Melatonin Confers Plant Cadmium Tolerance: An Update
Abstract
:1. Introduction
2. Role of Melatonin in Plant Abiotic Stress Responses
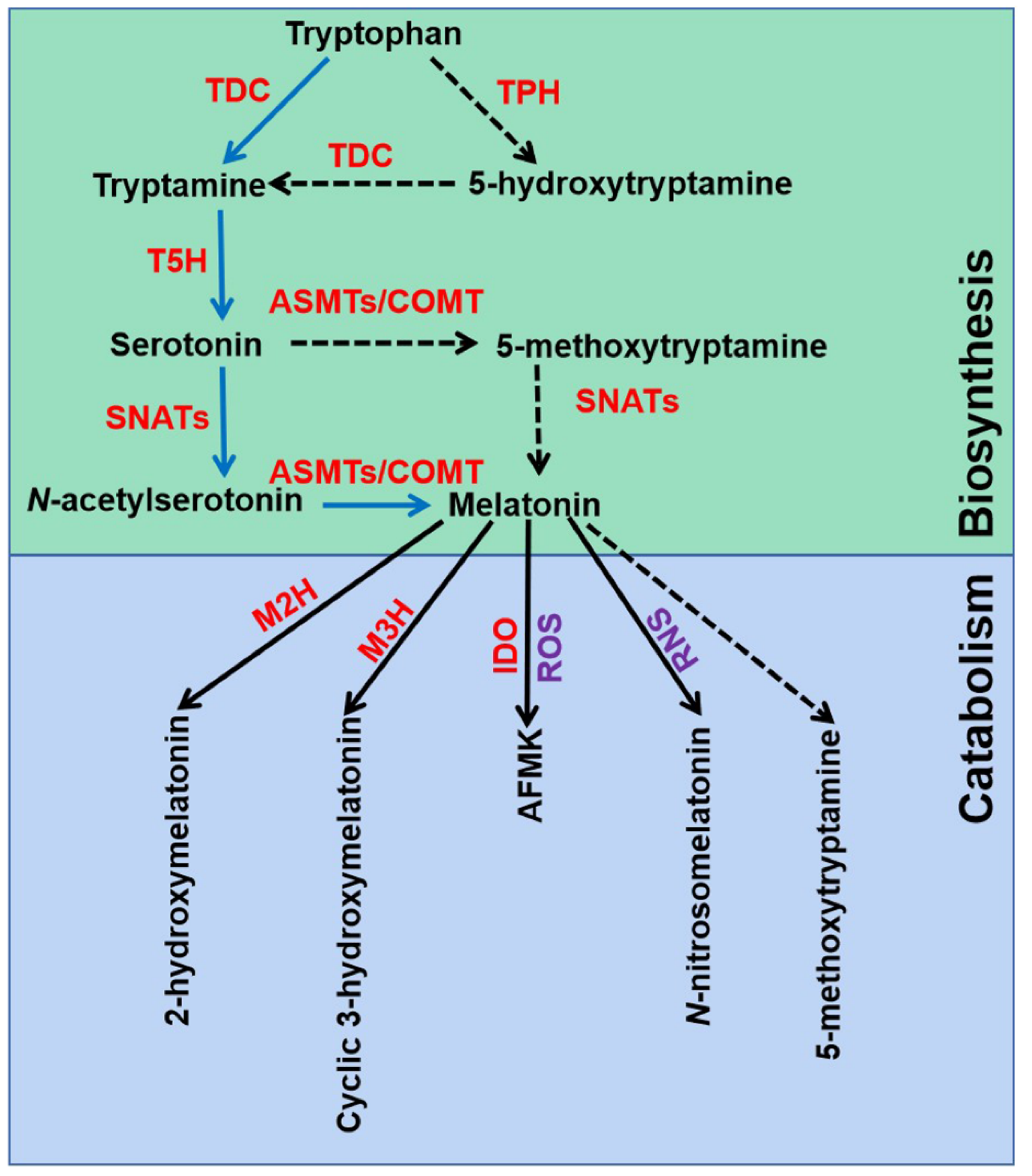
2.1. Melatonin Biosynthesis and Catabolism
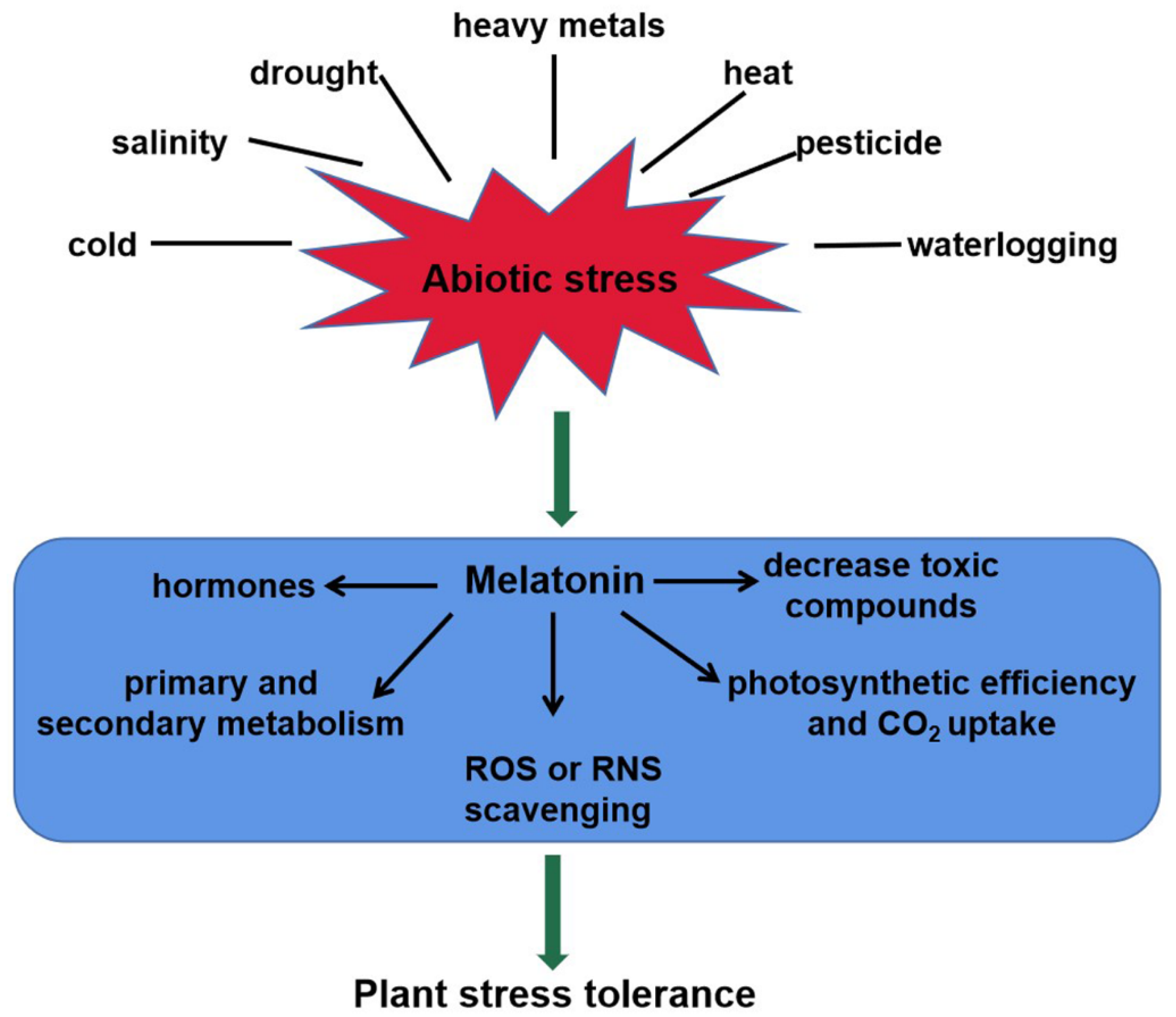
2.2. Melatonin Acts as a Master Regulator in Plant Abiotic Stress
3. Melatonin Improves Cd Tolerance in Plants
3.1. Melatonin Activates Antioxidant Defense Systems in Response to Cd Stress
3.2. Melatonin Regulates Cadmium Uptake and Translocation
3.3. Other Regulators Are Involved in Melatonin-Mediated Cd Tolerance
4. A Possible Role for H2S in Melatonin-Mediated Tolerance against Cd Stress
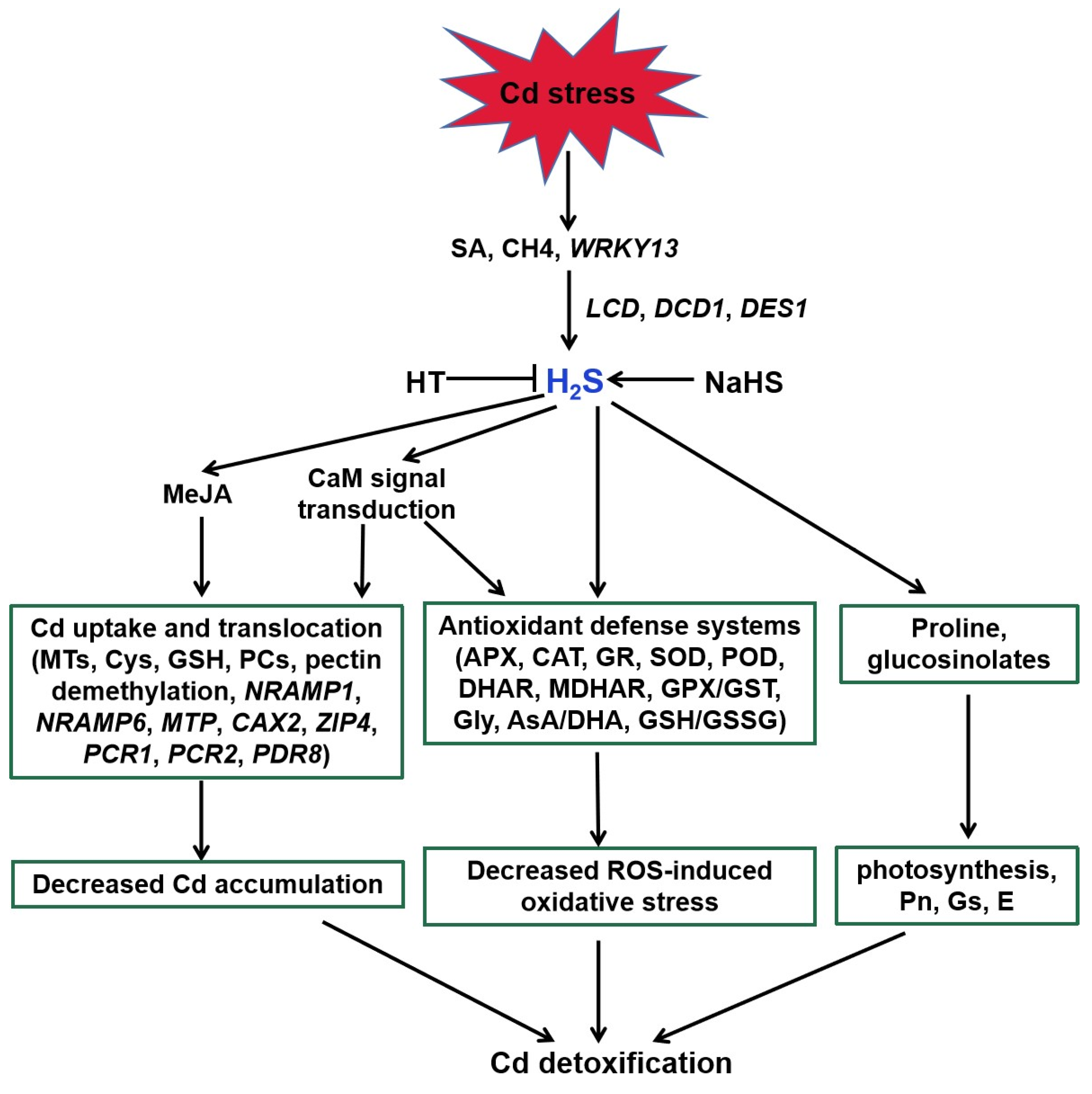
4.1. H2S Action in Plant Tolerance against Cd Stress
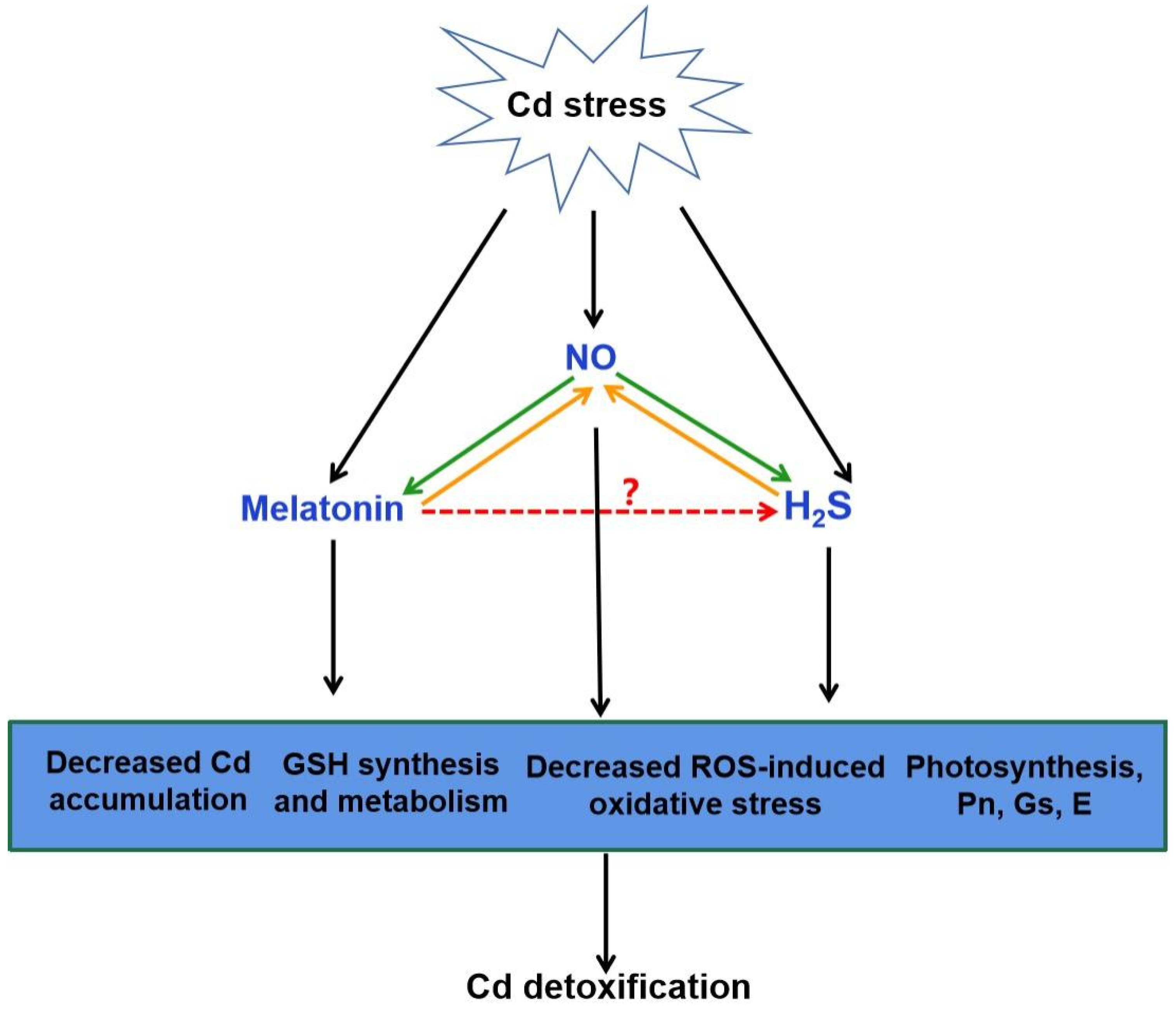
4.2. Crosstalk of Melatonin and H2S in Plants
5. Conclusions and Future Prospects
5.1. Pharmacological, Genetic and ‘Omics’ Approach to Understand the Crosstalk of H2S–Melatonin during Cd Stress
5.2. The Potential Role of Persulfidation Driven by H2S in Melatonin-Mediated Cd Tolerance
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Weissmannová, H.D.; Pavlovský, J. Indices of soil contamination by heavy metals-methodology of calculation for pollution assessment. Environ. Monit. Assess. 2017, 189, 616. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S. Safer food through plant science: Reducing toxic element accumulation in crops. J. Exp. Bot. 2019, 70, 5537–5557. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.; Aarts, M.G.; Thomine, S.; Verbruggen, N. Plant science: The key to preventing slow cadmium poisoning. Trends Plant Sci. 2013, 18, 92–99. [Google Scholar] [CrossRef]
- DalCorso, G.; Manara, A.; Furini, A. An overview of heavy metal challenge in plants: From roots to shoots. Metallomics 2013, 5, 1117–1132. [Google Scholar] [CrossRef]
- Ismael, M.A.; Elyamine, A.M.; Moussa, M.G.; Cai, M.; Zhao, X.H.; Hu, C.X. Cadmium in plants: Uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 2019, 11, 255–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Duan, S.; Zhou, Z.; Chen, S.; Wang, D. Foliar spraying of melatonin confers cadmium tolerance in Nicotiana tabacum L. Ecotoxicol. Environ. Saf. 2019, 170, 68–76. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.J. The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 2009, 14, 43–50. [Google Scholar] [CrossRef]
- Pérez-Chaca, M.V.; Rodríguez-Serrano, M.; Molina, A.S.; Pedranzani, H.E.; Zirulnik, F.; Sandalio, L.M.; Romero-Puertas, M.C. Cadmium induces two waves of reactive oxygen species in Glycine max (L.) roots. Plant Cell Environ. 2014, 37, 1672–1687. [Google Scholar] [CrossRef]
- Khaliq, M.A.; James, B.; Chen, Y.H.; Saqib, H.; Li, H.H.; Jayasuriya, P.; Guo, W. Uptake, translocation, and accumulation of Cd and its interaction with mineral nutrients (Fe, Zn, Ni, Ca, Mg) in upland rice. Chemosphere 2019, 215, 916–924. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2021, 35, 454–484. [Google Scholar] [CrossRef]
- Cobbett, C.; Goldsbrough, P. Phytochelatins and metallothioneins: Roles in heavy metal detoxification and homeostasis. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef] [Green Version]
- Rizwan, M.; Ali, S.; Adrees, M.; Rizvi, H.; Zia-ur-Rehman, M.; Hannan, F.; Qayyum, M.F.; Hafeez, F.; Ok, Y.S. Cadmium stress in rice: Toxic effects, tolerance mechanisms, and management: A critical review. Environ. Sci. Pollut. Res. Int. 2016, 23, 17859–17879. [Google Scholar] [CrossRef]
- Thao, N.P.; Khan, M.I.R.; Thu, N.B.A.; Hoang, X.L.T.; Asgher, M.; Khan, N.A.; Tran, L.S.P. Role of ethylene and its cross talk with other signaling molecules in plant responses to heavy metal stress. Plant Physiol. 2015, 169, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Rabia, A.; Faiza, M.; Ghulam, K.; Tooba, I.; Maryam, K. Plant signaling molecules and cadmium stress tolerance. Cadmium Toler. Plants 2019, 367–399. [Google Scholar] [CrossRef]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar]
- Arnao, M.B.; Hernandez-Ruiz, J. Melatonin: A New Plant Hormone and/or a Plant Master Regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef]
- Tousi, S.; Zoufan, P.; Ghahfarrokhie, A.R. Alleviation of cadmium-induced phytotoxicity and growth improvement by exogenous melatonin pretreatment in mallow (Malva parviflora) plants. Ecotoxicol. Environ. Saf. 2020, 206, 111403. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Chen, Z.; Yu, X.; Cui, W.; Pan, J.; Zhao, G.; Xu, S.; Wang, R.; Shen, W. Melatonin confers plant tolerance against cadmium stress via the decrease of cadmium accumulation and reestablishment of microRNA-mediated redox homeostasis. Plant Sci. 2017, 261, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Okant, M.; Ugurlar, F.; Alyemeni, M.N.; Ashraf, M.; Ahmad, P. Melatonin-mediated nitric oxide improves tolerance to cadmium toxicity by reducing oxidative stress in wheat plants. Chemosphere 2019, 225, 627–638. [Google Scholar] [CrossRef]
- He, J.; Zhuang, X.; Zhou, J.; Sun, L.; Wan, H.; Li, H.; Lyu, D. Exogenous melatonin alleviates cadmium uptake and toxicity in apple rootstocks. Tree Physiol. 2020, 40, 746–761. [Google Scholar] [CrossRef]
