Effect of N95 Respirator on Oxygen and Carbon Dioxide Physiologic Response: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Searching Strategy
2.2. Inclusion Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Search Study
3.2. Study Characteristics
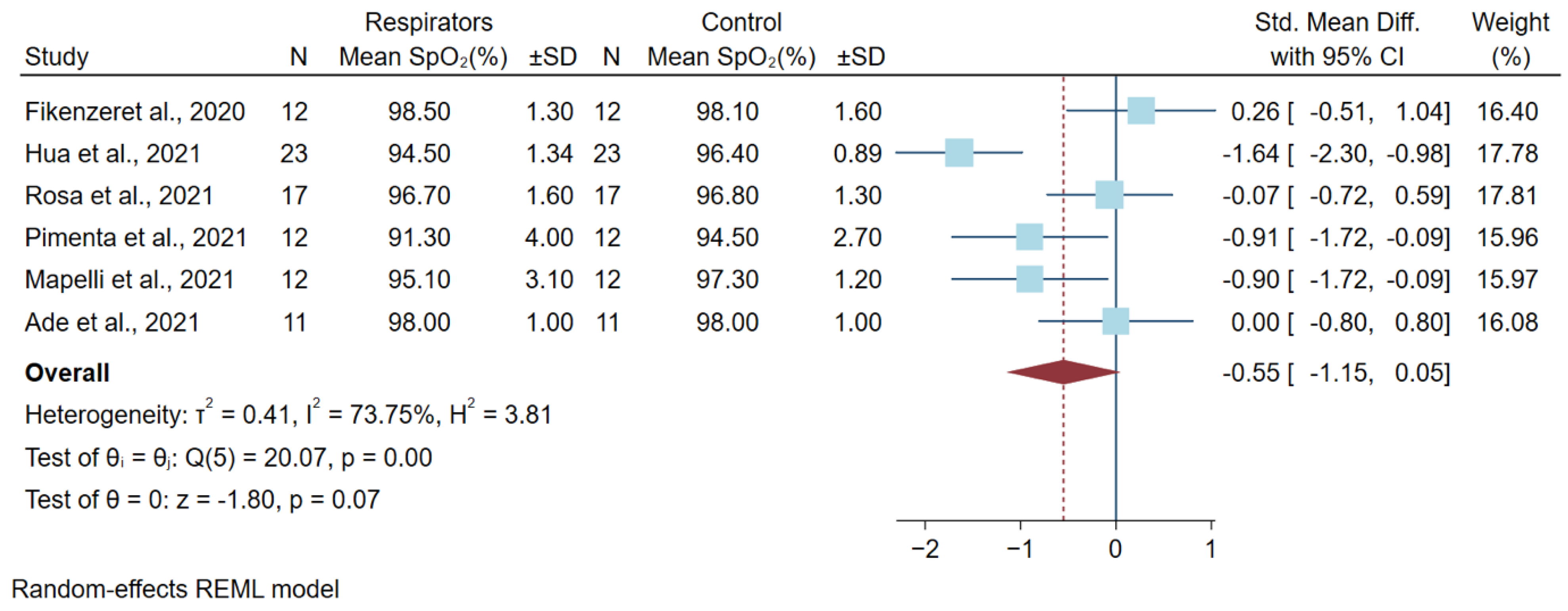
3.3. Differences in Oxygen Saturation Levels after a High Physical Workload
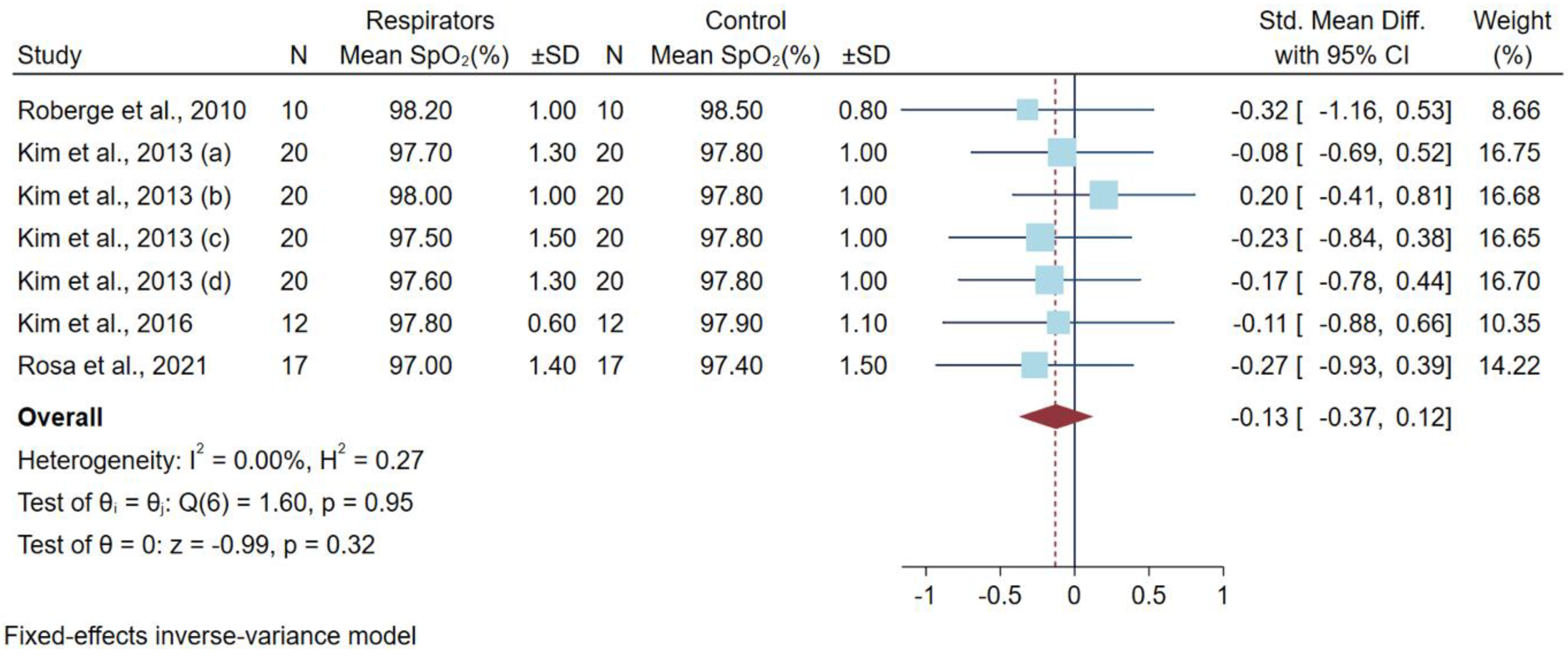
3.4. Differences in Oxygen Saturation Levels after a Low-to-Moderate Physical Workload
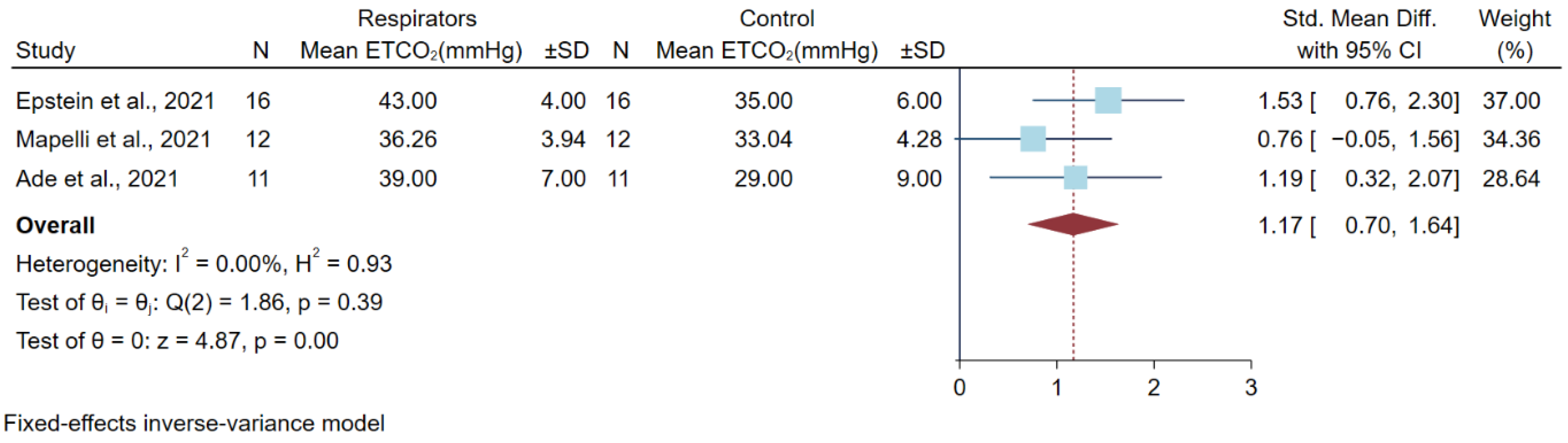
3.5. Differences in Partial Pressure of Carbon (PCO2) Dioxide after a High Physical Workload
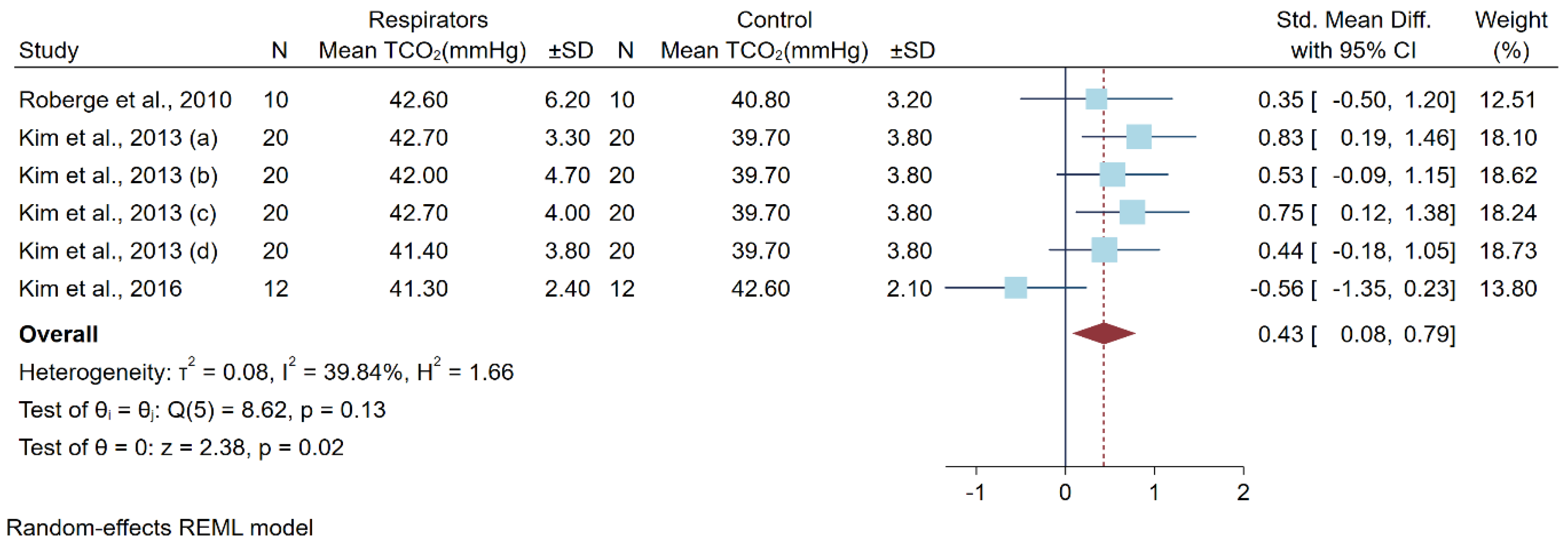
3.6. Differences in Carbon Dioxide Levels after a Low-to-Moderate Physical Workload
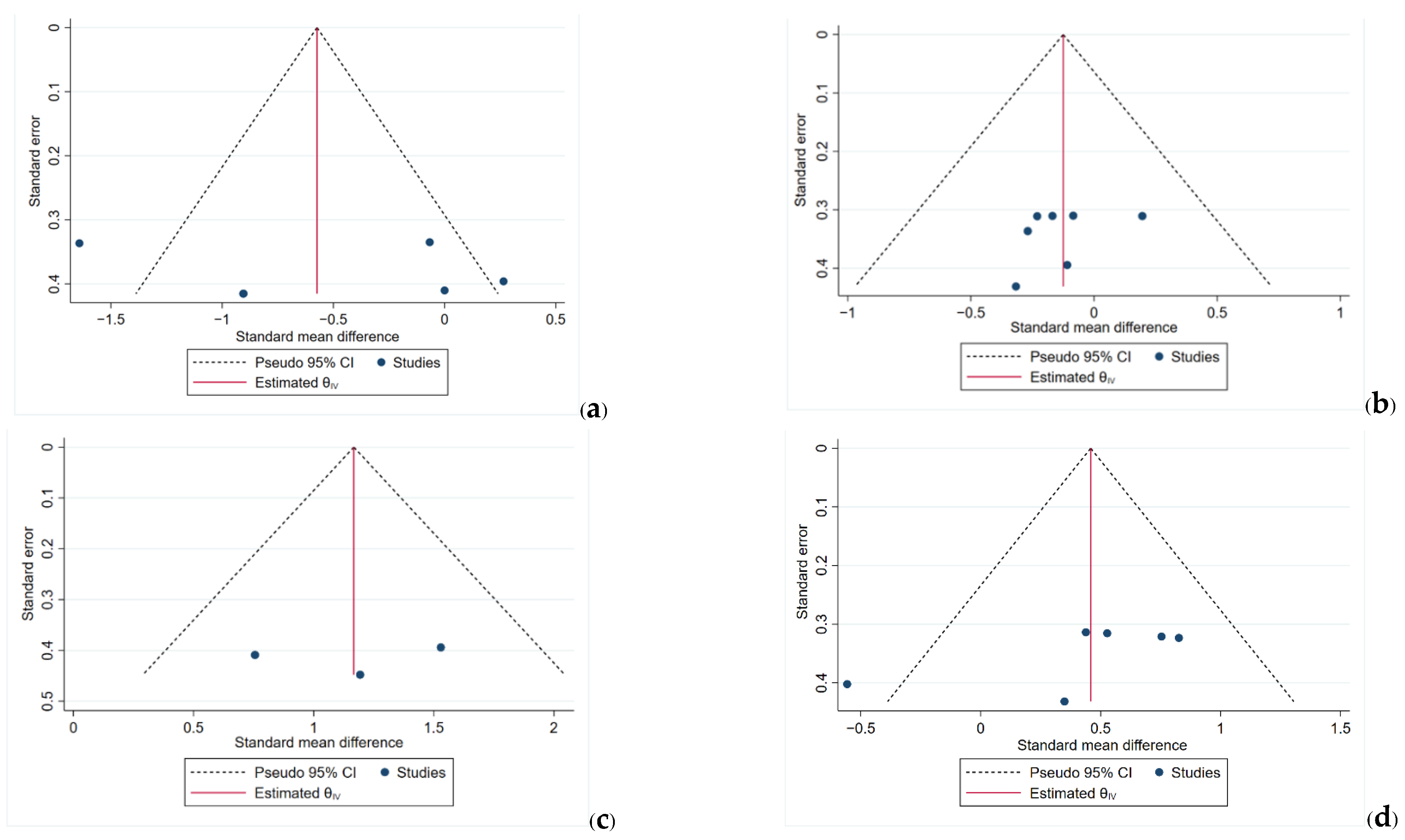
3.7. Funnel Plots
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rashid, T.U.; Sharmeen, S.; Biswas, S. Effectiveness of N95 Masks against SARS-CoV-2: Performance Efficiency, Concerns, and Future Directions. ACS Chem. Health Saf. 2022, 29, 135–164. [Google Scholar] [CrossRef]
- CDC Tnpptlini. NIOSH-Approved N95 Particulate Filtering Facepiece Respirators 2022. Available online: https://www.cdc.gov/niosh/npptl/topics/respirators/disp_part/n95list1.html (accessed on 15 May 2022).
