Effects of Multi-Component Exercise on Sleep Quality in Middle-Aged Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participant
2.2. Study Design
2.3. Intervention
2.3.1. Multi-Component Exercise Group (MCE)
2.3.2. Control Group
2.4. Outcome Measures
2.4.1. Pittsburgh Sleep Quality Index (PSQI)
2.4.2. Physical Fitness
2.4.3. Data Analysis
3. Results
3.1. Characteristics
3.2. Pittsburgh Sleep Quality Index (PSQI)
3.3. Physical Fitness
4. Discussion
4.1. Effects of the Intervention on Sleep Quality
4.2. Effects of the Intervention on Physical Fitness
4.3. Limitations and Directions for Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz, T.; García, L.; Álvarez, M.; Manzanero, A. Sleep quality and memory function in healthy ageing. Neurologia 2022, 37, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Vitiello, M.V.; Gooneratne, N.S. Sleep in normal aging. Sleep Med. Clin. 2018, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dumic, I.; Nordin, T.; Jecmenica, M.; Stojkovic Lalosevic, M.; Milosavljevic, T.; Milovanovic, T. Gastrointestinal tract disorders in older age. Can. J. Gastroenterol. Hepatol. 2019, 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Bond, J.; Dickinson, H.O.; Matthews, F.; Jagger, C.; Brayne, C. Self-rated health status as a predictor of death, functional and cognitive impairment: A longitudinal cohort study. Eur J. Ageing 2006, 3, 193–206. [Google Scholar] [CrossRef]
- Akishita, M.; Ishii, S.; Kojima, T.; Kozaki, K.; Kuzuya, M.; Arai, H.; Arai, H.; Eto, M.; Takahashi, R.; Endo, H. Priorities of health care outcomes for the elderly. J. Am. Med. Dir. Assoc. 2013, 14, 479–484. [Google Scholar] [CrossRef]
- Madrid-Valero, J.J.; Martínez-Selva, J.M.; Couto, B.R.D.; Sánchez-Romera, J.F.; Ordoñana, J.R. Age and gender effects on the prevalence of poor sleep quality in the adult population. Gac. Sanit. 2017, 31, 18–22. [Google Scholar] [CrossRef]
- Mukku, S.S.R.; Harbishettar, V.; Sivakumar, P.T. Insomnia in elderly: A neglected epidemic. J. Geriatr. Ment. Health (Online) 2018, 5, 84. [Google Scholar]
- Vanderlinden, J.; Boen, F.; Van Uffelen, J. Effects of physical activity programs on sleep outcomes in older adults: A systematic review. Int J. Behav. Nutr. Phys. Act. 2020, 17, 1–15. [Google Scholar] [CrossRef]
- Irwin, M.R.; Olmstead, R.; Motivala, S.J. Improving sleep quality in older adults with moderate sleep complaints: A randomized controlled trial of Tai Chi Chih. Sleep 2008, 31, 1001–1008. [Google Scholar]
