Carotenoid Biosynthesis and Plastid Development in Plants: The Role of Light
Abstract
1. Introduction
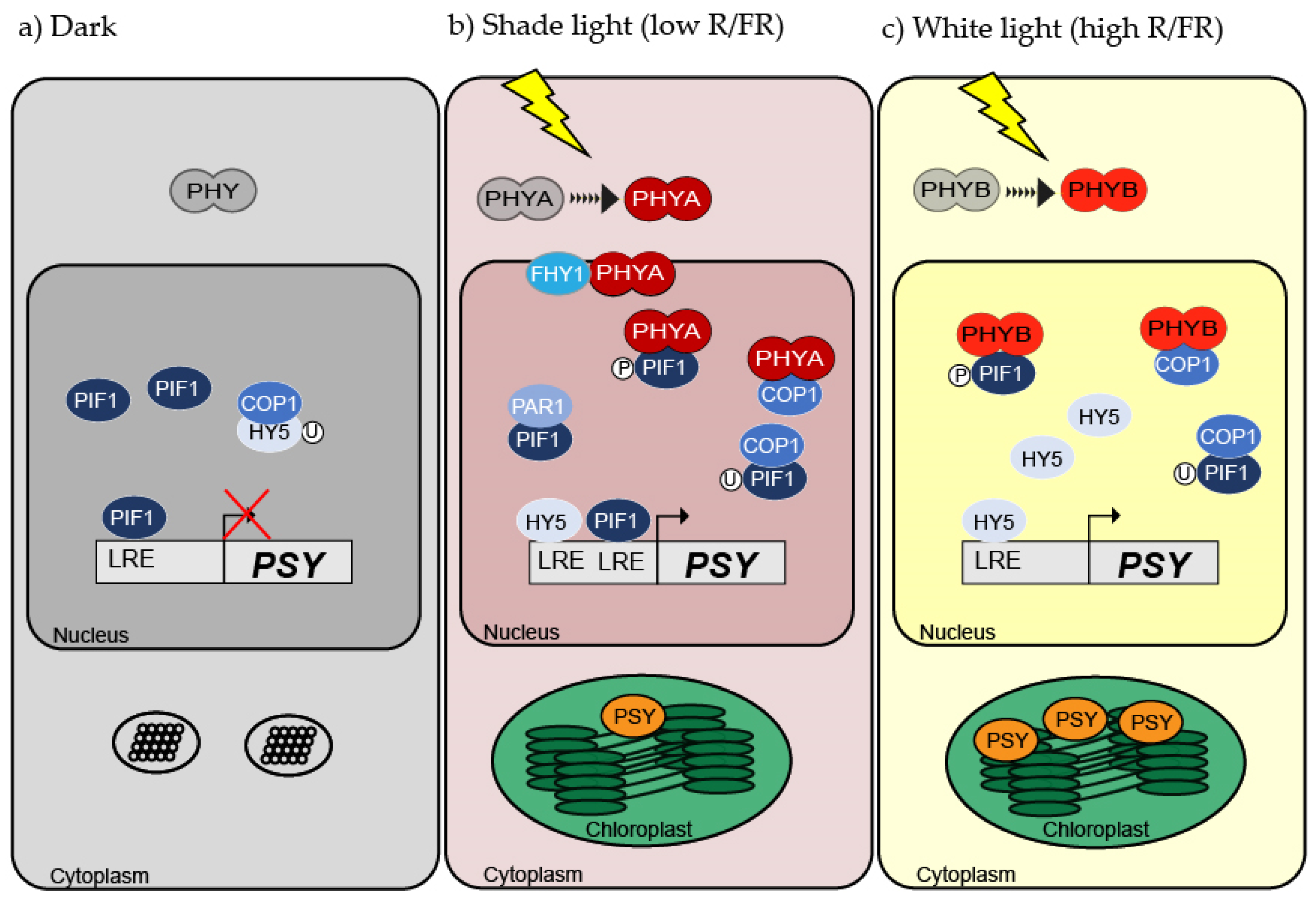
2. Phytochromes: The First Stage of Light Perception
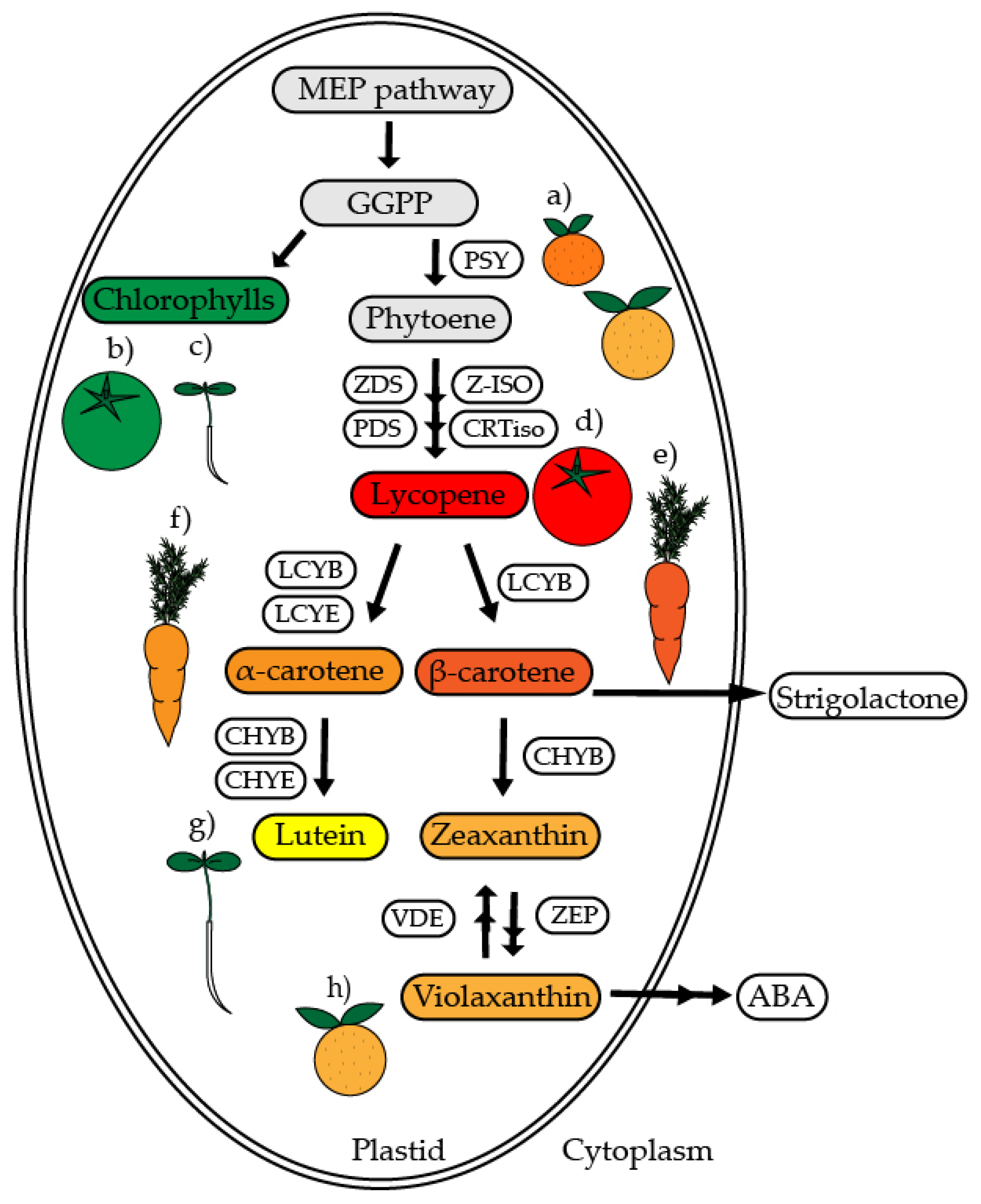
3. Carotenoid Synthesis in Plants
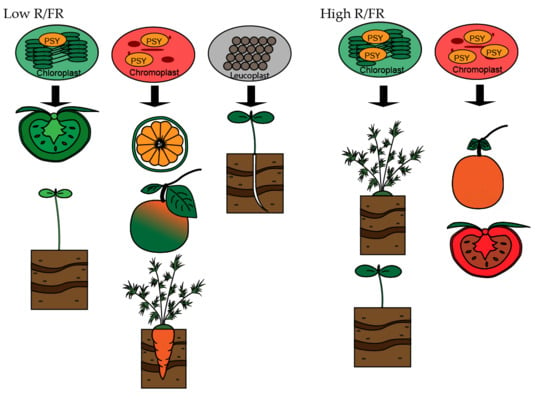
3.1. Leaves: Carotenoids Synthesis Takes Place in Chloroplasts
3.2. Fruits: Carotenoid Synthesis Takes Place in Chromoplasts
3.3. Roots: From Leucoplasts to Chromoplasts
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FR | Far red |
| R | Red |
| B | Blue |
| W | White |
| PHY | Phytochrome |
| CRY | Cryptochrome |
References
- Llorente, B.; Martinez-Garcia, J.F.; Stange, C.; Rodriguez-Concepcion, M. Illuminating colors: Regulation of carotenoid biosynthesis and accumulation by light. Curr. Opin. Plant Biol. 2017, 37, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, P.; Pizarro, L.; Moreno, J.C.; Handford, M.; Rodriguez-Concepcion, M.; Stange, C. Light-dependent changes in plastid differentiation influence carotenoid gene expression and accumulation in carrot roots. Plant Mol. Biol. 2012, 79, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Avalos, J.; Carmen Limón, M. Biological roles of fungal carotenoids. Curr. Genet. 2015, 61, 309–324. [Google Scholar] [CrossRef]
- Ram, S.; Mitra, M.; Shah, F.; Tirkey, S.R.; Mishra, S. Bacteria as an alternate biofactory for carotenoid production: A review of its applications, opportunities and challenges. J. Funct. Foods 2020, 67, 1038673. [Google Scholar] [CrossRef]
- Zhu, C.; Bai, C.; Sanahuja, G.; Yuan, D.; Farré, G.; Naqvi, S.; Shi, L.; Capell, T.; Christou, P. The regulation of carotenoid pigmentation in flowers. Arch. Biochem. Biophys. 2010, 504, 132–141. [Google Scholar] [CrossRef]
- Khoo, H.E.; Prasad, K.N.; Kong, K.W.; Jiang, Y.; Ismail, A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef]
- Kevan, P. Floral colors through the insect eye: What they are and what they mean. In The Handbook of Experimental Pollination Biology; Jones, C.E., Little, R.J., Eds.; Scientific and Academic Editions; Van Nostrand Reinhold: New York, NY, USA, 1983; pp. 3–30. [Google Scholar]
- Hashimoto, H.; Uragami, C.; Cogdell, R.J. Carotenoids and photosynthesis. In Carotenoids in Nature; Stange, C., Ed.; Springer: Cham, Switzerland, 2016; pp. 111–139. [Google Scholar]
- Havaux, M. Carotenoid oxidation products as stress signals in plants. Plant J. 2014, 79, 597–606. [Google Scholar] [CrossRef]
- Johnson, M.P.; Havaux, M.; Triantaphylidès, C.; Ksas, B.; Pascal, A.A.; Robert, B.; Davison, P.A.; Ruban, A.V.; Horton, P. Elevated zeaxanthin bound to oligomeric LHCII enhances the resistance of arabidopsis to photooxidative stress by a lipid-protective, antioxidant mechanism. J. Biol. Chem. 2007, 282, 22605–22618. [Google Scholar] [CrossRef]
- Havaux, M.; Dall’Osto, L.; Bassi, R. Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in arabidopsis leaves and functions independent of binding to PSII antennae. Plant Physiol. 2007, 145, 1506–1520. [Google Scholar] [CrossRef]
- Crozier, A.; Kamiya, Y.; Bishop, G.; Yolota, T. Biosynthesis of hormone and elicitor molecules. In Biochemistry and Molecular Biology of Plants; Buchanan, B., Gruissem, W., Jones, R., Eds.; American Society of Plant Physiologists: Rockille, MD, USA, 2000; pp. 865–872. [Google Scholar]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid Metabolism in Plants. Mol. Plant. 2015, 8, 68–82. [Google Scholar] [CrossRef]
- Matusova, R.; Rani, K.; Verstappen, F.W.A.; Franssen, M.C.R.; Beale, M.H.; Bouwmeester, H.J. The strigolactone germination stimulants of the plant-parasitic striga and orobanche spp. are derived from the carotenoid pathway. Plant Physiol. 2005, 139, 920–934. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Aspects Med. 2005, 26, 459–516. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I.; Wang, X.D.; Tang, G.; Russell, R.M. Mechanism of carotenoid cleavage to retinoids. Ann. N. Y. Acad. Sci. 1993, 691, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Sajilata, M.G.; Singhal, R.S.; Kamat, M.Y. The carotenoid pigment zeaxanthin—A review. Compr. Rev. Food Sci. Food Saf. 2008, 7, 29–49. [Google Scholar] [CrossRef]
- Mayne, S.T. Beta-carotene, carotenoids, and disease prevention in humans. FASEB J. 1996, 10, 690–701. [Google Scholar] [CrossRef]
- Grune, T.; Lietz, G.; Palou, A.; Ross, A.C.; Stahl, W.; Tang, G.; Thurnham, D.; Yin, S.A.; Biesalski, H.K. β-carotene is an important vitamin a source for humans. J. Nutr. 2010, 140, 2268S–2285S. [Google Scholar] [CrossRef]
- Xavier, A.A.O.; Pérez-Gálvez, A. Carotenoids as a source of antioxidants in the diet. Subcell. Biochem. 2016, 79, 359–375. [Google Scholar] [CrossRef]
