The Present and Future of Prostate Cancer Urine Biomarkers
Abstract
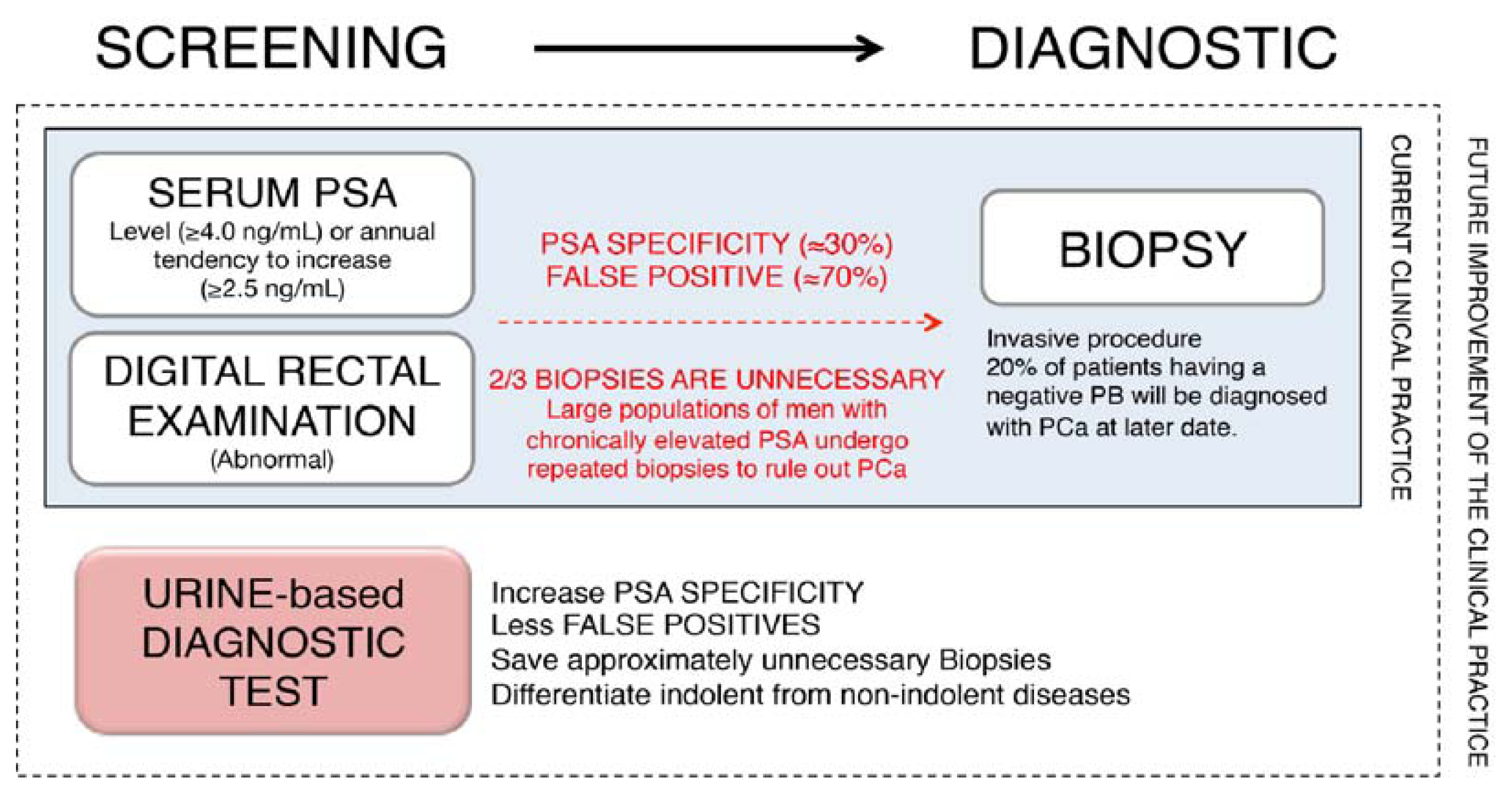
:1. Introduction
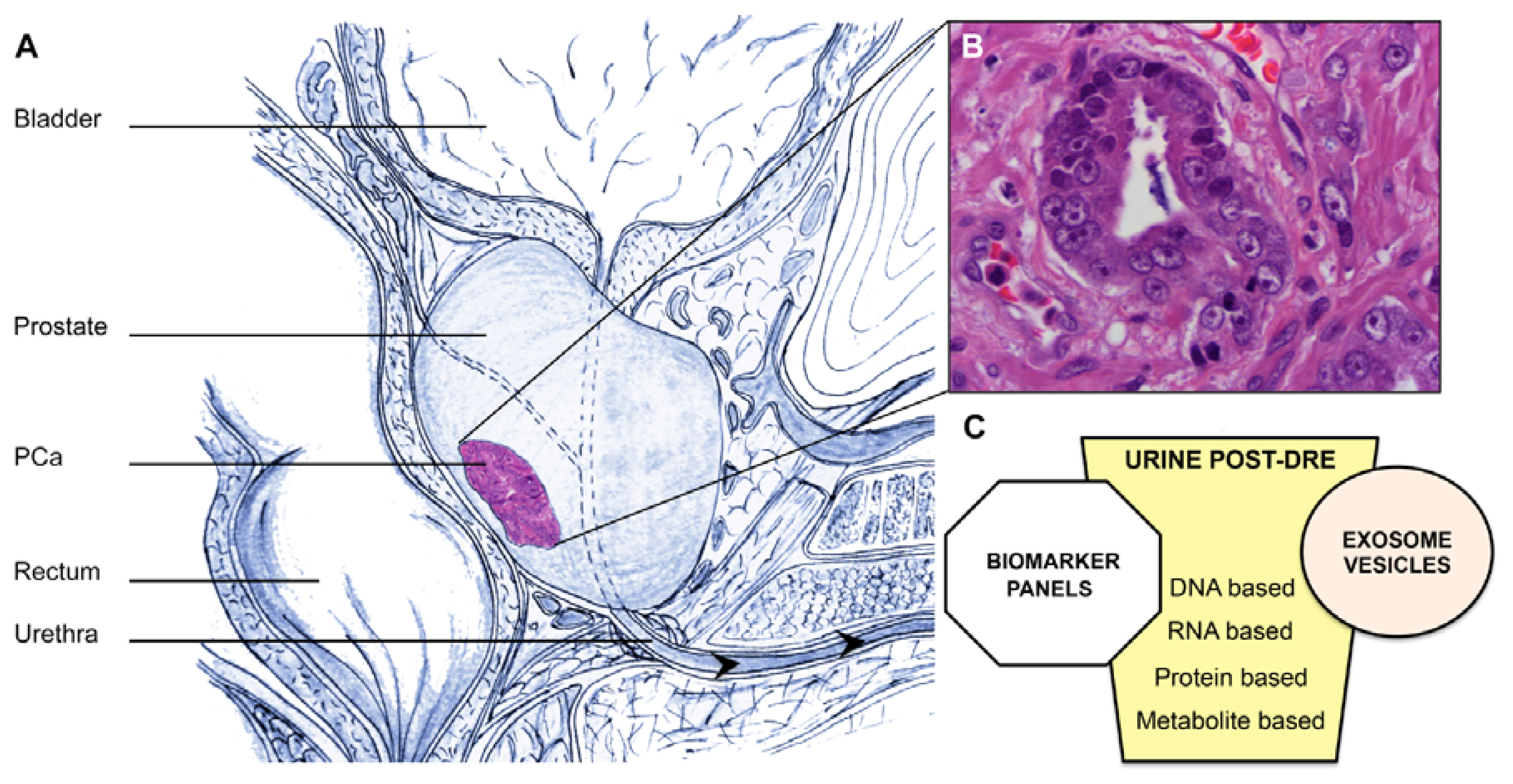
2. Urine: A Source of Prostate Cancer Biomarkers
2.1. DNA-Based Urinary Biomarkers
2.2. RNA-Based Urine Biomarkers
2.3. miRNA-Based Urine Biomarkers
2.4. Protein-Based Urine Biomarkers
2.5. Metabolite-Based Urine Biomarkers
2.6. Urine Biomarker Panels
2.7. Exosomes as a Source of Urine Biomarkers
3. Conclusions
Acknowledgments
Conflict of Interest
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin 2013, 63, 11–30. [Google Scholar]
- Ferlay, J.; Parkin, D.M.; Steliarova-Foucher, E. Estimates of cancer incidence and mortality in Europe in 2008. Eur. J. Cancer 2010, 46, 765–781. [Google Scholar]
- Strope, S.A.; Andriole, G.L. Prostate cancer screening: Current status and future perspectives. Nat. Rev. Urol 2010, 7, 487–493. [Google Scholar]
- Bretton, P.R. Prostate-specific antigen and digital rectal examination in screening for prostate cancer: A community-based study. South Med. J 1994, 87, 720–723. [Google Scholar]
- Catalona, W.J.; Richie, J.P.; Ahmann, F.R.; Hudson, M.A.; Scardino, P.T.; Flanigan, R.C.; deKernion, J.B.; Ratliff, T.L.; Kavoussi, L.R.; Dalkin, B.L.; et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: Results of a multicenter clinical trial of 6630 men. J. Urol 1994, 151, 1283–1290. [Google Scholar]
- Catalona, W.J.; Smith, D.S.; Ratliff, T.L.; Dodds, K.M.; Coplen, D.E.; Yuan, J.J.; Petros, J.A.; Andriole, G.L. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N. Engl. J. Med 1991, 324, 1156–1161. [Google Scholar]
- Tuma, R.S. New tests for prostate cancer may be nearing the clinic. J. Natl. Cancer Inst 2010, 102, 752–754. [Google Scholar]
- Thompson, I.M.; Ankerst, D.P.; Chi, C.; Lucia, M.S.; Goodman, P.J.; Crowley, J.J.; Parnes, H.L.; Coltman, C.A., Jr. Operating characteristics of prostate-specific antigen in men with an initial PSA level of 3.0 ng/mL or lower. JAMA 2005, 294, 66–70. [Google Scholar]
- Thompson, I.M.; Pauler, D.K.; Goodman, P.J.; Tangen, C.M.; Lucia, M.S.; Parnes, H.L.; Minasian, L.M.; Ford, L.G.; Lippman, S.M.; Crawford, E.D.; et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N. Engl. J. Med 2004, 350, 2239–2246. [Google Scholar]
- Schroder, F.H.; van der Cruijsen-Koeter, I.; de Koning, H.J.; Vis, A.N.; Hoedemaeker, R.F.; Kranse, R. Prostate cancer detection at low prostate specific antigen. J. Urol 2000, 163, 806–812. [Google Scholar]
- Gerszten, R.E.; Wang, T.J. The search for new cardiovascular biomarkers. Nature 2008, 451, 949–952. [Google Scholar]
- Boja, E.; Hiltke, T.; Rivers, R.; Kinsinger, C.; Rahbar, A.; Mesri, M.; Rodriguez, H. Evolution of Clinical Proteomics and its Role in Medicine. J. Proteome Res 2010, 10, 66–84. [Google Scholar]
- Anderson, L. Candidate-based proteomics in the search for biomarkers of cardiovascular disease. J. Physiol 2005, 563, 23–60. [Google Scholar]
- Drake, R.R.; White, K.Y.; Fuller, T.W.; Igwe, E.; Clements, M.A.; Nyalwidhe, J.O.; Given, R.W.; Lance, R.S.; Semmes, O.J. Clinical collection and protein properties of expressed prostatic secretions as a source for biomarkers of prostatic disease. J. Proteomics 2009, 72, 907–917. [Google Scholar]
- Duijvesz, D.; Luider, T.; Bangma, C.H.; Jenster, G. Exosomes as biomarker treasure chests for prostate cancer. Eur. Urol 2011, 59, 823–831. [Google Scholar]
- Foot, N.C.; Papanicolaou, G.N.; Holmquist, N.D.; Seybolt, J.F. Exfoliative cytology of urinary sediments; a review of 2829 cases. Cancer 1958, 11, 127–137. [Google Scholar]
- Krishnan, B.; Truong, L.D. Prostatic adenocarcinoma diagnosed by urinary cytology. Am. J. Clin. Pathol 2000, 113, 29–34. [Google Scholar]
- Rifai, N.; Gillette, M.A.; Carr, S.A. Protein biomarker discovery and validation: The long and uncertain path to clinical utility. Nat. Biotechnol 2006, 24, 971–983. [Google Scholar]
- You, J.; Cozzi, P.; Walsh, B.; Willcox, M.; Kearsley, J.; Russell, P.; Li, Y. Innovative biomarkers for prostate cancer early diagnosis and progression. Crit. Rev. Oncol. Hematol 2010, 73, 10–22. [Google Scholar]
- Zehentner, B.K.; Secrist, H.; Zhang, X.; Hayes, D.C.; Ostenson, R.; Goodman, G.; Xu, J.; Kiviat, M.; Kiviat, N.; Persing, D.H.; et al. Detection of alpha-methylacyl-coenzyme-A racemase transcripts in blood and urine samples of prostate cancer patients. Mol. Diagn Ther 2006, 10, 397–403. [Google Scholar]
- Sreekumar, A.; Laxman, B.; Rhodes, D.R.; Bhagavathula, S.; Harwood, J.; Giacherio, D.; Ghosh, D.; Sanda, M.G.; Rubin, M.A.; Chinnaiyan, A.M. Humoral immune response to alpha-methylacyl-CoA racemase and prostate cancer. J. Natl. Cancer Inst. 2004, 9(6), 834–843. [Google Scholar]
- Pisitkun, T.; Shen, R.F.; Knepper, M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar]
- Wozny, W.; Schroer, K.; Schwall, G.P.; Poznanovic, S.; Stegmann, W.; Dietz, K.; Rogatsch, H.; Schaefer, G.; Huebl, H.; Klocker, H.; et al. Differential radioactive quantification of protein abundance ratios between benign and malignant prostate tissues: Cancer association of annexin A3. Proteomics 2007, 7, 313–322. [Google Scholar]
- Gerke, V.; Creutz, C.E.; Moss, S.E. Annexins: Linking Ca2+ signalling to membrane dynamics. Nat. Rev. Mol. Cell Biol 2005, 6, 449–461. [Google Scholar]
- Chen, Y.; Li, J.; Yu, X.; Li, S.; Zhang, X.; Mo, Z.; Hu, Y. APC gene hypermethylation and prostate cancer: A systematic review and meta-analysis. Eur. J. Hum. Genet. 2013. [Google Scholar] [CrossRef]
- Foley, R.; Marignol, L.; Keane, J.P.; Lynch, T.H.; Hollywood, D. Androgen hypersensitivity in prostate cancer: Molecular perspectives on androgen deprivation therapy strategies. Prostate 2010, 71, 550–557. [Google Scholar]
- Massie, C.E.; Lynch, A.; Ramos-Montoya, A.; Boren, J.; Stark, R.; Fazli, L.; Warren, A.; Scott, H.; Madhu, B.; Sharma, N.; et al. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J 2011, 30, 2719–2733. [Google Scholar]
- Hu, R.; Isaacs, W.B.; Luo, J. A snapshot of the expression signature of androgen receptor splicing variants and their distinctive transcriptional activities. Prostate 2011, 71, 1656–1667. [Google Scholar]
- Gorlov, I.P.; Sircar, K.; Zhao, H.; Maity, S.N.; Navone, N.M.; Gorlova, O.Y.; Troncoso, P.; Pettaway, C.A.; Byun, J.Y.; Logothetis, C.J. Prioritizing genes associated with prostate cancer development. BMC Cancer 2010, 10, 599. [Google Scholar]
- Aparicio, A.; Logothetis, C.J.; Maity, S.N. Understanding the lethal variant of prostate cancer: Power of examining extremes. Cancer Discov 2011, 1, 466–468. [Google Scholar]
- Mosquera, J.M.; Beltran, H.; Park, K.; MacDonald, T.Y.; Robinson, B.D.; Tagawa, S.T.; Perner, S.; Bismar, T.A.; Erbersdobler, A.; Dhir, R.; et al. Concurrent AURKA and MYCN gene amplifications are harbingers of lethal treatment-related neuroendocrine prostate cancer. Neoplasia 2013, 15, 1–10. [Google Scholar]
- Katafigiotis, I.; Tyritzis, S.I.; Stravodimos, K.G.; Alamanis, C.; Pavlakis, K.; Vlahou, A.; Makridakis, M.; Katafigioti, A.; Garbis, S.D.; Constantinides, C.A. Zinc alpha2-glycoprotein as a potential novel urine biomarker for the early diagnosis of prostate cancer. BJU Int 2012, 11, 688–693. [Google Scholar]
