Mediterranean Diet Interventions for Depressive Symptoms in Adults with Depressive Disorders: A Protocol for a Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.1.1. Population
2.1.2. Intervention
2.1.3. Comparison
2.1.4. Outcome
2.1.5. Study Design
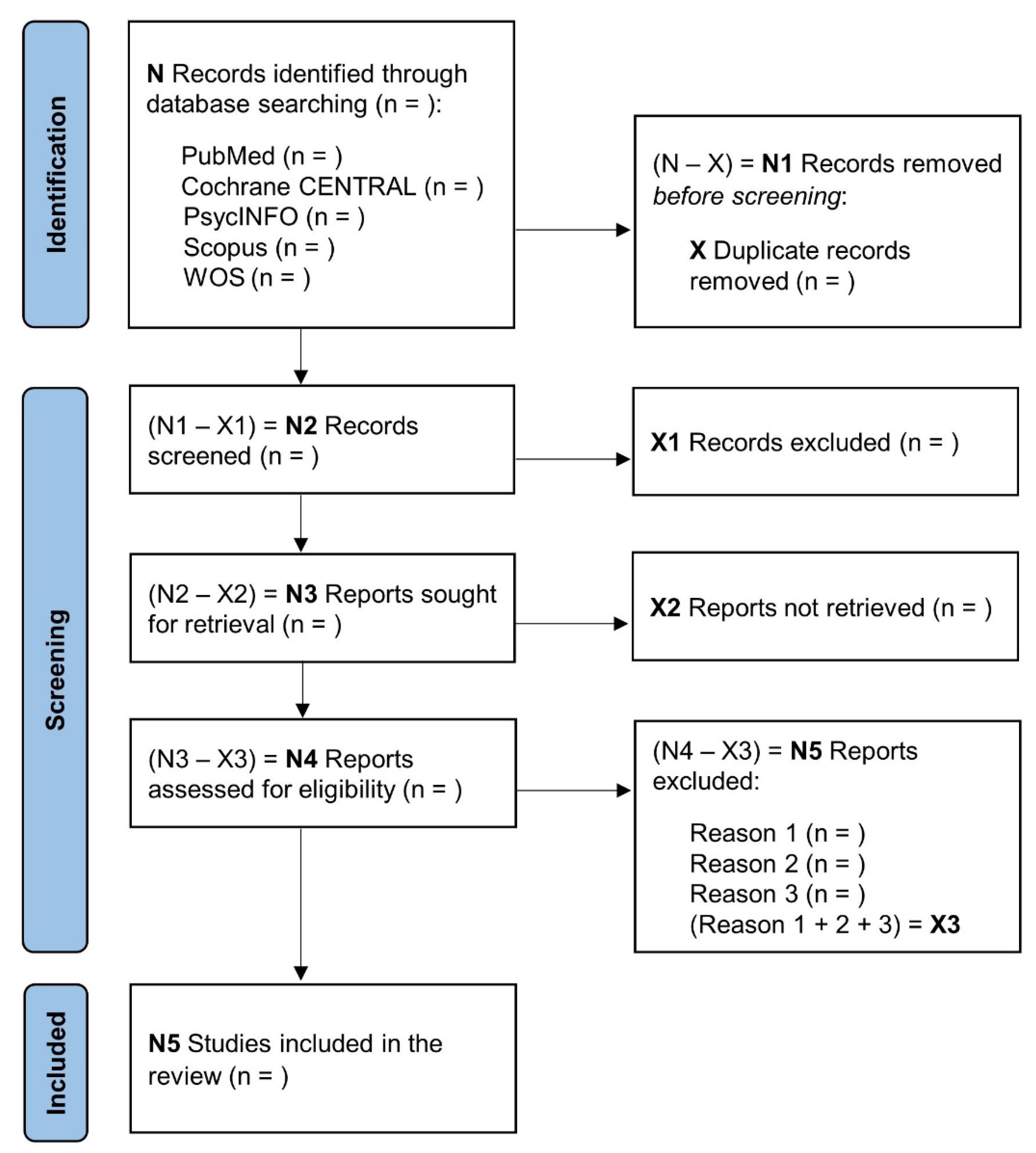
2.2. Search Methods for Study Identification
2.3. Data Collection and Analysis
2.3.1. Study Selection
2.3.2. Data Collection Process
2.3.3. Risk of Bias
2.3.4. Certainty of the Evidence
2.4. Synthesis of Data
3. Ethics and Dissemination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar] [CrossRef]
- Institute of Health Metrics and Evaluation. Global Health Data Exchange (GHDx). Available online: http://ghdx.healthdata.org/gbd-results-tool?params=gbd-api-2019-permalink/d780dffbe8a381b25e1416884959e88b (accessed on 20 September 2022).
