Smartphone Application for Smoking Cessation (Quit with US): A Randomized Controlled Trial among Young Adult Light Smokers in Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Recruitment
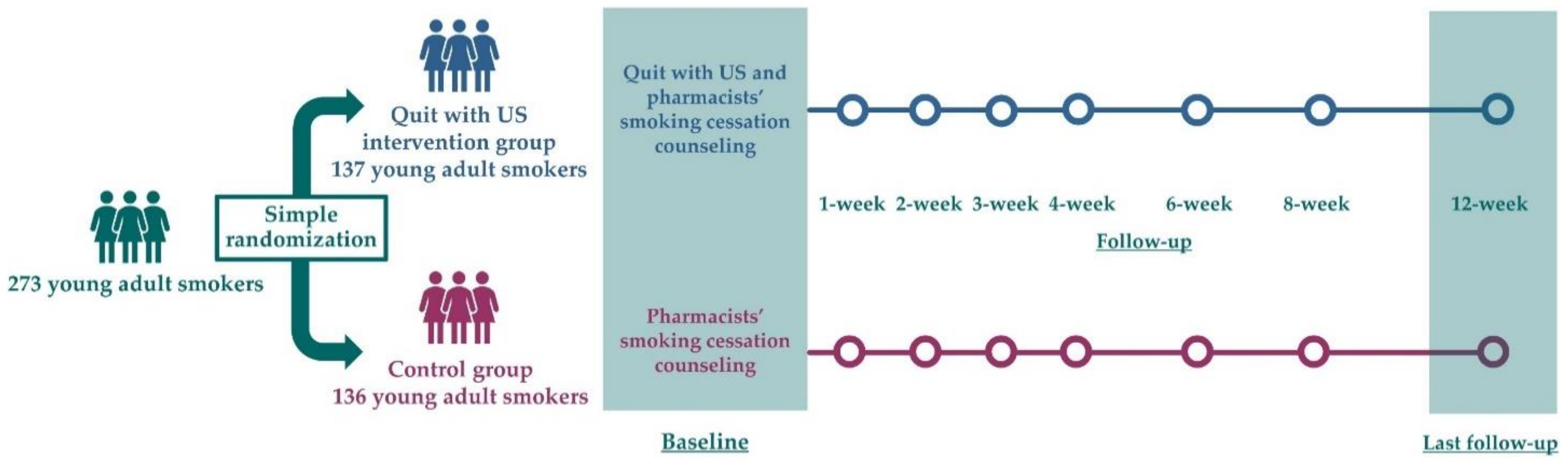
2.3. Randomization
2.4. Pharmacists’ Smoking Cessation Counseling
2.5. Quit with US Intervention Group
2.6. Control Group
2.7. Baseline and Follow-Up
2.8. Measures
2.8.1. Primary Outcome
2.8.2. Secondary Outcomes
2.9. Sample Size
2.10. Statistical Analysis
3. Results
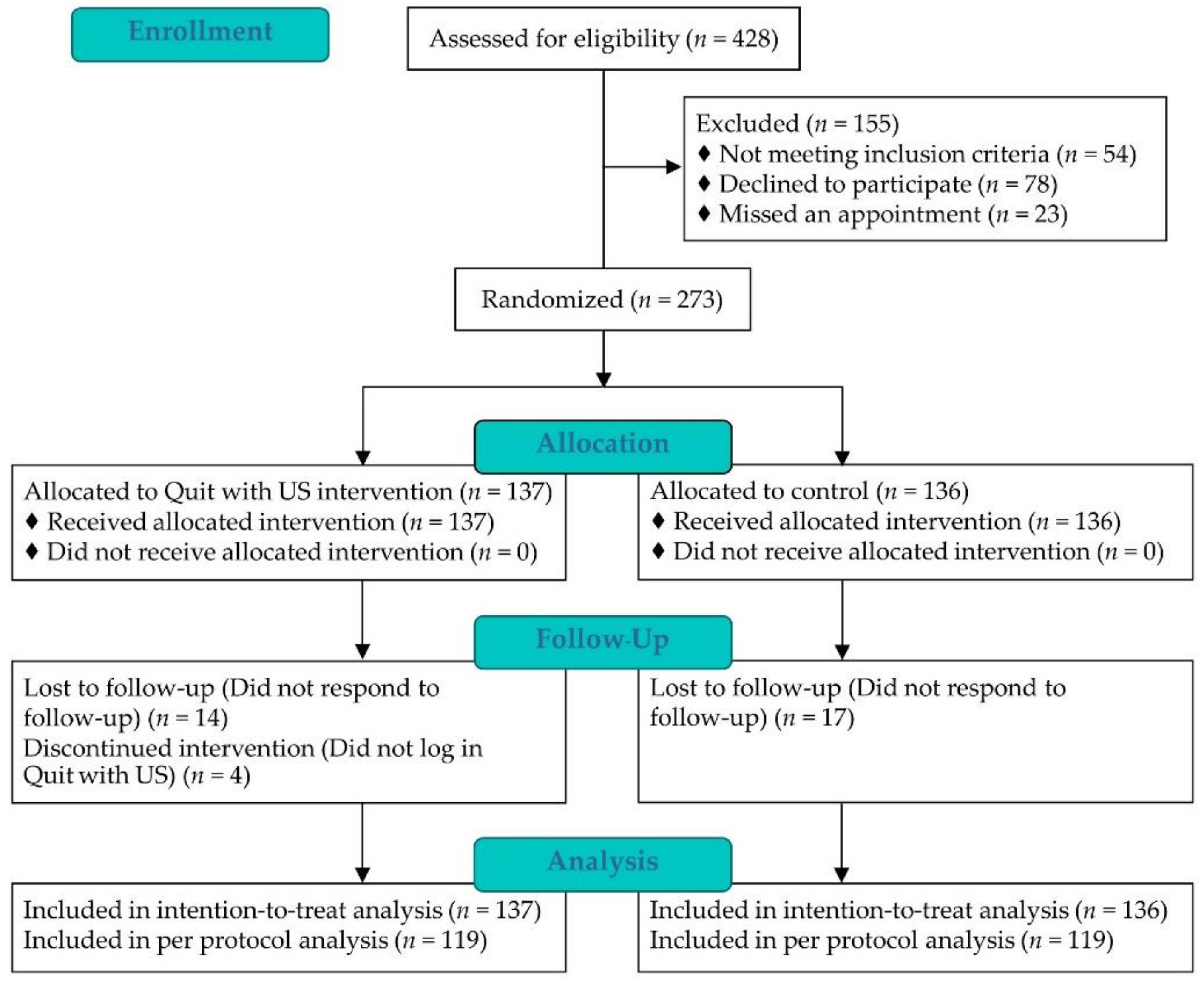
3.1. Recruitment and Baseline Characteristics
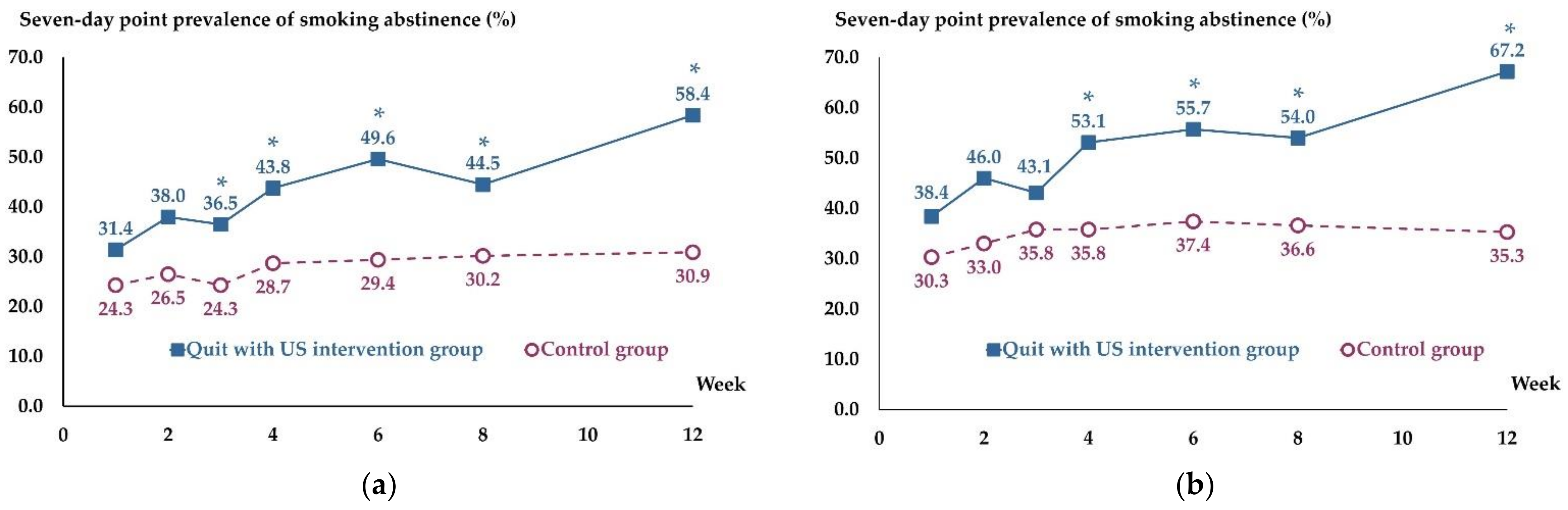
3.2. Smoking Abstinence Outcome
3.3. Secondary Outcomes
3.4. Use of Quit with US
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reitsma, M.B.; Fullman, N.; Ng, M.; Salama, J.S.; Abajobir, A.; Abate, K.H.; Abbafati, C.; Abera, S.F.; Abraham, B.; Abyu, G.Y.; et al. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: A systematic analysis from the Global Burden of Disease Study 2015. Lancet 2017, 389, 1885–1906. [Google Scholar] [CrossRef]
