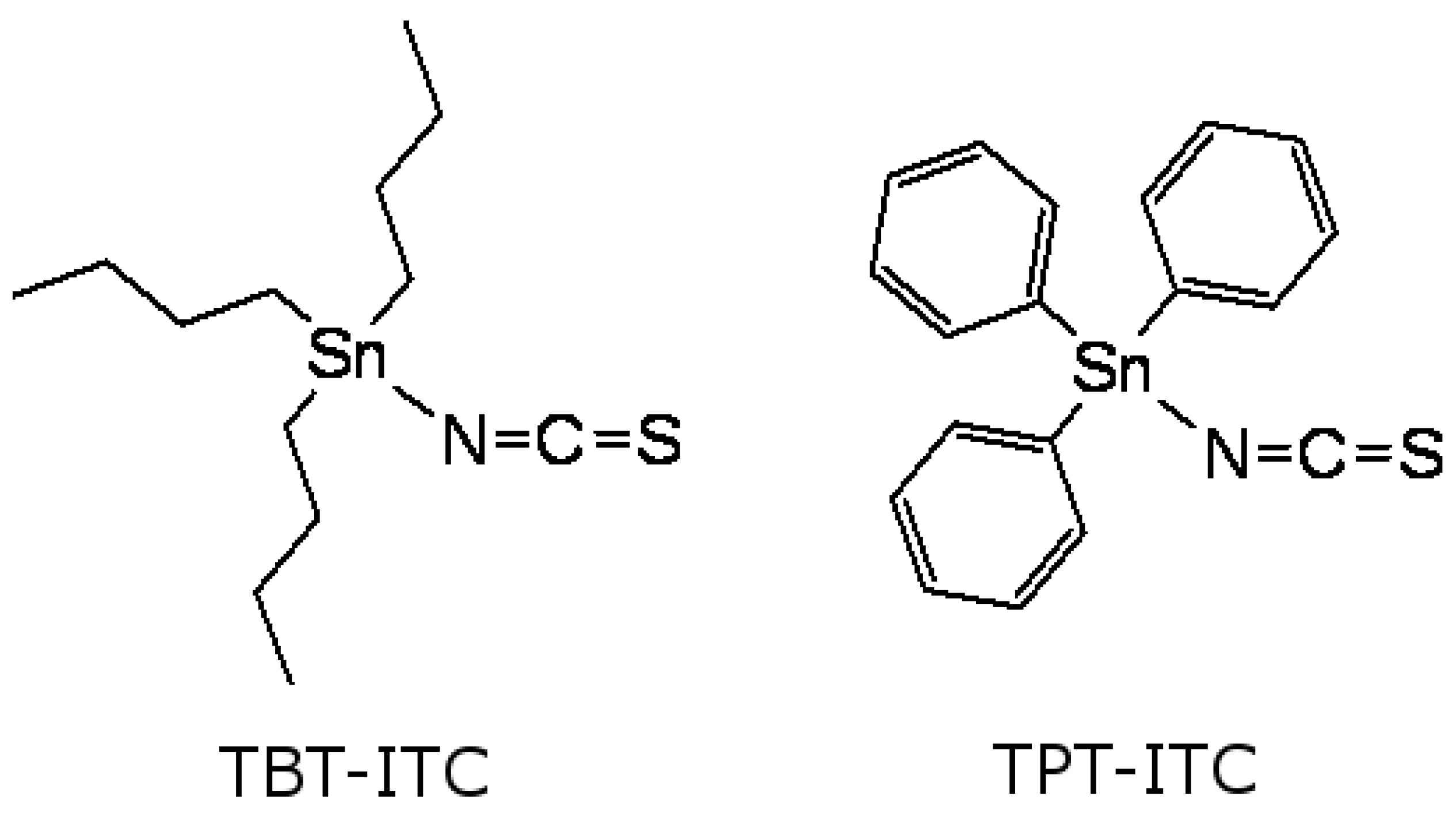
Genotoxic Effects of Tributyltin and Triphenyltin Isothiocyanates, Cognate RXR Ligands: Comparison in Human Breast Carcinoma MCF 7 and MDA-MB-231 Cells
Abstract
1. Introduction
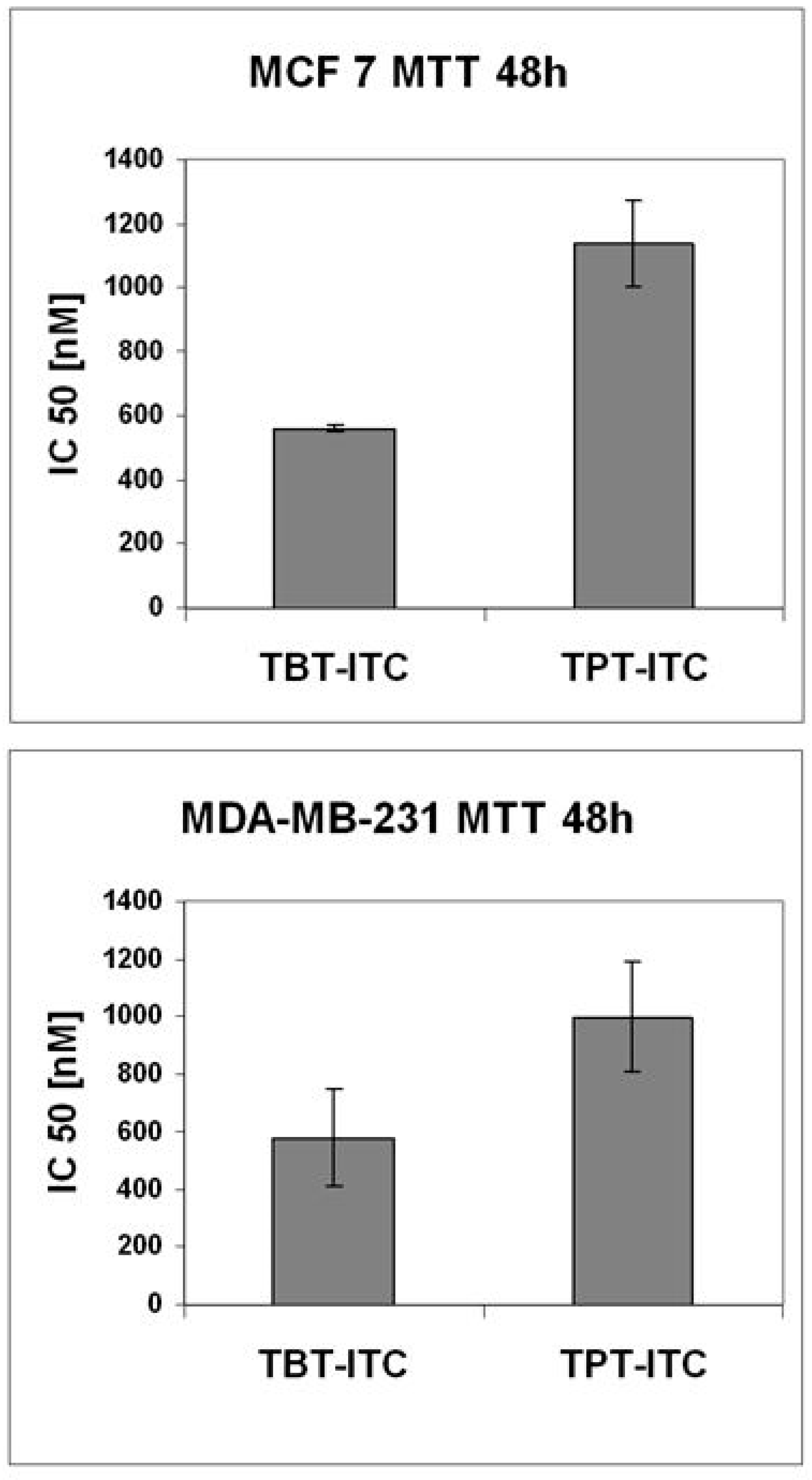
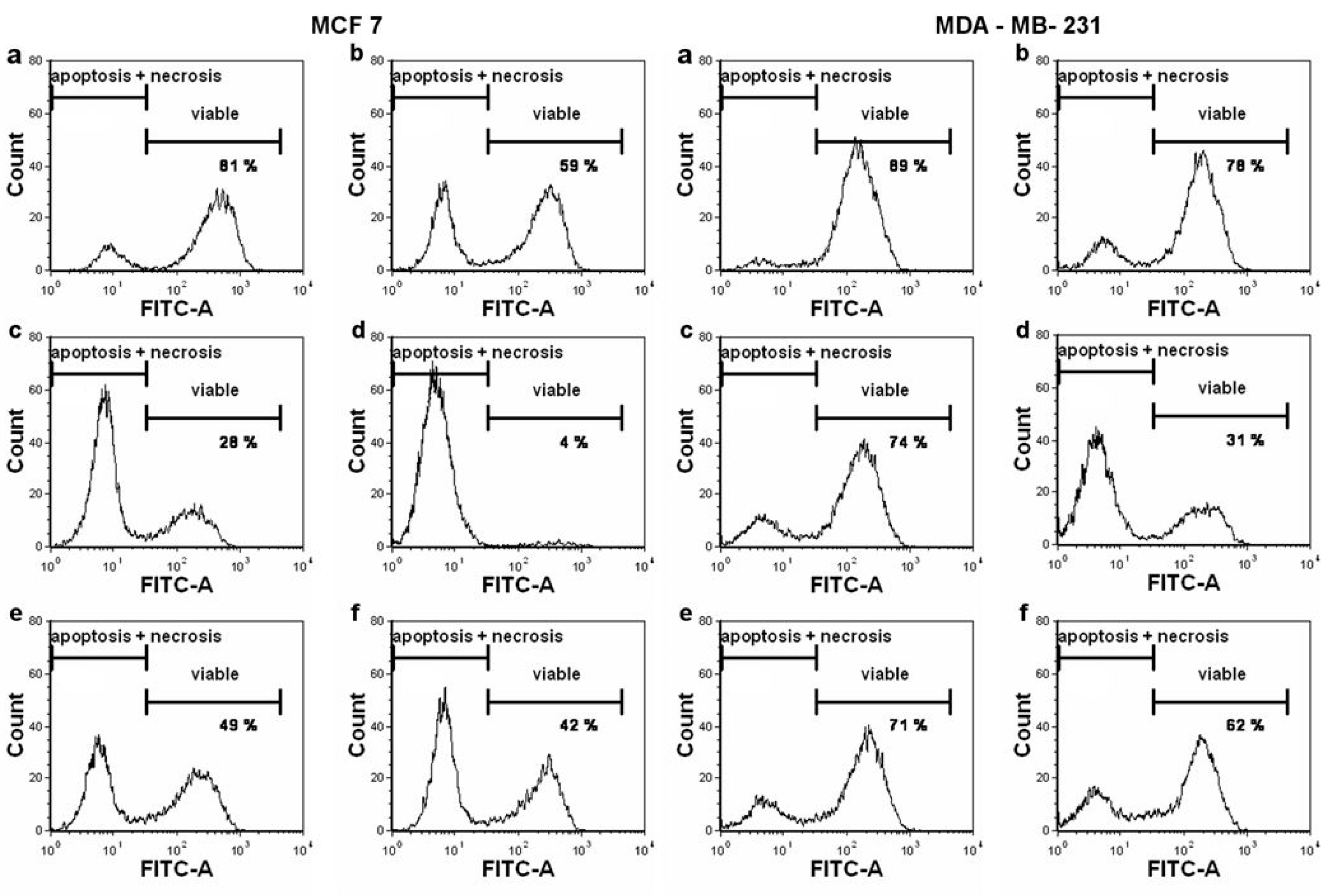
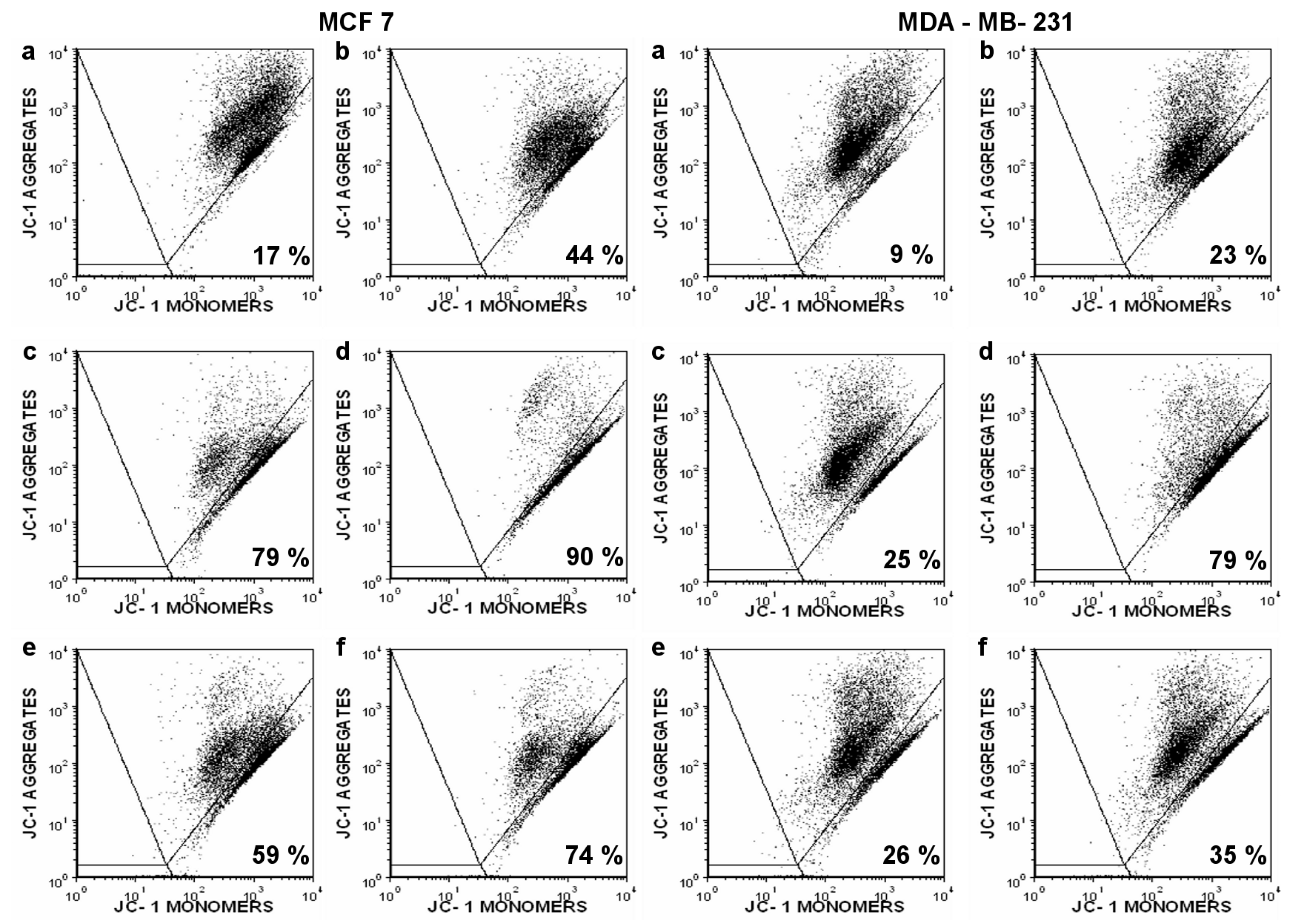
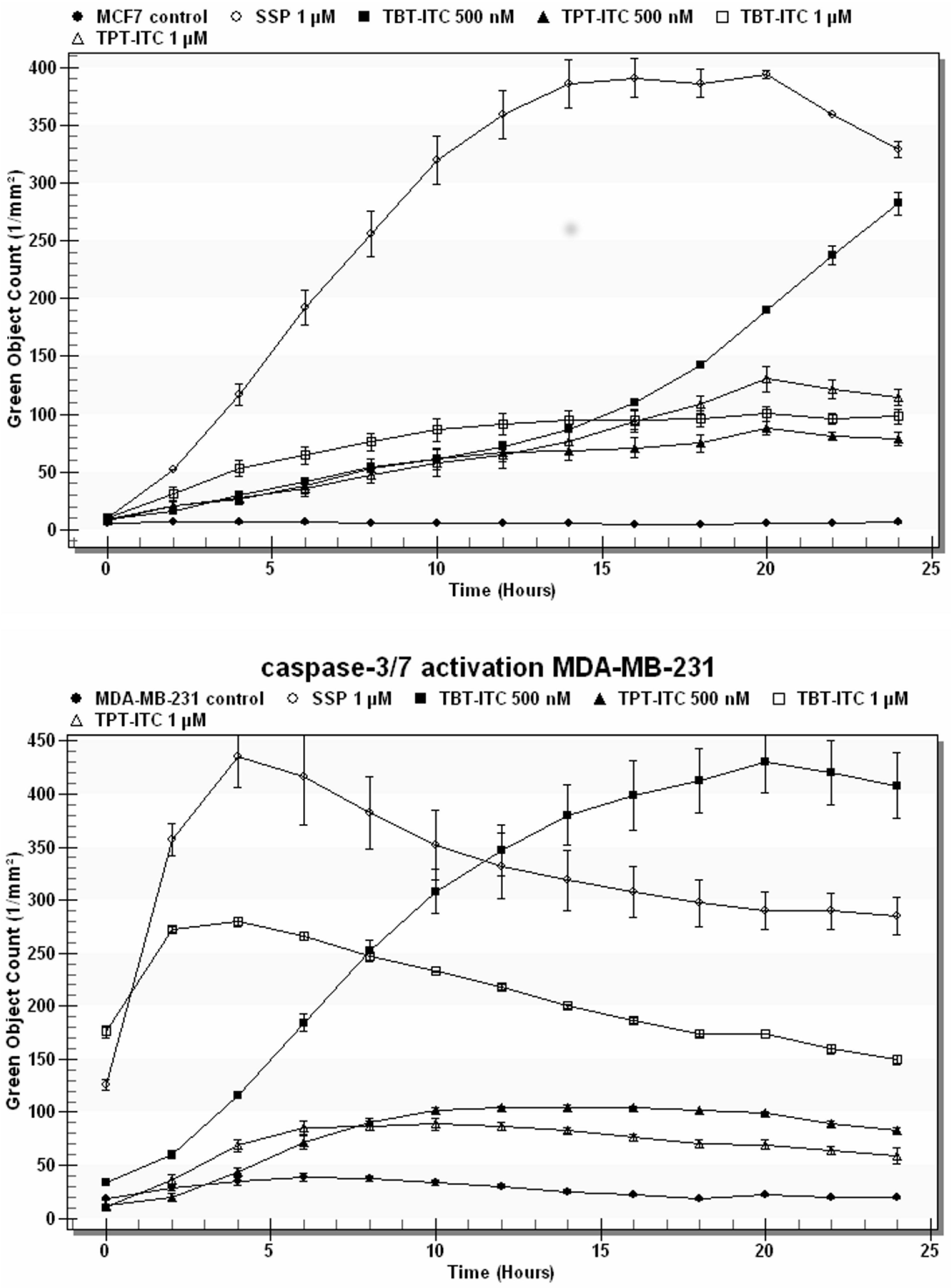
2. Results
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cells and Treatment
4.3. Cytotoxicity Assay
4.4. Alkaline Single-Cell Gel Electrophoresis (Alkaline Comet Assay)
4.5. Flow Cytometry
4.6. Caspase-3/7 Assay
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BSA | Bovine serum albumin |
| cisPt | Cisplatin |
| DMSO | Dimethylsulfoxide |
| EMT | Epithelial‒mesenchymal transition |
| ER | Estrogen receptor |
| EtBr | Ethidium bromide |
| FCS | Fetal calf serum |
| FDA | Fluorescein diacetate |
| FITC | Fluorescein isothiocyanate |
| ITC | Isothiocyanate |
| MCF 7 | Estrogen-receptor-positive human breast cancer cell line |
| MDA-MB-231 | Estrogen-receptor-negative human breast cancer cell line |
| MMP, Δψm | Mitochondrial membrane potential |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide |
| PBS | Phosphate-buffered saline |
| PI | Propidium iodide |
| RA | All-trans retinoic acid |
| RAR | Nuclear all-trans retinoic acid receptor |
| RXR | Nuclear retinoid X receptor |
| SSP | Staurosporin |
| StO | Styrene oxide |
| TBT-Cl | Tributyltin chloride |
| TBT-ITC | Tributyltin isothiocyanate |
