Interplay between Abscisic Acid and Gibberellins, as Related to Ethylene and Sugars, in Regulating Maturation of Non-Climacteric Fruit
Abstract
:1. Introduction
2. Introduction to Abscisic Acid in Fruit
2.1. Abscisic Acid Biosynthesis and Accumulation
2.2. Abscisic Acid Function During Fruit Maturation
3. GAs, ABA and Their Interplay during Fruit Development and Maturation
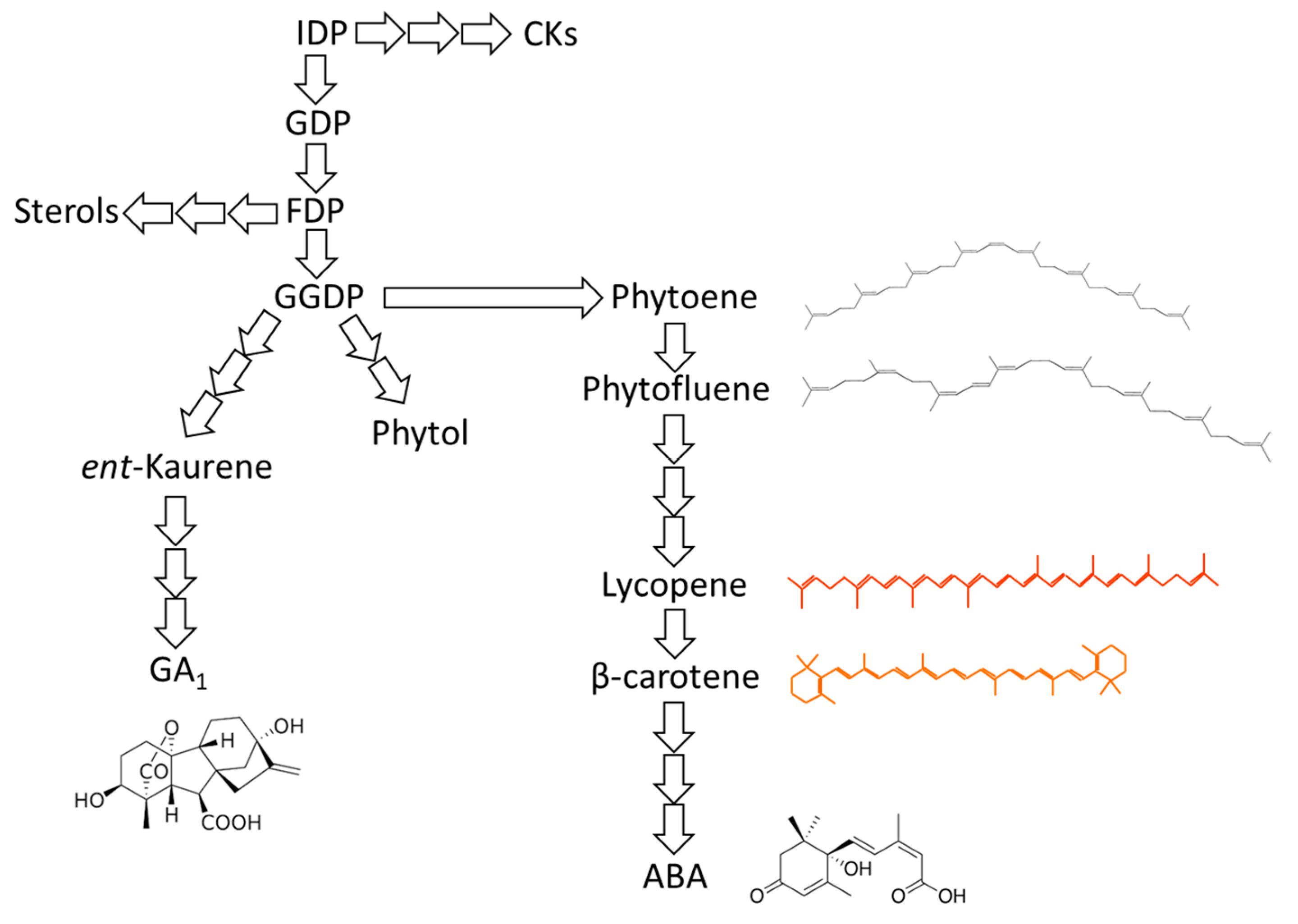
3.1. Integration of ABA and GAs Biosynthesis
3.2. GA Biosynthesis During Fruit Development and Maturation
3.3. Exogenous GA Affect Fruit Maturation and ABA Levels in Fruit
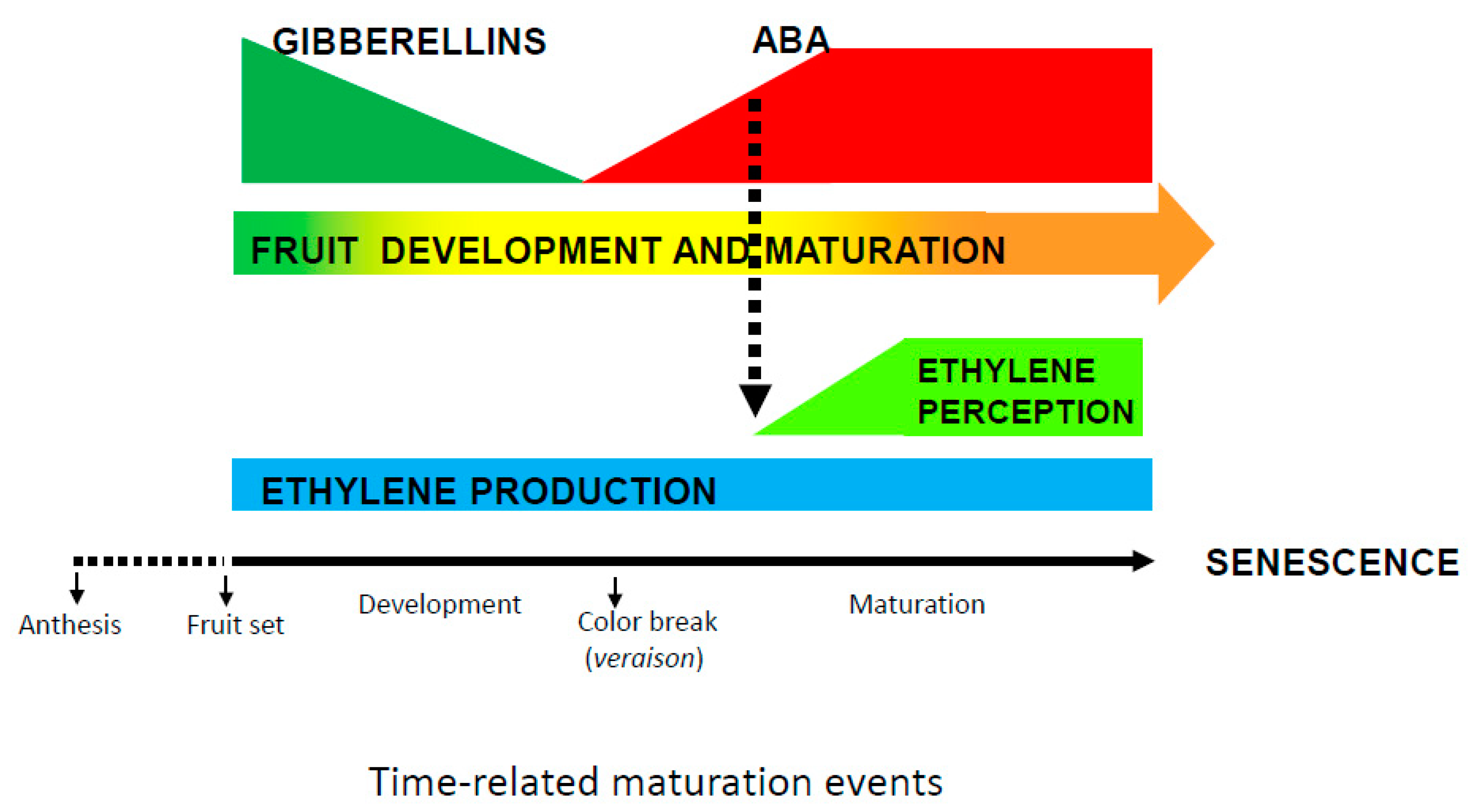
4. Integrating Signals to Regulate Maturation: GA, ABA, Sugars, and Ethylene Interaction
Citrus as a Model Plant for Non-Climacteric Maturation Studies
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cao, M.; Zheng, J.; Zhao, Y.; Zhang, Z.; Zheng, Z.-L. Network Analysis of Differentially Expressed Genes across Four Sweet Orange Varieties Reveals a Conserved Role of Gibberellin and Ethylene Responses and Transcriptional Regulation in Expanding Citrus Fruits. Trop. Plant Biol. 2018, 12, 12–20. [Google Scholar] [CrossRef]
- Klee, H.J.; Giovannoni, J.J. Genetics and Control of Tomato Fruit Ripening and Quality Attributes. Annu. Rev. Genet. 2011, 45, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Seymour, G.B.; Østergaard, L.; Chapman, N.H.; Knapp, S.; Martin, C. Fruit Development and Ripening. Annu. Rev. Plant Biol. 2013, 64, 219–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Khurana, A.; Sharma, A.K. Role of plant hormones and their interplay in development and ripening of fleshy fruits. J. Exp. Bot. 2013, 65, 4561–4575. [Google Scholar] [CrossRef] [Green Version]
- Rodrigo, M.J.; Marcos, J.F.; Alférez, F.; Mallent, M.D.; Zacarías, L. Characterization of Pinalate, a novel Citrus sinensis mutant with a fruit-specific alteration that results in yellow pigmentation and decreased ABA content. J. Exp. Bot. 2003, 54, 727–738. [Google Scholar] [CrossRef] [Green Version]
- Fraser, P.D.; Bramley, P. The biosynthesis and nutritional uses of carotenoids. Prog. Lipid Res. 2004, 43, 228–265. [Google Scholar] [CrossRef]
- Cutler, A.J.; Krochko, J.E. Formation and breakdown of ABA. Trends Plant Sci. 1999, 4, 472–478. [Google Scholar] [CrossRef]
- Liotenberg, S.; North, H.; Marion-Poll, A. Molecular biology and regulation of abscisic acid biosynthesis in plants. Plant Physiol. Biochem. 1999, 37, 341–350. [Google Scholar] [CrossRef]
- Taylor, I.B.; Burbidge, A.; Thompson, A.J. Control of abscisic acid synthesis. J. Exp. Bot. 2000, 51, 1563–1574. [Google Scholar] [CrossRef]