- Banno, M.; Harada, Y.; Taniguchi, M.; Tobita, R.; Tsujimoto, H.; Tsujimoto, Y.; Kataoka, Y.; Noda, A. Exercise can improve sleep quality: A systematic review and meta-analysis. PeerJ 2018, 6, e5172. [Google Scholar] [CrossRef]
- Wang, F.; Boros, S. The effect of physical activity on sleep quality: A systematic review. Eur J. Physiothe. 2021, 23, 11–18. [Google Scholar] [CrossRef]
- Hartescu, I.; Morgan, K.; Stevinson, C.D. Increased physical activity improves sleep and mood outcomes in inactive people with insomnia: A randomized controlled trial. J. Sleep Res. 2015, 24, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Szuhany, K.L.; Bugatti, M.; Otto, M.W. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J. Psychiatr. Res. 2015, 60, 56–64. [Google Scholar] [CrossRef]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The physical activity guidelines for americans. Jama 2018, 320, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Liguori, G.; Medicine, A.C.O.S. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2020. [Google Scholar]
- Timmons, J.F.; Minnock, D.; Hone, M.; Cogan, K.E.; Murphy, J.C.; Egan, B. Comparison of time-matched aerobic, resistance, or concurrent exercise training in older adults. Scand. J. Med. Sci. Sports 2018, 28, 2272–2283. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, S.; Chen, X.-J.; Yu, H.-H.; Yang, Y.; Wang, W. Effects of exercise on sleep quality and insomnia in adults: A systematic review and meta-analysis of randomized controlled trials. Front. Psychiatry 2021, 12, 676. [Google Scholar] [CrossRef]
- Larkey, L.; Jahnke, R.; Etnier, J.; Gonzalez, J. Meditative movement as a category of exercise: Implications for research. J. Phys. Act. Health 2009, 6, 230–238. [Google Scholar] [CrossRef]
- Lowe, H.; Haddock, G.; Mulligan, L.D.; Gregg, L.; Fuzellier-Hart, A.; Carter, L.-A.; Kyle, S.D. Does exercise improve sleep for adults with insomnia? A systematic review with quality appraisal. Clin. Psychol. Rev. 2019, 68, 1–12. [Google Scholar] [CrossRef]
- Harinath, K.; Malhotra, A.S.; Pal, K.; Prasad, R.; Kumar, R.; Kain, T.C.; Rai, L.; Sawhney, R.C. Effects of Hatha yoga and Omkar meditation on cardiorespiratory performance, psychologic profile, and melatonin secretion. J. Altern. Complement. Med. 2004, 10, 261–268. [Google Scholar] [CrossRef]
- Ozaki, H.; Nakagata, T.; Yoshihara, T.; Kitada, T.; Natsume, T.; Ishihara, Y.; Deng, P.; Kobayashi, H.; Machida, S.; Naito, H. Effects of progressive walking and stair-climbing training program on muscle size and strength of the lower body in untrained older adults. J. Sports Sci. Med. 2019, 18, 722. [Google Scholar]