- Cai, S.Y.; Zhang, Y.; Xu, Y.P.; Qi, Z.Y.; Li, M.Q.; Ahammed, G.J.; Xia, X.; Shi, K.; Zhou, Y.; Reiter, R.; et al. HsfA1a upregulates melatonin biosynthesis to confer cadmium tolerance in tomato plants. J. Pineal Res. 2017, 62, e12387. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Choi, G.H.; Back, K. Cadmium-induced melatonin synthesis in rice requires light, hydrogen peroxide, and nitric oxide: Key regulatory roles for tryptophan decarboxylase and caffeic acid O-methyltransferase. J. Pineal Res. 2017, 63, e12441. [Google Scholar] [CrossRef] [PubMed]
- Kyungjin, L.; Jin, H.O.; Reiter, R.J.; Kyoungwhan, B. Flavonoids inhibit both rice and sheep serotonin N-acetyltransferases and reduce melatonin levels in plants. J. Pineal Res. 2018, 65, e12512. [Google Scholar] [CrossRef]
- Lu, R.; Liu, Z.; Shao, Y.; Sun, F.; Zhang, Y.; Cui, J.; Zhou, T. Melatonin is responsible for rice resistance to rice stripe virus infection through a nitric oxide-dependent pathway. Virol. J. 2019, 16, 141. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Chen, Y.; Zhai, F.; Zhang, J.; Zhang, F.; Yuan, X.; Xie, Y. Hydrogen sulfide promotes rice drought tolerance via reestablishing redox homeostasis and activation of ABA biosynthesis and signaling. Plant Physiol. Biochem. 2020, 155, 213–220. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, M.; Zhou, H.; Zhao, D.; Gotor, C.; Romero, L.C.; Shen, J.; Ge, Z.; Zhang, Z.; Shen, W.; et al. Hydrogen sulfide, a signaling molecule in plant stress responses. J. Integr. Plant Biol. 2021, 63, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhou, H.; Shen, J.; Zhang, Z.; Xie, Y. H2S action in plant life cycle. Plant Growth Regul. 2021, 94, 1–9. [Google Scholar] [CrossRef]
- Zhou, H.; Guan, W.; Zhou, M.; Shen, J.; Liu, X.; Wu, D.; Yin, X.; Xie, Y. Cloning and characterization of a gene encoding true D-cysteine desulfhydrase from Oryza sativa. Plant Mol. Biol. Rep. 2020, 38, 95–113. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, W.; Ji, T.T.; Ye, L.; Lu, Y.T.; Yuan, T.T. WRKY13 enhances cadmium tolerance by promoting D-cysteine desulfhydrase and hydrogen sulfide production. Plant Physiol. 2020, 183, 345–357. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bhatla, S.C. Exogenous melatonin modulates endogenous H2S homeostasis and L-cysteine desulfhydrase activity in salt-stressed tomato (Solanum lycopersicum L. var. cherry) seedling cotyledons. J. Plant Growth Regul. 2020, 4, 1–13. [Google Scholar] [CrossRef]
- Huang, D.; Huo, J.; Liao, W. Hydrogen sulfide: Roles in plant abiotic stress response and crosstalk with other signals. Plant Sci. 2021, 302, 110733. [Google Scholar] [CrossRef]
- Wang, H.R.; Che, Y.H.; Wang, Z.H.; Zhang, B.N.; Ao, H. The multiple effects of hydrogen sulfide on cadmium toxicity in tobacco may be interacted with CaM signal transduction. J. Hazard. Mater. 2021, 403, 123651. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.; Khan, M.; Mukherjee, S.; Basahi, R.; Alamri, S.; Al-Amri, A.; Alsubaie, Q.; Ali, H.; Al-Munqedhi, B.; Almohisen, I. Exogenous melatonin-mediated regulation of K+/Na+ transport, H+-ATPase activity and enzymatic antioxidative defence operate through endogenous hydrogen sulphide signalling in NaCl-stressed tomato seedling roots. Plant Biol. 2021, 23, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ma, C.; Kang, X.; Zhang, L.; Wang, J.; Zheng, S.; Zhang, T. Hydrogen sulfide and nitric oxide are involved in melatonin-induced salt tolerance in cucumber. Plant Physiol. Biochem. 2021, 167, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Fatma, M.; Gautam, H.; Umar, S.; Khan, N.A. The crosstalk of melatonin and hydrogen sulfide determines photosynthetic performance by regulation of carbohydrate metabolism in wheat under heat stress. Plants 2021, 10, 1778. [Google Scholar] [CrossRef]
- Murch, S.J.; KrishnaRaj, S.; Saxena, P.K. Tryptophan is a precursor for melatonin and serotonin biosynthesis in in vitro regenerated St. John’s wort (Hypericum perforatum L. cv. Anthos) plants. Plant Cell Rep. 2000, 19, 698–704. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.; Kim, Y.S.; Back, K. Tryptamine 5-hydroxylase-deficient Sekiguchi rice induces synthesis of 5-hydroxytryptophan and N-acetyltryptamine but decreases melatonin biosynthesis during senescence process of detached leaves. J. Pineal Res. 2012, 52, 211–216. [Google Scholar] [CrossRef]
- Tan, D.X.; Reiter, R.J. An evolutionary view of melatonin synthesis and metabolism related to its biological functions in plants. J. Exp. Bot. 2020, 71, 4677–4689. [Google Scholar] [CrossRef]
- Ye, T.; Yin, X.; Yu, L.; Zheng, S.J.; Cai, W.J.; Wu, Y.; Feng, Y.Q. Metabolic analysis of the melatonin biosynthesis pathway using chemical labeling coupled with liquid chromatography-mass spectrometry. J. Pineal Res. 2019, 66, e12531. [Google Scholar] [CrossRef] [Green Version]
- Okazaki, M.; Higuchi, K.; Aouini, A.; Ezura, H. Lowering intercellular melatonin levels by transgenic analysis of indoleamine 2,3-dioxygenase from rice in tomato plants. J. Pineal Res. 2010, 49, 239–247. [Google Scholar] [CrossRef]