- Stanley, L.; Yuan, Y.W. Transcriptional regulation of carotenoid biosynthesis in plants: So many regulators, so little consensus. Front. Plant Sci. 2019, 10, 1017. [Google Scholar] [CrossRef]
- Sun, T.; Yuan, H.; Cao, H.; Yazdani, M.; Tadmor, Y.; Li, L. Carotenoid metabolism in plants: The role of plastids. Mol. Plant. 2018, 11, 58–74. [Google Scholar] [CrossRef]
- Ruiz-Sola, M.Á.; Rodríguez-Concepción, M. Carotenoid biosynthesis in arabidopsis: A colorful pathway. Arab. B 2012, 10, e0158. [Google Scholar] [CrossRef] [PubMed]
- Briggs, W.R.; Olney, M.A. Photoreceptors in plant photomorphogenesis to date. Five phytochromes, two cryptochromes, one phototropin, and one superchrome. Plant Physiol. 2001, 125, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Briggs, W.R.; Tseng, T.S.; Cho, H.Y.; Swartz, T.E.; Sullivan, S.; Bogomolni, R.A.; Kaiserli, E.; Christie, J.M. Phototropins and their LOV domains: Versatile plant blue-light receptors. J. Integr. Plant Biol. 2007, 49, 4–10. [Google Scholar] [CrossRef]
- Franklin, K.A.; Larner, V.S.; Whitelam, C.C. The signal transducing photoreceptors of plants. Int. J. Dev. Biol. 2005, 49, 653–664. [Google Scholar] [CrossRef]
- Rockwell, N.; Su, Y.-S.; Lagarias, J.C. Phytochome structure and signaling mechanisms. Annu. Rev. Plant Biol. 2006, 57, 837–858. [Google Scholar] [CrossRef]
- Shin, A.Y.; Han, Y.J.; Baek, A.; Ahn, T.; Kim, S.Y.; Nguyen, T.S.; Son, M.; Lee, K.W.; Shen, Y.; Song, P.S.; et al. Evidence that phytochrome functions as a protein kinase in plant light signalling. Nat. Commun. 2016, 7, 11545. [Google Scholar] [CrossRef]
- Wagner, D.; Fairchild, C.D.; Kuhn, R.M.; Quail, P.H. Chromophore-bearing NH2-terminal domains of phytochromes a and b determine their photosensory specificity and differential light lability. Proc. Natl. Acad. Sci. USA 1996, 93, 4011–4015. [Google Scholar] [CrossRef]
- Chen, M.; Tao, Y.; Lim, J.; Shaw, A.; Chory, J. Regulation of phytochrome B nuclear localization through light-dependent unmasking of nuclear-localization signals. Curr. Biol. 2005, 15, 637–642. [Google Scholar] [CrossRef]
- Oka, Y.; Matsushita, T.; Mochizuki, N.; Quail, P.H.; Nagatani, A. Mutant screen distinguishes between residues necessary for light-signal perception and signal transfer by phytochrome B. PLoS Genet. 2008, 4, e1000158. [Google Scholar] [CrossRef]
- Lagarias, J.C.; Rapoport, H. Chromopeptides from phytochrome. The structure and linkage of the PR Form of the phytochrome chromophore. J. Am. Chem. Soc. 1980, 102, 4821–4828. [Google Scholar] [CrossRef]
- Oka, Y.; Ono, Y.; Toledo-Ortiz, G.; Kokaji, K.; Matsui, M.; Mochizuki, N.; Nagatania, A. Arabidopsis phytochrome a is modularly structured to integrate the multiple features that are required for a highly sensitized phytochrome. Plant Cell 2012, 24, 2949–2962. [Google Scholar] [CrossRef] [PubMed]
- Whitelam, G.C.; Johnson, E.; Peng, J.; Carol, P.; Anderson, M.L.; Cowl, J.S.; Harberd, N.P. Phytochrome a null mutants of arabidopsis display a wild-type phenotype in white light. Plant Cell 1993, 5, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Elich, T.D.; Chory, J. Biochemical characterization of arabidopsis wild-type and mutant phytochrome B holoproteins. Plant Cell 1997, 9, 2271–2280. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Franklin, K.A.; Davis, S.J.; Stoddart, W.M.; Vierstra, R.D.; Whitelam, G.C. Mutant analyses define multiple roles for phytochrome C in arabidopsis photomorphogenesis. Plant Cell 2003, 15, 1981–1989. [Google Scholar] [CrossRef]
- Qin, M.; Kuhn, R.; Moran, S.; Quail, P.H. Overexpressed phytochrome C has similar photosensory specificity to phytochrome B but a distinctive capacity to enhance primary leaf expansion. Plant J. 1997, 12, 1163–1172. [Google Scholar] [CrossRef]
- Sheerin, D.J.; Hiltbrunner, A. Molecular mechanisms and ecological function of far-red light signalling. Plant Cell Environ. 2017, 40, 2509–2529. [Google Scholar] [CrossRef]
- Matsushita, T.; Mochizuki, N.; Nagatani, A. Dimers of the N-terminal domain of phytochrome B are functional in the nucleus. Nature 2003, 424, 571–574. [Google Scholar] [CrossRef]
- Sharrock, R.A.; Clack, T. Patterns of expression and normalized levels of the five arabidopsis phytochromes. Plant Physiol. 2002, 130, 442–456. [Google Scholar] [CrossRef]
- Huq, E.; Al-Sady, B.; Quail, P.H. Nuclear translocation of the photoreceptor phytochrome B is necessary for its biological function in seedling photomorphogenesis. Plant J. 2003, 35, 660–664. [Google Scholar] [CrossRef]
- Nagatani, A. Light-regulated nuclear localization of phytochromes. Curr. Opin. Plant Biol. 2004, 7, 708–711. [Google Scholar] [CrossRef]
- Nagy, F.; Schäfer, E. Phytochromes control photomorphogenesis by differentially regulated, interacting signaling pathways in higher plants. Annu. Rev. Plant Biol. 2002, 53, 329–355. [Google Scholar] [CrossRef] [PubMed]