- Thomas, E.; Battaglia, G.; Patti, A.; Brusa, J.; Leonardi, V.; Palma, A.; Bellafiore, M. Physical activity programs for balance and fall prevention in elderly: A systematic review. Medicine 2019, 98, e16218. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Shapiro, S.L.; Swanick, S.; Roesch, S.C.; Mills, P.J.; Bell, I.; Schwartz, G.E. A randomized controlled trial of mindfulness meditation versus relaxation training: Effects on distress, positive states of mind, rumination, and distraction. Ann. Behav. Med. 2007, 33, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Riemann, D.; Baglioni, C.; Bassetti, C.; Bjorvatn, B.; Dolenc Groselj, L.; Ellis, J.G.; Espie, C.A.; Garcia-Borreguero, D.; Gjerstad, M.; Gonçalves, M. European guideline for the diagnosis and treatment of insomnia. J. Sleep Res. 2017, 26, 675–700. [Google Scholar] [CrossRef] [PubMed]
- Buric, I.; Farias, M.; Jong, J.; Mee, C.; Brazil, I.A. What is the molecular signature of mind–body interventions? A systematic review of gene expression changes induced by meditation and related practices. Front. Immunol. 2017, 670. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.C.; Ulmer, C.S.; Manber, R. Improving sleep with mindfulness and acceptance: A metacognitive model of insomnia. Behav. Res. Ther. 2012, 50, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, J.R.; Bowe, M.; Kellezi, B.; Butcher, A.; Groeger, J.A. Longitudinal associations between family identification, loneliness, depression, and sleep quality. Br. J. Health Psychol. 2020, 25, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds III, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Grandner, M.A.; Kripke, D.F.; Yoon, I.-Y.; Youngstedt, S.D. Criterion validity of the Pittsburgh Sleep Quality Index: Investigation in a non-clinical sample. Sleep Biol. Rhythms 2006, 4, 129–136. [Google Scholar] [CrossRef]
- Alcántara-Cordero, F.J.; Gómez-Píriz, P.T.; Sánchez-López, A.M.; Cabeza-Ruiz, R. Feasibility and reliability of a physical fitness tests battery for adults with intellectual disabilities: The SAMU DIS-FIT battery. Disabil. Health J. 2020, 13, 100886. [Google Scholar] [CrossRef]
- Ujuagu, N.A.; Uzor, T.N. Optimising Female Secondary School Teachers’body Composition and Muscular Endurance Using Circuit Training Exercise. South. East. J. Res. Sustain. Dev. 2021, 5, 31–49. [Google Scholar]
- Lacy, A.C.; Williams, S.M. Measurement and Evaluation in Physical Education and Exercise Science; Routledge: Oxfordshire, UK, 2018. [Google Scholar]
- Bohannon, R.W. Single limb stance times: A descriptive meta-analysis of data from individuals at least 60 years of age. Top. Geriatr. Rehabil. 2006, 22, 70–77. [Google Scholar] [CrossRef]