- Byeon, Y.; Back, K. Molecular cloning of melatonin 2-hydroxylase responsible for 2-hydroxymelatonin production in rice (Oryza sativa). J. Pineal Res. 2015, 58, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Back, K. 2-Hydroxymelatonin promotes the resistance of rice plant to multiple simultaneous abiotic stresses (combined cold and drought). J. Pineal Res. 2016, 61, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Zawadzka, A.; Czarnocki, Z.; Reiter, R.J.; Back, K. Molecular cloning of melatonin 3-hydroxylase and its production of cyclic 3-hydroxymelatonin in rice (Oryza sativa). J. Pineal Res. 2016, 61, 470–478. [Google Scholar] [CrossRef]
- Singh, N.; Kaur, H.; Yadav, S.; Bhatla, S.C. Does N-nitrosomelatonin compete with S-nitrosothiols as a long distance nitric oxide carrier in plants? Biochem. Anal. Biochem. 2016, 5, 262. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S. Insights into nitric oxide-melatonin crosstalk and N-nitrosomelatonin functioning in plants. J. Exp. Bot. 2019, 70, 6035–6047. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, Y.; Gu, Q.; Zhao, G.; Zhang, Y.; Cui, W.; Xu, S.; Wang, R.; Shen, W. The AtrbohF-dependent regulation of ROS signaling is required for melatonin-induced salinity tolerance in Arabidopsis. Free Radical. Biol. Med. 2017, 108, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Shazad, R.; Bilal, S.; Imran, Q.M.; Lee, I.J. Exogenous melatonin mediates the regulation of endogenous nitric oxide in Glycine max L. to reduce effects of drought stress. Environ. Exp. Bot. 2021, 188, 104511. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Lan, Z.; Xu, K.; Chang, J.; Ahammed, G.J.; Ma, J.; Wei, C.; Zhang, X. Methyl jasmonate mediates melatonin-induced cold tolerance of grafted watermelon plants. Hortic. Res. 2021, 8, 57. [Google Scholar] [CrossRef]
- Xia, H.; Zhou, Y.; Deng, H.; Lin, L.; Deng, Q.; Wang, J.; Lv, X.; Zhang, X.; Liang, D. Melatonin improves heat tolerance in Actinidia deliciosa via carotenoid biosynthesis and heat shock proteins expression. Physiol Plant. 2021, 172, 1582–1593. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhou, J.; Tan, D.X.; Wang, N.; Wang, L.; Shan, D.; Kong, J. Melatonin improves waterlogging tolerance of Malus baccata (Linn.) Borkh. seedlings by maintaining aerobic respiration, photosynthesis and ROS migration. Front. Plant Sci. 2017, 8, 483. [Google Scholar] [CrossRef] [Green Version]
- Pang, Y.W.; Jiang, X.L.; Wang, Y.C.; Wang, Y.Y.; Hao, H.S.; Zhao, S.J.; Du, W.H.; Zhao, X.M.; Wang, L.; Zhu, H.B. Melatonin protects against paraquat-induced damage during in vitro maturation of bovine oocytes. J. Pineal Res. 2019, 66, e12532. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.F.; Xu, T.F.; Wang, Z.Z.; Fang, Y.L.; Xi, Z.M.; Zhang, Z.W. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: Antioxidant metabolites, leaf anatomy, and chloroplast morphology. J. Pineal Res. 2014, 57, 200–212. [Google Scholar] [CrossRef]
- Wang, P.; Yin, L.; Liang, D.; Li, C.; Ma, F.; Yue, Z. Delayed senescence of apple leaves by exogenous melatonin treatment: Toward regulating the ascorbate-glutathione cycle. J. Pineal Res. 2012, 53, 11–20. [Google Scholar] [CrossRef] [PubMed]
- DalCorso, G.; Farinati, S.; Maistri, S.; Furini, A. How plants cope with cadmium: Staking all on metabolism and gene expression. J. Integr. Plant Biol. 2008, 50, 1268–1280. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin against environmental plant stressors: A review. Curr. Protein Pept. Sci. 2021, 21, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Zelm, E.V.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.Y.; Back, K. Melatonin is required for H2O2- and NO-mediated defense signaling through MAPKKK3 and OXI1 in Arabidopsis thaliana. J. Pineal Res. 2017, 62, e12379. [Google Scholar] [CrossRef]
- Shi, H.; Jiang, C.; Ye, T.; Tan, D.X.; Reiter, R.J.; Zhang, H.; Liu, R.; Chan, Z. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin. J. Exp. Bot. 2015, 66, 681–694. [Google Scholar] [CrossRef] [Green Version]
- Li, M.Q.; Hasan, M.K.; Li, C.X.; Ahammed, G.J.; Xia, X.J.; Shi, K.; Zhou, Y.; Reiter, R.; Yu, J.; Xu, M.; et al. Melatonin mediates selenium-induced tolerance to cadmium stress in tomato plants. J. Pineal Res. 2016, 61, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wang, Q.; Shah, F.A.; Liu, W.; Wang, D.; Huang, S.; Fu, S.; Wu, L. Exogenous melatonin confers cadmium tolerance by counterbalancing the hydrogen peroxide homeostasis in wheat seedlings. Molecules 2018, 23, 799. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yao, Z.; Zhang, R.; Mou, Z.; Yin, H.; Xu, T.; Zhao, D.; Chen, S. Genome-wide identification and expression profile of the SNAT gene family in tobacco (Nicotiana tabacum). Front. Genet. 2020, 11, 591984. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, Y.; Qian, J.; Si, W.; Tan, Q.; Xu, J.; Zhao, Y. Melatonin enhances the cadmium tolerance of mushrooms through antioxidant-related metabolites and enzymes. Food Chem. 2020, 330, 127263. [Google Scholar] [CrossRef]
