HLA Polymorphisms Linked to the Severity and Extent of Periodontitis in Patients with Type 1 Diabetes from a Brazilian Mixed Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample
2.3. Demographic and General Health Data Collection
2.4. HLA Allele Determination
2.5. Autosomal Ancestry Percentage
2.6. Periodontal Clinical Examination
2.7. Statistical Analysis
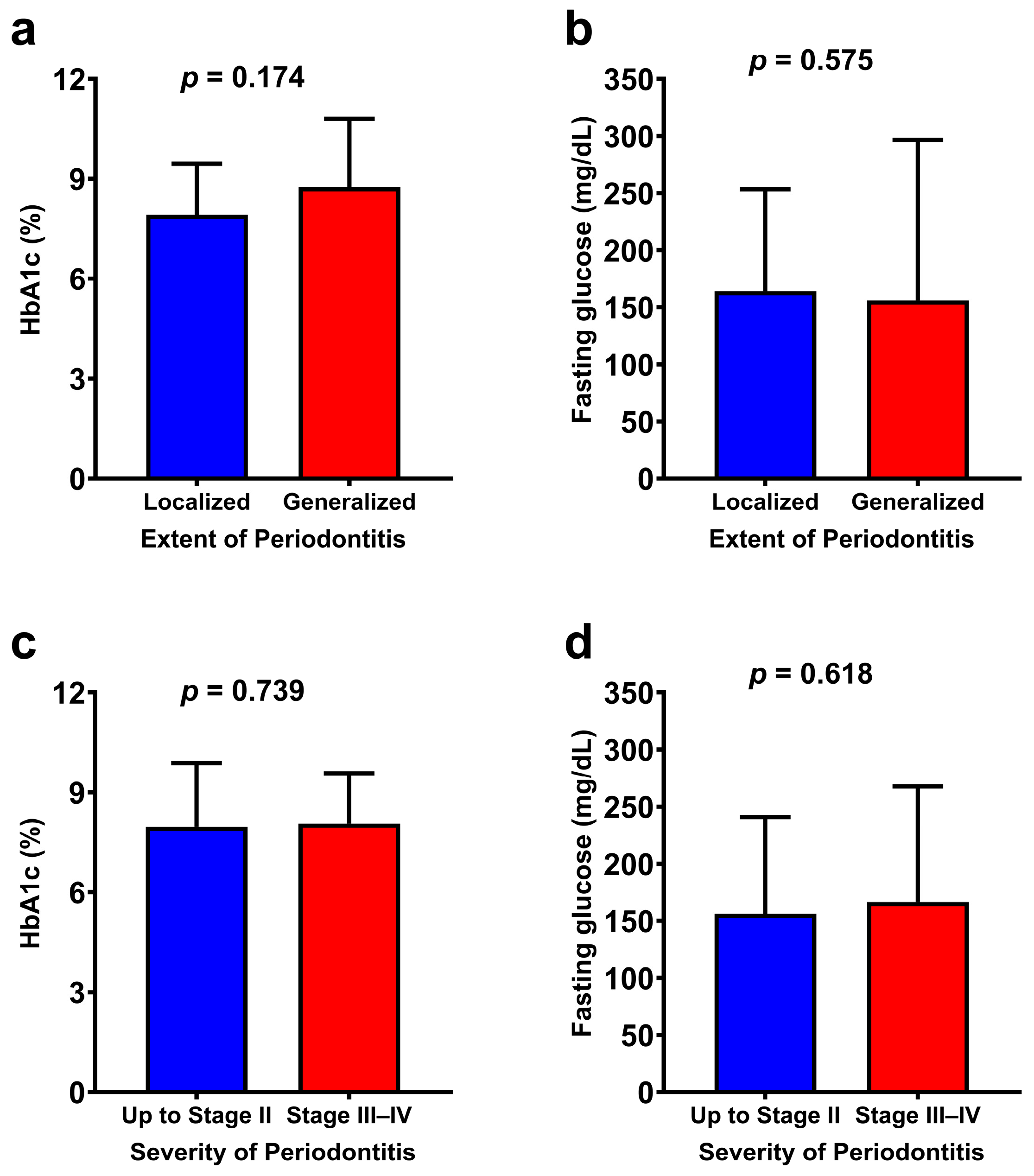
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | Confidence interval |
| CWD | Common and well-documented |
| GBI | Gingival bleeding index |
| HbA1c | glycated hemoglobin |
| HUUFMA | University Hospital of the Federal University of Maranhão |
| IL | Interleukin |
| OR | Odds ratio |
| SD | Standard deviation |
| T1D | Type 1 diabetes |
| VPI | Visible plaque index |
References
- Orlandi, M.; Aguilera, E.M.; Marletta, D.; Petrie, A.; Suvan, J.; D’Aiuto, F. Impact of the treatment of periodontitis on systemic health and quality of life: A systematic review. J. Clin. Periodontol. 2022, 49, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.C.; Dias-Pereira, A.C.; Branco-de-Almeida, L.S.; Martins, C.C.; Paiva, S.M. Impact of periodontal disease on quality of life: A systematic review. J. Periodontal. Res. 2017, 52, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Trindade, D.; Carvalho, R.; Machado, V.; Chambrone, L.; Mendes, J.J.; Botelho, J. Prevalence of periodontitis in dentate people between 2011 and 2020: A systematic review and meta-analysis of epidemiological studies. J. Clin. Periodontol. 2023, 50, 604–626. [Google Scholar] [CrossRef]
- Ray, R.R. Periodontitis: An Oral Disease with Severe Consequences. Appl. Biochem. Biotechnol. 2023, 195, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef]
- Atkinson, M.A. The pathogenesis and natural history of type 1 diabetes. Cold Spring Harb. Perspect. Med. 2012, 2, a007641. [Google Scholar] [CrossRef]
- Lindbladh, I.; Svärd, A.A.; Lernmark, Å. Autoimmune (type 1) diabetes. Autoimm. Dis. 2020, 1, 769–787. [Google Scholar] [CrossRef]
- Ilonen, J.; Lempainen, J.; Veijola, R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 635–650. [Google Scholar] [CrossRef]
- Jazi, M.M.; Solgi, G.; Aslroosta, H.; Noshad, S.; Moslemi, N.; Sadrimanesh, R.; Moradi, B.; Amirzargar, A.A. HLA-DRB and HLA-DQA/HLA-DQB allele and haplotype frequencies in Iranian patients with aggressive periodontitis. J. Periodontal. Res. 2013, 48, 533–539. [Google Scholar] [CrossRef]