| TPT-Cl | Triphenyltin chloride |
| TPT-ITC | Triphenyltin isothiocyanate |
| TX | Taxol |
References
- Nakanishi, T.; Nishikawa, J.; Hiromori, Y.; Yokoyama, H.; Koyanagi, M.; Takasuga, S.; Ishizaki, J.; Watanabe, M.; Isa, S.; Utoguchi, N.; et al. Trialkyltin compounds bind retinoid X receptor to alter human placental endocrine functions. Mol. Endocrinol. 2005, 19, 2502–2516. [Google Scholar] [CrossRef] [PubMed]
- leMaire, A.; Grimaldi, M.; Roecklin, D.; Dagnino, S.; Vivat-Hannah, V.; Balaguer, P.; Bourguet, W. Activation of RXR-PPAR heterodimers by organotin environmental endocrine disruptors. EMBO Rep. 2009, 10, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Toporova, L.; Macejova, D.; Brtko, J. Radioligand binding assay for accurate determination of nuclear retinoid X receptors: A case of triorganotin endocrine disrupting ligands. Toxicol. Lett. 2016, 254, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T. Endocrine disruption induced by organotin compounds: Organotins function as a powerful agonist for nuclear receptors rather than aromatase inhibitor. J. Toxicol. Sci. 2008, 33, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Brtko, J.; Dvorak, Z. Triorganotin compounds—Ligands for “rexinoid” inducible transcription factors: Biological effects. Toxicol. Lett. 2015, 234, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Novotny, L.; Sharaf, L.; Abdel-Hamid, M.E.; Brtko, J. Stability studies of endocrine disrupting tributyltin and triphenyltin compounds in an artificial sea water model. Gen. Physiol. Biophys. 