- Kondo, S.; Gemma, H. Relationship between Abscisic Acid (ABA) Content and Maturation of the Sweet Cherry. J. Jpn. Soc. Hortic. Sci. 1993, 62, 63–68. [Google Scholar] [CrossRef]
- Ren, J.; Sun, L.; Wu, J.; Zhao, S.; Wang, C.; Wang, Y.; Ji, K.; Leng, P. Cloning and expression analysis of cDNAs for ABA 8′-hydroxylase during sweet cherry fruit maturation and under stress conditions. J. Plant Physiol. 2010, 167, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Ponrod, W.; Kanlayanarat, S.; Hirai, N. Abscisic Acid Metabolism during Fruit Development and Maturation of Mangosteens. J. Am. Soc. Hortic. Sci. 2002, 127, 737–741. [Google Scholar] [CrossRef] [Green Version]
- Goldschmidt, E.E.; Goren, R.; Even-Chen, Z.; Bittner, S. Increase in Free and Bound Abscisic Acid during Natural and Ethylene-induced Senescence of Citrus Fruit Peel. Plant Physiol. 1973, 51, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.J.; Dugger, W.M. The Occurrence of Abscisic Acid and Abscisyl-β-d-Glucopyranoside in Developing and Mature Citrus Fruit as Determined by Enzyme Immunoassay. Plant Physiol. 1986, 82, 339–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, P.; Lafuente, M.T.; Rodrigo, M.J. The Citrus ABA signalosome: Identification and transcriptional regulation during sweet orange fruit ripening and leaf dehydration. J. Exp. Bot. 2012, 63, 4931–4945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alférez, F. Regulación Hormonal de la Maduración en Frutos Cítricos y Su relación Con Alteraciones Fisiológicas Durante la Postcosecha. Ph.D. Thesis, University of Valencia, Valencia, Spain, 2001. [Google Scholar]
- Aung, L.H.; Houck, L.G.; Norman, S.M. The Abscisic Acid Content of Citrus with Special Reference to Lemon. J. Exp. Bot. 1991, 42, 1083–1088. [Google Scholar] [CrossRef]
- Valero, D.; Martínez-Romero, D.; Serrano, M.; Riquelme, F. Influence of Postharvest Treatment with Putrescine and Calcium on Endogenous Polyamines, Firmness, and Abscisic Acid in Lemon (Citrus lemon L. Burm Cv. Verna). J. Agric. Food Chem. 1998, 46, 2102–2109. [Google Scholar] [CrossRef]
- Lafuente, M.T.; Martinez-Tellez, M.A.; Zacarias, L. Abscisic acid in the response of Fortune mandarin to chilling. Effects of maturity and high temperature conditioning. J. Sci. Food Agric. 1997, 73, 494–502. [Google Scholar] [CrossRef]
- Brisker, H.E.; Goldschmidt, E.E.; Goren, R. Ethylene-induced Formation of ABA in Citrus Peel as Related to Chloroplast Transformations. Plant Physiol. 1976, 58, 377–379. [Google Scholar] [CrossRef] [Green Version]
- Peppi, M.C.; Fidelibus, M.; Dokoozlian, N.; Walker, M.A. Abscisic acid applications improve the color of crimson seedless table grapes. Am. J. Enol. Vitic. 2006, 57, 388A. [Google Scholar]