- Byeon, Y.; Lee, H.Y.; Hwang, O.J.; Lee, H.J.; Back, K. Coordinated regulation of melatonin synthesis and degradation genes in rice leaves in response to cadmium treatment. J. Pineal Res. 2015, 58, 470–478. [Google Scholar] [CrossRef]
- Byeon, Y.; Lee, H.J.; Lee, H.Y.; Back, K. Cloning and functional characterization of the Arabidopsis N-acetylserotonin O-methyltransferase responsible for melatonin synthesis. J. Pineal Res. 2016, 60, 65–73. [Google Scholar] [CrossRef]
- Byeon, Y.; Lee, H.Y.; Back, K. Cloning and characterization of the serotoninn-acetyltransferase-2 gene (SNAT2) in rice (Oryza sativa). J. Pineal Res. 2016, 61, 198–207. [Google Scholar] [CrossRef]
- Wang, T.; Song, J.; Liu, Z.; Liu, Z.; Cui, J. Melatonin alleviates cadmium toxicity by reducing nitric oxide accumulation and IRT1 expression in Chinese cabbage seedlings. Environ. Sci. Pollut. Res. Int. 2021, 28, 15394–15405. [Google Scholar] [CrossRef]
- Asada, K. THE WATER-WATER CYCLE IN CHLOROPLASTS: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef]
- Considine, M.J.; Foyer, C.H. Oxygen and reactive oxygen species-dependent regulation of plant growth and development. Plant Physiol. 2020, 186, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abd_Allah, E.F.; Ahmad, P.; Corpas, F.J. Assessment of Subcellular ROS and NO Metabolism in Higher Plants: Multifunctional Signaling Molecules. Antioxidants 2019, 8, 641. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.P.; Dong, X.J.; Ma, H.H. Molecular mechanism for cadmium-induced anthocyanin accumulation in Azolla imbricata. Chemosphere 2012, 87, 319–325. [Google Scholar] [CrossRef]
- Alzahrani, Y.; Rady, M.M. Compared to antioxidants and polyamines, the role of maize grain-derived organic biostimulants in improving cadmium tolerance in wheat plants. Ecotoxicol. Environ. Saf. 2019, 182, 109378. [Google Scholar] [CrossRef]
- Kanu, A.S.; Ashraf, U.; Mo, Z.; Sabir, S.; Tang, X. Calcium amendment improved the performance of fragrant rice and reduced metal uptake under cadmium toxicity. Environ. Sci. Pollut. Res. 2019, 26, 24748–24757. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.K.; Ahammed, G.J.; Yin, L.; Shi, K.; Xia, X.; Zhou, Y.; Yu, J.; Zhou, J. Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front. Plant Sci. 2015, 6, 601. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.K.; Ahammed, G.J.; Sun, S.; Li, M.; Yin, H.; Zhou, J. Melatonin inhibits cadmium translocation and enhances plant tolerance by regulating sulfur uptake and assimilation in Solanum lycopersicum L. J. Agric. Food Chem. 2019, 67, 10563–10576. [Google Scholar] [CrossRef]
- Sami, A.; Shah, F.A.; Abdullah, M.; Zhou, X.Y.; Zhou, K.J. Melatonin mitigates cadmium and aluminium toxicity through modulation of antioxidant potential in Brassica napus L. Plant Biol. 2020, 22, 679–690. [Google Scholar] [CrossRef]
- Nabaei, M.; Amooaghaie, R. Melatonin and nitric oxide enhance cadmium tolerance and phytoremediation efficiency in Catharanthus roseus (L.) G. Don. Environ. Sci. Pollut. Res. Int. 2020, 27, 6981–6994. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Zhang, J.; Gong, X.; Wang, Y. Exogenous melatonin improves physiological characteristics and promotes growth of strawberry seedlings under cadmium stress. Hortic. Plant J. 2021, 7, 13–22. [Google Scholar] [CrossRef]
- Amjadi, Z.; Namdjoyan, S.; Soorki, A.A. Exogenous melatonin and salicylic acid alleviates cadmium toxicity in safflower (Carthamus tinctorius L.) seedlings. Ecotoxicology 2021, 30, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Dai, S.; Wang, B.; Xie, Z.; Li, J.; Wang, L.; Li, S.; Tan, Y.; Tian, B.; Shu, Q.; et al. Gold nanoparticles synthesized using melatonin suppress cadmium uptake and alleviate its toxicity in rice. Environ. Sci. Nano 2021, 8, 1042–1056. [Google Scholar] [CrossRef]
- Ma, L.; Huang, Z.; Li, S.; Ashraf, U.; Yang, W.; Liu, H.; Xu, D.; Li, W.; Mo, Z. Melatonin and nitrogen applications modulate early growth and related physio-biochemical attributes in Maize under Cd stress. J. Soil Sci. Plant Nutr. 2021, 21, 978–990. [Google Scholar] [CrossRef]
- Bao, Q.; Bao, W.; Li, Y.; Zhang, S.; Lian, F.; Huang, Y. Silicon combined with foliar melatonin for reducing the absorption and translocation of Cd and As by Oryza sativa L. in two contaminated soils. J. Environ. Manag. 2021, 287, 112343. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, F.; Tang, M.; Wang, Y.; Dong, J.; Ying, J.; Chen, Y.; Hu, B.; Li, C.; Liu, L. Melatonin confers cadmium tolerance by modulating critical heavy metal chelators and transporters in radish plants. J. Pineal Res. 2020, 69, e12659. [Google Scholar] [CrossRef]
- Tang, Y.; Lin, L.; Xie, Y.; Liu, J.; Sun, G.; Li, H.; Liao, M.; Wang, Z.; Liang, D.; Xia, H.; et al. Melatonin affects the growth and cadmium accumulation of Malachium aquaticum and Galinsoga parviflora. Int. J. Phytoremediation 2018, 20, 295–300. [Google Scholar] [CrossRef]