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association, Ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Santomauro, D.F.; Herrera, A.M.M.; Shadid, J.; Zheng, P.; Ashbaugh, C.; Pigott, D.M.; Abbafati, C.; Adolph, C.; Amlag, J.O.; Aravkin, A.Y.; et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef]
- Patel, V.; Chisholm, D.; Parikh, R.; Charlson, F.J.; Degenhardt, L.; Dua, T.; Ferrari, A.J.; Hyman, S.; Laxminarayan, R.; Levin, C.; et al. Addressing the burden of mental, neurological, and substance use disorders: Key messages from Disease Control Priorities, 3rd edition. Lancet 2016, 387, 1672–1685. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Zhao, Y.; Wang, Y.; Liu, L.; Zhang, X.; Li, B.; Cui, R. The Effects of Psychological Stress on Depression. Curr. Neuropharmacol. 2015, 13, 494–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuch, F.B.; Vancampfort, D.; Firth, J.; Rosenbaum, S.; Ward, P.B.; Silva, E.S.; Hallgren, M.; De Leon, A.P.; Dunn, A.L.; Deslandes, A.C.; et al. Physical Activity and Incident Depression: A Meta-Analysis of Prospective Cohort Studies. Am. J. Psychiatry 2018, 175, 631–648. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; Moseley, G.; Berk, M.; Jacka, F. Nutritional psychiatry: The present state of the evidence. Proc. Nutr. Soc. 2017, 76, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Sarris, J.; Logan, A.C.; Akbaraly, T.N.; Amminger, G.P.; Balanzá-Martínez, V.; Freeman, M.P.; Hibbeln, J.; Matsuoka, Y.; Mischoulon, D.; Mizoue, T.; et al. International Society for Nutritional Psychiatry Research consensus position statement: Nutritional medicine in modern psychiatry. World Psychiatry 2015, 14, 370–371. [Google Scholar] [CrossRef]
- Molendijk, M.; Molero, P.; Sánchez-Pedreño, F.O.; van der Does, W.; Martínez-González, M.A. Diet quality and depression risk: A systematic review and dose-response meta-analysis of prospective studies. J. Affect. Disord. 2018, 226, 346–354. [Google Scholar] [CrossRef] [Green Version]
- Marx, W.; Lane, M.; Hockey, M.; Aslam, H.; Berk, M.; Walder, K.; Borsini, A.; Firth, J.; Pariante, C.M.; Berding, K.; et al. Diet and depression: Exploring the biological mechanisms of action. Mol. Psychiatry 2020, 26, 134–150. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Sánchez-Villegas, A. Food patterns and the prevention of depression. Proc. Nutr. Soc. 2016, 75, 139–146. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, H.; Suzuki, K.; Ma, S.; Liu, C. Linking What We Eat to Our Mood: A Review of Diet, Dietary Antioxidants, and Depression. Antioxidants 2019, 8, 376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayes, J.; Schloss, J.; Sibbritt, D. Effects of Polyphenols in a Mediterranean Diet on Symptoms of Depression: A Systematic Literature Review. Adv. Nutr. 2020, 11, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Villegas, A.; Martínez-González, M.A.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Covas, M.I.; Arós, F.; Romaguera, D.; Gómez-Gracia, E.; Lapetra, J.; et al. Mediterranean dietary pattern and depression: The PREDIMED randomized trial. BMC Med. 2013, 11, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitó, M.; Guxens, M.; Corella, D.; Sáez, G.; Estruch, R.; De La Torre, R.; Francés, F.; Cabezas, C.; López-Sabater, M.D.C.; Marrugat, J.; et al. Effect of a traditional Mediterranean diet on lipoprotein oxidation: A randomized controlled trial. Arch. Intern. Med. 2007, 167, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Koelman, L.; Rodrigues, C.E.; Aleksandrova, K. Effects of Dietary Patterns on Biomarkers of Inflammation and Immune Responses: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2022, 13, 101–115. [Google Scholar] [CrossRef]