2018, 37, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Macejova, D.; Toporova, L.; Brtko, J. The role of retinoic acid receptors and their cognate ligands in reproduction in a context of triorganotin based endocrine disrupting chemicals. Endocr. Regul. 2016, 50, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Grün, F.; Blumberg, B. Environmental obesogens: Organotins and endocrine disruption via nuclear receptor signaling. Endocrinology 2006, 147, S50–S55. [Google Scholar] [CrossRef]
- Florea, A.M.; Dopp, E.; Obe, G. Genotoxicity of organometallic species. In Organic Metal and Metalloid Species in the Environment: Analysis, Distribution, Processes and Toxicology Evaluation; Springer: Heidelberg, Germany, 2004; pp. 205–219. [Google Scholar]
- Zuo, Z.; Wang, C.; Wu, M.; Wang, Y.; Cheng, Y. Exposure to tributyltin and triphenyltin induces DNA damage and alters nucleotide excision repair gene transcription in Sebastiscus marmoratus liver. Aquat. Toxicol. 2012, 122–123, 106–112. [Google Scholar] [CrossRef]
- Alama, A.; Tasso, B.; Novelli, F.; Sparatore, F. Organometallic compounds in oncology: Implications of novel organotins as antitumor agents. Drug Discov. Today 2009, 14, 500–508. [Google Scholar] [CrossRef]