- Primavera, M.; Giannini, C.; Chiarelli, F. Prediction and Prevention of Type 1 Diabetes. Front. Endocrinol. 2020, 11, 248. [Google Scholar] [CrossRef]
- Hassan, M.M.; Hussain, M.A.; Ali, S.S.; Mahdi, M.A. In Silico Analysis: HLA-DRB1 Gene’s Variants and Their Clinical Impact. Cell Transplant. 2023, 32, 9636897231184473. [Google Scholar] [CrossRef] [PubMed]
- Durge, K.; Baliga, V.; Dhadse, P.; Agrawal, D.; Sethiya, K.; Nibudey, A. Human leukocyte antigen and periodontal diseases. J. Datta Meghe Inst. Med. Sci. Univ. 2021, 16, 401–403. [Google Scholar] [CrossRef]
- Okada, Y.; Meguro, M.; Ohyama, H.; Yoshizawa, S.; Takeuchi-Hatanaka, K.; Kato, N.; Matsushita, S.; Takashiba, S.; Nishimura, F. Human leukocyte histocompatibility antigen class II-induced cytokines from human gingival fibroblasts promote proliferation of human umbilical vein endothelial cells: Potential association with enhanced angiogenesis in chronic periodontal inflammation. J. Periodontal. Res. 2009, 44, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, H.; Nishimura, F.; Meguro, M.; Takashiba, S.; Murayama, Y.; Matsushita, S. Counter-antigen presentation: Fibroblasts produce cytokines by signalling through HLA class II molecules without inducing T-cell proliferation. Cytokine 2002, 17, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Taba, M.; de Souza, S.L.S.; Mariguela, V.C. Periodontal disease: A genetic perspective. Braz. Oral. Res. 2012, 26, 32–38. [Google Scholar] [CrossRef]
- Bali, D.; Pandit, N.; Kathuria, R.; Bali, A. Genetics and Aggressive Periodontal Disease: An Update Review. J. Oral Health Community Dent. 2012, 6, 97–101. [Google Scholar] [CrossRef]
- Al-Ghurabi, B.H.; Mahmood, H. Frequency of HLA-DRB1 alleles in Iraqi patients with chronic periodontitis. Int. J. Immunol. Res. 2013, 3, 25–28. [Google Scholar]
- Cavalla, F.; Biguetti, C.C.; Melchiades, J.L.; Tabanez, A.P.; Azevedo, M.D.C.S.; Trombone, A.P.F.; Faveri, M.; Feres, M.; Garlet, G.P. Genetic Association with Subgingival Bacterial Colonization in Chronic Periodontitis. Genes 2018, 9, 271. [Google Scholar] [CrossRef]
- Fernando, M.M.A.; Stevens, C.R.; Walsh, E.C.; De Jager, P.L.; Goyette, P.; Plenge, R.M.; Vyse, T.J.; Rioux, J.D. Defining the role of the MHC in autoimmunity: A review and pooled analysis. PLoS Genet. 2008, 4, e1000024. [Google Scholar] [CrossRef]
- Shimomura-Kuroki, J.; Yamashita, K.; Shimooka, S. Tannerella forsythia and the HLA-DQB1 allele are associated with susceptibility to periodontal disease in Japanese adolescents. Odontology 2009, 97, 32–37. [Google Scholar] [CrossRef]