- Shubhanshu, K.; Singh, A. Prolonged Use of n95 Mask a Boon or Bane to Healthcare Workers during COVID–19 Pandemic. Indian J. Otolaryngol. Head Neck Surg. 2021, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, M.; Salvioni, E.; De Martino, F.; Mattavelli, I.; Gugliandolo, P.; Vignati, C.; Farina, S.; Palermo, P.; Campodonico, J.; Maragna, R.; et al. “You can leave your mask on”: Effects on cardiopulmonary parameters of different airway protective masks at rest and during maximal exercise. Eur. Respir. J. 2021, 58, 2004473. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, T.; Tavares, H.; Ramos, J.; Oliveira, M.; Reis, D.; Amorim, H.; Rocha, A. Facemasks during aerobic exercise: Implications for cardiac rehabilitation programs during the COVID-19 pandemic. Rev. Port. Cardiol. 2021, 40, 957–964. [Google Scholar] [CrossRef]
- Hopkins, S.R. Exercise Induced Arterial Hypoxemia: The Role of Ventilation-Perfusion Inequality and Pulmonary Diffusion Limitation; Springer: New York, NY, USA, 2006; pp. 17–30. [Google Scholar]
- Shaw, K.A.; Zello, G.A.; Butcher, S.J.; Ko, J.B.; Bertrand, L.; Chilibeck, P.D. The impact of face masks on performance and physiological outcomes during exercise: A systematic review and meta-analysis. Appl. Physiol. Nutr. Metab. 2021, 46, 693–703. [Google Scholar] [CrossRef]
- Brinkman, J.E.; Toro, F.; Sharma, S. Physiology, Respiratory Drive; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Wolters Kluwer; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2018. [Google Scholar]
- Rosa, B.V.; Rossi, F.E.; de Moura, H.P.D.N.; Santos, A.M.D.; Veras-Silva, A.S.; Ribeiro, S.L.G. Effects of FFP2/N95 face mask on low- and high-load resistance exercise performance in recreational weight lifters. Eur. J. Sport Sci. 2021. [Google Scholar] [CrossRef]