- Shinomura, T.; Nagatani, A.; Hanzawa, H.; Kubota, M.; Watanabe, M.; Furuya, M. Action Spectra for Phytochrome A- and B-Specific Photoinduction of Seed Germination in Arabidopsis Thaliana. Proc. Natl. Acad. Sci. USA 1996, 93, 8129–8133. [Google Scholar] [CrossRef]
- Trupkin, S.A.; Debrieux, D.; Hiltbrunner, A.; Fankhauser, C.; Casal, J.J. The serine-rich N-terminal region of arabidopsis phytochrome A is required for protein stability. Plant Mol. Biol. 2007, 63, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Casal, J.J. Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol. 2013, 64, 403–427. [Google Scholar] [CrossRef] [PubMed]
- Hiltbrunner, A.; Tscheuschler, A.; Viczián, A.; Kunkel, T.; Kircher, S.; Schäfer, E. FHY1 and FHL act together to mediate nuclear accumulation of the phytochrome A photoreceptor. Plant Cell Physiol. 2006, 47, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Hare, P.D.; Yang, S.W.; Zeidler, M.; Huang, L.F.; Chua, N.H. FHL is required for full phytochrome A signaling and shares overlapping functions with FHY1. Plant J. 2005, 43, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Genoud, T.; Schweizer, F.; Tscheuschler, A.; Debrieux, D.; Casal, J.J.; Schäfer, E.; Hiltbrunner, A.; Fankhauser, C. FHY1 mediates nuclear import of the light-activated phytochrome A photoreceptor. PLoS Genet. 2008, 4, e1000143. [Google Scholar] [CrossRef]
- Li, J.; Li, G.; Gao, S.; Martinez, C.; He, G.; Zhou, Z.; Huang, X.; Lee, J.H.; Zhang, H.; Shen, Y.; et al. Arabidopsis transcription factor ELONGATED HYPOCOTYL5 plays a role in the feedback regulation of phytochrome a signaling. Plant Cell 2010, 22, 3634–3649. [Google Scholar] [CrossRef]
- Sakamoto, K.; Nagatani, A. Nuclear localization activity of phytochrome B. Plant J. 1996, 10, 859–868. [Google Scholar] [CrossRef]
- Pfeiffer, A.; Nagel, M.K.; Popp, C.; Wüst, F.; Bindics, J.; Viczián, A.; Hiltbrunner, A.; Nagy, F.; Kunkel, T.; Schäfer, E. Interaction with plant transcription factors can mediate nuclear import of phytochrome B. Proc. Natl. Acad. Sci. USA 2012, 109, 5892–5897. [Google Scholar] [CrossRef]
- Chen, F.; Li, B.; Li, G.; Charron, J.B.; Dai, M.; Shi, X.; Deng, X.W. Arabidopsis phytochrome a directly targets numerous promoters for individualized modulation of genes in a wide range of pathways. Plant Cell 2014, 26, 1949–1966. [Google Scholar] [CrossRef]
- Casal, J.J.; Luccioni, L.G.; Oliverio, K.A.; Boccalandro, H.E. Light, Phytochrome Signalling and Photomorphogenesis in Arabidopsis. Photochem. Photobiol. Sci. 2003, 2, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Fankhauser, C.; Yeh, K.C.; Lagarias, J.C.; Zhang, H.; Elich, T.D.; Chory, J. PKS1, a substrate phosphorylated by phytochrome that modulates light signaling in arabidopsis. Science 1999, 284, 1539–1541. [Google Scholar] [CrossRef] [PubMed]
- Yeh, K.C.; Lagarias, J.C. Eukaryotic phytochromes: Light-regulated serine/threonine protein kinases with histidine kinase ancestry. Proc. Natl. Acad. Sci. USA 1998, 95, 13976–13981. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.A.; Quail, P.H. Phytochrome functions in arabidopsis development. J. Exp. Bot. 2010, 61, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Howitt, C.A.; Pogson, B.J. Carotenoid accumulation and function in seeds and non-green tissues. Plant Cell Environ. 2006, 29, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Tepperman, J.M.; Hwang, Y.S.; Quail, P.H. PhyA dominates in transduction of red-light signals to rapidly responding genes at the initiation of arabidopsis seedling de-etiolation. Plant J. 2006, 48, 728–742. [Google Scholar] [CrossRef]
- Welsch, R.; Beyer, P.; Hugueney, P.; Kleinig, H.; Von Lintig, J. Regulation and activation of phytoene synthase, a key enzyme in carotenoid biosynthesis, during photomorphogenesis. Planta 2000, 211, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Von Lintig, J.; Welsch, R.; Bonk, M.; Giuliano, G.; Batschauer, A.; Kleinig, H. Light-dependent regulation of carotenoid biosynthesis occurs at the level of phytoene synthase expression and is mediated by phytochrome in sinapis alba and arabidopsis thaliana seedlings. Plant J. 1997, 12, 625–634. [Google Scholar] [CrossRef]
- Welsch, R.; Medina, J.; Giuliano, G.; Beyer, P.; Von Lintig, J. Structural and functional characterization of the phytoene synthase promoter from arabidopsis thaliana. Planta 2003, 216, 523–534. [Google Scholar] [CrossRef]
- Rosas-Saavedra, C.; Stange, C. Biosynthesis of carotenoids in plants: Enzymes and color. In Carotenoids in Nature; Stange, C., Ed.; Springer: Cham, Switzerland, 2016; Volume 79, pp. 35–69. [Google Scholar] [CrossRef]
- Alcaíno, J.; Baeza, M.; Cifuentes, V. Carotenoid distribution in nature. In Carotenoids in Nature; Stange, C., Ed.; Springer: Cham, Switzerland, 2016; Volume 79, pp. 3–33. [Google Scholar] [CrossRef]