- Yip, P.Y.; Kench, J.G.; Rasiah, K.K.; Benito, R.P.; Lee, C.S.; Stricker, P.D.; Henshall, S.M.; Sutherland, R.L.; Horvath, L.G. Low AZGP1 expression predicts for recurrence in margin-positive, localized prostate cancer. Prostate 2011, 71, 1638–1645. [Google Scholar]
- Bondar, O.P.; Barnidge, D.R.; Klee, E.W.; Davis, B.J.; Klee, G.G. LC-MS/MS quantification of Zn-alpha2 glycoprotein: A potential serum biomarker for prostate cancer. Clin. Chem 2007, 53, 673–678. [Google Scholar]
- Palanisamy, N.; Ateeq, B.; Kalyana-Sundaram, S.; Pflueger, D.; Ramnarayanan, K.; Shankar, S.; Han, B.; Cao, Q.; Cao, X.; Suleman, K.; et al. Rearrangements of the RAF kinase pathway in prostate cancer, gastric cancer and melanoma. Nat. Med 2010, 16, 793–798. [Google Scholar]
- Wang, J.; Kobayashi, T.; Floc’h, N.; Kinkade, C.W.; Aytes, A.; Dankort, D.; Lefebvre, C.; Mitrofanova, A.; Cardiff, R.D.; McMahon, M.; et al. B-Raf activation cooperates with PTEN loss to drive c-Myc expression in advanced prostate cancer. Cancer Res 2012, 72, 4765–4776. [Google Scholar]
- Ren, G.; Liu, X.; Mao, X.; Zhang, Y.; Stankiewicz, E.; Hylands, L.; Song, R.; Berney, D.M.; Clark, J.; Cooper, C.; et al. Identification of frequent BRAF copy number gain and alterations of RAF genes in Chinese prostate cancer. Genes Chromosomes Cancer 2012, 51, 1014–1023. [Google Scholar]
- Tamura, K.; Furihata, M.; Tsunoda, T.; Ashida, S.; Takata, R.; Obara, W.; Yoshioka, H.; Daigo, Y.; Nasu, Y.; Kumon, H.; et al. Molecular features of hormone-refractory prostate cancer cells by genome-wide gene expression profiles. Cancer Res 2007, 67, 5117–5125. [Google Scholar]
- Karacosta, L.G.; Foster, B.A.; Azabdaftari, G.; Feliciano, D.M.; Edelman, A.M. A regulatory feedback loop between Ca2+/calmodulin-dependent protein kinase kinase 2 (CaMKK2) and the androgen receptor in prostate cancer progression. J. Biol. Chem 2012, 287, 24832–24843. [Google Scholar]
- Shima, T.; Mizokami, A.; Miyagi, T.; Kawai, K.; Izumi, K.; Kumaki, M.; Ofude, M.; Zhang, J.; Keller, E.T.; Namiki, M. Down-regulation of calcium/calmodulin-dependent protein kinase kinase 2 by androgen deprivation induces castration-resistant prostate cancer. Prostate 2012, 72, 1789–1801. [Google Scholar]
- Umbas, R.; Isaacs, W.B.; Bringuier, P.P.; Schaafsma, H.E.; Karthaus, H.F.; Oosterhof, G.O.; Debruyne, F.M.; Schalken, J.A. Decreased E-cadherin expression is associated with poor prognosis in patients with prostate cancer. Cancer Res 1994, 54, 3929–3933. [Google Scholar]
- Umbas, R.; Schalken, J.A.; Aalders, T.W.; Carter, B.S.; Karthaus, H.F.; Schaafsma, H.E.; Debruyne, F.M.; Isaacs, W.B. Expression of the cellular adhesion molecule E-cadherin is reduced or absent in high-grade prostate cancer. Cancer Res 1992, 52, 5104–5109. [Google Scholar]
- Chi, K.N.; Zoubeidi, A.; Gleave, M.E. Custirsen (OGX-011): A second-generation antisense inhibitor of clusterin for the treatment of cancer. Expert Opin. Investig Drugs 2008, 17, 1955–1962. [Google Scholar]
- Chen, M.; Wang, K.; Zhang, L.; Li, C.; Yang, Y. The discovery of putative urine markers for the specific detection of prostate tumor by integrative mining of public genomic profiles. PLoS One 2011, 6, e28552. [Google Scholar]
- Hosseini-Beheshti, E.; Pham, S.; Adomat, H.; Li, N.; Tomlinson Guns, E.S. Exosomes as biomarker enriched microvesicles: Characterization of exosomal proteins derived from a panel of prostate cell lines with distinct AR phenotypes. Mol. Cell Proteomics 2011, 11, 863–885. [Google Scholar]
- Lamoureux, F.; Thomas, C.; Yin, M.J.; Kuruma, H.; Beraldi, E.; Fazli, L.; Zoubeidi, A.; Gleave, M.E. Clusterin inhibition using OGX-011 synergistically enhances Hsp90 inhibitor activity by suppressing the heat shock response in castrate-resistant prostate cancer. Cancer Res 2011, 71, 5838–5849. [Google Scholar]
- Bjartell, A.S.; Al-Ahmadie, H.; Serio, A.M.; Eastham, J.A.; Eggener, S.E.; Fine, S.W.; Udby, L.; Gerald, W.L.; Vickers, A.J.; Lilja, H.; et al. Association of cysteine-rich secretory protein 3 and beta-microseminoprotein with outcome after radical prostatectomy. Clin. Cancer Res 2007, 13, 4130–4138. [Google Scholar]
- Bjartell, A.; Johansson, R.; Bjork, T.; Gadaleanu, V.; Lundwall, A.; Lilja, H.; Kjeldsen, L.; Udby, L. Immunohistochemical detection of cysteine-rich secretory protein 3 in tissue and in serum from men with cancer or benign enlargement of the prostate gland. Prostate 2006, 66, 591–603. [Google Scholar]
- Dhir, R.; Vietmeier, B.; Arlotti, J.; Acquafondata, M.; Landsittel, D.; Masterson, R.; Getzenberg, R.H. Early identification of individuals with prostate cancer in negative biopsies. J. Urol 2004, 171, 1419–1423. [Google Scholar]
- Paul, B.; Dhir, R.; Landsittel, D.; Hitchens, M.R.; Getzenberg, R.H. Detection of prostate cancer with a blood-based assay for early prostate cancer antigen. Cancer Res 2005, 65, 4097–4100. [Google Scholar]
- Leman, E.S.; Cannon, G.W.; Trock, B.J.; Sokoll, L.J.; Chan, D.W.; Mangold, L.; Partin, A.W.; Getzenberg, R.H. EPCA-2: A highly specific serum marker for prostate cancer. Urology 2007, 69, 714–720. [Google Scholar]
- Horoszewicz, J.S.; Kawinski, E.; Murphy, G.P. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res 1987, 7, 927–935. [Google Scholar]
- Zhang, Y.; Guo, Z.; Du, T.; Chen, J.; Wang, W.; Xu, K.; Lin, T.; Huang, H. Prostate specific membrane antigen (PSMA): A novel modulator of p38 for proliferation, migration, and survival in prostate cancer cells. Prostate 2012, 73, 835–841. [Google Scholar]
- Laxman, B.; Morris, D.S.; Yu, J.; Siddiqui, J.; Cao, J.; Mehra, R.; Lonigro, R.J.; Tsodikov, A.; Wei, J.T.; Tomlins, S.A.; et al. A first-generation multiplex biomarker analysis of urine for the early detection of prostate cancer. Cancer Res. 2008, 68, 645–649. [Google Scholar]
- Varambally, S.; Laxman, B.; Mehra, R.; Cao, Q.; Dhanasekaran, S.M.; Tomlins, S.A.; Granger, J.; Vellaichamy, A.; Sreekumar, A.; Yu, J.; et al. Golgi protein GOLM1 is a tissue and urine biomarker of prostate cancer. Neoplasia 2008, 10, 1285–1294. [Google Scholar]
- Gonzalgo, M.L.; Nakayama, M.; Lee, S.M.; de Marzo, A.M.; Nelson, W.G. Detection of GSTP1 methylation in prostatic secretions using combinatorial MSP analysis. Urology 2004, 63, 414–418. [Google Scholar]
- Crocitto, L.E.; Korns, D.; Kretzner, L.; Shevchuk, T.; Blair, S.L.; Wilson, T.G.; Ramin, S.A.; Kawachi, M.H.; Smith, S.S. Prostate cancer molecular markers GSTP1 and hTERT in expressed prostatic secretions as predictors of biopsy results. Urology 2004, 64, 821–825. [Google Scholar]
- Stephan, C.; Yousef, G.M.; Scorilas, A.; Jung, K.; Jung, M.; Kristiansen, G.; Hauptmann, S.; Kishi, T.; Nakamura, T.; Loening, S.A.; et al. Hepsin is highly over expressed in and a new candidate for a prognostic indicator in prostate cancer. J. Urol. 2004, 17(1), 187–191. [Google Scholar]
- Dhanasekaran, S.M.; Barrette, T.R.; Ghosh, D.; Shah, R.; Varambally, S.; Kurachi, K.; Pienta, K.J.; Rubin, M.A.; Chinnaiyan, A.M. Delineation of prognostic biomarkers in prostate cancer. Nature 2001, 412, 822–826. [Google Scholar]
- Shariat, S.F.; Andrews, B.; Kattan, M.W.; Kim, J.; Wheeler, T.M.; Slawin, K.M. Plasma levels of interleukin-6 and its soluble receptor are associated with prostate cancer progression and metastasis. Urology 2001, 58, 1008–1015. [Google Scholar]