- Peppi, M.C.; Walker, M.A.; Fidelibus, M.W. Application of abscisic acid rapidly upregulated UFGT gene expression and improved color of grape berries. Vitis 2008, 47, 11–14. [Google Scholar]
- Wheeler, S.; Loveys, B.; Ford, C.; Davies, C. The relationship between the expression of abscisic acid biosynthesis genes, accumulation of abscisic acid and the promotion of Vitis vinifera L. berry ripening by abscisic acid. Aust. J. Grape Wine Res. 2009, 15, 195–204. [Google Scholar] [CrossRef]
- Koyama, K.; Sadamatsu, K.; Goto-Yamamoto, N. Abscisic acid stimulated ripening and gene expression in berry skins of the Cabernet Sauvignon grape. Funct. Integr. Genom. 2009, 10, 367–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Zhang, M.; Ren, J.; Qi, J.; Zhang, G.; Leng, P. Reciprocity between abscisic acid and ethylene at the onset of berry ripening and after harvest. BMC Plant Biol. 2010, 10, 257. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, L.; Figueroa, C.R.; Valdenegro, M. Recent Advances in Hormonal Regulation and Cross-Talk during Non-Climacteric Fruit Development and Ripening. Horticulturae 2019, 5, 45. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Huang, H.; Huang, X. Differential effects of abscisic acid and ethylene on the fruit maturation of Litchi chinensis Sonn. Plant Growth Regul. 2007, 52, 189–198. [Google Scholar] [CrossRef]
- Rehman, M.; Singh, Z.; Khurshid, T. Pre-harvest spray application of abscisic acid (S-ABA) regulates fruit colour development and quality in early maturing M7 Navel orange. Sci. Hortic. 2018, 229, 1–9. [Google Scholar] [CrossRef]
- Wang, X.; Yin, W.; Wu, J.; Chai, L.; Yi, H. Effects of exogenous abscisic acid on the expression of citrus fruit ripening-related genes and fruit ripening. Sci. Hortic. 2016, 201, 175–183. [Google Scholar] [CrossRef]
- Iglesias, D.J.; Cercós, M.; Colmenero-Flores, J.M.; Naranjo, M.A.; Ríos, G.; Carrera, E.; Ruiz-Rivero, O.; Lliso, I.; Morillon, R.; Tadeo, F.R.; et al. Physiology of citrus fruiting. Braz. J. Plant Physiol. 2007, 19, 333–362. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, G.; Kato, M.; Yamawaki, K.; Takagi, T.; Kiriiwa, Y.; Ikoma, Y.; Matsumoto, H.; Yoshioka, T.; Nesumi, H. Regulation of carotenoid accumulation and the expression of carotenoid metabolic genes in citrus juice sacs in vitro. J. Exp. Bot. 2011, 63, 871–886. [Google Scholar] [CrossRef] [Green Version]
- Neill, S.J.; Horgan, R.; Parry, A.D. The carotenoid and abscisic acid content of viviparous kernels and seedlings of Zea mays L. Planta 1986, 169, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Paiva, R.; Kriz, A. Effect of abscisic acid on embryo-specific gene expression during normal and precocious germination in normal and viviparous maize (Zea mays) embryos. Planta 1994, 192, 332–339. [Google Scholar] [CrossRef]