- Lin, L.; Li, J.; Chen, F.; Liao, M.; Tang, Y.; Liang, D.; Xia, H.; Lai, Y.; Wang, X.; Chen, C.; et al. Effects of melatonin on the growth and cadmium characteristics of Cyphomandra betacea seedlings. Environ. Monit. Assess. 2018, 190, 119. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Shu, F.; Fujiwara, T.; Yoneyama, T.; Hayashi, H. Quantitative estimation of the contribution of the phloem in cadmium transport to grains in rice plants (Oryza sativa L.). Soil Sci. Plant Nutr. 2010, 53, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Raza, A.; Habib, M.; Kakavand, S.N.; Zahid, Z.; Zahra, N.; Sharif, R.; Hasanuzzaman, M. Phytoremediation of cadmium: Physiological, biochemical, and molecular mechanisms. Biology 2020, 9, 177. [Google Scholar] [CrossRef]
- Nevo, Y.; Nelson, N. The NRAMP family of metal-ion transporters. Biochim. Biophys. Acta Mol. Cell Res. 2006, 1763, 609–620. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Yamaji, N.; Yamane, M.; Kashino-Fujii, M.; Sato, K.; Ma, J.F. The HvNramp5 transporter mediates uptake of cadmium and manganese, but not iron. Plant Physiol. 2016, 172, 1899–1910. [Google Scholar] [CrossRef] [Green Version]
- Uraguchi, S.; Kamiya, T.; Sakamoto, T.; Kasai, K.; Sato, Y.; Nagamura, Y.; Yoshida, A.; Kyozuka, J.; Ishikawa, S.; Fujiwara, T. Low-affinity cation transporter (OsLCT1) regulates cadmium transport into rice grains. Proc. Natl. Acad. Sci. USA 2011, 108, 20959–20964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.Y.; Bovet, L.; Maeshima, M.; Martinoia, E.; Lee, Y. The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J. 2007, 50, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Migocka, M.; Kosieradzka, A.; Papierniak, A.; Maciaszczyk-Dziubinska, E.; Posyniak, E.; Garbiec, A.; Filleur, S. Two metal-tolerance proteins, MTP1 and MTP4, are involved in Zn homeostasis and Cd sequestration in cucumber cells. J. Exp. Bot. 2015, 66, 1001–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lekeux, G.; Crowet, J.-M.; Nouet, C.; Joris, M.; Jadoul, A.; Bosman, B.; Carnol, M.; Motte, P.; Lins, L.; Galleni, M. Homology modeling and in vivo functional characterization of the zinc permeation pathway in a heavy metal P-type ATPase. J. Exp. Bot. 2019, 70, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Song, W.Y.; Ko, D.; Eom, Y.; Hansen, T.H.; Schiller, M.; Lee, T.G.; Martinoia, E.; Lee, Y. The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J. 2012, 69, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, P.; Zanella, L.; De Paolis, A.; Di Litta, D.; Cecchetti, V.; Falasca, G.; Barbieri, M.; Altamura, M.M.; Costantino, P.; Cardarelli, M. Cadmium-inducible expression of the ABC-type transporter AtABCC3 increases phytochelatin-mediated cadmium tolerance in Arabidopsis. J. Exp. Bot. 2015, 66, 3815–3829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Zhao, H.; Wu, L.; Liu, A.; Zhao, F.J.; Xu, W. Heavy metal ATPase 3 (HMA3) confers cadmium hypertolerance on the cadmium/zinc hyperaccumulator Sedum plumbizincicola. New Phytol. 2017, 215, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Krämer, U.; Talke, I.N.; Hanikenne, M. Transition metal transport. FEBS Lett. 2007, 581, 2263–2272. [Google Scholar] [CrossRef]
- Rahman, M.F.; Ghosal, A.; Alam, M.F.; Kabir, A.H. Remediation of cadmium toxicity in field peas (Pisum sativum L.) through exogenous silicon. Ecotoxicol. Environ. Saf. 2017, 135, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Singh, P.; Tripathi, D.K.; Corpas, F.J.; Singh, V.P. Nitric oxide and hydrogen sulfide: An indispensable combination for plant functioning. Trends Plant Sci. 2021, 17, S1360–S1385. [Google Scholar] [CrossRef]
- Besson-Bard, A.; Gravot, A.; Richaud, P.; Auroy, P.; Duc, C.; Gaymard, F.; Taconnat, L.; Renou, J.P.; Pugin, A.; Wendehenne, D. Nitric oxide contributes to cadmium toxicity in Arabidopsis by promoting cadmium accumulation in roots and by up-regulating genes related to iron uptake. Plant Physiol. 2009, 149, 1302–1315. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Yang, Z.; Xie, Y.; Nie, L.; Jin, C.; Shen, W. Arabidopsis HY1 confers cadmium tolerance by decreasing nitric oxide production and improving iron homeostasis. Mol. Plant 2014, 7, 388–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehrawat, A.; Deswal, R. S-Nitrosylation in Abiotic Stress in Plants and Nitric Oxide Interaction with Plant Hormones; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 399–411. [Google Scholar]
- Gupta, K.J.; Hancock, J.T.; Petrivalsky, M.; Kolbert, Z.; Lindermayr, C.; Durner, J.; Barroso, J.B.; Palma, J.M.; Brouquisse, R.; Wendehenne, D.; et al. Recommendations on terminology and experimental best practice associated with plant nitric oxide research. New Phytol. 2020, 225, 1828–1834. [Google Scholar] [CrossRef] [Green Version]
- Metwally, A.; Finkemeier, I.; Georgi, M.; Dietz, K.J. Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol. 2003, 132, 272–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krantev, A.; Yordanova, R.; Janda, T.; Szalai, G.; Popova, L. Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J. Plant Physiol. 2008, 165, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Liang, Y.C.; Zhu, Y.G.; Zhao, F.J. Role of salicylic acid in alleviating oxidative damage in rice roots (Oryza sativa) subjected to cadmium stress. Environ. Pollut. 2007, 147, 743–749. [Google Scholar] [CrossRef]
