Impaired Responses to In Vitro Lipopolysaccharide-Induced Stimulation After Long-Term, Rotating Shift Work
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Groups
2.2. Biospecimen Collection and LPS Stimulation
2.3. Quantitative Assays
2.4. Data Analysis
3. Results
3.1. Group Characteristics and Assessment of Immune Activation
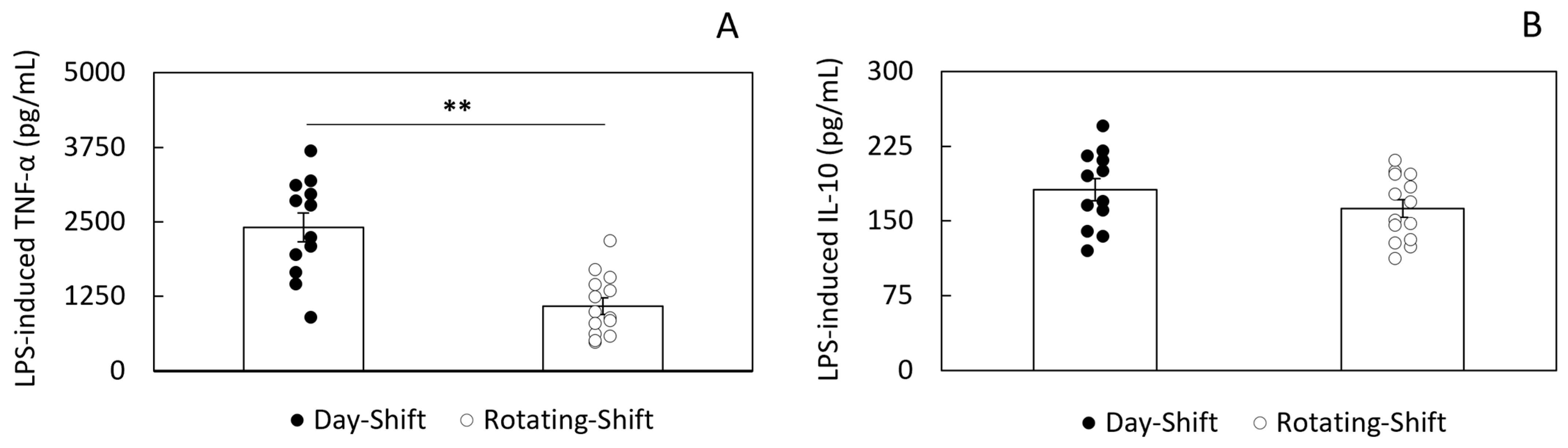
3.2. LPS-Induced Cytokine Secretion
3.3. Correlation Between Immune Activation and LPS-Induced Responsiveness
4. Discussion
5. Conclusions
Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LPS | Lipopolysaccharide |
| LBP | Lipopolysaccharide-binding protein |
| PBMCs | Peripheral blood mononuclear cells |
| sCD14 | Soluble CD14 |
References
- IARC Working Group on the Identification of Carcinogenic Hazards to Humans. Night Shift Work; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Caruso, C.C. Negative impacts of shiftwork and long work hours. Rehabil. Nurs. 2013, 39, 16–25. [Google Scholar] [CrossRef]
- Moreno, C.R.C.; Marqueze, E.C.; Sargent, C.; Wright, K.P., Jr.; Ferguson, S.A.; Tucker, P. Working Time Society consensus statements: Evidence-based effects of shift work on physical and mental health. Ind. Health 2019, 57, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC) Working Conditions and Employment Benefits (NHIS 2004–2013) Charts. National Health Interview Survey (NHIS 2004–2013) 2015. Available online: https://wwwn.cdc.gov/Niosh-whc/topic/work (accessed on 8 May 2025).