- Niyogi, K.K.; Björkman, O.; Grossman, A.R. The roles of specific xanthophylls in photoprotection. Proc. Natl. Acad. Sci. USA 1997, 94, 14162–14167. [Google Scholar] [CrossRef]
- Kimura, M.; Rodriguez-Amaya, D.B. Carotenoid composition of hydroponic leafy vegetables. J. Agric. Food Chem. 2003, 51, 2603–2607. [Google Scholar] [CrossRef] [PubMed]
- Žnidarčič, D.; Ban, D.; Šircelj, H. Carotenoid and chlorophyll composition of commonly consumed leafy vegetables in mediterranean countries. Food Chem. 2011, 129, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B. Carotenoids and photoprotection in plants: A role for the xanthophyll zeaxanthin. BBA Bioenerg. 1990, 1020, 1–24. [Google Scholar] [CrossRef]
- Cazzaniga, S.; Li, Z.; Niyogi, K.K.; Bassi, R.; Dall’Osto, L. The arabidopsis Szl1 mutant reveals a critical role of β-carotene in photosystem i photoprotection. Plant Physiol. 2012, 159, 1745–1758. [Google Scholar] [CrossRef] [PubMed]
- Stange, C.; Flores, C. Carotenoids and Photosynthesis—Regulation of carotenoid biosyntesis by photoreceptors. In Advances in Photosynthesis—Fundamental Aspects; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Wolf, I.; Kircher, S.; Fejes, E.; Kozma-Bognár, L.; Schäfer, E.; Nagy, F.; Ádám, É. Light-regulated nuclear import and degradation of arabidopsis phytochrome-a N-terminal fragments. Plant Cell Physiol. 2011, 52, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, R.; Nakamura, M.; Mochizuki, N.; Kay, S.A.; Nagatani, A. Light-dependent translocation of a phytochrome B-GFP fusion protein to the nucleus in transgenic arabidopsis. J. Cell Biol. 1999, 145, 437–445. [Google Scholar] [CrossRef]
- Toledo-Ortiz, G.; Kiryu, Y.; Kobayashi, J.; Oka, Y.; Kim, Y.; Nam, H.G.; Mochizuki, N.; Nagatani, A. Subcellular sites of the signal transduction and degradation of phytochrome A. Plant Cell Physiol. 2010, 51, 1648–1660. [Google Scholar] [CrossRef]
- Toledo-Ortiz, G.; Johansson, H.; Lee, K.P.; Bou-Torrent, J.; Stewart, K.; Steel, G.; Rodríguez-Concepción, M.; Halliday, K.J. The HY5-PIF regulatory module coordinates light and temperature control of photosynthetic gene transcription. PLoS Genet. 2014, 10, e1004416. [Google Scholar] [CrossRef]
- Zhu, L.; Bu, Q.; Xu, X.; Paik, I.; Huang, X.; Hoecker, U.; Deng, X.W.; Huq, E. CUL4 forms an E3 ligase with COP1 and SPA to promote light-induced degradation of PIF1. Nat. Commun. 2015, 6, 7245. [Google Scholar] [CrossRef]
- Shi, H.; Wang, X.; Mo, X.; Tang, C.; Zhong, S.; Deng, X.W. Arabidopsis DET1 degrades HFR1 but stabilizes PIF1 to precisely regulate seed germination. Proc. Natl. Acad. Sci. USA 2015, 112, 3817–3822. [Google Scholar] [CrossRef] [PubMed]
- Leivar, P.; Quail, P.H. PIFs: Pivotal components in a cellular signaling hub. Trends Plant Sci. 2011, 16, 19–28. [Google Scholar] [CrossRef]
- Moon, J.; Zhu, L.; Shen, H.; Huq, E. PIF1 directly and indirectly regulates chlorophyll biosynthesis to optimize the greening process in arabidopsis. Proc. Natl. Acad. Sci. USA 2008, 105, 9433–9438. [Google Scholar] [CrossRef] [PubMed]
- Ang, L.H.; Chattopadhyay, S.; Wei, N.; Oyama, T.; Okada, K.; Batschauer, A.; Deng, X.W. Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of arabidopsis development. Mol. Cell 1998, 1, 213–222. [Google Scholar] [CrossRef]
- Yang, H.Q.; Tang, R.H.; Cashmore, A.R. The signalling mechanism of arabidopsis CRY1 involves direct interaction with COP1. Plant Cell 2001, 13, 2573–2587. [Google Scholar] [CrossRef]
- Holm, M.; Ma, L.G.; Qu, L.J.; Deng, X.W. Two interacting BZIP proteins are direct targets of COP1-mediated control of light dependent gene expression in arabidopsis. Genes Dev. 2002, 16, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- Yanawaga, J.; Sullivan, J.; Komatsu, S.; Gusmaroli, G.; Suzuki, G.; Yin, J.; Ishibashi, T.; Saijo, Y.; Rubio, V.; Kimura, S.; et al. Arabidopsis COP10 forms a complex with DDB1 and DET1 in vivo and enhances the activity of ubiquitin conjugating enzymes. Genes Dev. 2004, 18, 2172–2181. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.C.; Yang, J.Y.; Seo, H.S.; Chua, N.H. HFRA is target by COP1 E3 ligase for post-transcriptional proteolysis during phytochrome A signaling. Genes Dev. 2005, 19, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Rausenberger, J.; Hussong, A.; Kircher, S.; Kirchenbauer, D.; Timmer, J.; Nagy, F.; Schäfer, E.; Fleck, C. An integrative model for phytochrome B mediated photomorphogenesis: From protein dynamics to physiology. PLoS ONE 2010, 5, e10721. [Google Scholar] [CrossRef]
- Sheerin, D.J.; Menon, C.; Oven-Krockhaus, S.Z.; Enderle, B.; Zhu, L.; Johnen, P.; Schleifenbaum, F.; Stierhof, Y.D.; Huq, E.; Hiltbrunner, A. Light-activated phytochrome A and B interact with members of the SPA family to promote photomorphogenesis in arabidopsis by reorganizing the COP1/SPA complex. Plant Cell 2015, 27, 189–201. [Google Scholar] [CrossRef]