- Burden of Disease Research Program Thailand (BOD Thailand). Thailand Burden of Diseases Attributable to Risk Factors—2014; International Health Policy Program, Ministry of Public Health: Bangkok, Thailand, 2018; pp. 15–17. Available online: http://bodthai.net/en/download/thailand-burden-of-disease-attributable-to-risk-factors-2014/ (accessed on 16 December 2021).
- Pitayarangsarit, S.; Punkrajang, P. (Eds.) Tobacco Consumption Control Situation in Thailand 2018; Tobacco Research Control and Knowledge Management Center (TRC): Bangkok, Thailand, 2018; pp. 3–4. (In Thai) [Google Scholar]
- Benjakul, S.; Kengganpanich, M.; Kengganpanich, T.; Sujirarat, D. Forecasting the Situation of Tobacco Consumption in Thailand 2014; Bureau of Tobacco Control, Department of Disease Control, Ministry of Public Health: Bangkok, Thailand, 2014; pp. 44–50. (In Thai) [Google Scholar]
- Solberg, L.I.; Boyle, R.G.; McCarty, M.; Asche, S.E.; Thoele, M.J. Young adult smokers: Are they different? Am. J. Manag. Care 2007, 13, 626–632. [Google Scholar] [PubMed]
- Hughes, J.R.; Cohen, B.; Callas, P.W. Treatment seeking for smoking cessation among young adults. J. Subst. Abus. Treat. 2009, 37, 211–213. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Curry, S.J.; Sporer, A.K.; Pugach, O.; Campbell, R.T.; Emery, S. Use of tobacco cessation treatments among young adult smokers: 2005 National Health Interview Survey. Am. J. Public Health 2007, 97, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Fanshawe, T.R.; Halliwell, W.; Lindson, N.; Aveyard, P.; Livingstone-Banks, J.; Hartmann-Boyce, J. Tobacco cessation interventions for young people. Cochrane Database Syst. Rev. 2017, 11, CD003289. [Google Scholar] [CrossRef] [PubMed]
- Suls, J.M.; Luger, T.M.; Curry, S.J.; Mermelstein, R.J.; Sporer, A.K.; An, L.C. Efficacy of smoking-cessation interventions for young adults: A meta-analysis. Am. J. Prev. Med. 2012, 42, 655–662. [Google Scholar] [CrossRef]
- Carson-Chahhoud, K.V.; Livingstone-Banks, J.; Sharrad, K.J.; Kopsaftis, Z.; Brinn, M.P.; To, A.N.R.; Bond, C.M. Community pharmacy personnel interventions for smoking cessation. Cochrane Database Syst. Rev. 2019, 2019, CD003698. [Google Scholar] [CrossRef]
- United States Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Smoking Cessation: A Report of the Surgeon General; US Department of Health and Human Services: Washington, DC, USA, 2020; pp. 501–575. Available online: https://www.hhs.gov/sites/default/files/2020-cessation-sgr-full-report.pdf (accessed on 16 December 2021).