- Nakashima, J.; Tachibana, M.; Horiguchi, Y.; Oya, M.; Ohigashi, T.; Asakura, H.; Murai, M. Serum interleukin 6 as a prognostic factor in patients with prostate cancer. Clin. Cancer Res 2000, 6, 2702–2706. [Google Scholar]
- Shariat, S.F.; Kattan, M.W.; Traxel, E.; Andrews, B.; Zhu, K.; Wheeler, T.M.; Slawin, K.M. Association of pre- and postoperative plasma levels of transforming growth factor beta(1) and interleukin 6 and its soluble receptor with prostate cancer progression. Clin. Cancer Res 2004, 10, 1992–1999. [Google Scholar]
- Han, Z.D.; Zhang, Y.Q.; He, H.C.; Dai, Q.S.; Qin, G.Q.; Chen, J.H.; Cai, C.; Fu, X.; Bi, X.C.; Zhu, J.G.; et al. Identification of novel serological tumor markers for human prostate cancer using integrative transcriptome and proteome analysis. Med. Oncol 2012, 29, 2877–2888. [Google Scholar]
- Darson, M.F.; Pacelli, A.; Roche, P.; Rittenhouse, H.G.; Wolfert, R.L.; Young, C.Y.; Klee, G.G.; Tindall, D.J.; Bostwick, D.G. Human glandular kallikrein 2 (hK2) expression in prostatic intraepithelial neoplasia and adenocarcinoma: A novel prostate cancer marker. Urology 1997, 49, 857–862. [Google Scholar]
- Haese, A.; Graefen, M.; Steuber, T.; Becker, C.; Pettersson, K.; Piironen, T.; Noldus, J.; Huland, H.; Lilja, H. Human glandular kallikrein 2 levels in serum for discrimination of pathologically organ-confined from locally-advanced prostate cancer in total PSA-levels below 10 ng/mL. Prostate 2001, 49, 101–109. [Google Scholar]
- Aprikian, A. PSA for prostate cancer detection: In serum, in urine or both? Can. Urol. Assoc. J 2007, 1, 382. [Google Scholar]
- Wang, W.; Mize, G.J.; Zhang, X.; Takayama, T.K. Kallikrein-related peptidase-4 initiates tumor-stroma interactions in prostate cancer through protease-activated receptor-1. Int. J. Cancer 2010, 126, 599–610. [Google Scholar]
- Avgeris, M.; Stravodimos, K.; Scorilas, A. Kallikrein-related peptidase 4 gene (KLK4) in prostate tumors: Quantitative expression analysis and evaluation of its clinical significance. Prostate 2011, 71, 1780–1789. [Google Scholar]
- Pressinotti, N.C.; Klocker, H.; Schafer, G.; Luu, V.D.; Ruschhaupt, M.; Kuner, R.; Steiner, E.; Poustka, A.; Bartsch, G.; Sultmann, H. Differential expression of apoptotic genes PDIA3 and MAP3K5 distinguishes between low- and high-risk prostate cancer. Mol. Cancer 2009, 8, 130. [Google Scholar]
- Bubendorf, L.; Tapia, C.; Gasser, T.C.; Casella, R.; Grunder, B.; Moch, H.; Mihatsch, M.J.; Sauter, G. Ki67 labeling index in core needle biopsies independently predicts tumor-specific survival in prostate cancer. Hum. Pathol 1998, 29, 949–954. [Google Scholar]
- Cuzick, J.; Swanson, G.P.; Fisher, G.; Brothman, A.R.; Berney, D.M.; Reid, J.E.; Mesher, D.; Speights, V.O.; Stankiewicz, E.; Foster, C.S.; et al. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: A retrospective study. Lancet Oncol 2009, 12, 245–255. [Google Scholar]
- Zellweger, T.; Gunther, S.; Zlobec, I.; Savic, S.; Sauter, G.; Moch, H.; Mattarelli, G.; Eichenberger, T.; Curschellas, E.; Rufenacht, H.; et al. Tumor growth fraction measured by immunohistochemical staining of Ki67 is an independent prognostic factor in preoperative prostate biopsies with small-volume or low-grade prostate cancer. Int. J. Cancer 2009, 124, 2116–2123. [Google Scholar]
- Riddick, A.C.; Shukla, C.J.; Pennington, C.J.; Bass, R.; Nuttall, R.K.; Hogan, A.; Sethia, K.K.; Ellis, V.; Collins, A.T.; Maitland, N.J.; et al. Identification of degradome components associated with prostate cancer progression by expression analysis of human prostatic tissues. Br. J. Cancer 2005, 92, 2171–2180. [Google Scholar]
- Lee, S.; Desai, K.K.; Iczkowski, K.A.; Newcomer, R.G.; Wu, K.J.; Zhao, Y.G.; Tan, W.W.; Roycik, M.D.; Sang, Q.X. Coordinated peak expression of MMP-26 and TIMP-4 in preinvasive human prostate tumor. Cell Res 2006, 16, 750–758. [Google Scholar]
- Shukla, C.J.; Pennington, C.J.; Riddick, A.C.; Sethia, K.K.; Ball, R.Y.; Edwards, D.R. Laser-capture microdissection in prostate cancer research: Establishment and validation of a powerful tool for the assessment of tumor-stroma interactions. BJU Int 2008, 101, 765–74. [Google Scholar]
- Zhao, Y.G.; Xiao, A.Z.; Ni, J.; Man, Y.G.; Sang, Q.X. Expression of matrix metalloproteinase-26 in multiple human cancer tissues and smooth muscle cells. Ai Zheng 2009, 28, 1168–1175. [Google Scholar]
- Moses, M.A.; Wiederschain, D.; Loughlin, K.R.; Zurakowski, D.; Lamb, C.C.; Freeman, M.R. Increased incidence of matrix metalloproteinases in urine of cancer patients. Cancer Res 1998, 58, 1395–1399. [Google Scholar]
- Roy, R.; Louis, G.; Loughlin, K.R.; Wiederschain, D.; Kilroy, S.M.; Lamb, C.C.; Zurakowski, D.; Moses, M.A. Tumor-specific urinary matrix metalloproteinase fingerprinting: Identification of high molecular weight urinary matrix metalloproteinase species. Clin. Cancer Res 2008, 14, 6610–6617. [Google Scholar]
- Xu, L.L.; Stackhouse, B.G.; Florence, K.; Zhang, W.; Shanmugam, N.; Sesterhenn, I.A.; Zou, Z.; Srikantan, V.; Augustus, M.; Roschke, V.; et al. PSGR, a novel prostate-specific gene with homology to a G protein-coupled receptor, is overexpressed in prostate cancer. Cancer Res 2000, 60, 6568–6572. [Google Scholar]
- Xu, L.L.; Sun, C.; Petrovics, G.; Makarem, M.; Furusato, B.; Zhang, W.; Sesterhenn, I.A.; McLeod, D.G.; Sun, L.; Moul, J.W.; et al. Quantitative expression profile of PSGR in prostate cancer. Prostate Cancer Prostatic Dis 2006, 9, 56–61. [Google Scholar]
- Rigau, M.; Morote, J.; Mir, M.C.; Ballesteros, C.; Ortega, I.; Sanchez, A.; Colas, E.; Garcia, M.; Ruiz, A.; Abal, M.; et al. PSGR and PCA3 as biomarkers for the detection of prostate cancer in urine. Prostate 2010, 70, 1760–1767. [Google Scholar]
- Gutman, A.B. The development of the acid phosphatase test for prostatic carcinoma: The Sixth Ferdinand C. Valentine Memorial Lecture. Bull. N. Y. Acad. Med 1968, 44, 63–76. [Google Scholar]
- Kim, Y.; Ignatchenko, V.; Yao, C.Q.; Kalatskaya, I.; Nyalwidhe, J.O.; Lance, R.S.; Gramolini, A.O.; Troyer, D.A.; Stein, L.D.; Boutros, P.C.; et al. Identification of differentially expressed proteins in direct expressed prostatic secretions of men with organ-confined versus extracapsular prostate cancer. Mol. Cell Proteomics 2012, 11, 1870–1884. [Google Scholar]
- Leyten, G.H.; Hessels, D.; Jannink, S.A.; Smit, F.P.; de Jong, H.; Cornel, E.B.; de Reijke, T.M.; Vergunst, H.; Kil, P.; Knipscheer, B.C.; et al. Prospective multicentre evaluation of PCA3 and TMPRSS2-ERG gene fusions as diagnostic and prognostic urinary biomarkers for prostate cancer. Eur. Urol. 2012. [Google Scholar] [CrossRef]
- Bussemakers, M.J.; van Bokhoven, A.; Verhaegh, G.W.; Smit, F.P.; Karthaus, H.F.; Schalken, J.A.; Debruyne, F.M.; Ru, N.; Isaacs, W.B. DD3: A new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res 1999, 5(9), 5975–5979. [Google Scholar]
- Day, J.R.; Jost, M.; Reynolds, M.A.; Groskopf, J.; Rittenhouse, H. PCA3: From basic molecular science to the clinical lab. Cancer Lett 2011, 301, 1–6. [Google Scholar]
- Hessels, D.; Schalken, J.A. The use of PCA3 in the diagnosis of prostate cancer. Nat. Rev. Urol 2009, 6, 255–261. [Google Scholar]