- Zhang, L.; Pei, Y.; Wang, H.; Jin, Z.; Liu, Z.; Qiao, Z.; Fang, H.; Zhang, Y. Hydrogen sulfide alleviates cadmium-induced cell death through restraining ROS accumulation in roots of Brassica rapa L. ssp. pekinensis. Oxidative Med. Cell. Longev. 2015, 2015, 804603. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Li, H.; Huang, S.; Wang, C.; Sun, W.J.; Mo, H.Z.; Shi, Z.Q.; Chen, J. Eugenol confers cadmium tolerance via intensifying endogenous hydrogen sulfide signaling in Brassica rapa. J. Agric. Food Chem. 2018, 66, 9914–9922. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, M.J.; Ge, Z.L.; Shen, J.; Zhou, C.; Gotor, C.; Romero, L.C.; Duan, X.L.; Liu, X.; Wu, D.L.; et al. Abscisic acid-triggered guard cell L-cysteine desulfhydrase function and in situ hydrogen sulfide production contributes to heme oxygenase-modulated stomatal closure. Plant Cell Environ. 2020, 43, 624–636. [Google Scholar] [CrossRef]
- Qiao, Z.; Tao, J.; Liu, Z.; Zhang, L.; Jin, Z.; Liu, D.; Pei, Y. H2S acting as a downstream signaling molecule of SA regulates Cd tolerance in Arabidopsis. Plant Soil 2015, 393, 137–146. [Google Scholar] [CrossRef]
- Yang, X.; Kong, L.; Wang, Y.; Su, J.; Shen, W. Methane control of cadmium tolerance in alfalfa roots requires hydrogen sulfide. Environ. Pollut. 2021, 284, 117123. [Google Scholar] [CrossRef]
- Sun, J.; Wang, R.; Zhang, X.; Yu, Y.; Zhao, R.; Li, Z.; Chen, S. Hydrogen sulfide alleviates cadmium toxicity through regulations of cadmium transport across the plasma and vacuolar membranes in Populus euphratica cells. Plant Physiol. Biochem. 2013, 65, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Chen, H.; Zhu, K.; Jin, Q.; Xie, Y.; Cui, J.; Xia, Y.; Zhang, J.; Shen, W. Cadmium-induced hydrogen sulphide synthesis is involved in cadmium tolerance in Medicago sativa by reestablishment of reduced (homo) glutathione and reactive oxygen species homeostases. PLoS ONE 2014, 9, e109669. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ye, T.; Chan, Z. Nitric oxide-activated hydrogen sulfide is essential for cadmium stress response in bermudagrass (Cynodon dactylon (L). pers.). Plant Physiol. Biochem. 2014, 74, 99–107. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Rahman, A.; Ansary, M.; Watanabe, A.; Fujita, M.; Tran, L.P. Hydrogen sulfide modulates cadmium-induced physiological and biochemical responses to alleviate cadmium toxicity in rice. Sci. Rep. 2015, 5, 14078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, H.; Wang, X.; Dou, Y.; Liu, D.; Si, W.; Fang, H.; Zhao, C.; Chen, S.; Xi, J.; Li, J. Hydrogen sulfide-cysteine cycle system enhances cadmium tolerance through alleviating cadmium-induced oxidative stress and ion toxicity in Arabidopsis roots. Sci. Rep. 2016, 6, 39702. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Responses of nitric oxide and hydrogen sulfide in regulating oxidative defence system in wheat plants grown under cadmium stress. Physiol. Plantarum 2020, 168, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Xu, J.; Bo, T.; Wang, W. Comparative transcriptome analysis uncovers roles of hydrogen sulfide for alleviating cadmium toxicity in Tetrahymena thermophila. BMC Genom. 2021, 22, 21. [Google Scholar] [CrossRef]
- Li, G.; Shah, A.A.; Khan, W.U.; Yasin, N.A.; Ahmad, A.; Abbas, M.; Ali, A.; Safdar, N. Hydrogen sulfide mitigates cadmium induced toxicity in Brassica rapa by modulating physiochemical attributes, osmolyte metabolism and antioxidative machinery. Chemosphere 2021, 263, 127999. [Google Scholar] [CrossRef]
- Jia, H.; Wang, X.; Shi, C.; Guo, J.; Ma, P.; Ren, X.; Wei, T.; Liu, H.; Li, J. Hydrogen sulfide decreases Cd translocation from root to shoot through increasing Cd accumulation in cell wall and decreasing Cd2+ influx in Isatis indigotica. Plant Physiol. Biochem. 2020, 155, 605–612. [Google Scholar] [CrossRef]
- Tian, B.; Zhang, Y.; Jin, Z.; Liu, Z.; Pei, Y. Role of hydrogen sulfide in the methyl jasmonate response to cadmium stress in foxtail millet. Front. Biosci. 2017, 22, 530–538. [Google Scholar] [CrossRef] [Green Version]
- Tian, B.; Qiao, Z.; Zhang, L.; Li, H.; Pei, Y. Hydrogen sulfide and proline cooperate to alleviate cadmium stress in foxtail millet seedlings. Plant Physiol. Biochem. 2016, 109, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Y.; Shen, W. Roles of hydrogen sulfide and nitric oxide in the alleviation of cadmium-induced oxidative damage in alfalfa seedling roots. Biometals 2012, 25, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Siddiqui, M.H.; AlSolami, M.A.; Alamri, S.; Hu, Y.; Ali, H.M.; Al-Amri, A.A.; Alsubaie, Q.D.; Al-Munqedhi, B.M.A.; Al-Ghamdi, A. Crosstalk of hydrogen sulfide and nitric oxide requires calcium to mitigate impaired photosynthesis under cadmium stress by activating defense mechanisms in Vigna radiata. Plant Physiol. Biochem. 2020, 156, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Ju, W.; Yang, C.; Jin, X.; Liu, D.; Li, M.; Yu, J.; Zhao, W.; Zhang, C. Exogenous application of signaling molecules to enhance the resistance of legume-rhizobium symbiosis in Pb/Cd-contaminated soils. Environ. Pollut. 2020, 265, 114744. [Google Scholar] [CrossRef]