- Fray, R.G.; Grierson, D. Identification and genetic analysis of normal and mutant phytoene synthase genes of tomato by sequencing, complementation and co-suppression. Plant Mol. Biol. 1993, 22, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Fray, R.G.; Wallace, A.; Fraser, P.; Valero, D.; Hedden, P.; Bramley, P.M.; Grierson, D. Constitutive expression of a fruit phytoene synthase gene in transgenic tomatoes causes dwarfism by redirecting metabolites from the gibberellin pathway. Plant J. 1995, 8, 693–701. [Google Scholar] [CrossRef]
- Talon, M.; Koornneef, M.; Zeevaart, J.A. Endogenous gibberellins in Arabidopsis thaliana and possible steps blocked in the biosynthetic pathways of the semidwarf ga4 and ga5 mutants. Proc. Natl. Acad. Sci. USA 1990, 87, 7983–7987. [Google Scholar]
- Milborrow, B.V. The pathway of biosynthesis of abscisic acid in vascular plants: A review of the present state of knowledge of ABA biosynthesis. J. Exp. Bot. 2001, 52, 1145–1164. [Google Scholar] [CrossRef]
- French, E.; Iyer-Pascuzzi, A.S. A role for the gibberellin pathway in biochar-mediated growth promotion. Sci. Rep. 2018, 8, 5389. [Google Scholar] [CrossRef]
- Macmillan, J. Occurrence of Gibberellins in Vascular Plants, Fungi, and Bacteria. J. Plant Growth Regul. 2001, 20, 387–442. [Google Scholar] [CrossRef]
- Yamaguchi, S. Gibberellin Metabolism and its Regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef]
- Csukasi, F.; Osorio, S.; Gutierrez, J.R.; Kitamura, J.; Giavalisco, P.; Nakajima, M.; Fernie, A.R.; Rathjen, J.P.; Botella, M.A.; Valpuesta, V.; et al. Gibberellin biosynthesis and signalling during development of the strawberry receptacle. New Phytol. 2011, 191, 376–390. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, M.; Wu, W.; Korir, N.K.; Qian, Y.; Wang, Z. Comparative transcriptome analysis of berry-sizing effects of gibberellin (GA3) on seedless Vitis vinifera L. Genes Genom. 2017, 39, 493–507. [Google Scholar] [CrossRef]
- Murcia, G.; Pontin, M.; Piccoli, P. Role of ABA and Gibberellin A3 on gene expression pattern of sugar transporters and invertases in Vitis vinifera cv. Malbec during berry ripening. Plant Growth Regul. 2017, 84, 275–283. [Google Scholar] [CrossRef]
- Fasoli, M.; Santo, S.D.; Zenoni, S.; Tornielli, G.B.; Farina, L.; Zamboni, A.; Porceddu, A.; Venturini, L.; Bicego, M.; Murino, V.; et al. The Grapevine Expression Atlas Reveals a Deep Transcriptome Shift Driving the Entire Plant into a Maturation Program. Plant Cell 2012, 24, 3489–3505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Z.; Su, Z.; Wang, W.; Guan, L.; Bai, Y.; Zhu, X.; Wang, X.; Jia, H.; Fang, J.; Wang, C. Characterization of VvSPL18 and Its Expression in Response to Exogenous Hormones during Grape Berry Development and Ripening. Cytogenet. Genome Res. 2019, 159, 97–108. [Google Scholar] [CrossRef]