- Amiri, S.; Hasani, J.; Satkin, M. Effect of exercise training on improving sleep disturbances: A systematic review and meta-analysis of randomized control trials. Sleep Med. 2021, 84, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Mireku, M.O.; Rodriguez, A. Sleep duration and waking activities in relation to the national sleep foundation’s recommendations: An analysis of US population sleep patterns from 2015 to 2017. Int J. Environ. Res. Public Health 2021, 18, 6154. [Google Scholar] [CrossRef] [PubMed]
- Cortez-Cooper, M.Y.; Anton, M.M.; DeVan, A.E.; Neidre, D.B.; Cook, J.N.; Tanaka, H. The effects of strength training on central arterial compliance in middle-aged and older adults. Eur. J. Cardiovasc. Prev. Rehabil. 2008, 15, 149–155. [Google Scholar] [CrossRef]
- Lee, H.; Kim, S.; Kim, D. Effects of exercise with or without light exposure on sleep quality and hormone reponses. J. Exerc. Nutrition Biochem. 2014, 18, 293–299. [Google Scholar] [CrossRef]
- Wirojanan, J.; Jacquemont, S.; Diaz, R.; Bacalman, S.; Anders, T.F.; Hagerman, R.J.; Goodlin-Jones, B.L. The efficacy of melatonin for sleep problems in children with autism, fragile X syndrome, or autism and fragile X syndrome. J. Clin. Sleep Med. 2009, 5, 145–150. [Google Scholar] [CrossRef]
- Maski, K.; Owens, J.A. Insomnia, parasomnias, and narcolepsy in children: Clinical features, diagnosis, and management. Lancet Neurol. 2016, 15, 1170–1181. [Google Scholar] [CrossRef]
- Uchida, S.; Shioda, K.; Morita, Y.; Kubota, C.; Ganeko, M.; Takeda, N. Exercise effects on sleep physiology. Front. Neurol. 2012, 3, 48. [Google Scholar] [CrossRef]
- Sato, M.; Betriana, F.; Tanioka, R.; Osaka, K.; Tanioka, T.; Schoenhofer, S. Healthy lifestyle, autonomic nervous system activity, and sleep status for healthy aging. Int. J. Environ. Res. Public Health 2022. [Google Scholar] [CrossRef]
- Jerath, R.; Edry, J.W.; Barnes, V.A.; Jerath, V. Physiology of long pranayamic breathing: Neural respiratory elements may provide a mechanism that explains how slow deep breathing shifts the autonomic nervous system. Med. Hypotheses 2006, 67, 566–571. [Google Scholar] [CrossRef]
