Five Days Periodic Fasting Elevates Levels of Longevity Related Christensenella and Sirtuin Expression in Humans
Abstract
:1. Introduction
2. Results
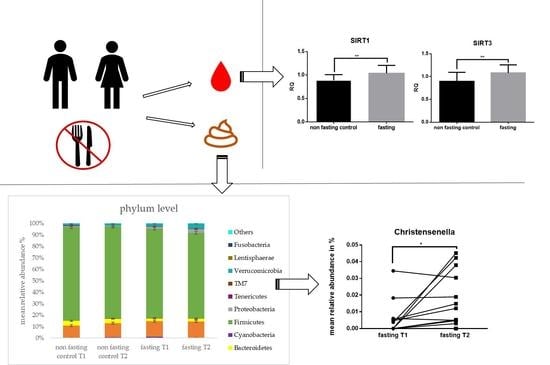
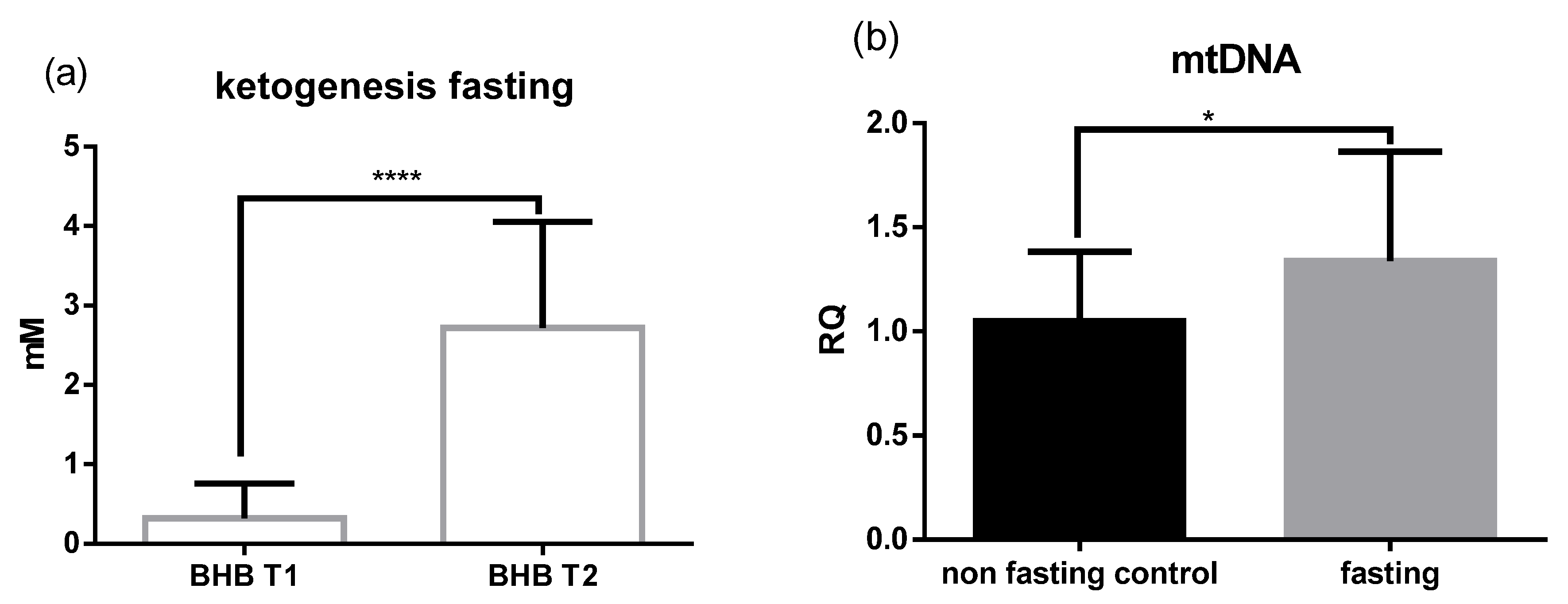
2.1. Fasting Results in Ketogenesis, Weight loss, and Increased Levels of mtDNA
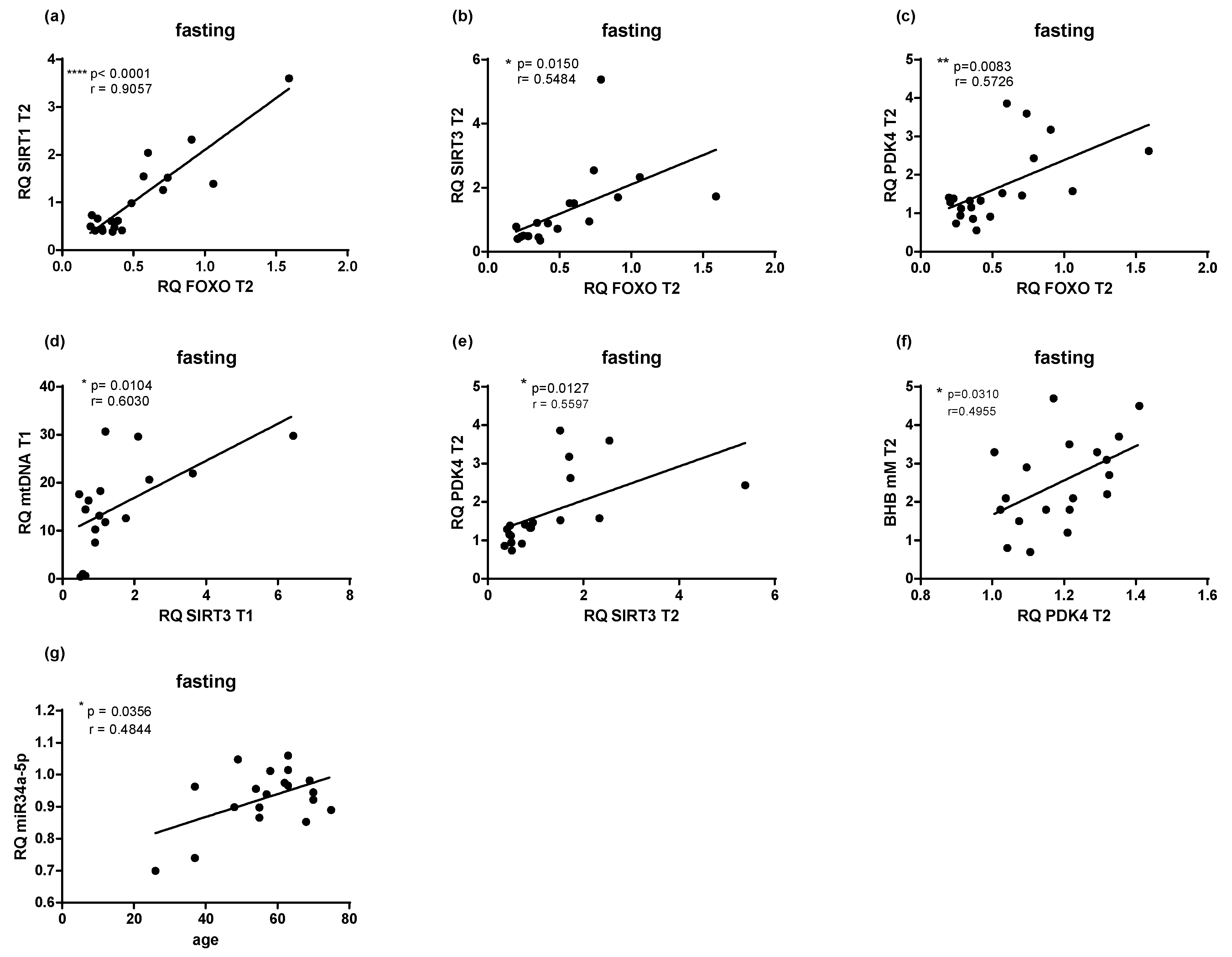
2.2. PF Affects mRNA and miRNA Expression
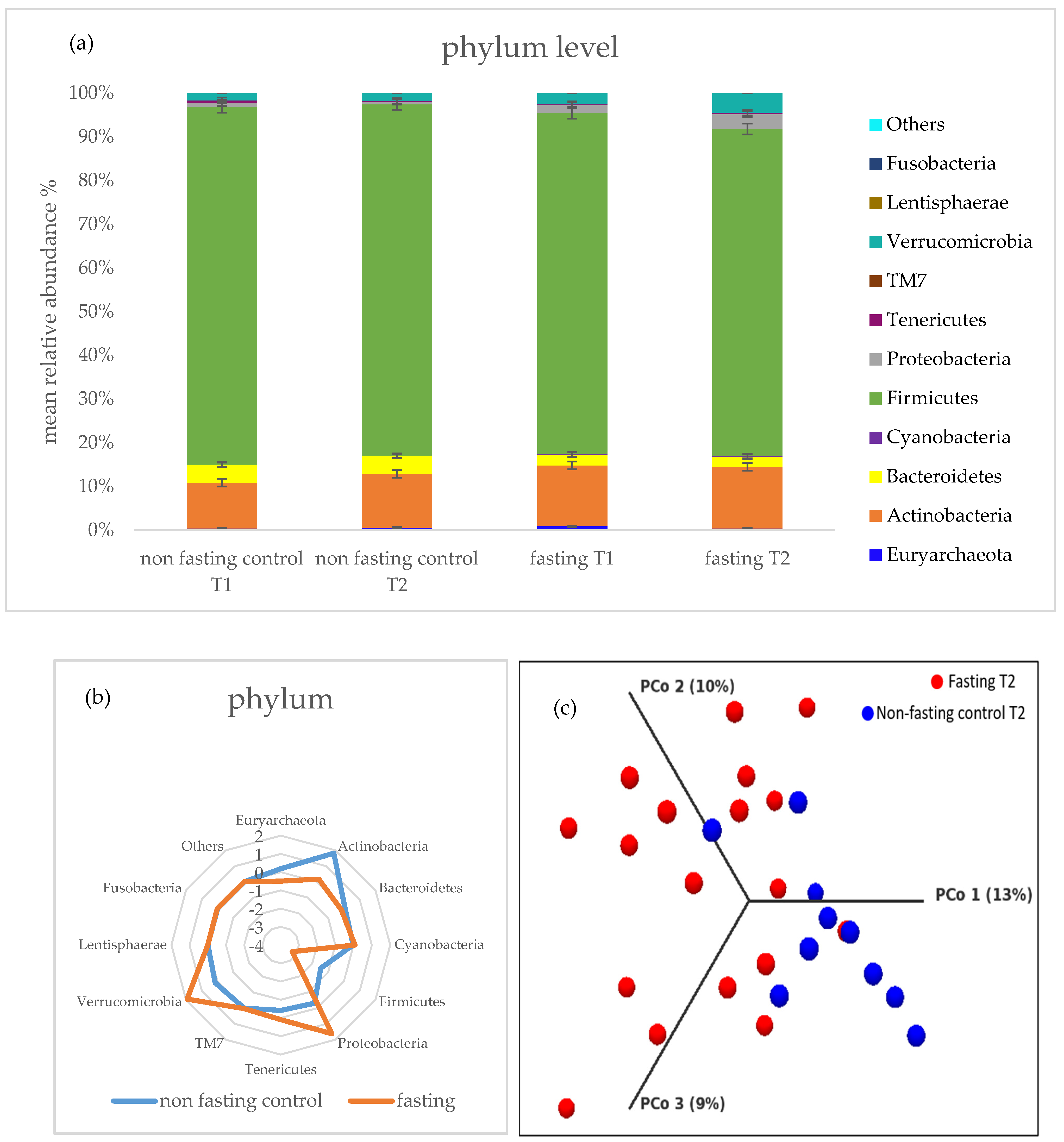
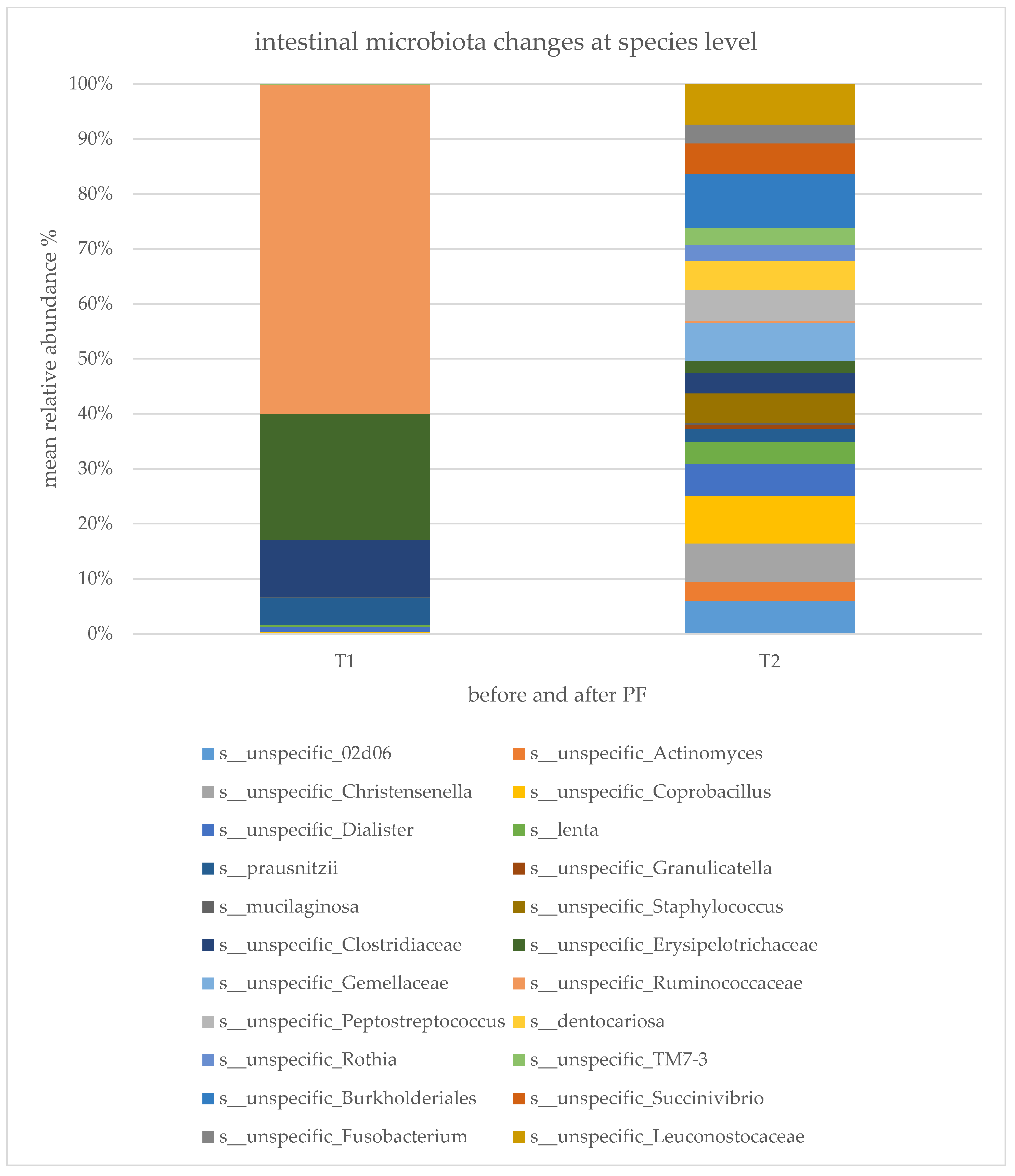
2.3. Gut Microbiota Composition Changes and Diversity
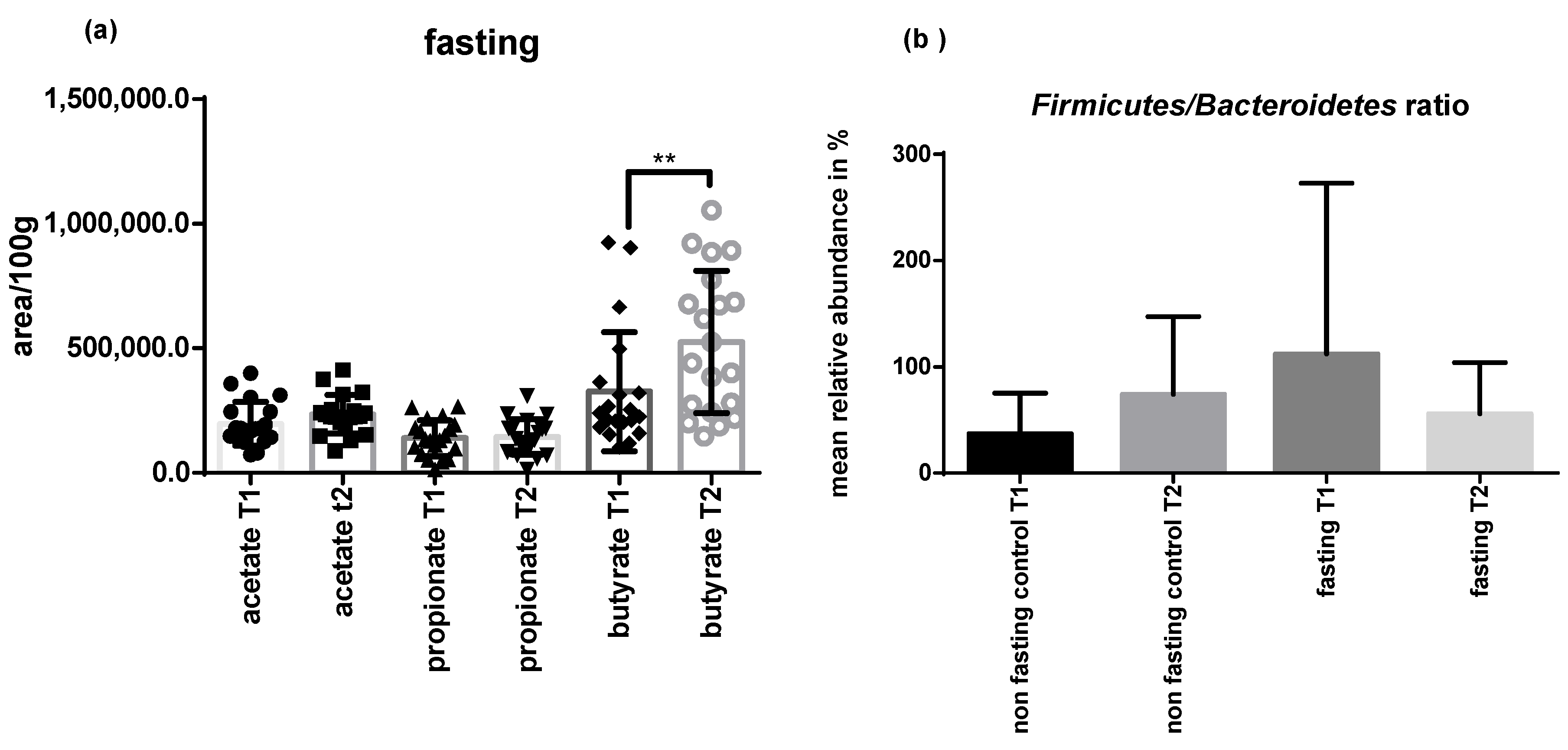
2.4. PF Decreases Firmicutes/Bacteroidetes Ratio and Addresses Microbial Metabolites
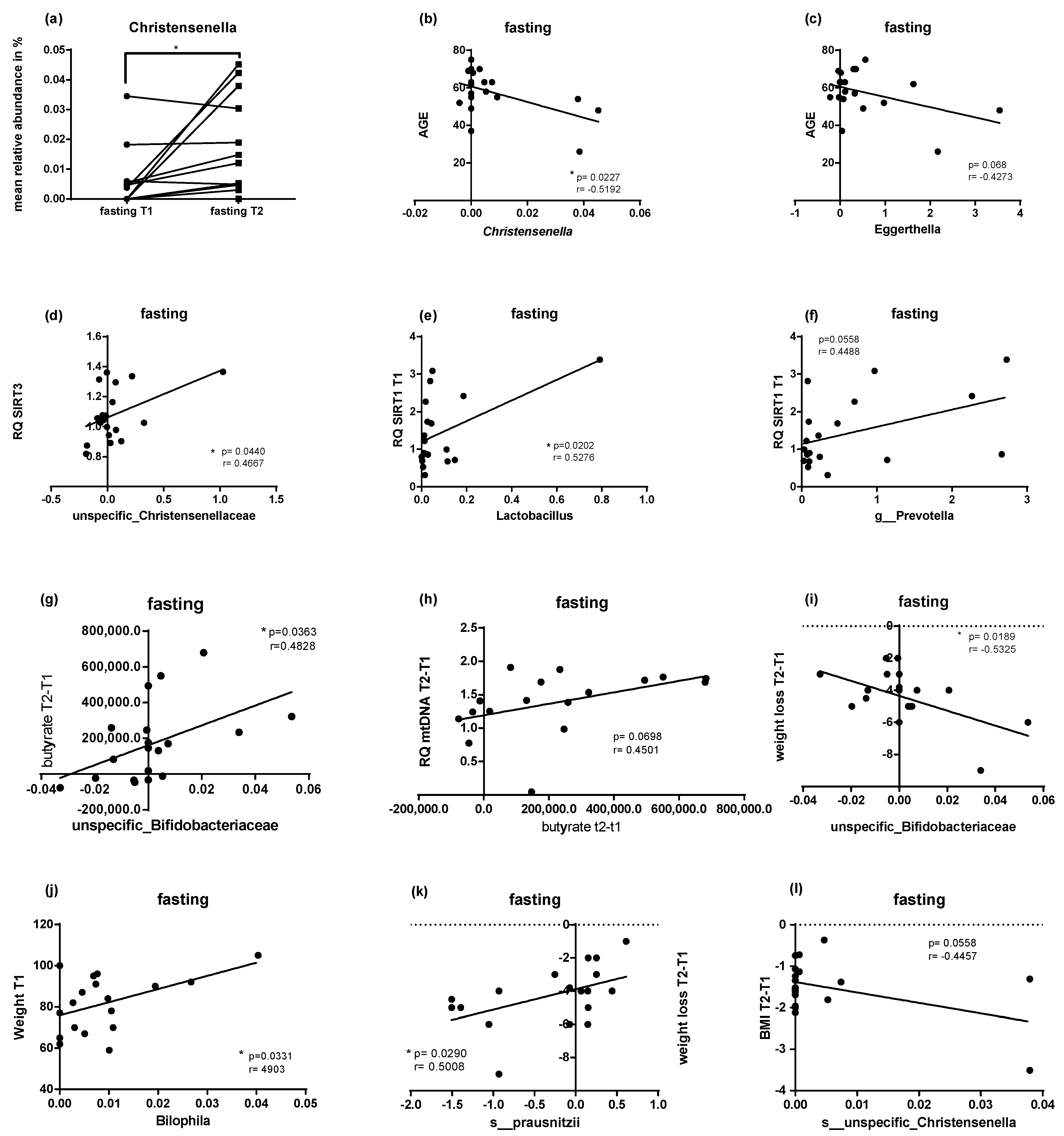
2.5. Microbial Changes at Genus Level and Correlations
3. Discussion
4. Material and method
4.1. Experimental Design and Dietary Intervention
4.2. Sample Collection
4.3. BHB Measurement
4.4. DNA and RNA Extraction
4.5. Mitochondrial DNA
4.6. mRNA and miRNA Expression
4.7. 16S rRNA Gene Amplification and Sequencing and Microbial Metabolites
4.8. Bioinformatics and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lakhan, S.E.; Kirchgessner, A. Gut microbiota and sirtuins in obesity-related inflammation and bowel dysfunction. J. Transl. Med. 2011, 9, 2020. [Google Scholar] [CrossRef] [Green Version]
- Jura, M.; Kozak, L.P. Obesity and related consequences to ageing. Age 2016, 38, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masoro, E.J. Overview of caloric restriction and ageing. Mech. Ageing Dev. 2005, 126, 913–922. [Google Scholar] [CrossRef]
- Heilbronn, L.K.; Ravussin, E. Calorie restriction and aging: Review of the literature and implications for studies in humans. Am. J. Clin. Nutr. 2003, 78, 361–369. [Google Scholar] [CrossRef]