- Vetter, C.; Devore, E.E.; Wegrzyn, L.R.; Massa, J.; Speizer, F.E.; Kawachi, I.; Rosner, B.; Stampfer, M.J.; Schernhammer, E.S. Association Between Rotating Night Shift Work and Risk of Coronary Heart Disease Among Women. JAMA 2016, 315, 1726–1734. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Li, Y.; Zong, G.; Guo, Y.; Li, J.; Manson, J.E.; Hu, F.B.; Willett, W.C.; Schernhammer, E.S.; Bhupathiraju, S.N. Rotating night shift work and adherence to unhealthy lifestyle in predicting risk of type 2 diabetes: Results from two large US cohorts of female nurses. BMJ 2018, 363, k4641. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Schernhammer, E.S.; Sun, Q.; Hu, F.B. Rotating night shift work and risk of type 2 diabetes: Two prospective cohort studies in women. PLoS Med. 2011, 8, e1001141. [Google Scholar] [CrossRef]
- Lawson, C.C.; Whelan, E.A.; Hibert, E.N.L.; Spiegelman, D.; Schernhammer, E.S.; Rich-Edwards, J.W. Rotating shift work and menstrual cycle characteristics. Epidemiology 2011, 22, 305–312. [Google Scholar] [CrossRef]
- Roman, P.; Perez-Cayuela, I.; Gil-Hernández, E.; Rodriguez-Arrastia, M.; Aparicio-Mota, A.; Ropero-Padilla, C.; Rueda-Ruzafa, L. Influence of Shift Work on The Health of Nursing Professionals. J. Pers. Med. 2023, 13, 627. [Google Scholar] [CrossRef]
- De Bacquer, D.; Van Risseghem, M.; Clays, E.; Kittel, F.; De Backer, G.; Braeckman, L. Rotating shift work and the metabolic syndrome: A prospective study. Leuk. Res. 2009, 38, 848–854. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.; Duan, P.; Liu, B.; Li, T.; Wang, C.; Li, H.; Yang, T.; Gan, Y.; Wang, X.; et al. Is shift work associated with a higher risk of overweight or obesity? A systematic review of observational studies with meta-analysis. Int. J. Epidemiol. 2018, 47, 1956–1971. [Google Scholar] [CrossRef]
- Grundy, A.; Cotterchio, M.; Kirsh, V.A.; Nadalin, V.; Lightfoot, N.; Kreiger, N. Rotating shift work associated with obesity in men from northeastern Ontario. Association entre le travail par quarts et l’obésité chez les hommes dans le nord-est de l’Ontario. Health Promot. Chronic Dis. Prev. Can. 2017, 37, 238–247. [Google Scholar] [CrossRef]
- Puttonen, S.; Viitasalo, K.; Härmä, M. Effect of shiftwork on systemic markers of inflammation. Chronobiol. Int. 2011, 28, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Alireza, S.; Khosro, S.; Omid, A.; Forough, S. Night work and inflammatory markers. Indian J. Occup. Environ. Med. 2011, 15, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-W.; Jang, E.-C.; Kwon, S.-C.; Han, W.; Kang, M.-S.; Nam, Y.-H.; Lee, Y.-J. Night shift work and inflammatory markers in male workers aged 20–39 in a display manufacturing company. Ann. Occup. Environ. Med. 2016, 28, 48. [Google Scholar] [CrossRef]
- Skogstad, M.; Aass, H.C.D.; Sirnes, P.A.; Mamen, A.; Skare, Ø.; Matre, D.; Hammer, S.E.; Goffeng, E.; Lunde, L.-K. Influence of Shift Work on Arterial Stiffness and Systemic Inflammation: A 3-Year Follow-up Study in Industry. J. Occup. Environ. Med. 2022, 65, 284–291. [Google Scholar] [CrossRef]
- Woo, S.-J.; Chae, C.-H.; Lim, J.-W. Association between shift work and inflammatory markers in workers at an electronics manufacturing company. Ann. Occup. Environ. Med. 2022, 34, e35. [Google Scholar] [CrossRef]