- Lu, X.D.; Zhou, C.M.; Xu, P.B.; Luo, Q.; Lian, H.L.; Yang, H.Q. Red-light-dependent interaction of PhyB with SPA1 promotes COP1-SPA1 dissociation and photomorphogenic development in arabidopsis. Mol. Plant. 2015, 8, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Al-Sady, B.; Ni, W.; Kircher, S.; Schäfer, E.; Quail, P.H. Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol. Cell 2006, 23, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Ortiz, G.; Huq, E.; Rodríguez-Concepción, M. Direct regulation of phytoene synthase gene expression and carotenoid biosynthesis by phytochrome-interacting factors. Proc. Natl. Acad. Sci. USA 2010, 107, 11626–11631. [Google Scholar] [CrossRef] [PubMed]
- Maass, D.; Arango, J.; Wüst, F.; Beyer, P.; Welsch, R. Carotenoid crystal formation in arabidopsis and carrot roots caused by increased phytoene synthase protein levels. PLoS ONE 2009, 4, e6373. [Google Scholar] [CrossRef]
- Rodríguez-Villalón, A.; Gas, E.; Rodríguez-Concepción, M. Phytoene synthase activity controls the biosynthesis of carotenoids and the supply of their metabolic precursors in dark-grown arabidopsis seedlings. Plant J. 2009, 60, 424–435. [Google Scholar] [CrossRef]
- Cazzonelli, C.I.; Pogson, B.J. Source to sink: Regulation of carotenoid biosynthesis in plants. Trends Plant Sci. 2010, 15, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, J.F.; Gallemí, M.; Molina-Contreras, M.J.; Llorente, B.; Bevilaqua, M.R.R.; Quail, P.H. The shade avoidance syndrome in arabidopsis: The antagonistic role of phytochrome A and B differentiates vegetation proximity and canopy shade. PLoS ONE 2014, 9, e109275. [Google Scholar] [CrossRef] [PubMed]
- Sng, B.J.R.; Singh, G.P.; Van Vu, K.; Chua, N.H.; Ram, R.J.; Jang, I.C. Rapid metabolite response in leaf blade and petiole as a marker for shade avoidance syndrome. Plant Methods 2020, 16, 144. [Google Scholar] [CrossRef]
- Saijo, Y.; Zhu, D.; Li, J.; Rubio, V.; Zhou, Z.; Shen, Y.; Hoecker, U.; Wang, H.; Deng, X.W. Arabidopsis COP1/SPA1 complex and FHY1/FHY3 associate with distinct phosphorylated forms of phytochrome A in balancing light signaling. Mol. Cell 2008, 31, 607–613. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, H.; Ma, M.; Li, Q.; Kong, D.; Sun, J.; Ma, X.; Wang, B.; Chen, C.; Xie, Y.; et al. Arabidopsis FHY3 and FAR1 regulate the balance between growth and defense responses under shade conditions. Plant Cell 2019, 31, 2089–2106. [Google Scholar] [CrossRef] [PubMed]
- Bou-Torrent, J.; Toledo-Ortiz, G.; Ortiz-Alcaide, M.; Cifuentes-Esquivel, N.; Halliday, K.J.; Martinez-García, J.F.; Rodriguez-Concepcion, M. Regulation of Carotenoid biosynthesis by shade relies on specific subsets of antagonistic transcription factors and cofactors. Plant Physiol. 2015, 169, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Lorrain, S.; Allen, T.; Duek, P.D.; Whitelam, G.C.; Fankhauser, C. Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting BHLH transcription factors. Plant J. 2008, 53, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Hornitschek, P.; Kohnen, M.V.; Lorrain, S.; Rougemont, J.; Ljung, K.; López-Vidriero, I.; Franco-Zorrilla, J.M.; Solano, R.; Trevisan, M.; Pradervand, S.; et al. Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signaling. Plant J. 2012, 71, 699–711. [Google Scholar] [CrossRef]
- Seymour, G.B.; Østergaard, L.; Chapman, N.H.; Knapp, S.; Martin, C. Fruit development and ripening. Annu. Rev. Plant Biol. 2013, 64, 219–241. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, S.M.; Christian, R.; Castro-Velasquez, N.; Hyden, B.; Lynch-Holm, V.; Dhingra, A. Comparative Ultrastructure of fruit plastids in three genetically diverse genotypes of apple (Malus × Domestica Borkh.) during development. Plant Cell Rep. 2017, 36, 1627–1640. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Cheng, Y.; Deng, X.X. Diversity of plastids in citrus fruit. Acta Hortic. 2018, 1203, 111–116. [Google Scholar] [CrossRef]
- Lu, P.; Wang, R.; Zhu, C.; Fu, X.; Wang, S.; Grierson, D.; Xu, C. Microscopic analyses of fruit cell plastid development in loquat (Eriobotrya Japonica) during fruit ripening. Molecules 2019, 24, 448. [Google Scholar] [CrossRef]
- Kahlau, S.; Bock, R. Plastid transcriptomics and translatomics of tomato fruit development and chloroplast-to-chromoplast differentiation: Chromoplast gene expression largely serves the production of a single protein. Plant Cell 2008, 20, 856–874. [Google Scholar] [CrossRef]
- Kimura, S.; Sinha, N. Tomato (Solanum Lycopersicum): A model fruit-bearing crop. Cold Spring Harb. Protoc. 2008, 3, pdb-emo105. [Google Scholar] [CrossRef]