- Sato, M.; Betriana, F.; Tanioka, R.; Osaka, K.; Tanioka, T.; Schoenhofer, S. Balance of autonomic nervous activity, exercise, and sleep status in older adults: A review of the literature. Int. J. Environ. Res. Public Health 2021, 18, 12896. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, E.K.; Creswell, J.D. Mechanisms of mindfulness training: Monitor and Acceptance Theory (MAT). Clin. Psychol. Rev. 2017, 51, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Desbordes, G.; Negi, L.T.; Pace, T.W.; Wallace, B.A.; Raison, C.L.; Schwartz, E.L. Effects of mindful-attention and compassion meditation training on amygdala response to emotional stimuli in an ordinary, non-meditative state. Front. Hum. Neurosci 2012, 6, 292. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Lauderdale, D.S.; Waite, L.J. Social participation and older adults’ sleep. Soc. Sci 2016, 149, 164–173. [Google Scholar] [CrossRef]
- Kent de Grey, R.G.; Uchino, B.N.; Trettevik, R.; Cronan, S.; Hogan, J.N. Social support and sleep: A meta-analysis. Health Psychol. 2018, 37, 787–798. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, Y.; Kong, D.; Li, S.; Yang, N. The effects of social support on sleep quality of medical staff treating patients with coronavirus disease 2019 (COVID-19) in January and February 2020 in China. Med. Sci. Monit. 2020, 26, e923549-e1. [Google Scholar] [CrossRef]
- Nuzzo, J.L. The case for retiring flexibility as a major component of physical fitness. Sports Med. 2020, 50, 853–870. [Google Scholar] [CrossRef]
- Govindaraj, R.; Karmani, S.; Varambally, S.; Gangadhar, B. Yoga and physical exercise—A review and comparison. Int. Rev. Psychiatry 2016, 28, 242–253. [Google Scholar] [CrossRef]
- Hurley, B.F.; Hanson, E.D.; Sheaff, A.K. Strength training as a countermeasure to aging muscle and chronic disease. Sports Med. 2011, 41, 289–306. [Google Scholar] [CrossRef]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta-analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 1–10. [Google Scholar] [CrossRef]
- Im, J.Y.; Bang, H.S.; Seo, D.Y. The effects of 12 weeks of a combined exercise program on physical function and hormonal status in elderly Korean women. Int. J. Environ. Res. Public Health 2019, 16, 4196. [Google Scholar] [CrossRef] [PubMed]
- Hoogendijk, E.O.; Afilalo, J.; Ensrud, K.E.; Kowal, P.; Onder, G.; Fried, L.P. Frailty: Implications for clinical practice and public health. Lancet 2019, 394, 1365–1375. [Google Scholar] [CrossRef]
| All Participants (24) | Multi-Component Exercise (n = 12) | Control (n = 12) | |
|---|---|---|---|
| Gender (male/female) | 1.83 ± 0.38 | 2/10 | 2/10 |
| Age (years) | 54.7 ± 7.54 | 54.17 ± 6.70 | 55.25 ± 8.57 |
| Height (cm) | 160.71 ± 5.44 | 160.96 ± 5.28 | 160.46 ± 5.81 |
| BMI (kg/m2) PSQI global score | 26.40 ± 4.18 7.83 ± 3.70 | 24.22 ± 3.50 9.00 ± 4.16 | 26.52 ± 3.00 6.67 ± 2.90 |
| Multi-Component Exercise (n = 12) | Control (n = 12) | |||