- Moyano-Cañete, E.; Bellido, M.L.; García-Caparrós, N.; Medina-Puche, L.; Amil-Ruiz, F.; González-Reyes, J.A.; Caballero, J.L.; Garciía-Limones, C.; Blanco-Portales, R. FaGAST2, a Strawberry Ripening-Related Gene, Acts Together with FaGAST1 to Determine Cell Size of the Fruit Receptacle. Plant Cell Physiol. 2012, 54, 218–236. [Google Scholar] [CrossRef] [Green Version]
- An, L.; Ma, J.; Wang, H.; Li, F.; Qin, D.; Wu, J.; Zhu, G.; Zhang, J.; Yuan, Y.; Zhou, L.; et al. NMR-based global metabolomics approach to decipher the metabolic effects of three plant growth regulators on strawberry maturation. Food Chem. 2018, 269, 559–566. [Google Scholar] [CrossRef]
- Gu, T.; Jia, S.; Huang, X.; Wang, L.; Fu, W.; Huo, G.; Gan, L.; Ding, J.; Li, Y. Transcriptome and hormone analyses provide insights into hormonal regulation in strawberry ripening. Planta 2019, 250, 145–162. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.G.; Hong, Y.; Lee, E.J. Analysis of eight phytohormone concentrations, expression levels of ABA biosynthesis genes, and ripening-related transcription factors during fruit development in strawberry. J. Plant Physiol. 2019, 239, 52–60. [Google Scholar] [CrossRef]
- Symons, G.M.; Chua, Y.-J.; Ross, J.J.; Quittenden, L.J.; Davies, N.W.; Reid, J.B. Hormonal changes during non-climacteric ripening in strawberry. J. Exp. Bot. 2012, 63, 4741–4750. [Google Scholar] [CrossRef] [Green Version]
- Martinez, G.A.; Chaves, A.R.; Añon, M.C. Effect of gibberellic acid on ripening of strawberry fruits (Fragaria annanassa Duch.). J. Plant Growth Regul. 1994, 13, 87–91. [Google Scholar] [CrossRef]
- Martinez, G.A.; Chaves, A.R.; Añon, M.C. Effect of exogenous application of gibberellic acid on color change and phenylalanine ammonia-lyase, chlorophyllase, and peroxidase activities during ripening of strawberry fruit (Fragaria x ananassa Duch.). J. Plant Growth Regul. 1996, 15, 139–146. [Google Scholar] [CrossRef]
- García-Luis, A.; Herrero-Villén, A.; Guardiola, J. Effects of applications of gibberellic acid on late growth, maturation and pigmentation of the Clementine mandarin. Sci. Hortic. 1992, 49, 71–82. [Google Scholar] [CrossRef]