- Loef, B.; Dollé, M.E.; Proper, K.I.; van Baarle, D.; Lifelines Corona Research Initiative; van Kerkhof, L.W. Night-shift work is associated with increased susceptibility to SARS-CoV-2 infection. Chronobiol. Int. 2022, 39, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Bjorvatn, B.; Waage, S.; Emberland, K.E.; Litleskare, S.; Rebnord, I.K.; Forthun, I.; Rortveit, G. The associations between different types of infections and circadian preference and shift work. Chronobiol. Int. 2024, 41, 259–266. [Google Scholar] [CrossRef]
- Loef, B.; van Baarle, D.; van der Beek, A.J.; Sanders, E.A.M.; Bruijning-Verhagen, P.; Proper, K.I. Shift Work and Respiratory Infections in Health-Care Workers. Am. J. Epidemiol. 2019, 188, 509–517. [Google Scholar] [CrossRef]
- Silva, I.; Costa, D. Consequences of Shift Work and Night Work: A Literature Review. Healthcare 2023, 11, 1410. [Google Scholar] [CrossRef]
- Vyas, M.V.; Garg, A.X.; Iansavichus, A.V.; Costella, J.; Donner, A.; Laugsand, L.E.; Janszky, I.; Mrkobrada, M.; Parraga, G.; Hackam, D.G. Shift work and vascular events: Systematic review and meta-analysis. BMJ 2012, 345, e4800. [Google Scholar] [CrossRef]
- Brown, J.P.; Martin, D.; Nagaria, Z.; Verceles, A.C.; Jobe, S.L.; Wickwire, E.M. Mental Health Consequences of Shift Work: An Updated Review. Curr. Psychiatry Rep. 2020, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Berge, L.A.M.; Liu, F.-C.; Grimsrud, T.K.; Babigumira, R.; Støer, N.C.; Kjærheim, K.; Robsahm, T.E.; Ghiasvand, R.; Hosgood, H.D.; Samuelsen, S.O.; et al. Night shift work and risk of aggressive prostate cancer in the Norwegian Offshore Petroleum Workers (NOPW) cohort. Leuk. Res. 2022, 52, 1003–1014. [Google Scholar] [CrossRef]
- Cooper, G.S.; Parks, C.G.; Treadwell, E.L.; Clair, E.W.S.; Gilkeson, G.S.; Dooley, M.A. Occupational risk factors for the development of systemic lupus erythematosus. J. Rheumatol. 2004, 31, 1928–1933. [Google Scholar]
- Gao, R.-C.; Sang, N.; Jia, C.-Z.; Zhang, M.-Y.; Li, B.-H.; Wei, M.; Wu, G.-C. Association Between Sleep Traits and Rheumatoid Arthritis: A Mendelian Randomization Study. Front. Public Health 2022, 10, 940161. [Google Scholar] [CrossRef]
- Ranjbaran, Z.; Keefer, L.; Farhadi, A.; Stepanski, E.; Sedghi, S.; Keshavarzian, A. Impact of sleep disturbances in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2007, 22, 1748–1753. [Google Scholar] [CrossRef] [PubMed]
- Olsson, T.; Barcellos, L.F.; Alfredsson, L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 2016, 13, 25–36. [Google Scholar] [CrossRef]
- Thorkildsen, M.S.; Gustad, L.T.; Damås, J.K. The Effects of Shift Work on the Immune System: A Narrative Review. Sleep Sci. 2023, 16, e368–e374. [Google Scholar] [CrossRef]
- Loef, B.; Nanlohy, N.M.; Jacobi, R.H.J.; van de Ven, C.; Mariman, R.; van der Beek, A.J.; Proper, K.I.; van Baarle, D. Immunological effects of shift work in healthcare workers. Sci. Rep. 2019, 9, 18220. [Google Scholar] [CrossRef]
- Streng, A.A.; Loef, B.; Dollé, M.E.T.; van der Horst, G.T.J.; Chaves, I.; Proper, K.I.; van Kerkhof, L.W.M. Night shift work characteristics are associated with several elevated metabolic risk factors and immune cell counts in a cross-sectional study. Sci. Rep. 2022, 12, 2022. [Google Scholar] [CrossRef]