- Hua, D.; Xu, Y.; Heiduschka, P.; Zhang, W.; Zhang, X.; Zeng, X.; Zhu, X.; He, T.; Zheng, H.; Xiao, X.; et al. Retina Vascular Perfusion Dynamics During Exercise with and without Face Masks in Healthy Young Adults: An OCT Angiography Study. Transl. Vis. Sci. Technol. 2021, 10, 23. [Google Scholar] [CrossRef]
- Ade, C.J.; Turpin, V.R.G.; Parr, S.K.; Hammond, S.T.; White, Z.; Weber, R.E. Does wearing a facemask decrease arterial blood oxygenation and impair exercise tolerance? Respir. Physiol. Neurobiol. 2021, 294, 103765. [Google Scholar] [CrossRef]
- Fikenzer, S.U.T.; Lavall, D.; Rudolph, U.; Falz, R.; Busse, M.; Hepp, P.; Laufs, U. Effects of surgical and FFP2/N95 face masks on cardiopulmonary exercise capacity. Clin. Res. Cardiol. 2020, 109, 1522–1530. [Google Scholar] [CrossRef]
- Kim, J.-H.; Wu, T.; Powell, J.B.; Roberge, R.J. Physiologic and fit factor profiles of N95 and P100 filtering facepiece respirators for use in hot, humid environments. Am. J. Infect. Control 2015, 44, 194–198. [Google Scholar] [CrossRef]
- Kim, J.-H.; Benson, S.M.; Roberge, R.J. Pulmonary and heart rate responses to wearing N95 filtering facepiece respirators. Am. J. Infect. Control 2013, 41, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Singh, M.; Khurana, D.K.; Mustafi, S.M.; Sharma, S. Physiological Effects of N95 FFP and PPE in Healthcare Workers in COVID Intensive Care Unit: A Prospective Cohort Study. Indian J. Crit. Care Med. 2020, 24, 1169–1173. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.B.; Kim, J.-H.; Roberge, R.J. Powered air-purifying respirator use in healthcare: Effects on thermal sensations and comfort. J. Occup. Environ. Hyg. 2017, 14, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Roberge, R.J.; Coca, A.; Williams, W.J.; Powell, J.B.; Palmiero, A.J. Physiological impact of the N95 filtering facepiece respirator on healthcare workers. Respir. Care 2010, 55, 569–577. [Google Scholar]
- Nwosu, A.D.G.; Ossai, E.N.; Onwuasoigwe, O.; Ahaotu, F. Oxygen saturation and perceived discomfort with face mask types, in the era of COVID-19: A hospital-based cross-sectional study. Pan Afr. Med. J. 2021, 39, 203. [Google Scholar] [CrossRef]
- Epstein, D.; Korytny, A.; Isenberg, Y.; Marcusohn, E.; Zukermann, R.; Bishop, B.; Minha, S.; Raz, A.; Miller, A. Return to training in the COVID-19 era: The physiological effects of face masks during exercise. Scand. J. Med. Sci. Sports 2020, 31, 70–75. [Google Scholar] [CrossRef]
- Brown, S.M.; Grissom, C.K.; Moss, M.; Rice, T.W.; Schoenfeld, D.; Hou, P.C. Nonlinear Imputation of Pao2/Fio2 from Spo2/Fio2 among Patients with Acute Respiratory Distress Syndrome. Chest 2016, 150, 307–313. [Google Scholar] [CrossRef]
- Li, Y.T.H.; Guo, Y.P.; Wong, A.S.W.; Wong, T.; Chung, J. Effects of wearing N95 and surgical facemasks on heart rate, thermal stress and subjective sensations. Int. Arch. Occup. Environ. Health 2005, 78, 9. [Google Scholar] [CrossRef]