- Zhang, S.J.; Yang, A.L.; Zhang, J.C.; Zhang, Y.H.; Tang, Y. Association of HLA-DRB1*1501 polymorphism with the susceptibility to severe chronic periodontitis in Chinese Han nationality. Shanghai J. Stomatol. 2004, 13, 182–185. [Google Scholar]
- Chowdhury, M.; Agrawal, N.; Kundu, D.; Biswas, N. Association of human leukocyte antigens Class I and Class II antigens with chronic periodontitis in East India. J. Indian Soc. Periodontol. 2017, 21, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Sippert, E.Â.; Silva, C.D.O.E.; Ayo, C.M.; Marques, S.B.D.; Visentainer, J.E.L.; Sell, A.M. HLA Haplotypes and Genotypes Frequencies in Brazilian Chronic Periodontitis Patients. Mediat. Inflamm. 2015, 2015, 481656. [Google Scholar] [CrossRef]
- Mauramo, M.; Ramseier, A.M.; Buser, A.; Tiercy, J.M.; Weiger, R.; Waltimo, T. Associations of HLA-A, -B and -DRB1 types with oral diseases in Swiss adults. PLoS ONE 2014, 9, e103527. [Google Scholar] [CrossRef]
- Alves, C.; Meyer, I.; Vieira, N.; Toralles, M.B.P.; LeMaire, D. Distribution and frequency of HLA alleles and haplotypes in Brazilians with type 1 diabetes mellitus. Arq. Bras. Endocrinol. Metabol. 2006, 50, 436–444. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas 2021; International Diabetes Federation: Brussels, Belgium, 2021; pp. 1–10. [Google Scholar]
- Dicembrini, I.; Serni, L.; Monami, M.; Caliri, M.; Barbato, L.; Cairo, F.; Mannucci, E. Type 1 diabetes and periodontitis: Prevalence and periodontal destruction-a systematic review. Acta Diabetol. 2020, 57, 1405–1412. [Google Scholar] [CrossRef]
- Reddy, M.; Gopalkrishna, P. Type 1 diabetes and periodontal disease: A literature review. Can. J. Dent. Hyg. 2022, 56, 22. [Google Scholar]
- Santos, D.C.; Porto, L.C.; Oliveira, R.V.; Secco, D.; Hanhoerderster, L.; Pizarro, M.H.; Barros, B.S.V.; Mello, L.G.N.; Muniz, L.H.; Silva, D.A.; et al. HLA class II genotyping of admixed Brazilian patients with type 1 diabetes according to self-reported color/race in a nationwide study. Sci. Rep. 2020, 10, 6628. [Google Scholar] [CrossRef]
- Hurley, C.K.; Kempenich, J.; Wadsworth, K.; Sauter, J.; Hofmann, J.A.; Schefzyk, D.; Schmidt, A.H.; Galarza, P.; Cardozo, M.B.R.; Dudkiewicz, M.; et al. Common, intermediate and well-documented HLA alleles in world populations: CIWD version 3.0.0. HLA 2020, 95, 516–531. [Google Scholar] [CrossRef]