- Hessels, D.; Klein Gunnewiek, J.M.; van Oort, I.; Karthaus, H.F.; van Leenders, G.J.; van Balken, B.; Kiemeney, L.A.; Witjes, J.A.; Schalken, J.A. DD3(PCA3)-based molecular urine analysis for the diagnosis of prostate cancer. Eur. Urol 2003, 44, 8–15, ; discussion 15–16.. [Google Scholar]
- Van Gils, M.P.; Hessels, D.; van Hooij, O.; Jannink, S.A.; Peelen, W.P.; Hanssen, S.L.; Witjes, J.A.; Cornel, E.B.; Karthaus, H.F.; Smits, G.A.; et al. The time-resolved fluorescence-based PCA3 test on urinary sediments after digital rectal examination; a Dutch multicenter validation of the diagnostic performance. Clin. Cancer Res 2007, 13, 939–943. [Google Scholar]
- Groskopf, J.; Aubin, S.M.; Deras, I.L.; Blase, A.; Bodrug, S.; Clark, C.; Brentano, S.; Mathis, J.; Pham, J.; Meyer, T.; et al. APTIMA PCA3 molecular urine test: Development of a method to aid in the diagnosis of prostate cancer. Clin. Chem 2006, 52, 1089–1095. [Google Scholar]
- Deras, I.L.; Aubin, S.M.; Blase, A.; Day, J.R.; Koo, S.; Partin, A.W.; Ellis, W.J.; Marks, L.S.; Fradet, Y.; Rittenhouse, H.; et al. PCA3: A molecular urine assay for predicting prostate biopsy outcome. J. Urol 2008, 179, 1587–1592. [Google Scholar]
- Ploussard, G.; Durand, X.; Xylinas, E.; Moutereau, S.; Radulescu, C.; Forgue, A.; Nicolaiew, N.; Terry, S.; Allory, Y.; Loric, S.; et al. Prostate cancer antigen 3 score accurately predicts tumor volume and might help in selecting prostate cancer patients for active surveillance. Eur. Urol 2011, 59, 422–429. [Google Scholar]
- Van Gils, M.P.; Cornel, E.B.; Hessels, D.; Peelen, W.P.; Witjes, J.A.; Mulders, P.F.; Rittenhouse, H.G.; Schalken, J.A. Molecular PCA3 diagnostics on prostatic fluid. Prostate 2007, 67, 881–887. [Google Scholar]
- Gu, Z.; Thomas, G.; Yamashiro, J.; Shintaku, I.P.; Dorey, F.; Raitano, A.; Witte, O.N.; Said, J.W.; Loda, M.; Reiter, R.E. Prostate stem cell antigen (PSCA) expression increases with high gleason score, advanced stage and bone metastasis in prostate cancer. Oncogene 2000, 19, 1288–1296. [Google Scholar]
- Gu, Z.; Yamashiro, J.; Kono, E.; Reiter, R.E. Anti-prostate stem cell antigen monoclonal antibody 1G8 induces cell death in vitro and inhibits tumor growth in vivo via a Fc-independent mechanism. Cancer Res 2005, 65, 9495–9500. [Google Scholar]
- Ameri, A.; Alidoosti, A.; Hosseini, S.Y.; Parvin, M.; Emranpour, M.H.; Taslimi, F.; Salehi, E.; Fadavip, P. Prognostic value of promoter hypermethylation of retinoic acid receptor beta (RARB) and CDKN2 (p16/MTS1) in prostate cancer. Chin. J. Cancer Res 2011, 23, 306–311. [Google Scholar]
- Daniunaite, K.; Berezniakovas, A.; Jankevicius, F.; Laurinavicius, A.; Lazutka, J.R.; Jarmalaite, S. Frequent methylation of RASSF1 and RARB in urine sediments from patients with early stage prostate cancer. Medicina (Kaunas) 2011, 47, 147–153. [Google Scholar]
- Sreekumar, A.; Poisson, L.M.; Rajendiran, T.M.; Khan, A.P.; Cao, Q.; Yu, J.; Laxman, B.; Mehra, R.; Lonigro, R.J.; Li, Y.; et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 2009, 457, 910–914. [Google Scholar]
- Tomlins, S.A.; Rhodes, D.R.; Yu, J.; Varambally, S.; Mehra, R.; Perner, S.; Demichelis, F.; Helgeson, B.E.; Laxman, B.; Morris, D.S.; et al. The role of SPINK1 in ETS rearrangement-negative prostate cancers. Cancer Cell 2008, 13, 519–528. [Google Scholar]
- Meid, F.H.; Gygi, C.M.; Leisinger, H.J.; Bosman, F.T.; Benhattar, J. The use of telomerase activity for the detection of prostatic cancer cells after prostatic massage. J. Urol 2001, 165, 1802–1805. [Google Scholar]
- March-Villalba, J.A.; Martinez-Jabaloyas, J.M.; Herrero, M.J.; Santamaria, J.; Alino, S.F.; Dasi, F. Plasma hTERT mRNA discriminates between clinically localized and locally advanced disease and is a predictor of recurrence in prostate cancer patients. Expert Opin. Biol. Ther 2012, 12, S69–S77. [Google Scholar]
- Shariat, S.F.; Walz, J.; Roehrborn, C.G.; Montorsi, F.; Jeldres, C.; Saad, F.; Karakiewicz, P.I. Early postoperative plasma transforming growth factor-beta1 is a strong predictor of biochemical progression after radical prostatectomy. J. Urol 2008, 179, 1593–1597. [Google Scholar]
- Ivanovic, V.; Melman, A.; Davis-Joseph, B.; Valcic, M.; Geliebter, J. Elevated plasma levels of TGF-beta 1 in patients with invasive prostate cancer. Nat. Med 1995, 1, 282–284. [Google Scholar]
- Hessels, D.; Smit, F.P.; Verhaegh, G.W.; Witjes, J.A.; Cornel, E.B.; Schalken, J.A. Detection of TMPRSS2-ERG fusion transcripts and prostate cancer antigen 3 in urinary sediments may improve diagnosis of prostate cancer. Clin. Cancer Res 2007, 13, 5103–5108. [Google Scholar]
- Tomlins, S.A.; Rhodes, D.R.; Perner, S.; Dhanasekaran, S.M.; Mehra, R.; Sun, X.W.; Varambally, S.; Cao, X.; Tchinda, J.; Kuefer, R.; et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005, 310, 644–648. [Google Scholar]
- Mosquera, J.M.; Mehra, R.; Regan, M.M.; Perner, S.; Genega, E.M.; Bueti, G.; Shah, R.B.; Gaston, S.; Tomlins, S.A.; Wei, J.T.; et al. Prevalence of TMPRSS2-ERG fusion prostate cancer among men undergoing prostate biopsy in the United States. Clin. Cancer Res 2009, 15, 4706–4711. [Google Scholar]
- McCabe, N.P.; Angwafo, F.F., III; Zaher, A.; Selman, S.H.; Kouinche, A.; Jankun, J. Expression of soluble urokinase plasminogen activator receptor may be related to outcome in prostate cancer patients. Oncol. Rep 2000, 7, 879–882. [Google Scholar]
- Shariat, S.F.; Roehrborn, C.G.; McConnell, J.D.; Park, S.; Alam, N.; Wheeler, T.M.; Slawin, K.M. Association of the circulating levels of the urokinase system of plasminogen activation with the presence of prostate cancer and invasion, progression, and metastasis. J. Clin. Oncol 2007, 25, 349–355. [Google Scholar]
- Ludwig, J.A.; Weinstein, J.N. Biomarkers in cancer staging, prognosis and treatment selection. Nat. Rev. Cancer 2005, 5, 845–856. [Google Scholar]
- Roupret, M.; Hupertan, V.; Yates, D.R.; Catto, J.W.; Rehman, I.; Meuth, M.; Ricci, S.; Lacave, R.; Cancel-Tassin, G.; de la Taille, A.; et al. Molecular detection of localized prostate cancer using quantitative methylation-specific PCR on urinary cells obtained following prostate massage. Clin. Cancer Res 2007, 13, 1720–1725. [Google Scholar]
- Nakayama, M.; Gonzalgo, M.L.; Yegnasubramanian, S.; Lin, X.; de Marzo, A.M.; Nelson, W.G. GSTP1 CpG island hypermethylation as a molecular biomarker for prostate cancer. J. Cell Biochem 2004, 91, 540–552. [Google Scholar]
- Hoque, M.O.; Topaloglu, O.; Begum, S.; Henrique, R.; Rosenbaum, E.; van Criekinge, W.; Westra, W.H.; Sidransky, D. Quantitative methylation-specific polymerase chain reaction gene patterns in urine sediment distinguish prostate cancer patients from control subjects. J. Clin. Oncol 2005, 23, 6569–6575. [Google Scholar]
- Costa, V.L.; Henrique, R.; Danielsen, S.A.; Eknaes, M.; Patricio, P.; Morais, A.; Oliveira, J.; Lothe, R.A.; Teixeira, M.R.; Lind, G.E.; et al. TCF21 and PCDH17 methylation: An innovative panel of biomarkers for a simultaneous detection of urological cancers. Epigenetics 2011, 6, 1120–1130. [Google Scholar]