- Lee, H.P.; Wang, D.Y. Objective Assessment of Increase in Breathing Resistance of N95 Respirators on Human Subjects. Ann. Occup. Hyg. 2011, 55, 917–921. [Google Scholar] [CrossRef][Green Version]
- Johnson, A.T.; Scott, W.H.; Lausted, C.G.; Coyne, K.M.; Sahota, M.S.; Johnson, M.M. Effect of external dead volume on performance while wearing a respirator. Am. Ind. Hyg. Assoc. 2000, 61, 678–684. [Google Scholar] [CrossRef]
- Williams, W.J. Physiological response to alterations in [O2] and [CO2]: Relevance to respiratory protective devices. J. Int. Soc. Respir. Prot. 2010, 27, 27–51. [Google Scholar]
- Anderson, G.; Rhodes, E. Relationship between blood lactate and excess CO2 in elite cyclists. J. Sports Sci. 1991, 9, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.W.; Mayberry, K.; Crapo, R.; Jensen, R.L. The accuracy of pulse oximetry in the emergency department. Am. J. Emerg. Med. 2000, 18, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, Y.; Farrero, E.; Córdoba, A.; González, N.; Dorca, J.; Prats, E. Transcutaneous Carbon Dioxide Monitoring in Subjects with Acute Respiratory Failure and Severe Hypercapnia. Respir. Care 2016, 61, 428–433. [Google Scholar] [CrossRef]
- Limited MSAtLC. Comparison of FFP2, KN95, and N95 Filtering Facepiece Respirator Classes 2021. Available online: https://multimedia.3m.com/mws/media/1791500O/comparison-ffp2-kn95-n95-filtering-facepiece-respirator-classes-tb.pdf (accessed on 15 May 2022).