- Alós, E.; Cercós, M.; Rodrigo, M.J.; Zacarías, L.; Talon, M. Regulation of Color Break in Citrus Fruits. Changes in Pigment Profiling and Gene Expression Induced by Gibberellins and Nitrate, Two Ripening Retardants. J. Agric. Food Chem. 2006, 54, 4888–4895. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, M.J.; Zacarias, L. Effect of postharvest ethylene treatment on carotenoid accumulation and the expression of carotenoid biosynthetic genes in the flavedo of orange (Citrus sinensis L. Osbeck) fruit. Postharvest Biol. Technol. 2007, 43, 14–22. [Google Scholar] [CrossRef]
- Gambetta, G.; Martínez-Fuentes, A.; Bentancur, O.; Mesejo, C.; Reig, C.; Gravina, A.; Agustí, M. Hormonal and nutritional changes in the flavedo regulatingrind color development in sweet orange [Citrus sinensis (L.) Osb.]. J. Plant Growth Regul. 2012, 31, 273–282. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Alquézar, B.; Alós, E.; Lado, J.; Zacarías, L. Biochemical bases and molecular regulation of pigmentation in the peel of Citrus fruit. Sci. Hortic. 2013, 163, 46–62. [Google Scholar] [CrossRef]
- Finkelstein, R.; Gibson, S.I. ABA and sugar interactions regulating development: Cross-talk or voices in a crowd? Curr. Opin. Plant Biol. 2002, 5, 26–32. [Google Scholar] [CrossRef]
- Li, Y.; Lee, K.K.; Walsh, S.; Smith, C.; Hadingham, S.; Sorefan, K.; Cawley, G.C.; Bevan, M. Establishing glucose- and ABA-regulated transcription networks in Arabidopsis by microarray analysis and promoter classification using a Relevance Vector Machine. Genome Res. 2006, 16, 414–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dekkers, B.J.W.; Schuurmans, J.A.M.J.; Smeekens, S.C.M. Interaction between sugar and abscisic acid signalling during early seedling development in Arabidopsis. Plant Mol. Biol. 2008, 67, 151–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, C.; Boss, P.K.; Robinson, S.P. Treatment of grape berries, a nonclimacteric fruit with a synthetic auxin, retards ripening and alters the expression of developmentally regulated genes. Plant Physiol. 1997, 115, 1155–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deluc, L.G.; Quilici, D.R.; Decendit, A.; Grimplet, J.; Wheatley, M.D.; Schlauch, K.; Mérillon, J.-M.; Cushman, J.C.; Cramer, G.R. Water deficit alters differentially metabolic pathways affecting important flavor and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC Genom. 2009, 10, 212–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, Z.; Ping, L.; Guanglian, Z.; Xiangxin, L. Cloning and functional analysis of 9-cis-epoxycarotenoid dioxygenase (NCED) genes encoding a key enzyme during abscisic acid biosynthesis from peach and grape fruits. J. Plant Physiol. 2009, 166, 1241–1252. [Google Scholar]