- Reinhardt, É.L.; Fernandes, P.A.C.M.; Markus, R.P.; Fischer, F.M. Night work effects on salivary cytokines TNF, IL-1β and IL-6. Chronobiol. Int. 2018, 36, 11–26. [Google Scholar] [CrossRef]
- Cuesta, M.; Boudreau, P.; Dubeau-Laramée, G.; Cermakian, N.; Boivin, D.B. Simulated Night Shift Disrupts Circadian Rhythms of Immune Functions in Humans. J. Immunol. 2016, 196, 2466–2475. [Google Scholar] [CrossRef] [PubMed]
- Atwater, A.Q.; Castanon-Cervantes, O. Uncovering Novel Biomarkers of Inflammation as Potential Screening Targets of Disease Risk in Healthcare Shift Workers: A Pilot Study. Int. J. Nurs. Health Care Res. 2023, 6, 101466. [Google Scholar] [CrossRef]
- Brooks, D.; Barr, L.C.; Wiscombe, S.; McAuley, D.F.; Simpson, A.J.; Rostron, A.J. Human lipopolysaccharide models provide mechanistic and therapeutic insights into systemic and pulmonary inflammation. Eur. Respir. J. 2020, 56, 1901298. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, D.M.; Lankelma, J.M.; Vachot, L.; Cerrato, E.; Pachot, A.; Wiersinga, W.J.; Textoris, J. Comparison of host immune responses to LPS in human using an immune profiling panel, in vivo endotoxemia versus ex vivo stimulation. Sci. Rep. 2020, 10, 9918. [Google Scholar] [CrossRef]
- Schildberger, A.; Rossmanith, E.; Eichhorn, T.; Strassl, K.; Weber, V. Monocytes, peripheral blood mononuclear cells, and THP-1 cells exhibit different cytokine expression patterns following stimulation with lipopolysaccharide. Mediat. Inflamm. 2013, 2013, 697972. [Google Scholar] [CrossRef]
- Ngkelo, A.; Meja, K.; Yeadon, M.; Adcock, I.; Kirkham, P.A. LPS induced inflammatory responses in human peripheral blood mononuclear cells is mediated through NOX4 and Giα dependent PI-3kinase signalling. J. Inflamm. 2012, 9, 1. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Kell, D.B.; Pretorius, E. On the translocation of bacteria and their lipopolysaccharides between blood and peripheral locations in chronic, inflammatory diseases: The central roles of LPS and LPS-induced cell death. Integr. Biol. 2015, 7, 1339–1377. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Kell, D.B.; Pretorius, E. The Role of Lipopolysaccharide-Induced Cell Signalling in Chronic Inflammation. Chronic Stress 2022, 6, 24705470221076390. [Google Scholar] [CrossRef]
- Zweigner, J.; Schumann, R.R.; Weber, J.R. The role of lipopolysaccharide-binding protein in modulating the innate immune response. Microbes Infect. 2006, 8, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C. The endotoxin hypothesis of neurodegeneration. J. Neuroinflamm. 2019, 16, 180. [Google Scholar] [CrossRef]
- Brown, G.C.; Heneka, M.T. The endotoxin hypothesis of Alzheimer’s disease. Mol. Neurodegener. 2024, 19, 30. [Google Scholar] [CrossRef]
- Morris, M.C.; Gilliam, E.A.; Li, L. Innate immune programing by endotoxin and its pathological consequences. Front. Immunol. 2015, 5, 680. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Quintela, A.; Alonso, M.; Campos, J.; Vizcaino, L.; Loidi, L.; Gude, F. Determinants of serum concentrations of lipopolysaccharide-binding protein (LBP) in the adult population: The role of obesity. PLoS ONE 2013, 8, e54600. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Cho, Y.S.; Baek, K.S.; Li, L.; Baek, K.