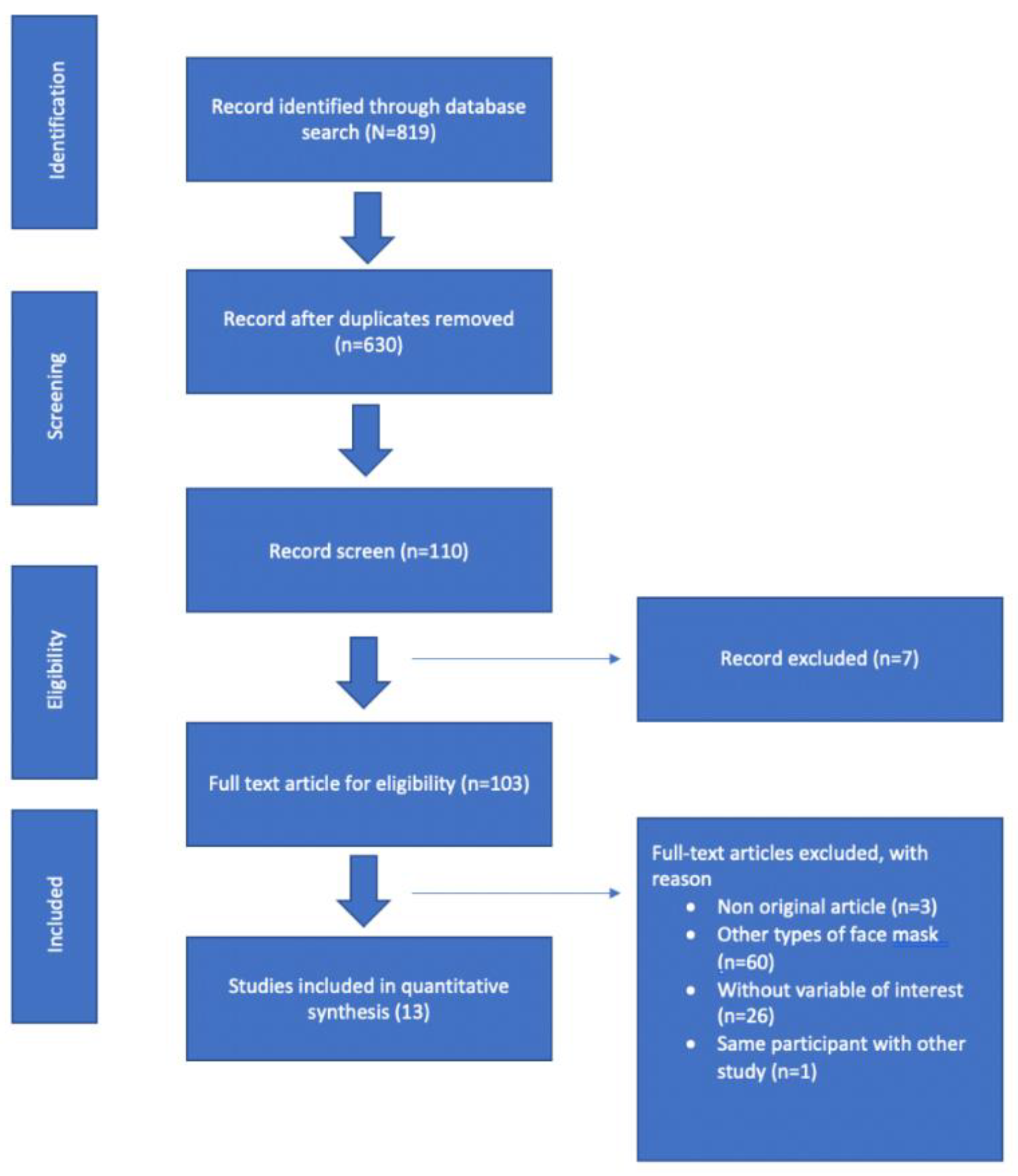
| Author | Population and Study Country | Study Design | Physical Load | Respirators | Outcome | Confounding | |||
|---|---|---|---|---|---|---|---|---|---|
| Respirators | Control | ||||||||
| Baseline | After a Workload | Baseline | After a Workload | ||||||
| Rosa et al., 2021 [10] | 17 male recreational weightlifters, Brazil | Cross-over randomized controlled trial | High-intensity bench press | FFP2/N95 | 96.1 ± 2.3 a | 96.7 ± 1.6 b | 97.1 ± 1.6 a | 96.8 ± 1.3 b | Training, experience of participant, and no fit testing |
| Pimenta et al., 2021 [5] | 12 professionals (8 men and 4 women) Portugal | Cross-over randomized controlled trial | Exercise testing followed Bruce treadmill protocol | KN95 (Gb2626-2006) | 95.6 ± 2.0 c | 91.3 ± 4.0 | 96.0 ± 1.6 c | 94.5 ± 2.7 | No fit testing, age, gender, physical activity, and habits |
| Mapelli et al., 2021 [4] | 12 subjects (6 men and 6 women) Italy | Prospective, randomized, double-blind, and cross-over design | Cardiopulmonary exercise to the peak level by cycle ergometer | FFP2 (KN95) | 96.9 ± 1.2 | 95.1 ± 3.1 | 97.2 ± 0.9 | 97.3 ± 1.2 | No fit testing |
| Hua et al., 2021 [11] | 23 participants (6 men and 17 women) China | Non-randomized controlled trial | Incremental continuous running test (ICRT) compared with no mask | N95 | 96.4 ± 1.41 | 94.5 ± 1.34 | 97.4 ± 0.78 | 96.4 ± 0.89 | No fit testing |
| Ade et al., 2021 [12] | 11 participants (5 men and 6 women) USA | Cross-over randomized controlled trial | Four incremental ramp exercise tests until exhaustion on a cycle ergometer | Vertical-fold N95 | 98.0 ± 1 | 98.0 ± 1 | 98.0 ± 1 | 98.0 ± 1 | No fit testing |
| Fikenzer et al., 2020 [13] | 12 men Germany | Cross-over randomized controlled trial | Incremental exertion test until exhaustion by cycle ergometer | FFP2/N95 | 98.7 ± 0.5 d | 98.5 ± 1.3 d | 98.3 ± 0.2 d | 98.1 ± 1.6 d | No fit testing, but seal check was performed |
| Author | Population and Study Country | Study Design | Physical Load | Respirators | Outcome | Confounding | |||
|---|---|---|---|---|---|---|---|---|---|
| Respirators | Control | ||||||||
| Baseline | After a Workload | Baseline | After a Workload | ||||||
| Rosa et al., 2021 [10] | 17 male recreational weightlifters, Brazil | Cross-over randomized controlled trial | Moderate-intensity bench press exercise | FFp2/N95 | 95.6 ± 2.4 a | 97.0 ± 1.4 b | 97.0 ± 1.5 a | 97.4 ± 1.5 b | Training experience of participant, no fit testing |
| Kim et al., 2016 [14] | 12 male adults USA | Cross-over randomized controlled trial | 1 h on treadmill speed 5.6 km/h | N95 | 98.2 ± 0.8 | 97.8 ± 0.6 | 98.1 ± 0.7 | 97.9 ± 1.1 | |