- Barbieri, F.; Viale, M.; Sparatore, F.; Schettini, G.; Favre, A.; Bruzzo, C.; Novelli, F.; Alama, A. Antitumor activity of a new orally active organotin compound: A preliminary study in murine tumor models. Anticancer Drugs 2002, 13, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Hunakova, L.; Macejova, D.; Toporova, L.; Brtko, J. Anticancer effects of tributyltin chloride and triphenyltin chloride in human breast cancer cell lines MCF 7 and MDA-MB-231. Tumor Biol. 2016, 37, 6701–6708. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Blanco, L.; Garrido, A.; Vieites, J.M.; Cabado, A.G. In vitro approaches to evaluate toxicity induced by organotin compounds tributyltin (TBT), dibutyltin (DBT), and monobutyltin (MBT) in neuroblastoma cells. J. Agric. Food Chem. 2013, 61, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Botelho, G.; Bernardini, C.; Zannoni, A.; Ventrella, V.; Bacci, M.L.; Forni, M. Effect of tributyltin on mammalian endothelial cell integrity. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2015, 176–177, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, S.; Pettinary, C. Chemical and biotechnological developments in organotin cancer chemotherapy. J. Organomet. Chem. 2006, 691, 1761–1766. [Google Scholar] [CrossRef]
- Hunakova, L.; Brtko, J. Sn- and Ge-triorganometallics exert different cytotoxicity and modulation of migration in triple-negative breast cancer cell line MDA-MB-231. Toxicol. Lett. 2017, 279, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Brtko, J.; Dvorak, Z. Nuclear retinoid/retinoid X receptors and their endogenous and xenobiotic ligands in metabolism, differentiation and cancer treatment. Toxicol. Lett. 2014, 229, 5–6. [Google Scholar] [CrossRef]