- Ainamo, J.; Bay, I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar]
- Salvi, G.E.; Roccuzzo, A.; Imber, J.C.; Stähli, A.; Klinge, B.; Lang, N.P. Clinical periodontal diagnosis. Periodontol. 2000 2023, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Clin. Periodontol. 2018, 45, S149–S161. [Google Scholar] [CrossRef] [PubMed]
- Gosho, M.; Ohigashi, T.; Nagashima, K.; Ito, Y.; Maruo, K. Bias in odds ratios from logistic regression methods with sparse data sets. J. Epidemiol. 2023, 33, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Azulay, R.S.D.S.; Porto, L.C.; Silva, D.A.; Tavares, M.D.G.; Reis, R.M.D.F.; Nascimento, G.C.; da Silva Pereira Damianse, S.; de Carvalho Rocha, V.C.; Magalhães, M.; Rodrigues, V.; et al. Genetic ancestry inferred from autosomal and Y chromosome markers and HLA genotypes in Type 1 Diabetes from an admixed Brazilian population. Sci. Rep. 2021, 11, 14157. [Google Scholar] [CrossRef]
- Takashiba, S.; Ohyama, H.; Oyaizu, K.; Kogoe-Kato, N.; Murayama, Y. HLA genetics for diagnosis of susceptibility to early-onset periodontitis. J. Periodontal Res. 1999, 34, 374–378. [Google Scholar] [CrossRef]
- Wang, H.; Pan, Y. Screening and analysis of multi-alleles in generalized aggressive periodontitis. Chin. J. Stomatol. 2008, 43, 406–409. [Google Scholar]
- Cruz-Tapias, P.; Pérez-Fernández, O.M.; Rojas-Villarraga, A.; Rodríguez-Rodríguez, A.; Arango, M.T.; Anaya, J.M. Shared HLA Class II in Six Autoimmune Diseases in Latin America: A Meta-Analysis. Autoimm. Dis. 2012, 2012, 569728. [Google Scholar] [CrossRef]
- Wysocki, T.; Olesińska, M.; Paradowska-Gorycka, A. Current understanding of an emerging role of HLA-DRB1 gene in rheumatoid arthritis–from research to clinical practice. Cells 2020, 9, 1127. [Google Scholar] [CrossRef]
- Wang, J.; Jelcic, I.; Mühlenbruch, L.; Haunerdinger, V.; Toussaint, N.C.; Zhao, Y.; Cruciani, C.; Faigle, W.; Naghavian, R.; Foege, M.; et al. HLA-DR15 molecules jointly shape an autoreactive T cell repertoire in multiple sclerosis. Cell 2020, 183, 1264–1281. [Google Scholar] [CrossRef]
- Silva, J.A.; Santos, M.D.S.; Guimarães, P.E.M.; Ferreira, A.C.S.; Bandelt, H.J.; Pena, S.D.J.; Prado, V.F. The Ancestry of Brazilian mtDNA Lineages. Am. J. Hum. Genet. 2000, 67, 444–461. [Google Scholar] [CrossRef]