- Duszka, K.; Gregor, A.; Guillou, H.; König, J.; Wahli, W. Peroxisome Proliferator-Activated Receptors and Caloric Restriction-Common Pathways Affecting Metabolism, Health, and Longevity. Cells 2020, 16, 1708. [Google Scholar] [CrossRef] [PubMed]
- De Cabo, R.; Carmona-Gutierrez, D.; Bernier, M.; Hall, M.N.; Madeoand, F. The search for anti-aging interventions: From elixirs to fasting regimens. Cell 2014, 157, 1515–1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesnage, R.; Grundler, F.; Schwiertz, A.; Le Maho, Y.; Wilhelmi de Toledo, F. Changes in human gut microbiota composition are linked to the energy metabolic switch during 10 d of Buchinger fasting. J. Nutr. Sci. 2019, 8, e36. [Google Scholar] [CrossRef] [Green Version]
- Higashino-Matsui, Y.; Shirato, K.; Suzuki, Y.; Kawashima, Y.; Someya, Y.; Sato, S.; Shiraishi, A.; Jinde, M.; Matsumoto, A.; Ideno, H. Age-related effects of fasting on ketone body production during lipolysis in rats. Environ. Health Prev. Med. 2012, 17, 157–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.M.; Ramprasath, T.; Zou, M.H. Β-Hydroxybutyrate and Its Metabolic Effects on Age-Associated Pathology. Exp. Mol. Med. 2020, 52, 548–555. [Google Scholar] [CrossRef] [Green Version]
- Cotter, D.G.; Schugar, R.C.; Crawford, P.A. Ketone body metabolism and cardiovascular disease. AJP Hear. Circ. Physiol. 2013, H1060–H1076. [Google Scholar] [CrossRef] [Green Version]
- Cantó, C.; Auwerx, J. Calorie restriction: Is AMPK a key sensor and effector? Physiology 2011, 26, 214–224. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.; Hardy, T.; Tollefsbol, T. Medicinal Chemistry of the Epigenetic Diet and Caloric Restriction. Curr. Med. Chem. 2013, 20, 4050–4059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruderman, N.B.; Xu, J.X.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef] [PubMed]