- Lassale, C.; Batty, G.D.; Baghdadli, A.; Jacka, F.; Sánchez-Villegas, A.; Kivimäki, M.; Akbaraly, T. Healthy dietary indices and risk of depressive outcomes: A systematic review and meta-analysis of observational studies. Mol. Psychiatry 2019, 24, 965–986. [Google Scholar] [CrossRef] [Green Version]
- Psaltopoulou, T.; Sergentanis, T.N.; Panagiotakos, D.B.; Sergentanis, I.N.; Kosti, R.; Scarmeas, N. Mediterranean diet, stroke, cognitive impairment, and depression: A meta-analysis. Ann. Neurol. 2013, 74, 580–591. [Google Scholar] [CrossRef]
- Shafiei, F.; Salari-Moghaddam, A.; Larijani, B.; Esmaillzadeh, A. Adherence to the Mediterranean diet and risk of depression: A systematic review and updated meta-analysis of observational studies. Nutr. Rev. 2019, 77, 230–239. [Google Scholar] [CrossRef]
- Matison, A.P.; Mather, K.A.; Flood, V.M.; Reppermund, S. Associations between nutrition and the incidence of depression in middle-aged and older adults: A systematic review and meta-analysis of prospective observational population-based studies. Ageing Res. Rev. 2021, 70, 101403. [Google Scholar] [CrossRef]
- Barton, S. Which clinical studies provide the best evidence? BMJ 2000, 321, 255–256. [Google Scholar] [CrossRef]
- Trepanowski, J.F.; Ioannidis, J.P.A. Perspective: Limiting Dependence on Nonrandomized Studies and Improving Randomized Trials in Human Nutrition Research: Why and How. Adv. Nutr. 2018, 9, 367–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altun, A.; Brown, H.; Szoeke, C.; Goodwill, A.M. The Mediterranean dietary pattern and depression risk: A systematic review. Neurol. Psychiatry Brain Res. 2019, 33, 1–10. [Google Scholar] [CrossRef]
- Opie, R.S.; O’Neil, A.; Itsiopoulos, C.; Jacka, F.N. The impact of whole-of-diet interventions on depression and anxiety: A systematic review of randomised controlled trials. Public Health Nutr. 2015, 18, 2074–2093. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, S.; Minehan, M.; Knight-Agarwal, C.R.; Turner, M. Depression, Is It Treatable in Adults Utilising Dietary Interventions? A Systematic Review of Randomised Controlled Trials. Nutrients 2022, 14, 1398. [Google Scholar] [CrossRef]
- Bot, M.; Brouwer, I.; Roca, M.; Kohls, E.; Penninx, B.W.J.H.; Watkins, E.; van Grootheest, G.; Cabout, M.; Hegerl, U.; Gili, M.; et al. Effect of Multinutrient Supplementation and Food-Related Behavioral Activation Therapy on Prevention of Major Depressive Disorder Among Overweight or Obese Adults With Subsyndromal Depressive Symptoms: The MooDFOOD Randomized Clinical Trial. JAMA 2019, 321, 858–868. [Google Scholar] [CrossRef] [Green Version]
- Bayes, J.; Schloss, J.; Sibbritt, D. The effect of a Mediterranean diet on the symptoms of depression in young males (the “AMMEND: A Mediterranean Diet in MEN with Depression” study): A randomized controlled trial. Am. J. Clin. Nutr. 2022, 116, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Francis, H.M.; Stevenson, R.J.; Chambers, J.R.; Gupta, D.; Newey, B.; Lim, C.K. A brief diet intervention can reduce symptoms of depression in young adults—A randomised controlled trial. PLoS ONE 2019, 14, e0222768. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 148–160. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021). Cochrane. 2021. Available online: www.training.cochrane.org/handbook (accessed on 6 September 2022).