- Gomes, M.B.; Rodrigues, V.; Santos, D.C.; Bôas, P.R.V.; Silva, D.A.; de Souza Azulay, R.S.; Dib, S.A.; Pavin, E.J.; Fernandes, V.O.; Montenegro Junior, R.M.; et al. Association between HLA class II alleles/haplotypes and genomic ancestry in Brazilian patients with type 1 diabetes: A nationwide exploratory study. Genes 2023, 14, 991. [Google Scholar] [CrossRef] [PubMed]


| Variables | Mean ± SD | n (%) |
|---|---|---|
| Demographic data | ||
| Sex | ||
| Male | 23 (46.9) | |
| Female | 26 (53.1) | |
| Age (in years) | 39.7 ± 9.9 | |
| Self-reported skin color/race | ||
| White | 8 (16.3) | |
| Black | 6 (12.2) | |
| Mixed race | 35 (71.4) | |
| Diabetes History | ||
| Age at diagnosis of T1D | 16.5 ± 10.0 | |
| Time since T1D diagnosis | 11.2 ± 7.9 | |
| HbA1c (%) | 8.0 ± 1.6 | |
| Fasting glucose | 167 ± 103 | |
| Periodontal Data | ||
| Periodontitis Stage | ||
| Stage I | 16 (32.7) | |
| Stage II | 23 (46.9) | |
| Stage III–IV | 10 (20.4) | |
| Extent of Periodontitis | ||
| Localized (<30% of teeth) | 43 (87.8) | |
| Generalized (≥30% of teeth) | 6 (12.2) | |
| GBI (%) | 4.7 ± 8.1 | |
| VPI (%) | 7.4 ± 8.8 |
| Variables | Mean ± SD | n (%) |
|---|---|---|
| % Autosomal ancestry | ||
| Native American | 24.2 ± 9.4 | |
| European | 44.6 ± 15.4 | |
| African | 31.2 ± 13.5 | |
| HLA-DRB1* haplogroup (2n) | ||
| DRB1*01 | 6 (6.1) | |
| DRB1*03 | 37 (37.8) | |
| DRB1*04 | 22 (22.4) | |
| DRB1*07 | 11 (11.2) | |
| DRB1*08 | 4 (4.1) | |
| DRB1*09 | 3 (3.1) | |
| DRB1*11 | 4 (4.1) | |
| DRB1*12 | 1 (1.0) | |
| DRB1*13 | 6 (6.1) | |
| DRB1*15 | 1 (1.0) | |
| DRB1*16 | 3 (3.1) | |
| HLA-DQA1* alleles (2n) | ||
| 01:01 | 6 (6.3) | |
| 01:02 | 7 (7.3) | |
| 01:03 | 1 (1.0) | |
| 02:01 | 11 (11.5) | |
| 03:01 | 25 (26.0) | |
| 03:02 | 3 (3.1) | |
| 04:01 | 4 (4.2) | |
| 05:01 | 35 (36.5) | |
| 05:05 | 4 (4.2) | |
| HLA-DQB1* alleles (2n) | ||
| 02:01 | 33 (33.7) | |
| 02:02 | 15 (15.3) | |
| 03:01 | 5 (5.1) | |
| 03:02 | 24 (24.5) | |
| 03:03 | 1 (1.0) | |
| 03:19 | 1 (1.0) | |
| 04:02 | 5 (5.1) | |
| 05:01 | 7 (7.1) | |
| 05:02 | 1 (1.0) | |
| 06:02 | 1 (1.0) | |
| 06:03 | 1 (1.0) | |
| 06:04 | 3 (3.1) | |
| 06:09 | 1 (1.0) |
| Variables | OR | (95% CI) | p Value |
|---|---|---|---|
| Outcome (Generalized Periodontitis) | |||
| HLA-DRB1* haplogroup (2n) | |||
| DRB1*01 | 0.23 | (0.01–4.29) | 0.167 |
| DRB1*03 | 19.8 | (1.14–346) | 0.003 * |
| DRB1*04 | 0.76 | (0.21–2.74) | 0.680 |
| DRB1*07 | 0.66 | (0.13–3.26) | 0.608 |
| DRB1*08 | 0.36 | (0.02–6.81) | 0.270 |
| DRB1*09 | 0.49 | (0.02–9.35) | 0.345 |
| DRB1*11 | 2.67 | (0.47–15.0) | 0.251 |
| DRB1*12 | 1.35 | (0.06–29.8) | 0.594 |