- Lilja, S.; Oldenburg, J.; Pointner, A.; Dewald, L.; Lerch, M.; Hippe, B.; Switzeny, O.; Haslberger, A. Epigallocatechin Gallate Effectively Affects Senescence and Anti- SASP via SIRT3 in 3T3-L1 Preadipocytes in Comparison with Other Bioactive Substances. Hindawi 2020, 2020, 4793125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.I.; Zhou, M.; Kang, C.; Lang, H.; Chen, M.; Hui, S.; Wang, B.; Mi, M. Crosstalk between gut microbiota and Sirtuin-3 in colonic inflammation and tumorigenesis. Exp. Mol. Med. 2018, 50, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulose, N.; Raju, R. Sirtuin regulation in aging and injury. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 2442–2455. [Google Scholar] [CrossRef] [Green Version]
- Nagpal, R.; Mainali, R.; Ahmadi, S.; Wang, S.; Singh, R.; Kavanagh, K.; Kitzman, D.W.; Kushugulova, A.; Marotta, F.; Yadav, H. Gut microbiome and aging: Physiological and mechanistic insights. Nutr. Health Aging 2018, 4, 267–285. [Google Scholar] [CrossRef] [Green Version]
- Makwana, K.; Patel, S.A.; Velingkaar, N.; Ebron, J.S.; Shukla, G.C.; Kondratov, R.V. Aging and calorie restriction regulate the expression of miR-125a-5p and its target genes Stat3 Casp2 and Stard13. Aging 2017, 9, 1825–1843. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Li, Y.; Stoll, M.L.; Tollefsbol, T.O. The Epigenetic Connection Between the Gut Microbiome in Obesity and Diabetes. Front. Genet. 2020, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Remely, M.; Hippe, B.; Geretschlaeger, I.; Stegmayer, S.; Hoefinger, I.; Haslberger, A. Increased gut microbiota diversity and abundance of Faecalibacterium prausnitzii and Akkermansia after fasting: A pilot study. Wien. Klin. Wochenschr 2015, 127, 394–398. [Google Scholar] [CrossRef] [Green Version]