- Gambettaa, G.A.; Matthews, M.A.; Shaghasi, T.H.; McElrone, A.J.; Castellarin, S.D. Sugar and abscisic acid signaling orthologs are activated at the onset of ripening in grape. Planta 2010, 232, 219–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çakir, B.; Agasse, A.; Gaillard, C.; Saumonneau, A.; Delrot, S.; Atanassova, R. A Grape ASR Protein Involved in Sugar and Abscisic Acid Signaling. Plant Cell 2003, 15, 2165–2180. [Google Scholar] [CrossRef] [Green Version]
- Pourtau, N.; Mares, M.; Purdy, S.; Quentin, N.; Ruël, A.; Wingler, A. Interactions of abscisic acid and sugar signaling in the regulation of leaf senescence. Planta 2004, 219, 765–772. [Google Scholar] [CrossRef]
- Hiratsuka, S.; Onodera, H.; Kawai, Y.; Kubo, T.; Itoh, H.; Wada, R. ABA and sugar effects on anthocyanin formation in grape berry cultured in vitro. Sci. Hortic. 2001, 90, 121–130. [Google Scholar] [CrossRef]
- Larronde, F.; Krisa, S.; Decendit, A.; Chèze, C.; Deffieux, G.; Mérillon, J.-M. Regulation of polyphenol production in Vitis vinifera cell suspension cultures by sugars. Plant Cell Rep. 1998, 17, 946–950. [Google Scholar] [CrossRef]
- Matsushima, J.; Hiratsuka, S.; Taniguchi, N.; Wada, R.; Suzaki, N. Anthocyanin accumulation and sugar content in the skin of grape cultivar Olympia treated with ABA. J. Jpn. Soc. Hortic. Sci. 1989, 58, 551–555. [Google Scholar] [CrossRef] [Green Version]
- Pirie, A.; Mullins, M.G. Changes in Anthocyanin and Phenolics Content of Grapevine Leaf and Fruit Tissues Treated with Sucrose, Nitrate, and Abscisic Acid. Plant Physiol. 1976, 58, 468–472. [Google Scholar] [CrossRef] [Green Version]
- Eveland, A.L.; Jackson, D. Sugars, signalling, and plant development. J. Exp. Bot. 2012, 63, 3367–3377. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.A.; Terol, J.; Ibanez, V.; López-García, A.; Pérez-Román, E.; Borredá, C.; Domingo, C.; Tadeo, F.R.; Carbonell-Caballero, J.; Alonso, R.; et al. Genomics of the origin and evolution of Citrus. Nat. Cell Biol. 2018, 554, 311–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alós, E.; Roca, M.; Iglesias, D.J.; Minguez-Mosquera, M.I.; Damasceno, C.M.; Thannhauser, T.W.; Rose, J.K.; Talon, M.; Cercos, M. An evaluation of the basis and consequences of a stay-green mutation in the navel negra (nan) citrus mutant using transcriptomic and proteomic profiling and metabolite analysis. Plant Physiol. 2008, 147, 1300–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peña, L.; Martín-Trillo, M.; Juárez, J.; Pina, J.A.; Navarro, L.; Martínez-Zapater, J.M. Constitutive expression of Arabidopsis LEAFY or APETALA1 genes in citrus reduces their generation time. Nat. Biotechnol. 2001, 19, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Orbovic, V.; Jones, J.B.; Wang, N. Modification of the PthA4 effector binding elements in Type I CsLOB1 promoter using Cas9/sgRNA to produce transgenic Duncan grapefruit alleviating XccDpthA4:dCsLOB1.3 infection. Plant Biotechnol. J. 2016, 14, 1291–1301. [Google Scholar] [CrossRef]