-H.; Kim, J.H.; Kim, H.-S.; Sheen, Y.H. Lipopolysaccharide-binding protein plasma levels as a biomarker of obesity-related insulin resistance in adolescents. Korean J. Pediatr. 2016, 59, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Chellappa, S.L.; Morris, C.J.; Scheer, F.A.J.L. Circadian misalignment increases mood vulnerability in simulated shift work. Sci. Rep. 2020, 10, 18614. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.J.; Yang, J.N.; Garcia, J.I.; Myers, S.; Bozzi, I.; Wang, W.; Buxton, O.M.; Shea, S.A.; Scheer, F.A.J.L. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc. Natl. Acad. Sci. USA 2015, 112, E2225–E2234. [Google Scholar] [CrossRef]
- Morris, C.J.; Purvis, T.E.; Mistretta, J.; Hu, K.; Scheer, F.A.J.L. Circadian Misalignment Increases C-Reactive Protein and Blood Pressure in Chronic Shift Workers. J. Biol. Rhythm. 2017, 32, 154–164. [Google Scholar] [CrossRef]
- Bøggild, H.; Knutsson, A. Shift work, risk factors and cardiovascular disease. Scand. J. Work Environ. Health 1999, 25, 85–99. [Google Scholar] [CrossRef]
- Gu, F.; Han, J.; Laden, F.; Pan, A.; Caporaso, N.E.; Stampfer, M.J.; Kawachi, I.; Rexrode, K.M.; Willett, W.C.; Hankinson, S.E.; et al. Total and cause-specific mortality of U.S. nurses working rotating night shifts. Am. J. Prev. Med. 2015, 48, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Stock, D.; Knight, J.A.; Raboud, J.; Cotterchio, M.; Strohmaier, S.; Willett, W.; Eliassen, A.H.; Rosner, B.; Hankinson, S.E.; Schernhammer, E. Rotating night shift work and menopausal age. Hum. Reprod. 2019, 34, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Papantoniou, K.; Devore, E.E.; Massa, J.; Strohmaier, S.; Vetter, C.; Yang, L.; Shi, Y.; Giovannucci, E.; Speizer, F.; Schernhammer, E.S. Rotating night shift work and colorectal cancer risk in the nurses’ health studies. Int. J. Cancer 2018, 143, 2709–2717. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Chen, W.; Lin, X. Night-shift work, breast cancer incidence, and all-cause mortality: An updated meta-analysis of prospective cohort studies. Sleep Breath. 2021, 26, 1509–1526. [Google Scholar] [CrossRef]
- Gao, Y.; Gan, T.; Jiang, L.; Yu, L.; Tang, D.; Wang, Y.; Li, X.; Ding, G. Association between shift work and risk of type 2 diabetes mellitus: A systematic review and dose-response meta-analysis of observational studies. Chronobiol. Int. 2019, 37, 29–46. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- O’Rourke, R.W. Inflammation in obesity-related diseases. Surgery 2008, 145, 255–259. [Google Scholar] [CrossRef]
- Brandes-Leibovitz, R.; Riza, A.; Yankovitz, G.; Pirvu, A.; Dorobantu, S.; Dragos, A.; Streata, I.; Ricaño-Ponce, I.; de Nooijer, A.; Dumitrescu, F.; et al. Sepsis pathogenesis and outcome are shaped by the balance between the transcriptional states of systemic inflammation and antimicrobial response. Cell Rep. Med. 2024, 5, 101829. [Google Scholar] [CrossRef]
- de Punder, K.; Pruimboom, L. Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Front. Immunol. 2015, 6, 223. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Rowe, D.C.; Golenbock, D.T. Endotoxin recognition and signal transduction by the TLR4/MD2-complex. Microbes Infect. 2004, 6, 1361–1367. [Google Scholar] [CrossRef]