| Kim et al., 2013 (a) [15] | 20 young subjects (13 men and 7 women) USA | Non-randomized controlled trial | 1 h on treadmill speed 5.6 km/h 0-degree incline | N95 Moldex 2200 | 98.4 ± 0.9 | 97.7 ± 1.3 | 98.1 ± 1.3 | 97.8 ± 1.0 | |
| Kim et al., 2013 (b) [15] | N95 Moldex 2300 | 98.1 ± 1.3 | 98.0 ± 1.0 | ||||||
| Kim et al., 2013 (c) [15] | N95 3M 9210 | 97.9 ± 1.4 | 97.5 ± 1.5 | ||||||
| Kim et al., 2013 (d) [15] | N95 3M 9211 | 98.4 ± 0.9 | 97.6 ± 1.3 | ||||||
| Choudhury et al., 2020 [16] | 75 health care workers (35 men and 40 women) India | Prospective cohort study | 4 h work in ICU | N95 | 97.87 ± 1.17 | 97.73 ± 1.12 | N/A | N/A | Testing environment |
| Powell et al., 2017 [17] | 12 adults (6 women and 6 men) USA | Non-randomized controlled trial | 1 h on treadmill speed 5.6 km/h 0-degree incline | N95 | 98.9 ± 0.7 | 98.8 ± 0.7 | N//A | N/A | No fit testing |
| Roberge et al., 2010 [18] | 10 healthcare workers (7 women and 3 men) USA | Cross-over randomized controlled trial | 1 h on treadmill speed 2.5 mile/h (4.02 km·h) | N95 | 98.1 ± 1.2 c | 98.2 ± 1.0 | 98.5 ± 0.8 | 98.5 ± 0.8 | |
| Nwosu et al., 2021 [19] | 28 healthcare workers (15 men and 13 women) Nigeria | Cross-sectional | Intra-operation room, work average 210 min | N95 (various models) | 97.9 ± 0.8 | 97.8 ± 0.8 | - | - | No fit testing |
| Author | Population and Study Country | Study Design | Physical Load | Respirators | Outcome | Confounding | |||
|---|---|---|---|---|---|---|---|---|---|
| Respirators | Control | ||||||||
| Baseline | After a Workload | Baseline | After a Workload | ||||||
| Mapelli et al., 2021 [4] | 12 subjects (6 men and 6 women) Italy | Prospective, randomized, double-blind, and cross-over design | Cardiopulmonary exercise to the peak level by cycle ergometer | FFP2 (KN95) | 36.85 ± 6.14 a | 36.26 ± 3.94 a | 35.6 ± 5.7 a | 33.04 ± 4.28 a | No fit testing |
| Epstein et al., 2021 [20] | 16 male adults Israel | Multiple cross-over, self-control trial | Ramp exercise tests until exhaustion on a cycle ergometer | N95 | 41 ± 3 a | 43 ± 4 a | 39 ± 2 a | 35 ± 6 a | The resting time between each test, no fit testing |
| Ade et al., 2021 [12] | 11 adults (5 men and 6 women) USA | Randomized cross-over study | Four incremental ramp exercise tests until exhaustion on a cycle ergometer | vertical-fold N95 | 36 ± 4 a | 39 ± 7 a | 29 ± 7 a | 29 ± 9 a | No fit testing |
| Fikenzer et al., 2020 [13] | 12 men Germany | Cross-over randomized controlled trial | Incremental exertion test until exhaustion by cycle ergometer | FFP2/N95 | 39.3 ± 2.2 b | 34.9 b (missing SD) | 40.2 ± 3.4 b | 34.2 ± 3.8 b | No fit testing, but seal check was performed |
