvatinib | Hypertension 77.59% Diarrhea 68.97% Fatigue 60.34% Decreased appetite 55.17% Nausea 51.72% | Hypotension 6.9% Dehydration 6.9% Hypertension 3.45% Renal failure 3.45 Pneumonia 3.45% |
| A Trial of Lenvatinib (E7080) in Subjects with Iodine-131 Refractory Differentiated Thyroid Cancer to Evaluate Whether an Oral Starting Dose of 18 Milligram (mg) Daily Will Provide Comparable Efficacy to a 24 mg Starting Dose, But Have a Better Safety Profile NCT02702388 | 24 mg or 18 mg lenvatinib (starting dose) | 24 mg: Hypertension 57.33% Diarrhea 56% Proteinuria 42.67% Nausea 40.00% Weight loss 36.00% 18 mg: Hypertension 51.95% Diarrhea 51.95% Weight loss 42.86% Stomatitis 38.57% Nausea 35.06% | 24 mg: Total 33.33% Malignant neoplasm progression 4% Pneumothorax 2.67% Hypertension 1.33% 18 mg: Total 40.26% Osteoarthritis 2.60% Pathological fracture 2.60% Malignant neoplasm progression 2.60% Malignant pleural effusion 2.60% |
| A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Trial of Lenvatinib (E7080) in 131I-Refractory Differentiated Thyroid Cancer (DTC) (SELECT) NCT01321554 [58] | 24 mg lenvatinib Placebo matching the lenvatinib treatment. | Hypertension 69.35% Diarrhea 69.73% Loss of appetite 56.70% Weight loss 54.41% Nausea 48.66% | Pneumonia 4.6% Hypertension 3.83 Dehydration 3.45% General physical health deterioration 2.68% Pulmonary embolism 2.3% |
| A Study of E7080 in Subjects with Advanced Thyroid Cancer NCT01728623 | 24 mg lenvatinib | Hypertension 90.20% Palmar-plantar erythrodysaesthesia syndrome 76.47% Loss of appetite 76.47% Fatigue 72.55% Proteinuria 60.78% | Decreased appetite 13.73% Malignant neoplasm progression 7.84% Pneumonia 5.88% Nausea 3.92% Laryngeal stenosis 3.92% |
| Cabozantinib-S-Malate in Treating Patients with Refractory Thyroid Cancer NCT01811212 [31] | 60 mg cabozantinib S-malate | Liver transaminase elevation 80% Palmar-plantar erythrodysesthesia 76% Fatigue 76% Diarrhea 72% Nausea 64% … Hypertension 48% | Left ventricular systolic dysfunction 4% Osteonecrosis of the jaw 4% Asymptomatic increased lipase 4% Meningitis 4% Pneumonia 4% |
| A Study of Cabozantinib Compared with Placebo in Subjects with Radioiodine-refractory Differentiated Thyroid Cancer Who Have Progressed After Prior VEGFR-targeted Therapy (COSMIC-311 Trial) NCT03690388 [54] | 60 mg or 20 mg cabozantinib or placebo equivalent once daily | Diarrhea 44% Palmar-plantar erythrodysesthesia 35% Alanine aminotransferase increased 23% Aspartate aminotransferase increased 23% Nausea 21% … Hypertension 19% | Palmar-plantar erythrodysesthesia 10% Hypertension 9% Fatigue 8% Diarrhoea 7% Hypocalcaemia 7% |
| Safety and Efficacy of Sorafenib in Patients with Advanced Thyroid Cancer: a Phase II Clinical Study NCT02084732 [84] | 800 mg/day sorafenib | Hypertension 42.1% Hand/food Erythema 36.8% Diarrhea 31.5% Muscle pain 21% Rash 21% | Hypertension 56,8% Hand/food Erythema 31.5% Diarrhea 26.2% Rash 15.7% Acute myocardial infarction 5.2% |
| Sorafenib as Adjuvant to Radioiodine Therapy in Non-Medullary Thyroid Carcinoma NCT00887107 [85] | 800 mg/day sorafenib | Weight loss 47.6% Diarrhea 50% Alopecia 47% Rash 47% Hand foot syndrome 43.6% … Hypertension 25.4% | Hand foot syndrome 21.8% Hypertension 15% Weight loss 8.4% Myocardial infarction 3% Congestive heart disease 3% |
| A Double-Blind Randomized Phase III Study Evaluating the Efficacy and Safety of Sorafenib Compared to Placebo in Locally Advanced/Metastatic RAI-Refractory Differentiated Thyroid Cancer (DECISISON) NCT00984282 [56] | 800 mg/day sorafenib | Alopecia 67.1% Diarrhea 62.8% Hand–foot skin reaction 56% Rash 45.4% Fatigue 44% Hypertension 30.9% | Hand–foot skin reaction 20.3% Hypertension 9.7% Hypocalcemia 9.2 Diarrhea 5.8% Fatigue 5.8% |
| Drug | TE-HTN Grades 1–2 (%) | TE-HTN ≥ Grade 3 (%) | Dose Reductions (%) | Dose Interruptions (%) | Discontinuations (%) | TE-HTN-Related Deaths (%) |
|---|---|---|---|---|---|---|
| Lenvatinib [17,58] | 26 | 41.8 | 13 | 13 | 1 | 0 |
| Sorafenib [55,56] | 30.9 | 9.7 | 5.8 | 7.7 | 0.5 | 0 |
| Cabozantinib [54] | 19 | 9 | <7 | n/a | 0.8 | 0 |
| BP Level (mmHg) | Grade of AE | Half-life in plasma |
|---|---|---|
| SBP < 140, DBP < 90 | Grade 1 AE | No treatment needed |
| SBP 140–159, DBP 90–99 | Grade 2 AE | Need for antihypertensive medication |