- Thongphiew, A. (Ed.) Thailand Clinical Practice Recommendation on Smoking Cessation 2018, 2nd ed.; Thai Health Professional Alliance against Tobacco: Bangkok, Thailand, 2018; pp. 17–64. [Google Scholar]
- Lertsinudom, S.; Kaewketthong, P.; Chankaew, T.; Chinwong, D.; Chinwong, S. Smoking Cessation Services by Community Pharmacists: Real-World Practice in Thailand. Int. J. Environ. Res. Public Health 2021, 18, 11890. [Google Scholar] [CrossRef]
- Lertsinudom, S.; Hansuri, N.; Theeranut, A.; Dilokthornsakul, P. The effect and cost of smoking cessation services provided by community pharmacists in Thailand: A descriptive study approach. Thai J. Pharm. Sci. 2020, 44, 205–209. [Google Scholar]
- Chinwong, S.; Chinwong, D. A National Survey of Community Pharmacists on Smoking Cessation Services in Thailand. Pharmacy 2018, 6, 101. [Google Scholar] [CrossRef]
- Asayut, N.; Olson, P.S.; Kanjanasilp, J.; Thanarat, P.; Senkraigul, B.; Sittisarn, C.; Suksawat, S. A community pharmacist-led smoking cessation intervention using a smartphone app (PharmQuit): A randomized controlled trial. PLoS ONE 2022, 17, e0265483. [Google Scholar] [CrossRef]
- Masaki, K.; Tateno, H.; Nomura, A.; Muto, T.; Suzuki, S.; Satake, K.; Hida, E.; Fukunaga, K. A randomized controlled trial of a smoking cessation smartphone application with a carbon monoxide checker. NPJ Digit. Med. 2020, 3, 35. [Google Scholar] [CrossRef]
- Garrison, K.A.; Pal, P.; O’Malley, S.S.; Pittman, B.P.; Gueorguieva, R.; Rojiani, R.; Scheinost, D.; Dallery, J.; Brewer, J.A. Craving to Quit: A Randomized Controlled Trial of Smartphone App-Based Mindfulness Training for Smoking Cessation. Nicotine Tob. Res. 2020, 22, 324–331. [Google Scholar] [CrossRef]
- Bricker, J.B.; Watson, N.L.; Mull, K.E.; Sullivan, B.M.; Heffner, J.L. Efficacy of Smartphone Applications for Smoking Cessation: A Randomized Clinical Trial. JAMA Intern. Med. 2020, 180, 1472–1480. [Google Scholar] [CrossRef]
- O’Connor, M.; Whelan, R.; Bricker, J.; McHugh, L. Randomized Controlled Trial of a Smartphone Application as an Adjunct to Acceptance and Commitment Therapy for Smoking Cessation. Behav. Ther. 2020, 51, 162–177. [Google Scholar] [CrossRef]
- Herbec, A.; Brown, J.; Shahab, L.; West, R.; Raupach, T. Pragmatic randomised trial of a smartphone app (NRT2Quit) to improve effectiveness of nicotine replacement therapy in a quit attempt by improving medication adherence: Results of a prematurely terminated study. Trials 2019, 20, 547. [Google Scholar] [CrossRef]
- BinDhim, N.F.; McGeechan, K.; Trevena, L. Smartphone Smoking Cessation Application (SSC App) trial: A multicountry double-blind automated randomised controlled trial of a smoking cessation decision-aid ‘app’. BMJ Open 2018, 8, e017105. [Google Scholar] [CrossRef]
- Crane, D.; Ubhi, H.K.; Brown, J.; West, R. Relative effectiveness of a full versus reduced version of the ‘Smoke Free’ mobile application for smoking cessation: An exploratory randomised controlled trial. F1000Res 2018, 7, 1524. [Google Scholar] [CrossRef]