| Author | Population and Study Country | Study Design | Physical Load | Respirators | Outcome | Confounding | |||
|---|---|---|---|---|---|---|---|---|---|
| Respirators | Control | ||||||||
| Baseline | After a Workload | Baseline | After a Workload | ||||||
| Kim et al., 2016 [14] | 12 male adults USA | Cross-over randomized controlled trial | 1 h on treadmill speed 5.6 km/h | N95 | 39.3 ± 4.0 a | 41.3 ± 2.4 a | 41.2 ± 1.3 a | 42.6 ± 2.1a | |
| Kim et al., 2013 (a) [15] | 20 young subjects (13 men and 7 women) USA | Non-randomized controlled trial | 1 h on treadmill speed 5.6 km/h 0-degree incline | N95 Moldex 2200 | 98.4 ± 0.9 a | 97.7 ± 1.3 a | 39.0 ± 3.4 a | 39.7 ± 3.8 a | |
| Kim et al., 2013 (b) [15] | N95 Moldex 2300 | 98.1 ± 1.3 a | 98.0 ± 1.0 a | ||||||
| Kim et al., 2013 (c) [15] | N95 3M 9210 | 97.9 ± 1.4 a | 97.5 ± 1.5 a | ||||||
| Kim et al., 2013 (d) [15] | N95 3M 9211 | 98.4 ± 0.9 a | 97.6 ± 1.3 a | ||||||
| Powell et al., 2017 [17]. | 12 adults (6 women and 6 men) USA | Non-randomized controlled trial | 1 h on treadmill speed 5.6 km/h 0-degree incline | N95 | 36.8 ± 2.0 a | 38.0 ± 1.9 a | - | - | No fit testing |
| Roberge et al., 2010 [18] | 10 health care workers (7 women and 3 men) USA | Cross-over randomized controlled trial | 1 h on treadmill speed 2.5 mile/h (4.02 km·h) | N95 | 39.7 ± 2.6 a,b | 42.6 ± 6.2 a | 40.8 ± 3.2 a | 40.8 ± 3.2 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wangsan, K.; Sapbamrer, R.; Sirikul, W.; Panumasvivat, J.; Surawattanasakul, V.; Assavanopakun, P. Effect of N95 Respirator on Oxygen and Carbon Dioxide Physiologic Response: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 8646. https://doi.org/10.3390/ijerph19148646
Wangsan K, Sapbamrer R, Sirikul W, Panumasvivat J, Surawattanasakul V, Assavanopakun P. Effect of N95 Respirator on Oxygen and Carbon Dioxide Physiologic Response: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(14):8646. https://doi.org/10.3390/ijerph19148646
Chicago/Turabian StyleWangsan, Kampanat, Ratana Sapbamrer, Wachiranun Sirikul, Jinjuta Panumasvivat, Vithawat Surawattanasakul, and Pheerasak Assavanopakun. 2022. "Effect of N95 Respirator on Oxygen and Carbon Dioxide Physiologic Response: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 14: 8646. https://doi.org/10.3390/ijerph19148646
APA StyleWangsan, K., Sapbamrer, R., Sirikul, W., Panumasvivat, J., Surawattanasakul, V., & Assavanopakun, P. (2022). Effect of N95 Respirator on Oxygen and Carbon Dioxide Physiologic Response: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 19(14), 8646. https://doi.org/10.3390/ijerph19148646