- De Toledo, F.W.; Grundler, F.; Bergouignan, A.; Drinda, S.; Michalsen, A. Safety, health improvement and well-being during a 4 to 21-day fasting period in an observational study including 1422 subjects. PLoS ONE 2019, 14, 1–23. [Google Scholar]
- Chai, J.T.; Digby, J.E.; Choudhury, R.P. GPR109A and vascular inflammation. Curr. Atheroscler. Rep. 2013, 15, 325. [Google Scholar] [CrossRef] [Green Version]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Cannataro, R.; Perri, M.; Gallelli, L.; Caroleo, M.C.; De Sarro, G.; Cione, E. Ketogenic Diet Acts on Body Remodeling and MicroRNAs Expression Profile. MicroRNA 2018, 8, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Cavaleri, F.; Bashar, E. Potential Synergies of β -Hydroxybutyrate and Butyrate on the Modulation of Metabolism, Inflammation, Cognition, and General Health. J. Nutr. Metab. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Wen, F.; An, C.; Wu, X.; Yang, Y.; Xu, J.; Liu, Y.; Wang, C.; Nie, L.; Fang, H.; Yang, Z. MiR-34a regulates mitochondrial content and fat ectopic deposition induced by resistin through the AMPK/PPARα pathway in HepG2 cells. Int. J. Biochem. Cell Biol. 2018, 94, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Fomison-Nurse, I.; Saw, E.E.L.; Gandhi, S.; Munasinghe, P.E.; Van Hout, I.; Williams, M.J.A.; Galvin, I.; Bunton, R.; Davis, P.; Cameron, V.; et al. Diabetes induces the activation of pro-ageing MIR-34a in the heart, but has differential effects on cardiomyocytes and cardiac progenitor cells. Cell Death Differ. 2018, 25, 1336–1349. [Google Scholar] [CrossRef] [Green Version]