- Dutt, M.; Barthe, G.; Irey, M.; Grosser, J. Transgenic Citrus Expressing an Arabidopsis NPR1 Gene Exhibit Enhanced Resistance against Huanglongbing (HLB.; Citrus Greening). PLoS ONE 2015, 10, e0137134. [Google Scholar] [CrossRef]
- Romero, P.; Rodrigo, M.J.; Alférez, F.; Ballester, A.-R.; González-Candelas, L.; Zacarías, L.; Lafuente, M.T. Unravelling molecular responses to moderate dehydration in harvested fruit of sweet orange (Citrus sinensis L. Osbeck) using a fruit-specific ABA-deficient mutant. J. Exp. Bot. 2012, 63, 2753–2767. [Google Scholar] [CrossRef] [Green Version]
- Romero, P.; Lafuente, M.T.; Alferez, F. A transcriptional approach to unravel the connection between phospholipases A2 and D and ABA signal in citrus under water stress. Plant Physiol. Biochem. 2014, 80, 23–32. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Alquézar, B.; Zacarías, L. Cloning and characterization of two9-cis-epoxycarotenoid dioxygenase genes, differentially regulated during fruitmaturation and under stress conditions, from orange (Citrus sinensis L. Osbeck). J. Exp. Bot. 2006, 57, 633–643. [Google Scholar] [CrossRef] [Green Version]
- Alferez, F.; Zacarias, L. Interaction between ethylene and abscisic acid in the regulation of citrus fruit maturation. In Biology and Biotechnology of the Plant Hormone Ethylene II; Kanellis, A.K., Chang, C., Klee, H., Bleecker, A.B., Pech, J.C., Grierson, D., Eds.; Kluwer Academic Publishers: Doordrecht, The Netherlands, 1999. [Google Scholar]
- Romero, P.; Gandía, M.; Alferez, F. Interplay between ABA and phospholipases A2 and D in the response of citrus fruit to postharvest dehydration. Plant Physiol. Biochem. 2013, 70, 287–294. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alferez, F.; de Carvalho, D.U.; Boakye, D. Interplay between Abscisic Acid and Gibberellins, as Related to Ethylene and Sugars, in Regulating Maturation of Non-Climacteric Fruit. Int. J. Mol. Sci. 2021, 22, 669. https://doi.org/10.3390/ijms22020669
Alferez F, de Carvalho DU, Boakye D. Interplay between Abscisic Acid and Gibberellins, as Related to Ethylene and Sugars, in Regulating Maturation of Non-Climacteric Fruit. International Journal of Molecular Sciences. 2021; 22(2):669. https://doi.org/10.3390/ijms22020669
Chicago/Turabian StyleAlferez, Fernando, Deived Uilian de Carvalho, and Daniel Boakye. 2021. "Interplay between Abscisic Acid and Gibberellins, as Related to Ethylene and Sugars, in Regulating Maturation of Non-Climacteric Fruit" International Journal of Molecular Sciences 22, no. 2: 669. https://doi.org/10.3390/ijms22020669