| DRB1*13 | 0.23 | (0.01–4.29) | 0.167 |
| DRB1*15 | 41.2 | (1.85–917) | <0.001 * |
| DRB1*16 | 0.49 | (0.02–935) | 0.345 |
| Outcome (Periodontitis Stage III–IV) | |||
| HLA-DRB1* haplogroup (2n) | |||
| DRB1*01 | 0.75 | (0.15–3.76) | 0.171 |
| DRB1*03 | 7.71 | (1.68–35.5) | 0.003 * |
| DRB1*04 | 0.61 | (0.21–1.77) | 0.367 |
| DRB1*07 | 0.32 | (0.06–1.51) | 0.135 |
| DRB1*08 | 0.20 | (0.01–3.66) | 0.135 |
| DRB1*09 | 0.27 | (0.01–5.03) | 0.200 |
| DRB1*11 | 1.33 | (0.24–7.17) | 0.737 |
| DRB1*12 | 0.74 | (0.03–16.2) | 0.469 |
| DRB1*13 | 0.75 | (0.15–3.76) | 0.731 |
| DRB1*15 | 21.2 | (0.97–461) | 0.005 * |
| DRB1*16 | 0.27 | (0.01–5.03) | 0.200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menezes, C.F.S.; Lage, L.M.; Santos, L.G.S.; Nascimento, G.C.; Magalhães, M.; Facundo, A.; Silva, D.A.; Porto, L.C.; Gomes, M.B.; Faria, M.d.S.; et al. HLA Polymorphisms Linked to the Severity and Extent of Periodontitis in Patients with Type 1 Diabetes from a Brazilian Mixed Population. Int. J. Environ. Res. Public Health 2025, 22, 512. https://doi.org/10.3390/ijerph22040512
Menezes CFS, Lage LM, Santos LGS, Nascimento GC, Magalhães M, Facundo A, Silva DA, Porto LC, Gomes MB, Faria MdS, et al. HLA Polymorphisms Linked to the Severity and Extent of Periodontitis in Patients with Type 1 Diabetes from a Brazilian Mixed Population. International Journal of Environmental Research and Public Health. 2025; 22(4):512. https://doi.org/10.3390/ijerph22040512
Chicago/Turabian StyleMenezes, Carlos Felipe Sousa, Lucas Meneses Lage, Luís Gustavo Souza Santos, Gilvan Cortês Nascimento, Marcelo Magalhães, Alexandre Facundo, Dayse Aparecida Silva, Luís Cristóvão Porto, Marília Brito Gomes, Manuel dos Santos Faria, and et al. 2025. "HLA Polymorphisms Linked to the Severity and Extent of Periodontitis in Patients with Type 1 Diabetes from a Brazilian Mixed Population" International Journal of Environmental Research and Public Health 22, no. 4: 512. https://doi.org/10.3390/ijerph22040512
APA StyleMenezes, C. F. S., Lage, L. M., Santos, L. G. S., Nascimento, G. C., Magalhães, M., Facundo, A., Silva, D. A., Porto, L. C., Gomes, M. B., Faria, M. d. S., Azulay, R. S., & Rodrigues, V. (2025). HLA Polymorphisms Linked to the Severity and Extent of Periodontitis in Patients with Type 1 Diabetes from a Brazilian Mixed Population. International Journal of Environmental Research and Public Health, 22(4), 512. https://doi.org/10.3390/ijerph22040512