- Dzakovich, M.P.; Gas-Pascual, E.; Orchard, C.J.; Sari, E.N.; Riedl, K.M.; Schwartz, S.J.; Francis, D.M.; Cooperstone, J.L. Analysis of tomato carotenoids: Comparing extraction and chromatographic methods. J. AOAC Int. 2019, 102, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Ronen, G.; Cohen, M.; Zamir, D.; Hirschberg, J. Regulation of Carotenoid biosynthesis during tomato fruit development: Expression of the gene for lycopene epsilon-cyclase is down-regulated during ripening and is elevated in the mutant delta. Plant J. 1999, 17, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, G.; Bartley, G.E.; Scolnik, P.A. Regulation of carotenoid biosynthesis during tomato development. Plant Cell 1993, 5, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Giorio, G.; Stigliani, A.L.; D’Ambrosio, C. Phytoene synthase genes in tomato (Solanumlycopersicum L.)—New data on the structures, the deduced amino acid sequences and the expression patterns. FEBS J. 2008, 275, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Stauder, R.; Welsch, R.; Camagna, M.; Kohlen, W.; Balcke, G.U.; Tissier, A.; Walter, M.H. Strigolactone levels in dicot roots are determined by an ancestral symbiosis-regulated clade of the PHYTOENE SYNTHASE gene family. Front. Plant Sci. 2018, 9, 255. [Google Scholar] [CrossRef] [PubMed]
- Fantini, E.; Falcone, G.; Frusciante, S.; Giliberto, L.; Giuliano, G. Dissection of Tomato lycopene biosynthesis through virus-induced gene silencing. Plant Physiol. 2013, 163, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Schofield, A.; Paliyath, G. Modulation of carotenoid biosynthesis during tomato fruit ripening through phytochrome regulation of phytoene synthase activity. Plant Physiol. Biochem. 2005, 43, 1052–1060. [Google Scholar] [CrossRef]
- Hauser, B.A.; Cordonnier-Pratt, M.M.; Daniel-Vedele, F.; Pratt, L.H. The phytochrome gene family in tomato includes a novel subfamily. Plant Mol. Biol. 1995, 29, 1143–1155. [Google Scholar] [CrossRef]
- Hauser, B.A.; Cordonnier-Pratt, M.M.; Pratt, L.H. Temporal and photoregulated expression of five tomato phytochrome genes. Plant J. 1998, 14, 431–439. [Google Scholar] [CrossRef]
- Pratt, L.H.; Cordonnier-Pratt, M.M.; Kelmenson, P.M.; Lazarova, G.I.; Kubota, T.; Alba, R.M. The phytochrome gene family in tomato (Solanum lycopersicum L.). Plant Cell Environ. 1997, 20, 672–677. [Google Scholar] [CrossRef]
- Husaineid, S.S.H.; Kok, R.A.; Schreuder, M.E.L.; Hanumappa, M.; Cordonnier-Pratt, M.M.; Pratt, L.H.; van Der Plas, L.H.W.; van Der Krol, A.R. Overexpression of homologous phytochrome genes in tomato: Exploring the limits in photoperception. J. Exp. Bot. 2007, 58, 615–626. [Google Scholar] [CrossRef]
- Alba, R.; Cordonnier-Pratt, M.M.; Pratt, L.H. Fruit-localized phytochromes regulate lycopene accumulation independently of ethylene production in tomato. Plant Physiol. 2000, 123, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Bianchetti, R.E.; Cruz, A.B.; Oliveira, B.S.; Demarco, D.; Purgatto, E.; Peres, L.E.; Rossi, M.; Freschi, L. Phytochromobilin deficiency impairs sugar metabolism through the regulation of cytokinin and auxin signaling in tomato fruits. Sci. Rep. 2017, 7, 7822. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Sharma, S.; Santisree, P.; Kilambi, H.V.; Appenroth, K.; Sreelakshmi, Y.; Sharma, R. Complex and shifting interactions of phytochromes regulate fruit development in tomato. Plant Cell Environ. 2014, 37, 1688–1702. [Google Scholar] [CrossRef] [PubMed]
- Bianchetti, R.E.; Lira, B.S.; Monteiro, S.; Demarco, D.; Purgatto, E.; Rothan, C.; Rossi, M.; Freschi, L. Fruit-localized phytochromes regulate plastid biogenesis, starch synthesis, and carotenoid metabolism in tomato. J. Exp. Bot. 2018, 69, 3573–3586. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.R.R.; Lira, B.S.; Pikart, F.C.; Monteiro, S.S.; Furlan, C.M.; Purgatto, E.; Pascoal, G.B.; Andrade, S.C.; Demarco, D.; Rossi, M.; et al. Beyond the limits of photoperception: Constitutively active PHYTOCHROME B2 overexpression as a means of improving fruit nutritional quality in tomato. Plant Biotechnol. J. 2020, 18, 2027–2041. [Google Scholar] [CrossRef]
- Weller, J.L.; Schreuder, M.E.L.; Smith, H.; Koornneef, M.; Kendrick, R.E. Physiological interactions of phytochromes A, B1 and B2 in the control of development in tomato. Plant J. 2000, 24, 345–356. [Google Scholar] [CrossRef]
- Liu, Y.; Roof, S.; Ye, Z.; Barry, C.; van Tuinent, A.; Vrebalov, J.; Bowler, C.; Giovannoni, J. Manipulation of light signal transduction as a means of modifying fruit nutritional quality in tomato. Proc. Natl. Acad. Sci. USA 2004, 101, 9897–9902. [Google Scholar] [CrossRef]
- Wang, A.; Chen, D.; Ma, Q.; Rose, J.K.C.; Fei, Z.; Liu, Y.; Giovannoni, J.J. The tomato HIGH PIGMENT1/DAMAGED DNA BINDING PROTEIN 1 gene contributes to regulation of fruit ripening. Hortic. Res. 2019, 6, 15. [Google Scholar] [CrossRef]