| SBP ≥ 160, DBP ≥ 100 | Grade 3 AE | Need for antihypertensive medication If persistent for 3 consecutive months, dose reductions are required |
| Life-threatening | Grade 4 AE | Urgent intervention is indicated, possibly treatment interruption |
| Drug | Clinical Trial | Dose Level | Daily Dose | References |
|---|---|---|---|---|
| Lenvatinib (Lenvima®) | SELECT (NCT01321554) | Recommended daily dose | 24 mg p.o. once daily: 2 × 10 mg and 1 × 4 mg in capsules | [44,58,72] |
| Dose reduction No. 1 | 20 mg p.o. once daily: 2 × 10 mg in capsules | |||
| Dose reduction No. 2 | 14 mg orally once daily (1 × 10 mg capsule plus 1 × 4 mg capsule) | |||
| Dose reduction No. 3 | 10 mg p.o. once daily: 1 × 10 mg capsule | |||
| Lenvatinib (Lenvima®) | Lenvatinib in Chinese patients with RR-DTC | Starting dose | 24 mg/day p.o. | [123] |
| In case of intolerable grade 2 or grade 3 HTN; lenvatinib treatment interruption until the toxicity had resolved to grade ≤1 or baseline. | ||||
| Lenvatinib treatment was then resumed at a reduced dose: | ||||
| Dose reduction No. 1 | 20 mg/day | |||
| Dose reduction No. 2 | 14 mg/day | |||
| Dose reduction No. 3 | 10 mg/day | |||
| Lenvatinib (Lenvima®) Sorafenib (NEXAVAR®) | Lenvatinib and Sorafenib in South Korean patients with advanced and metastatic RR-DTC | Lenvatinib arm: starting dose | 20 mg/day capsules | [124] |
| Sorafenib arm: starting dose | 800 mg/day tablets | |||
| Final lenvatinib doses: | ||||
| N = 7 (30.4%) | 10 mg daily | |||
| N = 2 (8.7%) | 14 mg daily | |||
| N = 14 (60.9%) | 20 mg daily | |||
| Dose reduction for AEs: | ||||
| N = 8 (34.8%) | ||||
| Discontinuation of lenvatinib: | ||||
| AEs | ||||
| N = 1 (4.3%) | ||||
| Disease progression: | ||||
| N = 0 | ||||
| Death: | ||||
| N = 2 (8.7%) Financial issues: | ||||
| N = 1 (4.3%) | ||||
| Initial sorafenib dose: | ||||
| N = 12 (25.0) | ≤400 mg dail | |||
| N = 16 (33.3) | 600 mg daily | |||
| N = 20 (41.7) | 800 mg daily | |||
| Final sorafenib dose: | ||||
| N = 20 (41.7) | ≤400 mg daily | |||
| N = 21 (43.8) | 600 mg daily | |||
| N = 7 (14.6) | 800 mg daily | |||
| Dose reduction for AEs: | ||||
| N = 27 (56.3) | ||||
| Discontinuation of sorafenib: | ||||
| AE | ||||
| N = 4 (8.3) | ||||
| Disease progression: | ||||
| N = 17 (35.4) | ||||
| Death: | ||||
| N = 5 (10.4) | ||||
| Sorafenib (NEXAVAR®) | DECISION (NCT00984282) | Recommended daily dose | 2 × 400 mg p.o. | [56] |
| Study drug dose interruption or sequential reduction No. 1 | 600 mg (divided doses: 400 and 200) p.o. daily | |||
| Dose reduction No. 2 | 400 mg (divided 2 × 200) p.o. daily | |||
| Dose reduction No. 3 | 200 mg daily | |||
| Cabozantinib | COSMIC-311 (NCT03690388) | Cabozantinib | [54] | |
| AEs managed by dose modifications: | 60 mg/day tablets p.o. | |||
| The median daily dose was 42.0 mg (IQR 32.2–54.5) with cabozantinib and 60.0 mg (52.9–60.0) with placebo. | Reductions from 60 mg to 40 mg and then to 20 mg daily | |||
| Cabozantinib | EXAM (NCT00704730) MTC (medullary thyroid carcinoma) patients | Cabozantinib starting dose: | 140 mg/day | [125,126] |
| AEs managed with concomitant medications, dose interruptions, and dose reductions; | ||||
| SAEs were more frequent in cabozantinib- versus placebo-treated patients. For hypertension: 2.3% (5 of 214 cabozantinib) vs. 0% (0 of 109 placebo). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaae, A.C.; Kreissl, M.C.; Krüger, M.; Infanger, M.; Grimm, D.; Wehland, M. Kinase-Inhibitors in Iodine-Refractory Differentiated Thyroid Cancer—Focus on Occurrence, Mechanisms, and Management of Treatment-Related Hypertension. Int. J. Mol. Sci. 2021, 22, 12217. https://doi.org/10.3390/ijms222212217
Kaae AC, Kreissl MC, Krüger M, Infanger M, Grimm D, Wehland M. Kinase-Inhibitors in Iodine-Refractory Differentiated Thyroid Cancer—Focus on Occurrence, Mechanisms, and Management of Treatment-Related Hypertension. International Journal of Molecular Sciences. 2021; 22(22):12217. https://doi.org/10.3390/ijms222212217
Chicago/Turabian StyleKaae, Anne Christine, Michael C. Kreissl, Marcus Krüger, Manfred Infanger, Daniela Grimm, and Markus Wehland. 2021. "Kinase-Inhibitors in Iodine-Refractory Differentiated Thyroid Cancer—Focus on Occurrence, Mechanisms, and Management of Treatment-Related Hypertension" International Journal of Molecular Sciences 22, no. 22: 12217. https://doi.org/10.3390/ijms222212217