- Tong, N.; Jin, R.; Zhou, Z.; Wu, X. Involvement of microRNA-34a in age-related susceptibility to oxidative stress in ARPE-19 cells by targeting the silent mating type information regulation 2 homolog 1/p66shc pathway: Implications for age-related macular degeneration. Front. Aging Neurosci. 2019, 11, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Owczarz, M.; Budzinska, M.; Domaszewska-Szostek, A.; Borkowska, J.; Polosak, J.; Gewartowska, M.; Slusarczyk, P.; Puzianowska-Kuznicka, M. miR-34a and miR-9 are overexpressed and SIRT genes are downregulated in peripheral blood mononuclear cells of aging humans. Exp. Biol. Med. 2017, 242, 1453–1461. [Google Scholar] [CrossRef] [Green Version]
- Newman, J.C.; Verdin, E. Ketone bodies as signaling metabolites. Trends Endocrinol. Metab. 2014, 25, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Shimazu, T.; Hirschey, M.D.; Hua, L.; Dittenhafer-Reed, K.E.; Schwer, B.; Lombard DBLi, Y.; Bunkenborg, J.; Alt, F.W.; Denu, J.M.; Jacobson, M.P.; et al. SIRT3 deacetylates mitochondrial 3-hydroxy-3-methylglutaryl CoA synthase 2 and regulates ketone body production. Cell Metab. 2010, 12, 654–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouwens, M.; Afman, L.A.; Müller, M. Fasting induces changes in peripheral blood mononuclear cell gene expression profiles related to increases in fatty acid β-oxidation: Functional role of peroxisome proliferator-activated receptor α in human peripheral blood mononuclear cells. Am. J. Clin. Nutr. 2007, 86, 1515–1523. [Google Scholar] [CrossRef]