- Macejova, D.; Toporova, L.; Brtko, J. Effects of natural ligands and synthetic triorganotin compounds of nuclear retinoid X receptors in human MCF 7 breast cancer cell line. Gen. Physiol. Biophys. 2017, 36, 481–484. [Google Scholar] [CrossRef]
- Gupta, P.; Kim, B.; Kim, S.H.; Srivastava, S.K. Molecular targets of isothiocyanates in cancer: Recent advances. Mol. Nutr. Food Res. 2014, 58, 1685–1707. [Google Scholar] [CrossRef]
- Hunakova, L.; Gronesova, P.; Horvathova, E.; Chalupa, I.; Cholujova, D.; Duraj, J.; Sedlak, J. Modulation of cisplatin sensitivity in human ovarian carcinoma A2780 and SKOV3 cell lines by sulforaphane. Toxicol. Lett. 2014, 230, 479–486. [Google Scholar] [CrossRef]
- Bodo, J.; Hunakova, L.; Kvasnicka, P.; Jakubikova, J.; Duraj, J.; Kasparkova, J.; Sedlak, J. Sensitization for cisplatin-induced apoptosis by isothiocyanate E-4IB leads to signaling pathways alterations. Br. J. Cancer 2006, 95, 1348–1353. [Google Scholar] [CrossRef] [PubMed]
- Stehlik, P.; Paulikova, H.; Hunakova, L. Synthetic isothiocyanate indole-3-ethyl isothiocyanate (homoITC) enhances sensitivity of human ovarian carcinoma cell lines A2780 and A2780/CP to cisplatin. Neoplasma 2010, 57, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Hunakova, L.; Sedlakova, O.; Cholujova, D.; Gronesova, P.; Duraj, J.; Sedlak, J. Modulation of markers associated with aggressive phenotype in MDA-MB-231 breast carcinoma cells by sulforaphane. Neoplasma 2009, 56, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Metsios, A.; Verginadis, I.; Simos, Y.; Batistatou, A.; Peschos, D.; Ragos, V.; Vezyraki, P.; Evangelou, A.; Karkabounas, S. Cytotoxic and anticancer effects of the triorganotin compound [(C6H5)3Sn(cmbzt)]: An in vitro, ex vivo and in vivo study. Eur. J. Pharm. Sci. 2012, 47, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Bohacova, V.; Seres, M.; Pavlikova, L.; Kontar, S.; Cagala, M.; Bobal, P.; Otevrel, J.; Brtko, J.; Sulova, Z.; Breier, A. Triorganotin derivatives induce cell death effects on L1210 leukemia cells at submicromolar concentrations independently of P-glycoprotein expression. Molecules 2018, 23, 1053. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, Y.; Costa, S.; Collins, A.R.; Azqueta, A. The comet assay, DNA damage, DNA repair and cytotoxicity: Hedgehogs are not always dead. Mutagenesis 2013, 28, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Unger, F.T.; Klasen, H.A.; Tchartchian, G.; de Wilde, R.L.; Witte, I. DNA damage induced by cis- and carboplatin as indicator for in vitro sensitivity of ovarian carcinoma cells. BMC Cancer 2009, 9, 359. [Google Scholar] [CrossRef]
- Plunkett, W.; Huang, P.; Searcy, C.E.; Gandhi, V. Gemcitabine: Pharmacology and mechanisms of action. Sem. Oncol. 1996, 23, 3–15. [Google Scholar]
- Gewirtz, D.A. A Critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol. 1999, 57, 727–741. [Google Scholar] [CrossRef]