- Grammatikopoulou, M.G.; Nigdelis, M.P.; Theodoridis, X.; Gkiouras, K.; Tranidou, A.; Papamitsou, T.; Bogdanos, D.P.; Goulis, D.G. How fragile are Mediterranean diet interventions? A research-on-research study of randomised controlled trials. BMJ Nutr. Prev. Health 2021, 4, 115–131. [Google Scholar] [CrossRef]
- Trichopoulou, A.; A Martínez-González, M.; Tong, T.Y.; Forouhi, N.G.; Khandelwal, S.; Prabhakaran, D.; Mozaffarian, D.; de Lorgeril, M. Definitions and potential health benefits of the Mediterranean diet: Views from experts around the world. BMC Med. 2014, 12, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef] [PubMed]
- Fadnes, L.T.; Økland, J.-M.; Haaland, A.; Johansson, K.A. Estimating impact of food choices on life expectancy: A modeling study. PLoS Med. 2022, 19, e1003889. [Google Scholar] [CrossRef]
- Grosso, G.; Marventano, S.; Yang, J.; Micek, A.; Pajak, A.; Scalfi, L.; Galvano, F.; Kales, S.N. A comprehensive meta-analysis on evidence of Mediterranean diet and cardiovascular disease: Are individual components equal? Crit. Rev. Food Sci. Nutr. 2017, 57, 3218–3232. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Cusin, C.; Yang, H.; Yeung, A.; Fava, M. Rating Scales for Depression. In Handbook of Clinical Rating Scales and Assessment in Psychiatry and Mental Health; Baer, L., Blais, M.A., Eds.; Humana Press: Totowa, NJ, USA, 2009; pp. 7–35. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Goldet, G.; Howick, J. Understanding GRADE: An introduction. J. Evid. Based Med. 2013, 6, 50–54. [Google Scholar] [CrossRef]
- Jackson, D.; Turner, R. Power analysis for random-effects meta-analysis. Res. Synth. Methods 2017, 8, 290–302. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- DerSimonian, R.; Kacker, R. Random-effects model for meta-analysis of clinical trials: An update. Contemp. Clin. Trials 2007, 28, 105–114. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Egger, M.; Smith, G.D. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ 2001, 323, 101–105. [Google Scholar] [CrossRef]
- Serra-Majem, L.; Román-Viñas, B.; Sanchez-Villegas, A.; Guasch-Ferré, M.; Corella, D.; La Vecchia, C. Benefits of the Mediterranean diet: Epidemiological and molecular aspects. Mol Aspects Med. 2019, 67, 1–55. [Google Scholar] [CrossRef]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
| PICO Component | Keywords |
|---|---|
| #1 Population | Adult* OR “young adult” OR “middle aged” OR aged OR elderly OR olde* OR patient |
| #2 Intervention | “Mediterranean diet” OR “Med-Diet” OR “Mediterranean-style diet” OR “Mediterranean food” OR “dietary pattern” OR “diet quality” OR “healthy diet” OR “diet intervention” OR “diet improvement” OR “food therapy” |
| #3 Outcome | Depress* OR dysthymi* OR “dysphoric disorder” OR “mood disorder” OR “affective disorder” OR “affective symptoms” |
| #4 Study design | (random* OR clinical OR controlled OR intervention* OR experimental) AND (trial OR study OR allocation) |
| Search Strategy | [(#1) AND (#2) AND (#3) AND (#4)] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bizzozero-Peroni, B.; Godoy-Cumillaf, A.; Fernández-Rodríguez, R.; Rodríguez-Gutiérrez, E.; Jiménez-López, E.; Giakoni-Ramírez, F.; Duclos-Bastías, D.; Mesas, A.E. Mediterranean Diet Interventions for Depressive Symptoms in Adults with Depressive Disorders: A Protocol for a Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 14437. https://doi.org/10.3390/ijerph192114437
Bizzozero-Peroni B, Godoy-Cumillaf A, Fernández-Rodríguez R, Rodríguez-Gutiérrez E, Jiménez-López E, Giakoni-Ramírez F, Duclos-Bastías D, Mesas AE. Mediterranean Diet Interventions for Depressive Symptoms in Adults with Depressive Disorders: A Protocol for a Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(21):14437. https://doi.org/10.3390/ijerph192114437
Chicago/Turabian StyleBizzozero-Peroni, Bruno, Andrés Godoy-Cumillaf, Rubén Fernández-Rodríguez, Eva Rodríguez-Gutiérrez, Estela Jiménez-López, Frano Giakoni-Ramírez, Daniel Duclos-Bastías, and Arthur Eumann Mesas. 2022. "Mediterranean Diet Interventions for Depressive Symptoms in Adults with Depressive Disorders: A Protocol for a Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 21: 14437. https://doi.org/10.3390/ijerph192114437