- Meng, H.; Yan, W.Y.; Lei, Y.H.; Wan, Z.; Hou, Y.Y.; Sun, L.K.; Zhou, J.P. SIRT3 Regulation of Mitochondrial Quality Control in Neurodegenerative Diseases. Front. Aging Neurosci. 2019, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Harada, G.; Pattarawat, P.; Ito, K.; Matsumoto, T.; Hasegawa, T.; Katakura, Y. Lactobacillus brevis T2102 suppresses the growth of colorectal cancer cells by activating SIRT1. J. Funct. Foods 2016, 23, 444–452. [Google Scholar] [CrossRef]
- Kahleova, H.; Rembert, E.; Alwarith, J.; Yonas, W.N.; Tura, A.; Holubkov, A.; Agnello, M.; Chutkan, R.; Barnard, N.D. Effects of a low-fat vegan diet on gut microbiota in overweight individuals and relationships with body weight, body composition, and insulin sensitivity. A randomized clinical trial. Nutrients 2020, 12, 2917. [Google Scholar] [CrossRef]
- Xu, C.; Zhu, H.; Qiu, P. Aging progression of human gut microbiota. BMC Microbiol. 2019, 19, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Biagi, E.; Franceschi, C.; Rampelli, S.; Ostan, R.; Turroni, S.; Consolandi, S.; Scurti, M.; Quercia, S.; Monti, D.; Capri, M. Gut Microbiota and Extreme Longevity. Curr. Biol. 2016, 26, 1480–1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waters, J.L.; Ley, R.E. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 2019, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Bennuri, S.C.; Davis, J.E.; Wynne, B.; Slattery, J.C.; Tippett, M.; Delhey, L.; Melnyk, S.; Kahler, S.G.; MacFabe, D.F. Butyrate enhances mitochondrial function during oxidative stress in cell lines from boys with autism. Transl. Psychiatry 2018, 8, 42. [Google Scholar] [CrossRef]
- Hu, S.; Kuwabara, R.; De Haan, B.J.; Smink, A.M.; De Vos, P. Acetate and butyrate improve β-cell metabolism and mitochondrial respiration under oxidative stress. Int. J. Mol. Sci. 2020, 21, 1542. [Google Scholar] [CrossRef] [Green Version]