- You, M.K.; Kim, H.J.; Kook, J.H.; Kim, H.A.S. John’s wort regulates proliferation and apoptosis in MCF 7 human breast cancer cells by inhibiting AMPK/mTOR and activating the mitochondrial pathway. Int. J. Mol. Sci. 2018, 19, 966. [Google Scholar] [CrossRef]
- Arcidiacono, P.; Ragonese, F.; Stabile, A.; Pistilli, A.; Kuligina, E.; Rende, M.; Bottoni, U.; Calvieri, S.; Crisanti, A.; Spaccapelo, R. Antitumor activity and expression profiles of genes induced by sulforaphane in human melanoma cells. Eur. J. Nutr. 2018, 57, 2547–2569. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, R. The reactions of organotin chlorides with the cyanodithioimidocarbonate anion. J. Org. Chem. 1968, 33, 3896–3900. [Google Scholar] [CrossRef]
- Wharf, I. Studies in aryltin chemistry. Part 5. Tin-119 and carbon-13 NMR spectra of some tetra- and triaryltin compounds. Inorg. Chim. Acta 1989, 159, 41–48. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Slamenova, D.; Gabelova, A.; Ruzekova, L.; Chalupa, I.; Horvathova, E.; Farkasova, T.; Bozsakyova, E.; Stetina, R. Detection of MNNG-induced DNA lesions in mammalian cells: Validation of comet assay against DNA unwinding technique, alkaline elution of DNA and chromosomal aberrations. Mutat. Res. 1997, 383, 243–252. [Google Scholar] [CrossRef]
- Bartkowiak, D.; Hogner, S.; Baust, H.; Nothdurft, W.; Rottinger, E.M. Comparative analysis of apoptosis in HL60 detected by annexin V and fluorescein diacetate. Cytometry 1999, 37, 191–196. [Google Scholar] [CrossRef]