- Glaros, T.G.; Chang, S.; Gilliam, E.A.; Maitra, U.; Deng, H.; Li, L. Causes and consequences of low grade endotoxemia and inflammatory diseases. Front. Biosci. 2013, 5, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.; Thiemermann, C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front. Immunol. 2021, 11, 594150. [Google Scholar] [CrossRef] [PubMed]
- Barchetta, I.; Cimini, F.A.; Sentinelli, F.; Chiappetta, C.; Di Cristofano, C.; Silecchia, G.; Leonetti, F.; Baroni, M.G.; Cavallo, M.G. Reduced Lipopolysaccharide-Binding Protein (LBP) Levels Are Associated with Non-Alcoholic Fatty Liver Disease (NAFLD) and Adipose Inflammation in Human Obesity. Int. J. Mol. Sci. 2023, 24, 17174. [Google Scholar] [CrossRef]
- Shive, C.L.; Jiang, W.; Anthony, D.D.; Lederman, M.M. Soluble CD14 is a nonspecific marker of monocyte activation. AIDS 2015, 29, 1263–1265. [Google Scholar] [CrossRef]
- Fuster, D.; Garcia-Calvo, X.; Farré, O.; Zuluaga, P.; Bolao, F.; Leis, A.; Hernández-Rubio, A.; Rivas, I.; Muga, R. Markers of Monocyte Activation, Inflammation, and Microbial Translocation Are Associated with Liver Fibrosis in Alcohol Use Disorder. J. Clin. Med. 2021, 10, 3496. [Google Scholar] [CrossRef]
- Plevin, R.E.; Knoll, M.; McKay, M.; Arbabi, S.; Cuschieri, J. The Role of Lipopolysaccharide Structure in Monocyte Activation and Cytokine Secretion. Shock 2016, 45, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.C.; Andersen, M.N.; Møller, H.J. Monocyte isolation techniques significantly impact the phenotype of both isolated monocytes and derived macrophages in vitro. Immunology 2019, 159, 63–74. [Google Scholar] [CrossRef]
- Anderson, J.; Toh, Z.Q.; Reitsma, A.; Do, L.A.H.; Nathanielsz, J.; Licciardi, P.V. Effect of peripheral blood mononuclear cell cryopreservation on innate and adaptive immune responses. J. Immunol. Methods 2019, 465, 61–66. [Google Scholar] [CrossRef]
- Martikainen, M.-V.; Roponen, M. Cryopreservation affected the levels of immune responses of PBMCs and antigen-presenting cells. Toxicol. In Vitro 2020, 67, 104918. [Google Scholar] [CrossRef]
- Linder, A.; Portmann, K.; Eyer, K. The impact of cryopreservation on cytokine secretion and polyfunctionality in human PBMCs: A comparative study. Front. Immunol. 2024, 15, 1478311. [Google Scholar] [CrossRef]
- Kapellos, T.S.; Bonaguro, L.; Gemünd, I.; Reusch, N.; Saglam, A.; Hinkley, E.R.; Schultze, J.L. Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front. Immunol. 2019, 10, 2035. [Google Scholar] [CrossRef]
- Zhou, D.; Huang, C.; Lin, Z.; Zhan, S.; Kong, L.; Fang, C.; Li, J. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell. Signal. 2014, 26, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Cavaillon, J.-M.; Adib-Conquy, M. Bench-to-bedside review: Endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit. Care 2006, 10, 233. [Google Scholar] [CrossRef]
- Seeley, J.J.; Ghosh, S. Tolerization of inflammatory gene expression. Cold Spring Harb. Symp. Quant. Biol. 2013, 78, 69–79. [Google Scholar] [CrossRef]
- Byrne, A.; Reen, D.J. Lipopolysaccharide induces rapid production of IL-10 by monocytes in the presence of apoptotic neutrophils. J. Immunol. 2002, 168, 1968–1977. [Google Scholar] [CrossRef] [PubMed]