- Kumarasamy, S.; Gopalakrishnan, K.; Abdul-Majeed, S.; Partow-Navid, R.; Farms, P.; Joe, B. Construction of two novel reciprocal conplastic rat strains and characterization of cardiac mitochondria. Am. J. Physiol. Hear. Circ. Physiol. 2013, 304, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Tyrka, A.R.; Parade, S.H.; Price, L.H.; Kao, H.T.; Porton, B.; Philip, N.S.; Welch, E.S.; Carpenter, L.L. Alterations of Mitochondrial DNA Copy Number and Telomere Length with Early Adversity and Psychopathology. Biol Psychiatry. 2016, 79, 78–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, X.; Yang, Y.; Wang, S.; Feng, X.; Wang, T.; Wang, P.; Liu, S.; Li, L.; Yao, W.; Cui, L.; et al. Changes in the expression of genes involved in cell cycle regulation and the relative telomere length in the process of canceration induced by omethoate. Tumor Biol. 2017, 39, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glockner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.; Hellenbrand, J.; Mengerink, Y.; Van Der Wal, S. On-line determination of carboxylic acids, aldehydes and ketones by high-performance liquid chromatography-diode array detection-atmospheric pressure chemical ionisation mass spectrometry after derivatization with 2-nitrophenylhydrazine. J. Chromatogr. A 2004, 1031, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. Available online: https://www.nature.com/articles/nmeth.f.303 (accessed on 11 April 2010). [CrossRef] [Green Version]
- Edgar, R.C. Search and Clustering Orders of Magnitude Faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Characteristics | Total Study Population n (%) | Fasting Population n (%) | Non-Fasting Control n (%) |
| Population size | 51 (100) | 20 (39.2) | 31 (60.8) |