- Haugland, R.P.; Spence, M.T.Z.; Johnson, I.D. Handbook of Fluorescent Probes & Research Chemicals; Molecular Probes Inc.: Eugene, OR, USA, 1996; p. 269. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hunakova, L.; Horvathova, E.; Majerova, K.; Bobal, P.; Otevrel, J.; Brtko, J. Genotoxic Effects of Tributyltin and Triphenyltin Isothiocyanates, Cognate RXR Ligands: Comparison in Human Breast Carcinoma MCF 7 and MDA-MB-231 Cells. Int. J. Mol. Sci. 2019, 20, 1198. https://doi.org/10.3390/ijms20051198
Hunakova L, Horvathova E, Majerova K, Bobal P, Otevrel J, Brtko J. Genotoxic Effects of Tributyltin and Triphenyltin Isothiocyanates, Cognate RXR Ligands: Comparison in Human Breast Carcinoma MCF 7 and MDA-MB-231 Cells. International Journal of Molecular Sciences. 2019; 20(5):1198. https://doi.org/10.3390/ijms20051198
Chicago/Turabian StyleHunakova, Luba, Eva Horvathova, Karolina Majerova, Pavel Bobal, Jan Otevrel, and Julius Brtko. 2019. "Genotoxic Effects of Tributyltin and Triphenyltin Isothiocyanates, Cognate RXR Ligands: Comparison in Human Breast Carcinoma MCF 7 and MDA-MB-231 Cells" International Journal of Molecular Sciences 20, no. 5: 1198. https://doi.org/10.3390/ijms20051198
APA StyleHunakova, L., Horvathova, E., Majerova, K., Bobal, P., Otevrel, J., & Brtko, J. (2019). Genotoxic Effects of Tributyltin and Triphenyltin Isothiocyanates, Cognate RXR Ligands: Comparison in Human Breast Carcinoma MCF 7 and MDA-MB-231 Cells. International Journal of Molecular Sciences, 20(5), 1198. https://doi.org/10.3390/ijms20051198