- Beckett, M.L.; Cazares, L.H.; Vlahou, A.; Schellhammer, P.F.; Wright, G.L., Jr. Prostate-specific membrane antigen levels in sera from healthy men and patients with benign prostate hyperplasia or prostate cancer. Clin. Cancer Res 1999, 5, 4034–4040. [Google Scholar]
- Sokoloff, R.L.; Norton, K.C.; Gasior, C.L.; Marker, K.M.; Grauer, L.S. A dual-monoclonal sandwich assay for prostate-specific membrane antigen: Levels in tissues, seminal fluid and urine. Prostate 2000, 43, 150–157. [Google Scholar]
- Xiao, Z.; Adam, B.L.; Cazares, L.H.; Clements, M.A.; Davis, J.W.; Schellhammer, P.F.; Dalmasso, E.A.; Wright, G.L., Jr. Quantitation of serum prostate-specific membrane antigen by a novel protein biochip immunoassay discriminates benign from malignant prostate disease. Cancer Res 2001, 61, 6029–6033. [Google Scholar]
- Talesa, V.N.; Antognelli, C.; del Buono, C.; Stracci, F.; Serva, M.R.; Cottini, E.; Mearini, E. Diagnostic potential in prostate cancer of a panel of urinary molecular tumor markers. Cancer Biomark 2009, 5, 241–251. [Google Scholar]
- Mitchell, P.J.; Welton, J.; Staffurth, J.; Court, J.; Mason, M.D.; Tabi, Z.; Clayton, A. Can urinary exosomes act as treatment response markers in prostate cancer? J. Transl. Med 2009, 7, 4. [Google Scholar]
- Rigau, M.; Ortega, I.; Mir, M.C.; Ballesteros, C.; Garcia, M.; Llaurado, M.; Colas, E.; Pedrola, N.; Montes, M.; Sequeiros, T.; et al. A Three-Gene panel on urine increases PSA specificity in the detection of prostate cancer. Prostate 2011, 71, 1736–1745. [Google Scholar]
- Magi-Galluzzi, C.; Tsusuki, T.; Elson, P.; Simmerman, K.; LaFargue, C.; Esgueva, R.; Klein, E.; Rubin, M.A.; Zhou, M. TMPRSS2-ERG gene fusion prevalence and class are significantly different in prostate cancer of Caucasian, African-American and Japanese patients. Prostate 2011, 71, 489–497. [Google Scholar]
- Rostad, K.; Hellwinkel, O.J.; Haukaas, S.A.; Halvorsen, O.J.; Oyan, A.M.; Haese, A.; Budaus, L.; Albrecht, H.; Akslen, L.A.; Schlomm, T.; et al. TMPRSS2:ERG fusion transcripts in urine from prostate cancer patients correlate with a less favorable prognosis. APMIS 2009, 117, 575–582. [Google Scholar]
- Demichelis, F.; Fall, K.; Perner, S.; Andren, O.; Schmidt, F.; Setlur, S.R.; Hoshida, Y.; Mosquera, J.M.; Pawitan, Y.; Lee, C.; et al. TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene 2007, 26, 4596–4599. [Google Scholar]
- Bader, A.G.; Brown, D.; Stoudemire, J.; Lammers, P. Developing therapeutic microRNAs for cancer. Gene Ther 2011, 18, 1121–1126. [Google Scholar]
- Catto, J.W.; Miah, S.; Owen, H.C.; Bryant, H.; Myers, K.; Dudziec, E.; Larre, S.; Milo, M.; Rehman, I.; Rosario, D.J.; et al. Distinct microRNA alterations characterize high- and low-grade bladder cancer. Cancer Res 2009, 69, 8472–8481. [Google Scholar]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar]
- Bushati, N.; Cohen, S.M. microRNA functions. Annu. Rev. Cell. Dev. Biol 2007, 23, 175–205. [Google Scholar]
- Stefani, G.; Slack, F.J. Small non-coding RNAs in animal development. Nat. Rev. Mol. Cell. Biol 2008, 9, 219–230. [Google Scholar]
- Gangaraju, V.K.; Lin, H. MicroRNAs: Key regulators of stem cells. Nat. Rev. Mol. Cell. Biol 2009, 10, 116–125. [Google Scholar]
- Croce, C.M. Causes and consequences of microRNA dysregulation in cancer. Nat. Rev. Genet 2009, 10, 704–714. [Google Scholar]
- Kuner, R.; Brase, J.C.; Sultmann, H.; Wuttig, D. microRNA biomarkers in body fluids of prostate cancer patients. Methods 2013, 59, 132–137. [Google Scholar]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838. [Google Scholar]
- Gordanpour, A.; Nam, R.K.; Sugar, L.; Seth, A. MicroRNAs in prostate cancer: From biomarkers to molecularly-based therapeutics. Prostate Cancer Prostatic Dis 2012, 15, 314–319. [Google Scholar]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem 2010, 56, 1733–1741. [Google Scholar]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar]
- Brase, J.C.; Johannes, M.; Schlomm, T.; Falth, M.; Haese, A.; Steuber, T.; Beissbarth, T.; Kuner, R.; Sultmann, H. Circulating miRNAs are correlated with tumor progression in prostate cancer. Int. J. Cancer 2011, 128, 608–616. [Google Scholar]
- Yamada, Y.; Enokida, H.; Kojima, S.; Kawakami, K.; Chiyomaru, T.; Tatarano, S.; Yoshino, H.; Kawahara, K.; Nishiyama, K.; Seki, N.; et al. MiR-96 and miR-183 detection in urine serve as potential tumor markers of urothelial carcinoma: Correlation with stage and grade, and comparison with urinary cytology. Cancer Sci 2011, 102, 522–529. [Google Scholar]
- Bryant, R.J.; Pawlowski, T.; Catto, J.W.; Marsden, G.; Vessella, R.L.; Rhees, B.; Kuslich, C.; Visakorpi, T.; Hamdy, F.C. Changes in circulating microRNA levels associated with prostate cancer. Br. J. Cancer 2012, 106, 768–774. [Google Scholar]
- Sapre, N.; Selth, L.A. Circulating MicroRNAs as biomarkers of prostate cancer: The state of play. Prostate Cancer 2013, 2013, 539680, :1–539680:10.. [Google Scholar]
- Costa, F.F. Non-coding RNAs: New players in eukaryotic biology. Gene 2005, 357, 83–94. [Google Scholar]
- Mattick, J.S.; Makunin, I.V. Non-coding RNA. Hum. Mol. Genet 2006, 15, R17–R29. [Google Scholar]
- Szell, M.; Bata-Csorgo, Z.; Kemeny, L. The enigmatic world of mRNA-like ncRNAs: Their role in human evolution and in human diseases. Semin. Cancer Biol 2008, 18, 141–148. [Google Scholar]
- Turner, A.M.; Morris, K.V. Controlling transcription with noncoding RNAs in mammalian cells. Biotechniques 2010, 48, ix–xvi. [Google Scholar]
- Pavlou, M.P.; Diamandis, E.P. The cancer cell secretome: A good source for discovering biomarkers? J. Proteomics 2010, 73, 1896–1906. [Google Scholar]
- Makridakis, M.; Vlahou, A. Secretome proteomics for discovery of cancer biomarkers. J. Proteomics 2010, 73, 2291–2305. [Google Scholar]
- Xue, H.; Lu, B.; Zhang, J.; Wu, M.; Huang, Q.; Wu, Q.; Sheng, H.; Wu, D.; Hu, J.; Lai, M. Identification of serum biomarkers for colorectal cancer metastasis using a differential secretome approach. J. Proteome Res 2009, 9, 545–555. [Google Scholar]
- Bolduc, S.; Lacombe, L.; Naud, A.; Gregoire, M.; Fradet, Y.; Tremblay, R.R. Urinary PSA: A potential useful marker when serum PSA is between 2.5 ng/mL and 10 ng/mL. Can. Urol. Assoc. J 2007, 1, 377–381. [Google Scholar]
- Takayama, T.K.; Vessella, R.L.; Brawer, M.K.; True, L.D.; Noteboom, J.; Lange, P.H. Urinary prostate specific antigen levels after radical prostatectomy. J. Urol 1994, 151, 82–87. [Google Scholar]
- DeVere White, R.W.; Meyers, F.J.; Soares, S.E.; Miller, D.G.; Soriano, T.F. Urinary prostate specific antigen levels: Role in monitoring the response of prostate cancer to therapy. J. Urol 1992, 147, 947–951. [Google Scholar]
- Pannek, J.; Rittenhouse, H.G.; Evans, C.L.; Finlay, J.A.; Bruzek, D.J.; Cox, J.L.; Chan, D.W.; Subong, E.N.; Partin, A.W. Molecular forms of prostate-specific antigen and human kallikrein 2 (hK2) in urine are not clinically useful for early detection and staging of prostate cancer. Urology 1997, 50, 715–721. [Google Scholar]
- Malavaud, B.; Salama, G.; Miedouge, M.; Vincent, C.; Rischmann, P.; Sarramon, J.P.; Serre, G. Influence of digital rectal massage on urinary prostate-specific antigen: Interest for the detection of local recurrence after radical prostatectomy. Prostate 1998, 34, 23–28. [Google Scholar]