|---|---|---|---|---|
| PSQI | Pre-Test | Post-Test | Pre-Test | Post-Test |
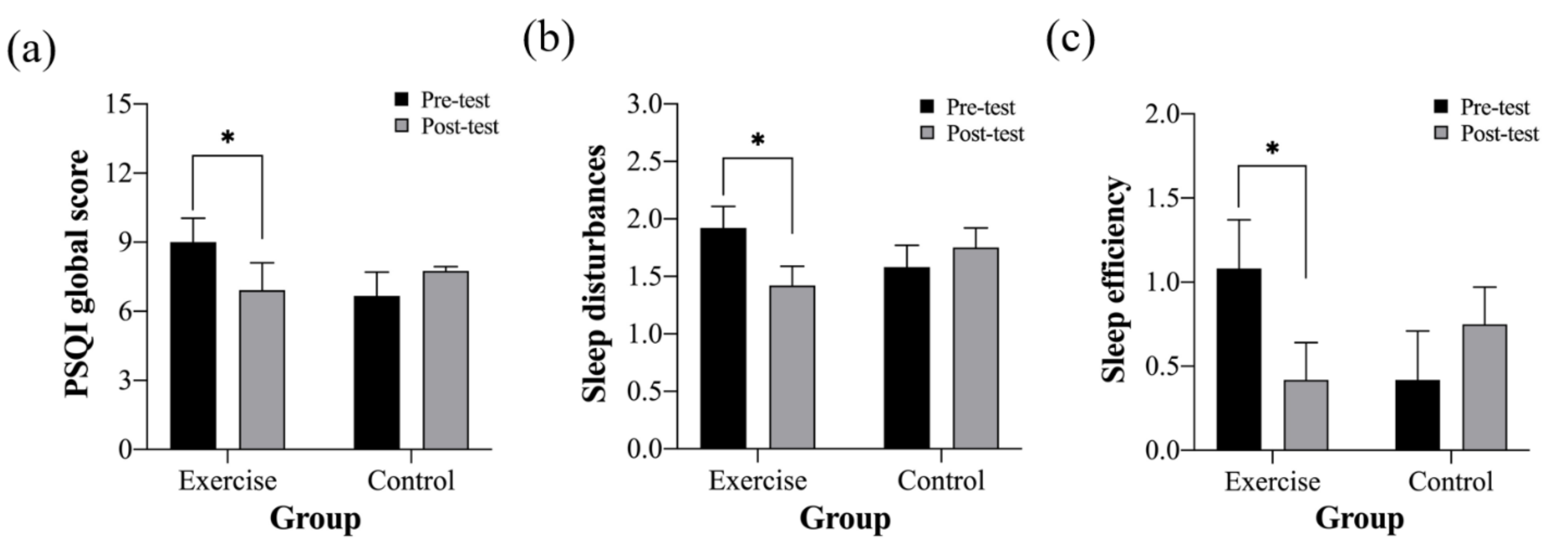
| PSQI global score | 9.00 ± 4.16 | 6.92 ± 4.10 * | 6.67 ± 2.90 | 7.75 ± 4.14 |
| Subjective sleep quality | 1.58 ± 0.79 | 1.08 ± 0.79 | 1.42 ± 0.79 | 1.42 ± 0.79 |
| Daytime dysfunction | 1.25 ± 0.75 | 0.75 ± 0.62 | 1.00 ± 0.74 | 0.92 ± 0.90 |
| Use sleeping medications | 0.58 ± 1.17 | 0.67 ± 1.12 | 0.00 ± 0.00 | 0.42 ± 1.00 |
| Sleep disturbances | 1.92 ± 0.79 | 1.42 ± 0.52 * | 1.58 ± 0.52 | 1.75 ± 0.62 |
| Sleep latency | 1.50 ± 1.09 | 1.25 ± 1.06 | 1.50 ± 0.80 | 1.25 ± 0.87 |
| Sleep efficiency | 1.08 ± 1.24 | 0.42 ± 0.67 * | 0.42 ± 0.67 | 0.75 ± 0.87 |
| Total sleep time | 1.08 ± 0.90 | 0.83 ± 0.72 | 1.08 ± 0.67 | 1.42 ± 0.79 |
| Multi-Component Exercise (n = 12) | Control (n = 12) | |||
|---|---|---|---|---|
| Physical Fitness | Pre-Test | Post-Test | Pre-Test | Post-Test |
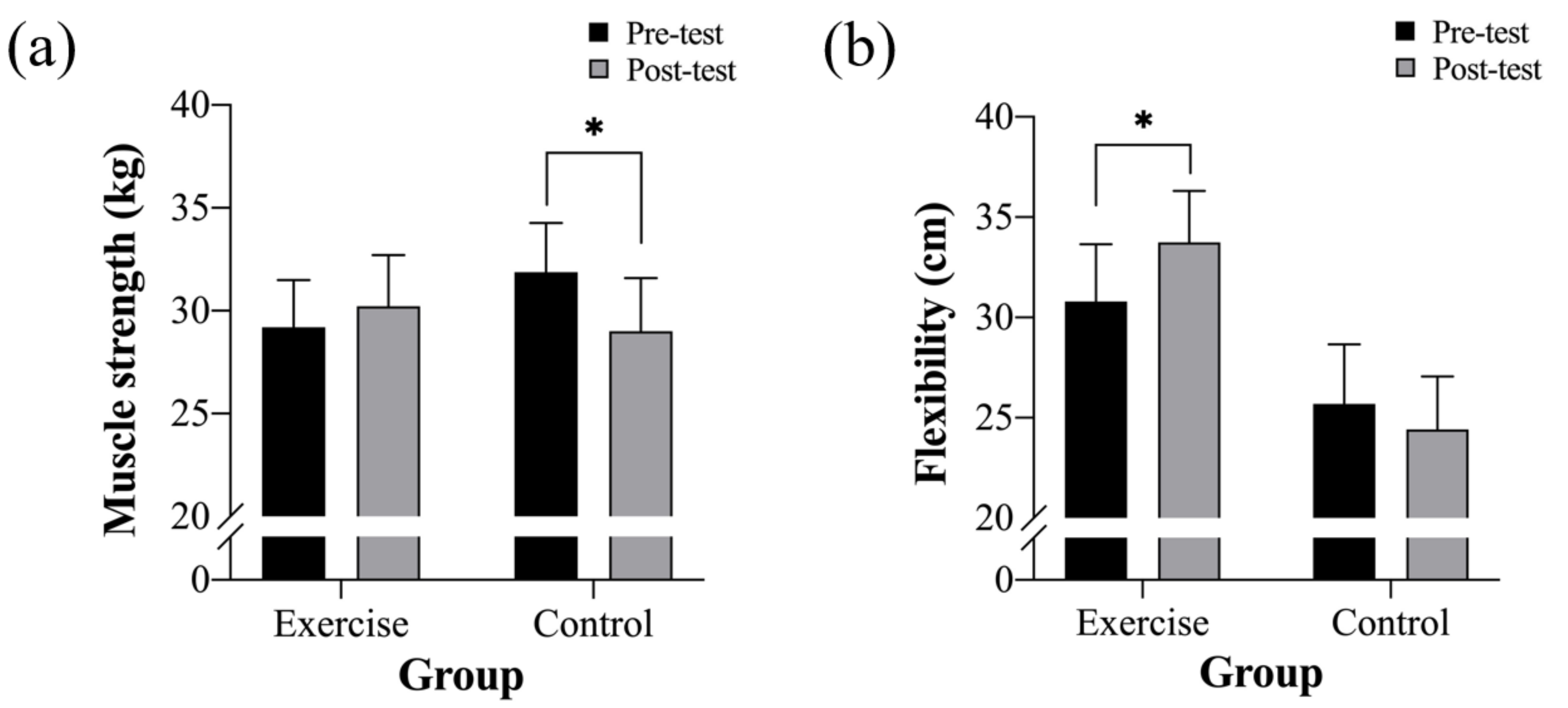
| Muscle strength (kg) | 29.21 ± 9.14 | 30.22 ± 10.20 | 31.88 ± 7.92 | 29.00 ± 8.25 * |
| Muscle endurance (reps) | 16.00 ± 10.26 | 16.27 ± 10.07 | 12.05 ± 6.46 | 13.63 ± 7.15 |
| Balance (s) | 14.36 ± 13.90 | 24.79 ± 25.11 | 10.85 ± 9.14 | 16.43 ± 20.89 |
| Flexibility (cm) | 30.79 ± 11.06 | 33.75 ± 8.51 * | 25.69 ± 10.38 | 24.42 ± 10.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ai, J.-Y.; Kuan, G.; Juang, L.Y.-T.; Lee, C.-H.; Kueh, Y.-C.; Chu, I.-H.; Geng, X.-L.; Chang, Y.-K. Effects of Multi-Component Exercise on Sleep Quality in Middle-Aged Adults. Int. J. Environ. Res. Public Health 2022, 19, 15472. https://doi.org/10.3390/ijerph192315472
Ai J-Y, Kuan G, Juang LY-T, Lee C-H, Kueh Y-C, Chu I-H, Geng X-L, Chang Y-K. Effects of Multi-Component Exercise on Sleep Quality in Middle-Aged Adults. International Journal of Environmental Research and Public Health. 2022; 19(23):15472. https://doi.org/10.3390/ijerph192315472
Chicago/Turabian StyleAi, Jing-Yi, Garry Kuan, Linda Ya-Ting Juang, Ching-Hsiu Lee, Yee-Cheng Kueh, I-Hua Chu, Xiao-Ling Geng, and Yu-Kai Chang. 2022. "Effects of Multi-Component Exercise on Sleep Quality in Middle-Aged Adults" International Journal of Environmental Research and Public Health 19, no. 23: 15472. https://doi.org/10.3390/ijerph192315472
APA StyleAi, J.-Y., Kuan, G., Juang, L. Y.-T., Lee, C.-H., Kueh, Y.-C., Chu, I.-H., Geng, X.-L., & Chang, Y.-K. (2022). Effects of Multi-Component Exercise on Sleep Quality in Middle-Aged Adults. International Journal of Environmental Research and Public Health, 19(23), 15472. https://doi.org/10.3390/ijerph192315472