- Cruz, A.B.; Bianchetti, R.E.; Alves, F.; Purgatto, E.; Peres, L.E.P.; Rossi, M.; Freschi, L. Light, ethylene and auxin signaling interaction regulates carotenoid biosynthesis during tomato fruit ripening. Front. Plant Sci. 2018, 9, 1370. [Google Scholar] [CrossRef]
- Lieberman, M.; Segev, O.; Gilboa, N.; Lalazar, A.; Levin, I. The tomato homolog of the gene encoding UV-damaged DNA binding protein 1 (DDB1) underlined as the gene that causes the high pigment-1 mutant phenotype. Theor. Appl. Genet. 2004, 108, 1574–1581. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, G.; Guo, X.; Yin, W.; Yu, X.; Hu, J.; Hu, Z. Overexpression of SlPRE2, an atypical BHLH transcription factor, affects plant morphology and fruit pigment accumulation in tomato. Sci. Rep. 2017, 7, 5786. [Google Scholar] [CrossRef] [PubMed]
- Llorente, B.; D’Andrea, L.; Ruiz-Sola, M.A.; Botterweg, E.; Pulido, P.; Andilla, J.; Loza-Alvarez, P.; Rodriguez-Concepcion, M. Tomato fruit carotenoid biosynthesis is adjusted to actual ripening progression by a light-dependent mechanism. Plant J. 2016, 85, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Ikoma, Y.; Matsumoto, H.; Sugiura, M.; Hyodo, H.; Yano, M. Accumulation of carotenoids and expression of carotenoid biosynthetic genes during maturation in citrus fruit. Plant Physiol. 2004, 134, 824–837. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Vandercook, C. Citrus carotenoids. I. Comparison of carotenoids of mature-green and yellow lemons. J. Food Sci. 1967, 32, 42–48. [Google Scholar] [CrossRef]
- Petry, F.C.; de Nadai, F.B.; Cristofani-Yaly, M.; Latado, R.R.; Mercadante, A.Z. Carotenoid biosynthesis and quality characteristics of new hybrids between tangor (Citrus Reticulata × C. Sinensis) Cv. ‘Murcott’ and sweet orange (C. Sinensis) Cv. ‘Pêra.’ Food Res. Int. 2019, 122, 461–470. [Google Scholar] [CrossRef]
- Tatmala, N.; Ma, G.; Zhang, L.; Kato, M.; Kaewsuksaeng, S. Characterization of carotenoid accumulation and carotenogenic gene expression during fruit ripening in red colored pulp of ‘Siam Red Ruby’ pumelo (Citrus Grandis) cultivated in Thailand. Hortic. J. 2019, 89, 237–243. [Google Scholar] [CrossRef]
- Lado, J.; Cronje, P.; Alquézar, B.; Page, A.; Manzi, M.; Gómez-Cadenas, A.; Stead, A.D.; Zacarías, L.; Rodrigo, M.J. Fruit shading enhances peel color, carotenes accumulation and chromoplast differentiation in red grapefruit. Physiol. Plant. 2015, 154, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Zhang, L.; Kato, M.; Yamawaki, K.; Kiriiwa, Y.; Yahata, M.; Ikoma, Y.; Matsumoto, H. Effect of the combination of ethylene and red LED light irradiation on carotenoid accumulation and carotenogenic gene expression in the flavedo of citrus fruit. Postharvest Biol. Technol. 2015, 99, 99–104. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, L.; Yungyuen, W.; Sato, Y.; Furuya, T.; Yahata, M.; Yamawaki, K.; Kato, M. Accumulation of carotenoids in a novel citrus cultivar ‘Seinannohikari’ during the fruit maturation. Plant Physiol. Biochem. 2018, 129, 349–356. [Google Scholar] [CrossRef]
- Lado, J.; Zacarías, L.; Gurrea, A.; Page, A.; Stead, A.; Rodrigo, M.J. Exploring the diversity in citrus fruit colouration to decipher the relationship between plastid ultrastructure and carotenoid composition. Planta 2015, 242, 645–661. [Google Scholar] [CrossRef]
- Tlili, I.; Hdider, C.; Lenucci, M.S.; Ilahy, R.; Jebari, H.; Dalessandro, G. Bioactive compounds and antioxidant activities during fruit ripening of watermelon cultivars. J. Food Compos. Anal. 2011, 24, 923–928. [Google Scholar] [CrossRef]
- Bangalore, D.V.; McGlynn, W.G.; Scott, D.D. Effects of fruit maturity on watermelon ultrastructure and intracellular lycopene distribution. J. Food Sci. 2008, 73, S222–S228. [Google Scholar] [CrossRef] [PubMed]
- Rumainum, I.M.; Worarad, K.; Srilaong, V.; Yamane, K. Fruit quality and antioxidant capacity of six thai mango cultivars. Agric. Nat. Resour. 2018, 52, 208–214. [Google Scholar] [CrossRef]
- Corrales-Bernal, A.; Maldonado Camila, M.E.; Urango, L.A.; Franco, M.E.; Rojano, B.A. Mango de azúcar (Mangifera Indica), variedad de Colombia: Características antioxidantes, nutricionales y sensoriales. Rev. Chil. Nutr. 2014, 41, 312–318. [Google Scholar] [CrossRef]
- Lado, J.; Alós, E.; Manzi, M.; Cronje, P.J.R.; Gómez-Cadenas, A.; Rodrigo, M.J.; Zacarías, L. Light regulation of carotenoid biosynthesis in the peel of mandarin and sweet orange fruits. Front. Plant Sci. 2019, 10, 1288. [Google Scholar] [CrossRef]
- Yoo, H.J.; Kim, J.H.; Park, K.S.; Son, J.E.; Lee, J.M. Light-controlled fruit pigmentation and flavor volatiles in tomato and bell pepper. Antioxidants 2020, 9, 14. [Google Scholar] [CrossRef]
- Hodge, A.; Berta, G.; Doussan, C.; Merchan, F.; Crespi, M. Plant root growth, architecture and function. Plant Soil 2009, 321, 153–187. [Google Scholar] [CrossRef]