- Schostak, M.; Schwall, G.P.; Poznanovic, S.; Groebe, K.; Muller, M.; Messinger, D.; Miller, K.; Krause, H.; Pelzer, A.; Horninger, W.; et al. Annexin A3 in urine: A highly specific noninvasive marker for prostate cancer early detection. J. Urol 2009, 181, 343–353. [Google Scholar]
- Kulasingam, V.; Diamandis, E.P. Strategies for discovering novel cancer biomarkers through utilization of emerging technologies. Nat. Clin. Pract. Oncol 2008, 5, 588–599. [Google Scholar]
- Decramer, S.; Gonzalez de Peredo, A.; Breuil, B.; Mischak, H.; Monsarrat, B.; Bascands, J.L.; Schanstra, J.P. Urine in clinical proteomics. Mol. Cell Proteomics 2008, 7, 1850–1862. [Google Scholar]
- Rehman, I.; Azzouzi, A.R.; Catto, J.W.; Allen, S.; Cross, S.S.; Feeley, K.; Meuth, M.; Hamdy, F.C. Proteomic analysis of voided urine after prostatic massage from patients with prostate cancer: A pilot study. Urology 2004, 64, 1238–1243. [Google Scholar]
- Theodorescu, D.; Fliser, D.; Wittke, S.; Mischak, H.; Krebs, R.; Walden, M.; Ross, M.; Eltze, E.; Bettendorf, O.; Wulfing, C.; et al. Pilot study of capillary electrophoresis coupled to mass spectrometry as a tool to define potential prostate cancer biomarkers in urine. Electrophoresis 2005, 26, 2797–2808. [Google Scholar]
- Theodorescu, D.; Schiffer, E.; Bauer, H.W.; Douwes, F.; Eichhorn, F.; Polley, R.; Schmidt, T.; Schofer, W.; Zurbig, P.; Good, D.M.; et al. Discovery and validation of urinary biomarkers for prostate cancer. Proteomics Clin. Appl 2008, 2, 556–570. [Google Scholar]
- M’Koma, A.E.; Blum, D.L.; Norris, J.L.; Koyama, T.; Billheimer, D.; Motley, S.; Ghiassi, M.; Ferdowsi, N.; Bhowmick, I.; Chang, S.S.; et al. Detection of pre-neoplastic and neoplastic prostate disease by MALDI profiling of urine. Biochem. Biophys. Res. Commun 2007, 353, 829–834. [Google Scholar]
- Okamoto, A.; Yamamoto, H.; Imai, A.; Hatakeyama, S.; Iwabuchi, I.; Yoneyama, T.; Hashimoto, Y.; Koie, T.; Kamimura, N.; Mori, K.; et al. Protein profiling of post-prostatic massage urine specimens by surface-enhanced laser desorption/ionization time-of-flight mass spectrometry to discriminate between prostate cancer and benign lesions. Oncol. Rep 2009, 21, 73–79. [Google Scholar]
- Gamagedara, S.; Kaczmarek, A.T.; Jiang, Y.; Cheng, X.; Rupasinghe, M.; Ma, Y. Validation study of urinary metabolites as potential biomarkers for prostate cancer detection. Bioanalysis 2012, 4, 1175–1183. [Google Scholar]
- Jentzmik, F.; Stephan, C.; Miller, K.; Schrader, M.; Erbersdobler, A.; Kristiansen, G.; Lein, M.; Jung, K. Sarcosine in urine after digital rectal examination fails as a marker in prostate cancer detection and identification of aggressive tumors. Eur. Urol 2010, 58, 12–18, ; discussion 20–21.. [Google Scholar]
- Landers, K.A.; Burger, M.J.; Tebay, M.A.; Purdie, D.M.; Scells, B.; Samaratunga, H.; Lavin, M.F.; Gardiner, R.A. Use of multiple biomarkers for a molecular diagnosis of prostate cancer. Int. J. Cancer 2005, 114, 950–956. [Google Scholar]
- Schmidt, U.; Fuessel, S.; Koch, R.; Baretton, G.B.; Lohse, A.; Tomasetti, S.; Unversucht, S.; Froehner, M.; Wirth, M.P.; Meye, A. Quantitative multi-gene expression profiling of primary prostate cancer. Prostate 2006, 66, 1521–1534. [Google Scholar]
- Etzioni, R.; Kooperberg, C.; Pepe, M.; Smith, R.; Gann, P.H. Combining biomarkers to detect disease with application to prostate cancer. Biostatistics 2003, 4, 523–538. [Google Scholar]
- Baden, J.; Green, G.; Painter, J.; Curtin, K.; Markiewicz, J.; Jones, J.; Astacio, T.; Canning, S.; Quijano, J.; Guinto, W.; et al. Multicenter evaluation of an investigational prostate cancer methylation assay. J. Urol 2009, 182, 1186–1193. [Google Scholar]
- Vener, T.; Derecho, C.; Baden, J.; Wang, H.; Rajpurohit, Y.; Skelton, J.; Mehrotra, J.; Varde, S.; Chowdary, D.; Stallings, W.; et al. Development of a multiplexed urine assay for prostate cancer diagnosis. Clin. Chem 2008, 54, 874–882. [Google Scholar]
- Payne, S.R.; Serth, J.; Schostak, M.; Kamradt, J.; Strauss, A.; Thelen, P.; Model, F.; Day, J.K.; Liebenberg, V.; Morotti, A.; et al. DNA methylation biomarkers of prostate cancer: Confirmation of candidates and evidence urine is the most sensitive body fluid for non-invasive detection. Prostate 2009, 69, 1257–1269. [Google Scholar]
- Ouyang, B.; Bracken, B.; Burke, B.; Chung, E.; Liang, J.; Ho, S.M. A duplex quantitative polymerase chain reaction assay based on quantification of alpha-methylacyl-CoA racemase transcripts and prostate cancer antigen 3 in urine sediments improved diagnostic accuracy for prostate cancer. J. Urol 2009, 181, 2508–2513, ; discussion 2513–2514.. [Google Scholar]
- Salami, S.S.; Schmidt, F.; Laxman, B.; Regan, M.M.; Rickman, D.S.; Scherr, D.; Bueti, G.; Siddiqui, J.; Tomlins, S.A.; Wei, J.T.; et al. Combining urinary detection of TMPRSS2:ERG and PCA3 with serum PSA to predict diagnosis of prostate cancer. Urol. Oncol 2011. [Google Scholar] [CrossRef]
- Jamaspishvili, T.; Kral, M.; Khomeriki, I.; Student, V.; Kolar, Z.; Bouchal, J. Urine markers in monitoring for prostate cancer. Prostate Cancer Prostatic Dis 2011, 13, 12–19. [Google Scholar]
- Nguyen, P.N.; Violette, P.; Chan, S.; Tanguay, S.; Kassouf, W.; Aprikian, A.; Chen, J.Z. A panel of TMPRSS2:ERG fusion transcript markers for urine-based prostate cancer detection with high specificity and sensitivity. Eur. Urol 2011, 59, 407–414. [Google Scholar]
- Tomlins, S.A.; Aubin, S.M.; Siddiqui, J.; Lonigro, R.J.; Sefton-Miller, L.; Miick, S.; Williamsen, S.; Hodge, P.; Meinke, J.; Blase, A.; et al. Urine TMPRSS2:ERG fusion transcript stratifies prostate cancer risk in men with elevated serum PSA. Sci. Transl. Med 2011, 3, 94r, a72.. [Google Scholar]
- Cao, D.L.; Ye, D.W.; Zhang, H.L.; Zhu, Y.; Wang, Y.X.; Yao, X.D. A multiplex model of combining gene-based, protein-based, and metabolite-based with positive and negative markers in urine for the early diagnosis of prostate cancer. Prostate 2011, 71, 700–710. [Google Scholar]
- Prior, C.; Guillen-Grima, F.; Robles, J.E.; Rosell, D.; Fernandez-Montero, J.M.; Agirre, X.; Catena, R.; Calvo, A. Use of a combination of biomarkers in serum and urine to improve detection of prostate cancer. World J. Urol. 2010, 2(8), 681–686. [Google Scholar]
- Thery, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol 2002, 2, 569–579. [Google Scholar]
- Simpson, R.J.; Jensen, S.S.; Lim, J.W. Proteomic profiling of exosomes: Current perspectives. Proteomics 2008, 8, 4083–4099. [Google Scholar]
- Guescini, M.; Genedani, S.; Stocchi, V.; Agnati, L.F. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J. Neural Transm 2010, 117, 1–4. [Google Scholar]
- Keller, S.; Ridinger, J.; Rupp, A.K.; Janssen, J.W.; Altevogt, P. Body fluid derived exosomes as a novel template for clinical diagnostics. J. Transl. Med 2011, 9, 86. [Google Scholar]
- Zhou, H.; Yuen, P.S.; Pisitkun, T.; Gonzales, P.A.; Yasuda, H.; Dear, J.W.; Gross, P.; Knepper, M.A.; Star, R.A. Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int 2006, 69, 1471–1476. [Google Scholar]
- Nilsson, J.; Skog, J.; Nordstrand, A.; Baranov, V.; Mincheva-Nilsson, L.; Breakefield, X.O.; Widmark, A. Prostate cancer-derived urine exosomes: A novel approach to biomarkers for prostate cancer. Br. J. Cancer 2009, 100, 1603–1607. [Google Scholar]
- Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal miRNAs as biomarkers for prostate cancer. Front Genet 2013, 4, 36. [Google Scholar]
- Laxman, B.; Tomlins, S.A.; Mehra, R.; Morris, D.S.; Wang, L.; Helgeson, B.E.; Shah, R.B.; Rubin, M.A.; Wei, J.T.; Chinnaiyan, A.M. Noninvasive detection of TMPRSS2:ERG fusion transcripts in the urine of men with prostate cancer. Neoplasia 2006, 8, 885–888. [Google Scholar]
- Makarov, D.V.; Loeb, S.; Getzenberg, R.H.; Partin, A.W. Biomarkers for prostate cancer. Annu. Rev. Med 2009, 60, 139–151. [Google Scholar]
- Ploussard, G.; de la Taille, A. Urine biomarkers in prostate cancer. Nat. Rev. Urol 2010, 7, 101–109. [Google Scholar]
| Gene | Description | Gene type | Expression | Type of biomarker | Sample | References |
|---|---|---|---|---|---|---|
| AMACR (P504) | Alpha-Methylacyl-CoA Racemase | Enzyme involved in branched chain fatty acid oxidation | Over-expressed in PCa (also in HGPIN) and in some other carcinomas, both at RNA and protein level | Diagnostic (in gray zone) and prognostic | Tissue, blood and urine | [20,21] |
| ANXA3 | Annexin A3 | Calcium and phospholipid binding protein | Presence in urinary exosomes and proteasomes. Lower production in PCa than in BPH, HGPIN and benign | Prognostic (able to stratify a large group of intermediate-risk patients into high- and low-risk subgroups) | Tissue and urine | [22–24] |
| APC | Adenomatous polyposis coli | Tumor suppressor. Promotes rapid degradation of CTNNB1 and participates in Wnt signaling as a negative regulator. | APC methylation higher in PCa than in BPH. Methylation level correlates positively with Gleason score | Diagnostic and prognostic | Tissue and Urine DNA | [25] |
| AR | Androgen receptor | Receptor for androgen stimulation of prostate. | Over-expression associated with poor prognosis prostate cancer and metastasis | Prognostic | Tissue RNA and IHC | [26–28] |
| AURKA | Aurora kinase. | Aurora kinase. AURKA is a centrosome-associated serine/threonine kinase involved in mitotic chromosomal segregation. | Amplified and over-expressed in certain types of poor prognosis prostate cancer | Prognostic | Tissue RNA and DNA | [29–31] |
| AZGP1 | Alpha-2-glycoprotein 1, zinc binding. Alias. ZAG | Stimulates lipid degradation in adipocytes and causes the extensive fat losses associated with some advanced cancers. May bind polyunsaturated fatty acids. | Over-expressed in PCa. Low AZGP1 expression predicts for recurrence in margin-positive, localized PCa | Diagnostic, prognostic | Tissue, blood and urine | [32–34] |
| BRAF | v-raf murine sarcoma viral oncogene homolog B1 | Belongs to the raf/mil family of serine/threonine protein kinases and is involved in the regulation of the MAP kinase/ERKs signaling pathway, which affects cell division, differentiation. | SLC45A3-BRAF fusion gene, mutations and gain in prostate cancer | Diagnostic and therapeutic target | Tissue RNA and DNA | [35–37] |
| CAMKK2 | Calcium/calmodulindependent protein kinase kinase 2. | AR target gene promoting biosynthesis and glycolysis | Down-regulation of calcium/calmodulin-dependent protein kinase kinase 2 by androgen deprivation induces castration-resistant prostate cancer. | Prognostic | Tissue RNA | [38–40] |
| CDH1 | Cadherin 1, type 1, E-cadherin (epithelial) | Epithelial cell - cell adhesion molecule | Reduced production in 50% of tumors. E-cadherin production by epithelial cells has been shown to predict PCa prognosis | Prognostic (correlated with grade, tumor stage, and survival) | Tissue | [41,42] |
| CLU | Clusterin | Function unknown, but is thought to be involved in several basic biological events such as cell death and tumor progression. | Developed as a potential therapeutic target | Therapeutic target | Tissue, exosome protein | [43–46] |
| CRISP-3 | Cysteine-Rich Secretory Protein 3 | Secreted protein produced in the male reproductive tract, is involved in sperm maturation | Large amounts have been detected in seminal plasma. Over-expressed in HGPIN and PCa. | Prognostic | Tissue | [47,48] |
| EPCA | Early Prostate Cancer Antigen | Nuclear matrix protein | Over-expressed in PCa | Diagnostic (for predicting repeated BP) | Tissue and blood | [49,50] |
| EPCA-2 | Early Prostate Cancer Antigen 2 | Nuclear matrix protein | Over-expressed in PCa | Diagnostic and Prognostic (differentiate localized PCa from metastatic PCa) | Blood | [51] |
| FOLH1/PS MA | Folate hydrolase 1/Prostate Specific Membrane Antigen | Type II membrane protein. 1/N-acetylated-alpha-linked acidic dipeptidase | Over-expressed in PCa compared to BPH and normal | Diagnostic. Imaging marker and target for therapy | Tissue, blood and urine | [52,53] |
| GOLM1 | Golgi membrane protein 1 (GOLPH2) | Cis-Golgi membrane protein of unknown function | Over-expressed in PCa | Diagnostic | Urine | [54,55] |
| GSTP1 | Glutathione S-transferase P1 | Enzyme involved in protecting DNA from free radicals | Loss of GSTP1 expression due to the promoter hypermethylation (>90% of PCa). Correlates with the number of cores found to contain PCa | Diagnostic (indicator for repeat biopsy) | Tissue and urine DNA | [56,57] |
| HPN | Hepsin | Membrane serine protease | Over-expressed in 90% PCa tumors (highly produced in HGPIN and PCa compared with BPH) | Diagnostic | Tissue | [58,59] |
| IL-6 | Interleukin-6 | Cytokine secreted by a variety of cell types, is involved in the immune and acute-phase response | Increased concentrations of IL-6 and IL-6R in metastatic and androgen-independent PCa | Diagnosis and Prognostic | Blood | [60–62] |
| IMPDH2 | IMP (inosine 5′-monophosphate) dehydrogenase 2 | Myc target gene associated with nucleotide biosynthesis | Increased serum level associated with the clinicopathological features of the patients with PCa | Diagnostic | Blood | [63] |
| KLK2 | Human Kallikrein 2 | Secreted serine protease | Over-expressed during PCa progression | Diagnostic and Prognostic | Tissue and blood | [64,65] |
| KLK3 (PSA) | Kallikrein-related peptidase 3 (Prostate-Specific Antigen) | Secreted serine protease. Serum level of this protein, called PSA in the clinical setting, is useful in the diagnosis and monitoring of PCa. | Increased expression associated with malignant PCa | Diagnostic | Blood, urine | [66] |
| KLK4 | Kallikrein-related peptidase 4 | One of fifteen kallikrein subfamily members located in a cluster on chromosome 19 | Increased expression associated with malignant PCa | Prognostic | Tissue RNA and IHC | [67,68]. |
| MAP3K5 | Mitogen-activated protein kinase kinase kinase 5 | Signaling cascade | Increased expression associated with PCa | Prognostic | Tissue RNA and IHC | [69] |
| MKI67 | Encoding antigen identified by monoclonal antibody Ki-67 | Tumor growth marker, encodes a nuclear protein that is associated with and may be necessary for cellular proliferation | Increased expression associated with malignant prostate cancer | Prognostic | Tissue | [70–72] |
| MMP26 | Matrix metallo peptidase 26 | Involved in the breakdown of extracellular matrix in normal physiological processes and cancer metastasis. | Highest expression in HGPIN and decline in cancer, possible involvement in formation of early cancer. | Progression | Tissue RNA | [73–76] |