- Graham, N.; Arisi, A.M.; Lise, J.; Kunert, K.J.; Heinz, R.; Foyer, C.H. Glutathione: Biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J. Exp. Bot. 1998, 49, 623–647. [Google Scholar] [CrossRef]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione is a key player in metal-induced oxidative stress defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Yang, L.; Yan, X.; Liu, Y.; Wang, R.; Fan, T.; Ren, Y.; Tang, X.; Xiao, F.; Liu, Y.; et al. Zinc-finger Transcription Factor ZAT6 positively regulates cadmium tolerance through the glutathione-dependent pathway in Arabidopsis. Plant Physiol. 2016, 171, 707–719. [Google Scholar] [CrossRef]
- Shen, J.; Su, Y.; Zhou, C.; Zhang, F.; Yuan, X. A putative rice L-cysteine desulfhydrase encodes a true L-cysteine synthase that regulates plant cadmium tolerance. Plant Growth Regul. 2019, 89, 217–226. [Google Scholar] [CrossRef]
- Gu, Q.; Chen, Z.; Cui, W.; Zhang, Y.; Hu, H.; Yu, X.; Wang, Q.; Shen, W. Methane alleviates alfalfa cadmium toxicity via decreasing cadmium accumulation and reestablishing glutathione homeostasis. Ecotoxicol. Environ. Saf. 2018, 147, 861–871. [Google Scholar] [CrossRef]
- Kok, L.; Bosma, W.; Maas, F.M.; Kuiper, P. The effect of short-term H2S fumigation on water-soluble sulphydryl and glutathione levels in spinach. Plant Cell Environ. 1985, 8, 189–194. [Google Scholar] [CrossRef]
- Anastasis, C.; Manganaris, G.A.; Ioannis, P.; Vasileios, F. Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J. Exp. Bot. 2013, 7, 1953–1966. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, X.; Cai, B.; Pan, D.; Ai, X. Physiological response and transcription profiling analysis reveal the role of glutathione in H2S-induced chilling stress tolerance of cucumber seedlings. Plant Sci. 2020, 291, 110363. [Google Scholar] [CrossRef]
- Wei, J.; Li, D.X.; Zhang, J.R.; Shan, C.; Rengel, Z.; Song, Z.B.; Chen, Q. Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J. Pineal Res. 2018, 65, e12500. [Google Scholar] [CrossRef]
- Lee, H.Y.; Back, K. The phytomelatonin receptor (PMRT1) Arabidopsis Cand2 is not a bona fide G protein-coupled melatonin receptor. Melatonin Res. 2020, 3, 177–186. [Google Scholar] [CrossRef]
- Chen, T.; Tian, M.; Han, Y. Hydrogen sulfide: A multi-tasking signal molecule in the regulation of oxidative stress responses. J. Exp. Bot. 2020, 71, 2862–2869. [Google Scholar] [CrossRef]
- Aroca, A.; Benito, J.M.; Gotor, C.; Romero, L.C. Persulfidation proteome reveals the regulation of protein function by hydrogen sulfide in diverse biological processes in Arabidopsis. J. Exp. Bot. 2017, 68, 4915–4927. [Google Scholar] [CrossRef] [Green Version]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical biology of H2S signaling through persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, J.; Zhou, M.; Zhou, H.; Cui, B.; Gotor, C.; Romero, L.C.; Fu, L.; Yang, J.; Foyer, C.H.; et al. Persulfidation-based modification of cysteine desulfhydrase and the NADPH oxidase RBOHD controls guard cell abscisic acid signaling. Plant Cell 2020, 32, 1000–1017. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, J.; Shen, J.; Zhou, H.; Zhao, D.; Gotor, C.; Romero, L.C.; Fu, L.; Li, Z.; Yang, J.; et al. Hydrogen sulfide-linked persulfidation of ABI4 controls ABA responses through the transactivation of MAPKKK18 in Arabidopsis. Mol. Plant 2021, 14, 921–936. [Google Scholar] [CrossRef]
- Chen, S.S.; Jia, H.L.; Wang, X.F.; Shi, C.; Wang, X.; Ma, P.; Wang, J.; Wang, M.J.; Li, J. Hydrogen sulfide positively regulates abscisic acid signaling through persulfidation of SnRK2.6 in guard cells. Mol. Plant 2020, 13, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, H.; Xie, Y. SnRK2.6 phosphorylation/persulfidation: Where ABA and H2S signaling meet. Trends Plant Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Aroca, Á.; Serna, A.; Gotor, C.; Romero, L.C. S-sulfhydration: A cysteine posttranslational modification in plant systems. Plant Physiol. 2015, 168, 334–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Plant Species | Cd Stress and Duration | Impact on Genes Related to Melatonin Metabolic Pathway | References |
|---|---|---|---|
| Solanum lycopersicum | 100 μM Cd2+ for 15 d | TDC, T5H, COMT genes (leaves) | [22] |
| Oryza sativa L. | 500 μM Cd2+ for 3 d | TDC1, TDC3, SNAT1, SNAT2, ASMT, COMT, M2H, M3H genes (seedlings) | [23] |
| Triticum aestivum L. | 200 μM Cd2+ for 1 d | ASMT, COMT, TDC genes (root and shoot) | [62] |
| Nicotiana tabacum L. | 10 mg/kg Cd2+ for 1, 4, and 7 d | SNAT1 gene (leaves) | [63] |
| Agaricus campestris | 2, 5, or 8 μM Cd2+ for 5 d | TDC, T5H, SNAT, ASMT, COMT genes | [64] |
| Oryza sativa L. | 200 μM Cd2+ for 6, 12, 24, 72 h | SNAT, ASMT, COMT, TDC, T5H genes (leaves) | [65,67] |
| Arabidopsis thaliana | 300 μM Cd2+ for 2, 3, 4 d | SNAT, COMT genes (leaves) | [66] |
| Plant Names | Treatments | Impact on Oxidative Stress Markers and Antioxidative Defense Systems | References |
|---|---|---|---|