- Ito, R.; Fujiwara, M.T. Regulation of leucoplat morphology in roots: Interorganellar signaling from mitochondria? Plant Signal. Behav. 2010, 6, 856–859. [Google Scholar] [CrossRef]
- Ruiz-Sola, M.Á.; Arbona, V.; Gómez-Cadenas, A.; Rodríguez-Concepción, M.; Rodríguez-Villalón, A. A root specific induction of carotenoid biosynthesis contributes to ABA production upon salt stress in arabidopsis. PLoS ONE 2014, 9, e90765. [Google Scholar] [CrossRef]
- Deng, X.-W.; Quail, P.H. Genetic and phenotypic characterization of Cop1 mutants of arabidopsis thaliana. Plant J. 1992, 2, 83–95. [Google Scholar] [CrossRef]
- Chory, J.; Peto, C.A. Mutations in the DET1 gene affect cell-type-specific expression of light-regulated genes and chloroplast development in arabidopsis. Proc. Natl. Acad. Sci. USA 1990, 87, 8776–8780. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Ha, J.H.; Kim, S.G.; Choi, H.K.; Kim, Z.H.; Han, Y.J.; Kim, J.I.; Oh, Y.; Fragoso, V.; Shin, K.; et al. Stem-piped light activates phytochrome B to trigger light responses in arabidopsis thaliana roots. Sci. Signal. 2016, 9, ra106. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, F.J.; Hall, A.; Grierson, C.S.; Halliday, K.J. Phytochrome coordinates arabidopsis shoot and root development. Plant J. 2007, 50, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Van Gelderen, K.; Kang, C.; Paalman, R.; Keuskamp, D.; Hayes, S.; Pierik, R. Far-red light detection in the shoot regulates lateral root development through the HY5 transcription factor. Plant Cell 2018, 30, 101–116. [Google Scholar] [CrossRef]
- Usami, T.; Mochizuki, N.; Kondo, M.; Nishimura, M.; Nagatani, A. Cryptochromes and phytochromes synergistically regulate arabidopsis root greening under blue light. Plant Cell Physiol. 2004, 45, 1798–1808. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Sasaki, D.; Noguchi, K.; Fujinuma, D.; Komatsu, H.; Kobayashi, M.; Sato, M.; Toyooka, K.; Sugimoto, K.; Niyogi, K.K.; et al. Photosynthesis of root chloroplasts developed in arabidopsis lines overexpressing GOLDEN2-LIKE transcription factors. Plant Cell Physiol. 2013, 54, 1365–1377. [Google Scholar] [CrossRef]
- Kobayashi, K.; Obayashi, T.; Masuda, T. Role of the G-box element in regulation of chlorophyll biosynthesis in arabidopsis roots. Plant Signal. Behav. 2012, 7, 922–926. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ohnishi, A.; Sasaki, D.; Fujii, S.; Iwase, A.; Sugimoto, K.; Masuda, T.; Wada, H. Shoot removal induces chloroplast development in roots via cytokinin signaling. Plant Physiol. 2017, 173, 2340–2355. [Google Scholar] [CrossRef]
- Sun, Q.; Yoda, K.; Suzuki, M.; Suzuki, H. Vascular tissue in the stem and roots of woody plants can conduct light. J. Exp. Bot. 2003, 54, 1627–1635. [Google Scholar] [CrossRef]
- Sun, Q.; Yoda, K.; Suzuki, H. Internal axial light conduction in the stems and roots of herbaceous plants. J. Exp. Bot. 2005, 56, 191–203. [Google Scholar] [CrossRef]
- Stange, C.; Fuentes, P.; Handford, M.; Pizarro, L. Daucus carota as a novel model to evaluate the effect of light on carotenogenic gene expression. Biol. Res. 2008, 41, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shaul, Y.; Klein, S. Development and structure of carotene bodies in carrot roots. Bot. Gaz. 1965, 126, 79–85. [Google Scholar] [CrossRef]
- Kujala, T.S.; Vienola, M.S.; Klika, K.D.; Loponen, J.M.; Pihlaja, K. Betalain and phenolic compositions of four beetroot (Beta Vulgaris) cultivars. Eur. Food Res. Technol. 2002, 214, 505–510. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization of red radish anthocyanins. J. Food Sci. 1996, 61, 322–326. [Google Scholar] [CrossRef]
- Arias, D.; Maldonado, J.; Silva, H.; Stange, C. A de novo transcriptome analysis revealed that photomorphogenic genes are required for carotenoid synthesis in the dark-grown carrot taproot. Mol. Genet. Genom. 2020, 295, 1379–1392. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quian-Ulloa, R.; Stange, C. Carotenoid Biosynthesis and Plastid Development in Plants: The Role of Light. Int. J. Mol. Sci. 2021, 22, 1184. https://doi.org/10.3390/ijms22031184
Quian-Ulloa R, Stange C. Carotenoid Biosynthesis and Plastid Development in Plants: The Role of Light. International Journal of Molecular Sciences. 2021; 22(3):1184. https://doi.org/10.3390/ijms22031184
Chicago/Turabian StyleQuian-Ulloa, Rocio, and Claudia Stange. 2021. "Carotenoid Biosynthesis and Plastid Development in Plants: The Role of Light" International Journal of Molecular Sciences 22, no. 3: 1184. https://doi.org/10.3390/ijms22031184
APA StyleQuian-Ulloa, R., & Stange, C. (2021). Carotenoid Biosynthesis and Plastid Development in Plants: The Role of Light. International Journal of Molecular Sciences, 22(3), 1184. https://doi.org/10.3390/ijms22031184