- López-Collazo, E.; del Fresno, C. Endotoxin tolerance and trained immunity: Breaking down immunological memory barriers. Front. Immunol. 2024, 15, 1393283. [Google Scholar] [CrossRef] [PubMed]
- Kitchens, R.L.; Thompson, P.A. Modulatory effects of sCD14 and LBP on LPS-host cell interactions. J. Endotoxin Res. 2005, 11, 225–229. [Google Scholar] [CrossRef]
- Wright, S.D.; Ramos, R.A.; Tobias, P.S.; Ulevitch, R.J.; Mathison, J.C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 1990, 249, 1431–1433. [Google Scholar] [CrossRef]
- Hiki, N.; Berger, D.; Prigl, C.; Boelke, E.; Wiedeck, H.; Seidelmann, M.; Staib, L.; Kaminishi, M.; Oohara, T.; Beger, H.G. Endotoxin binding and elimination by monocytes: Secretion of soluble CD14 represents an inducible mechanism counteracting reduced expression of membrane CD14 in patients with sepsis and in a patient with paroxysmal nocturnal hemoglobinuria. Infect. Immun. 1998, 66, 1135–1141. [Google Scholar] [CrossRef]
- Heinzelmann, M.; Mercer-Jones, M.; Cheadle, W.G.; Polk, H.C. CD14 expression in injured patients correlates with outcome. Ann. Surg. 1996, 224, 91–96. [Google Scholar] [CrossRef]
- Landmann, R.; Zimmerli, W.; Sansano, S.; Link, S.; Hahn, A.; Glauser, M.P.; Calandra, T. Increased circulating soluble CD14 is associated with high mortality in gram-negative Septic Shock. J. Infect. Dis. 1995, 171, 639–644. [Google Scholar] [CrossRef]
- Fingerle-Rowson, G.; Auersa, J.; Kreuzer, E.; Labeta, M.; Schmidta, B.; Samtleben, W.; Ziegler-Heitbrock, H.; Blumenstein, M. Down-regulation of surface monocyte lipopolysaccharide-receptor cd14 in patients on cardiopulmonary bypass undergoing aorta-coronary bypass operation. J. Thorac. Cardiovasc. Surg. 1998, 115, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Mascia, C.; Pozzetto, I.; Kertusha, B.; Marocco, R.; Del Borgo, C.; Tieghi, T.; Vita, S.; Savinelli, S.; Iannetta, M.; Vullo, V.; et al. Persistent high plasma levels of sCD163 and sCD14 in adult patients with measles virus infection. PLoS ONE 2018, 13, e0198174. [Google Scholar] [CrossRef]
- Kitchens, R.L.; Thompson, P.A.; Viriyakosol, S.; O’keefe, G.E.; Munford, R.S. Plasma CD14 decreases monocyte responses to LPS by transferring cell-bound LPS to plasma lipoproteins. J. Clin. Investig. 2001, 108, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Frey, E.A.; Miller, D.S.; Jahr, T.G.; Sundan, A.; Bazil, V.; Espevik, T.; Finlay, B.B.; Wright, S.D. Soluble CD14 participates in the response of cells to lipopolysaccharide. J. Exp. Med. 1992, 176, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef]
- Rey Nores, J.E.; Bensussan, A.; Vita, N.; Stelter, F.; Arias, M.A.; Jones, M.; Lefort, S.; Borysiewicz, L.K.; Ferrara, P.; Labéta, M.O. Soluble CD14 acts as a negative regulator of human T cell activation and function. Eur. J. Immunol. 1999, 29, 265–276. [Google Scholar] [CrossRef]
- Richard, C.; Wadowski, M.; Goruk, S.; Cameron, L.; Sharma, A.M.; Field, C.J. Individuals with obesity and type 2 diabetes have additional immune dysfunction compared with obese individuals who are metabolically healthy. BMJ Open Diabetes Res. Care 2017, 5, e000379. [Google Scholar] [CrossRef]
- Khondkaryan, L.; Margaryan, S.; Poghosyan, D.; Manukyan, G. Impaired Inflammatory Response to LPS in Type 2 Diabetes Mellitus. Int. J. Inflamm. 2018, 2018, 2157434. [Google Scholar] [CrossRef]