| MMP9 | Matrix metallo proteinase 9 | Implicated in invasion and metastasis of human malignancies | Over-expressed in PCa | Diagnostic | Urine | [77,78] |
| OR51E2/PS GR | Prostate Specific G-coupled Receptor | Receptors coupled to heterotrimeric GTP-binding proteins | Over-expressed in PCa | Diagnostic | Tissue and urine | [79–81] |
| PAP | Human Prostatic acid phosphatase | Enzyme | Over-expressed in PCa and in bone metastasis | Diagnostic and Prognostic of PCa bone metastasis | Blood and urine | [82,83] |
| PCA3 | Prostate Cancer Gene 3 | Non coding mRNA | Prostate specific and highly up-regulated in PCa | Diagnostic (indicator for repeat biopsy) | Tissue and urine | [84–93] |
| PDIA3 | Protein disulfide isomerase family A, member 3. | Endoplasmic reticulum that interacts with lectin chaperones calreticulin and calnexin to modulate folding of newly synthesized glycoproteins. | Increased expression associated with malignant PCa | Prognostic | Tissue RNA and IHC | [69] |
| PSCA | Prostate Stem Cell Antigen | Membrane glycoprotein | Specific production in the prostate and possible target for therapy | Prognostic (correlated with higher Gleason score, higher stage, and the presence of metastasis) | Tissue and blood | [94,95] |
| RARB | Retinoic acid receptor, beta | Binds retinoic acid. Mediates signalling in embryonic morphogenesis, cell growth and differentiation. | DNA methylation | Prognostic | Tissue and urine DNA | [96,97] |
| RASSF1A | Ras association (RalGDS/AF-6) domain family member 1 | Potential tumor suppressor. Required for death receptor-dependent apoptosis | DNA methylation | Prognostic | Tissue and urine DNA | [97] |
| Sarcosine | Sarcosine | N-methyl derivative of the amino-acid glycine | Seems to be differentially expressed metabolite elevated during PCa progression to metastasis | Prognostic | Urine and blood | [98] |
| SPINK1 | Serine peptidase inhibitor, Kazal type 1 | Serine peptidase inhibitor | Overexressed in a portion of non-ETS translocated tumors | Diagnostic | Urine, tissue | [54,99] |
| TERT | Telomerase reverse transcriptase | Maintains the telomeric ends of chromosomes and if telomerase is active, cancer cells may escape cell cycle arrest and replicative senscence | Amplification in PCa, significative association with Gleason score | Prognostic | Urine and blood | [57,100,101] |
| TGFB1 | Transforming growth factor-b1 | Growth factor involved in cellular differentiation, immune response, angiogenesis, and proliferation | Role of TGFβ1 in PCa progression. | Prognostic (Correlation with tumor grade and stage and lymph node metastasis) | Tissue and blood | [62,102,103] |
| TIMP4 | TIMP metallopeptidase inhibitor 4 | Inhibitors of the matrix metallo proteinases | Highest expression in HGPIN and decline in cancer, possible involvement in formation of early cancer. | Progression | Tissue RNA | [73–75] |
| TMPRSS2:E RG | 5′ UTR of the prostate-specific androgen regulated transmembrane protease serine2 and v-ETS erythroblostosis virus E26 oncogene homolog | Gene fusion; androgen drives the expression of ETS-TF and causes tumor proliferation | The most common gene fusion in PCa. Over-expressed PCa and related to PCa aggressiveness | Prognostic for aggressive PCa and detection of PCa | Tissue and urine | [104–106] |
| PLAU and UPAR | Plasminogen Activator, Urokinase and Receptor | Degradation of extra cellular matrix | Over-expressed in BPH and PCa vs benign | Prognostic (increased uPA and uPAR in PCa patients with bone metastasis) | Tissue and blood | [107,108] |
| Biomarker type | Study | Marker | PCa/study | Sens. | Spec. | AUC |
|---|---|---|---|---|---|---|
| DNA | Hoque et al., 2005 [112] | p16, ARF, MGMT, GSTP1 | 73 | 87% | 100% | ND |
| Rouprêt et al., 2007 [110] | GSTP1, RASSF1A, RARB, and APC | 95/133 | 87% | 89% | ND | |
| Vener et al., 2008 [165] | GSTP1, RARB and APC | 54/121 | 55% | 80% | 0.69 | |
| Payne et al., 2009 [166] | GSTP1, RASSF2, HIS1H4K, TFAP2E | 192 | 94% | 27% | ||
| Baden et al., 2009 [164] | GSTP1, RARB and APC | 178/159 | ND | ND | 0.72 | |
| Costa et al., 2011 [113] | PCDH17, TCF21 | 318 | 26% | 100% | ||
| mRNA | Hessels et al., 2007 [104] | PCA3 and TMPRSS2:ERG | 78/108 | 73% | 52% | ND |
| Laxman et al., 2008 [54] | PCA3, GOLPH2, SPINK1 and TMPRSS2:ERG | 152/257 | 66% | 76% | 0.76 | |
| Ouyang et al., 2009 [167] | AMACR and PCA3 | 43/92 | 72% | 53% | ND | |
| Talesa et al., 2009 [117] | PSMA, HPN, PCA3, GalNAC-T3 and serum PSA | 49% | ND | ND | ||
| Rigau et al., 2010 [81] | PCA3 and PSGR | 73/215 | 96% | 34% | 0.73 | |
| Rigau et al., 2011 [119] | PSMA, PSGR, PCA3 and serum PSA | 57/154 | 96% | 50% | 0.82 | |
| Salami et al., 2011 [168] | PCA3, TMPRSS2:ERG and serum PSA | 15/45 | 80% | 90% | 0.88 | |
| Jamasphvili et al., 2011 [169] | PCA3, AMACR, TRMP8, SMSB | 104 | 72% | 71% | ||
| Nguyen et al., 2011 [170] | TMPRSS2:ERG subtypes | 101 | 35% | 100% | ||
| Tomlins et al., 2011 [171] | PCA3 and TMPRSS2:ERG | 463 (acad.) and 439 (biopsy) | a_0.64 and b_0.66 | |||
| Protein | Rehman et al., 2004 [154] | ENO1, IDH3B, B2M, A1M, PRO2044 and S100A9 (Calgranulin_B/MRP-14) | 6 PC (12) | |||
| Theodorescu et al., 2005 [155] | Proteinpolypeptide | 26/47 | 92% | 96% | ||
| M’Koma et al., 2007 [157] | 130 m/z | 89/407 | 81% | 80% | ||
| Theodorescu et al., 2008 [156] | 12 protein pannel + age + serum PSA | 86 Training set + 213 validation set | 91% | 62% | ||
| Okamoto et al., 2008 [158] | 72 masspicks | 57/113 | 91% | 83% | ||
| Mixture | Cao et al., 2010 [172] | mRNA, protein and metabolite (PCA3, TMPRSS2: ERG, ANXA3, Sarcosine, and urine PSA) | 86/131 | 95% | 50% | 0.86 |
| Prior et al., 2010 [173] | mRNA (AMACR/MMP2), DNA (GSTP1/RASSF1A) and PSA in serum and urine | 34/113 | 57% | 97% | 0.79 | |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rigau, M.; Olivan, M.; Garcia, M.; Sequeiros, T.; Montes, M.; Colás, E.; Llauradó, M.; Planas, J.; De Torres, I.; Morote, J.; et al. The Present and Future of Prostate Cancer Urine Biomarkers. Int. J. Mol. Sci. 2013, 14, 12620-12649. https://doi.org/10.3390/ijms140612620
Rigau M, Olivan M, Garcia M, Sequeiros T, Montes M, Colás E, Llauradó M, Planas J, De Torres I, Morote J, et al. The Present and Future of Prostate Cancer Urine Biomarkers. International Journal of Molecular Sciences. 2013; 14(6):12620-12649. https://doi.org/10.3390/ijms140612620
Chicago/Turabian StyleRigau, Marina, Mireia Olivan, Marta Garcia, Tamara Sequeiros, Melania Montes, Eva Colás, Marta Llauradó, Jacques Planas, Inés De Torres, Juan Morote, and et al. 2013. "The Present and Future of Prostate Cancer Urine Biomarkers" International Journal of Molecular Sciences 14, no. 6: 12620-12649. https://doi.org/10.3390/ijms140612620
APA StyleRigau, M., Olivan, M., Garcia, M., Sequeiros, T., Montes, M., Colás, E., Llauradó, M., Planas, J., De Torres, I., Morote, J., Cooper, C., Reventós, J., Clark, J., & Doll, A. (2013). The Present and Future of Prostate Cancer Urine Biomarkers. International Journal of Molecular Sciences, 14(6), 12620-12649. https://doi.org/10.3390/ijms140612620