| Male | 16 (31.4) | 5 (25) | 11 (35.5) |
| Female | 35 (68.6) | 15 (75) | 20 (64.5) |
| BMI < 25 kg/m2 T1 | 27 (52.9) | 6 (30) | 21 (67.7) |
| BMI > 25 kg/m2 T1 | 18 (35.3) | 9 (45) | 9 (29) |
| BMI > 30 kg/m2 T1 | 6 (11.8) | 5 (25) | 1 (3.2) |
| Characteristics | Total study population mean ± SD | Fasting population mean ± SD | Non-fasting control mean ± SD |
| Age | 45.24 ± 14.625 | 56.55 ± 12.576 | 37.94 ± 10.767 |
| Weight kg at T1 | 75.882 ± 12.8509 | 80.550 ± −13.7093 | 72.871 ± 11.5029 |
| BMI kg/m2 at T1 | 25.9298 ± −3.932 | 27.7610 ± 4.32706 | 24.7484 ± 3.20067 |
| Weight loss kg T2–T1 | −2.0067 ± 2.66021 | −4.265 ± 1.77535 | −0.2 ± 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lilja, S.; Stoll, C.; Krammer, U.; Hippe, B.; Duszka, K.; Debebe, T.; Höfinger, I.; König, J.; Pointner, A.; Haslberger, A. Five Days Periodic Fasting Elevates Levels of Longevity Related Christensenella and Sirtuin Expression in Humans. Int. J. Mol. Sci. 2021, 22, 2331. https://doi.org/10.3390/ijms22052331
Lilja S, Stoll C, Krammer U, Hippe B, Duszka K, Debebe T, Höfinger I, König J, Pointner A, Haslberger A. Five Days Periodic Fasting Elevates Levels of Longevity Related Christensenella and Sirtuin Expression in Humans. International Journal of Molecular Sciences. 2021; 22(5):2331. https://doi.org/10.3390/ijms22052331
Chicago/Turabian StyleLilja, Stephanie, Carina Stoll, Ulrike Krammer, Berit Hippe, Kalina Duszka, Tewodros Debebe, Ingrid Höfinger, Jürgen König, Angelika Pointner, and Alexander Haslberger. 2021. "Five Days Periodic Fasting Elevates Levels of Longevity Related Christensenella and Sirtuin Expression in Humans" International Journal of Molecular Sciences 22, no. 5: 2331. https://doi.org/10.3390/ijms22052331