- del Fresno, C.; Gómez-Piña, V.; Lores, V.; Soares-Schanoski, A.; Fernández-Ruiz, I.; Rojo, B.; Alvarez-Sala, R.; Caballero-Garrido, E.; García, F.; Veliz, T.; et al. Monocytes from cystic fibrosis patients are locked in an LPS tolerance state: Down-regulation of TREM-1 as putative underlying mechanism. PLoS ONE 2008, 3, e2667. [Google Scholar] [CrossRef]
- Dorneles, G.P.; Teixeira, P.C.; Peres, A.; Júnior, L.C.R.; da Fonseca, S.G.; Monteiro, M.C.; Eller, S.; Oliveira, T.F.; Wendland, E.M.; Romão, P.R.T. Endotoxin tolerance and low activation of TLR-4/NF-κB axis in monocytes of COVID-19 patients. J. Mol. Med. 2023, 101, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Carlini, V.; Noonan, D.M.; Abdalalem, E.; Goletti, D.; Sansone, C.; Calabrone, L.; Albini, A. The multifaceted nature of IL-10: Regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front. Immunol. 2023, 14, 1161067. [Google Scholar] [CrossRef] [PubMed]
- Fekedulegn, D.; Burchfiel, C.M.; Hartley, T.A.; Andrew, M.E.; Charles, L.E.; Tinney-Zara, C.A.; Violanti, J.M. Shiftwork and sickness absence among police officers: The BCOPS study. Chronobiol. Int. 2013, 30, 930–941. [Google Scholar] [CrossRef]
- Costa, G. Shift work and health: Current problems and preventive actions. Saf. Health Work 2010, 1, 112–123. [Google Scholar] [CrossRef] [PubMed]
| Day Shift (n = 12) | Rotating Shift (n = 14) | |
|---|---|---|
| BMI (Kg/m2) | 25.97 ± 0.42 | 26.1 ± 0.26 |
| Age (years) | 47.75 ± 1.95 | 45.64 ± 1.71 |
| Gender (% female) | 83.3 | 78.6 |
| Shift-work exposure (years) ** | 0 | 20.86 ± 1.11 |
| Day Shift (n = 12) | Rotating Shift (n = 14) | |
|---|---|---|
| CRP (mg/L) ** | 1.85 ± 0.16 | 3.08 ± 0.21 |
| LBP (µg/mL) ** | 5.95 ± 0.2 | 7.6 ± 0.26 |
| sCD14 (µg/mL) ** | 2.54 ± 0.34 | 4.55 ± 0.33 |
| TNF-α (pg/mL) ** | 14.93 ± 0.68 | 19.64 ± 0.91 |
| IL-10 (pg/mL) ** | 2.23 ± 0.18 | 5.71 ± 0.29 |
| LPS-Induced TNF-α | LPS-Induced IL-10 | |
|---|---|---|
| Plasma LBP | Day Shift: r (10) = 0.87, p =0.0002 ** Rotating Shift: r (12) = 0.18, p = 0.54 | Day Shift: r (10) = 0.78 p = 0.003 ** Rotating Shift: r (12) = 0.67, p = 0.009 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, D.M.; Castanon-Cervantes, O. Impaired Responses to In Vitro Lipopolysaccharide-Induced Stimulation After Long-Term, Rotating Shift Work. Int. J. Environ. Res. Public Health 2025, 22, 791. https://doi.org/10.3390/ijerph22050791
Jackson DM, Castanon-Cervantes O. Impaired Responses to In Vitro Lipopolysaccharide-Induced Stimulation After Long-Term, Rotating Shift Work. International Journal of Environmental Research and Public Health. 2025; 22(5):791. https://doi.org/10.3390/ijerph22050791
Chicago/Turabian StyleJackson, Denise M., and Oscar Castanon-Cervantes. 2025. "Impaired Responses to In Vitro Lipopolysaccharide-Induced Stimulation After Long-Term, Rotating Shift Work" International Journal of Environmental Research and Public Health 22, no. 5: 791. https://doi.org/10.3390/ijerph22050791
APA StyleJackson, D. M., & Castanon-Cervantes, O. (2025). Impaired Responses to In Vitro Lipopolysaccharide-Induced Stimulation After Long-Term, Rotating Shift Work. International Journal of Environmental Research and Public Health, 22(5), 791. https://doi.org/10.3390/ijerph22050791