| Nicotiana tabacum L. | 0, 25, 50, 100, and 250 μM melatonin; 100 μM Cd2+ for 7 d | H2O2, O2·−; APX, SOD, CAT (leaves) | [6] |
| Malva parviflora | 0, 15, 50, and 100 μM melatonin; 50 μM Cd2+ for 8 d | H2O2, MDA, SOD, CAT, GPX, PAL, flavonoid, anthocyanins (shoots) | [18] |
| Medicago sativa L. | 0, 10, 50, and 200 μM melatonin; 100 μM Cd2+ for 1, 3 d | H2O2, O2·−; SOD (roots) | [19] |
| Triticum aestivum L. | 0, 50, and 100 μM melatonin; 100 μM Cd2+ for 28 d | H2O2, MDA; SOD, CAT, POD (leaves) | [20] |
| Triticum aestivum L. | 0, 50, and 100 μM melatonin; 100 μM Cd2+ for 12, 24, 48 h | H2O2; APX, SOD, CAT, POD, GSH/GSSG (leaves and roots) | [62] |
| Agaricus campestris | 0, 50, 100, and 200 μM melatonin; 2, 5, and 8 μM Cd2+ for 5 d | H2O2, MDA; SOD, CAT, POD, APX, GR, proline, sugars | [64] |
| Solanum lycopersicum | 0, 25, 50, 100, 250, and 500 μM melatonin; 100 μM Cd2+ for 14 d | H2O2, MDA, O2·−; SOD, CAT, GR, POD, APX (leaves) | [75] |
| Solanum lycopersicum | 100 μM melatonin; 100 μM Cd2+ for 15 d | H2O2; APX, SOD, CAT, POD (leaves and roots) | [76] |
| Brassica napus L. | 0, 50, and 100 μM melatonin; 20 μM Cd2+ for 5 d | H2O2, MDA; APX, SOD, CAT, POD, proline, anthocyanins (seedlings) | [77] |
| Catharanthus roseus (L.) | 100 μM melatonin; 0, 50, 100, and 200 mg Cd kg−1 soil for 30 d | H2O2; CAT, POD (leaves) | [78] |
| Fragaria x ananassa (Duch.) | 0, 10, 50, 100, 150, and 200 μM melatonin; 300 mL of 1 mmol·L−1 Cd2+ for 5, 10 d | MDA; SOD, CAT, POD, APX, soluble protein, anthocyanins (leaves and roots) | [79] |
| Carthamus tinctorius L. | 100 μM melatonin; 100 μM Cd2+ for 21 d | H2O2, MDA, LOX; ASA, DHA, GSH, GSSG, SOD, APX, DHAR, CAT, GR, MDHAR, Gly (leaves) | [80] |
| Oryza sativa L. | 0, 50, 100, and 200 μM melatonin; 100 μM Cd2+ for 10 d | H2O2, MDA; SOD, CAT, POD (leaves and roots) | [81] |
| Zea mays | 200 μM melatonin; 150 μM Cd2+ for 3 d | MDA; SOD, CAT, POD (root, stem, and leaf) | [82] |
| Oryza sativa L. | 100 μM melatonin | MDA; SOD, CAT, POD (shoots) | [83] |
| Raphanus sativus L. | 0, 10, 25, 50, 100, and 200 μM melatonin; 50 μM Cd2+ for 24 h | SOD, CAT, POD, APX, GR (roots and shoots) | [84] |
| Malachium aquaticum, Galinsoga parviflora | 0, 50, 100, 150, and 200 μM melatonin; 10 mg/L Cd for 40 d | SOD, POD, CAT (leaves) | [85] |
| Cyphomandra betacea | 0, 50, 100, 150, and 200 μM melatonin; 10 mg/L Cd for 40 d | SOD, POD, CAT (leaves) | [86] |
| Plant Names | Treatments | Impact on Cd in Subcellular Compartment | References |
|---|---|---|---|
| Nicotiana tabacum L. | 0, 25, 50, 100, and 250 μM melatonin; 100 μM Cd2+ for 7 d | Cd content in leaves; H+-ATPase activity, IRT1, IRT2, Nramp1, HMA2, HMA3, HMA4 | [6] |
| Malva parviflora | 0, 15, 50, and 100 μM melatonin; 50 μM Cd2+ for 8 d | Cd content in shoots | [18] |
| Medicago sativa L. Arabidopsis | 0, 10, 50, and 200 μM melatonin; 100 μM Cd2+ for 1, 3 d | Cd content in leaves; PCR2, Nramp6, PDR8, HMA4 | [19] |
| Solanum lycopersicum | 1 μM melatonin; 100 μM Cd2+ for 15 d | Cd content in leaves; GSH and PCs | [61] |
| Brassica pekinensis (Lour.) Rupr. | 100 μM melatonin; 20 μM Cd2+ for 24 h | Cd contents in roots and leaves; IRT1/2 | [68] |
| Solanum lycopersicum | 0, 25, 50, 100, 250, and 500 μM melatonin; 100 μM Cd2+ for 14 d | Cd content in leaves; H+-ATPase activity, GSH and PCs; SlGSH1, SlPCS, SlMT2, and SlABC1 | [75] |
| Solanum lycopersicum | 100 μM melatonin; 100 μM Cd2+ for 15 d | Cd content in leaves; Cys, γ-glutamyl cysteine, GSH and PCs | [76] |
| Brassica napus L. | 0, 50, and 100 μM melatonin; 20 μM Cd2+ for 5 d | Cd content; H+-ATPase activity | [77] |
| Carthamus tinctorius L. | 100 μM melatonin; 100 μM Cd2+ for 21 d | Cd content in roots, stems and leaves; PCs | [80] |
| Oryza sativa L. | 0, 50, 100, and 200 μM melatonin; 100 μM Cd2+ for 10 d | Cd content in leaves; OsIRT1, OsIRT2, OsHMA2, OsHMA3, OsNramp1, OsNramp5, and OsLCT1 | [81] |
| Oryza sativa L. | 100 μM melatonin | Cd content in roots and shoots; Nramp1, Nramp5, IRT1, IRT2, HMA2, HMA3 | [83] |
| Raphanus sativus L. | 0, 10, 25, 50, 100, and 200 μM melatonin; 50 μM Cd2+ for 24 h | Cd content in roots and leaves; PCS; MT, CAX4, ZIP12, HMA4, YSL2, YSL7 | [84] |
| Malachium aquaticum, Galinsoga parviflora | 0, 50, 100, 150, and 200 μM melatonin; 10 mg/L Cd for 40 d | Cd content in leaves | [85] |
| Cyphomandra betacea | 0, 50, 100, 150, and 200 μM melatonin; 10 mg/L Cd for 40 d | Cd contents in stems, leaves, and shoots | [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Q.; Wang, C.; Xiao, Q.; Chen, Z.; Han, Y. Melatonin Confers Plant Cadmium Tolerance: An Update. Int. J. Mol. Sci. 2021, 22, 11704. https://doi.org/10.3390/ijms222111704
Gu Q, Wang C, Xiao Q, Chen Z, Han Y. Melatonin Confers Plant Cadmium Tolerance: An Update. International Journal of Molecular Sciences. 2021; 22(21):11704. https://doi.org/10.3390/ijms222111704
Chicago/Turabian StyleGu, Quan, Chuyan Wang, Qingqing Xiao, Ziping Chen, and Yi Han. 2021. "Melatonin Confers Plant Cadmium Tolerance: An Update" International Journal of Molecular Sciences 22, no. 21: 11704. https://doi.org/10.3390/ijms222111704