- Baskerville, N.B.; Struik, L.L.; Guindon, G.E.; Norman, C.D.; Whittaker, R.; Burns, C.; Hammond, D.; Dash, D.; Brown, K.S. Effect of a Mobile Phone Intervention on Quitting Smoking in a Young Adult Population of Smokers: Randomized Controlled Trial. JMIR Mhealth Uhealth 2018, 6, e10893. [Google Scholar] [CrossRef]
- Whittaker, R.; McRobbie, H.; Bullen, C.; Rodgers, A.; Gu, Y.; Dobson, R. Mobile phone text messaging and app-based interventions for smoking cessation. Cochrane Database Syst. Rev. 2019, 10, CD006611. [Google Scholar] [CrossRef]
- National Statistical Office. The 2018 Household Survey on the Use of Information and Communication Technology; Ministry of Digital Economy and Society: Bangkok, Thailand, 2018; p. 6, (In Thai). ISBN 9789749765746. [Google Scholar]
- Barroso-Hurtado, M.; Suarez-Castro, D.; Martinez-Vispo, C.; Becona, E.; Lopez-Duran, A. Smoking Cessation Apps: A Systematic Review of Format, Outcomes, and Features. Int. J. Environ. Res. Public Health 2021, 18, 11664. [Google Scholar] [CrossRef]
- Hoeppner, B.B.; Hoeppner, S.S.; Seaboyer, L.; Schick, M.R.; Wu, G.W.; Bergman, B.G.; Kelly, J.F. How Smart are Smartphone Apps for Smoking Cessation? A Content Analysis. Nicotine Tob. Res. 2016, 18, 1025–1031. [Google Scholar] [CrossRef]
- Rajani, N.B.; Weth, D.; Mastellos, N.; Filippidis, F.T. Adherence of popular smoking cessation mobile applications to evidence-based guidelines. BMC Public Health 2019, 19, 743. [Google Scholar] [CrossRef]
- Lee, J.; Dallery, J.; Laracuente, A.; Ibe, I.; Joseph, S.; Huo, J.; Salloum, R.G. A content analysis of free smoking cessation mobile applications in the USA. J. Smok. Cessat. 2019, 14, 195–202. [Google Scholar] [CrossRef]
- World Health Organization. Toolkit for Delivering the 5A’s and 5R’s Brief Tobacco Interventions in Primary Care. Available online: https://apps.who.int/iris/bitstream/handle/10665/112835/9789241506953_eng.pdf?sequence=1 (accessed on 16 December 2021).
- Chulasai, P.; Chinwong, D.; Chinwong, S.; Hall, J.J.; Vientong, P. Feasibility of a Smoking Cessation Smartphone App (Quit with US) for Young Adult Smokers: A Single Arm, Pre-Post Study. Int. J. Environ. Res. Public Health 2021, 18, 9376. [Google Scholar] [CrossRef]
- Kruger, J.; O’Halloran, A.; Rosenthal, A.C.; Babb, S.D.; Fiore, M.C. Receipt of evidence-based brief cessation interventions by health professionals and use of cessation assisted treatments among current adult cigarette-only smokers: National Adult Tobacco Survey, 2009–2010. BMC Public Health 2016, 16, 141. [Google Scholar] [CrossRef]
- Puschel, K.; Thompson, B.; Coronado, G.; Huang, Y.; Gonzalez, L.; Rivera, S. Effectiveness of a brief intervention based on the ‘5A’ model for smoking cessation at the primary care level in Santiago, Chile. Health Promot. Int. 2008, 23, 240–250. [Google Scholar] [CrossRef]
- Bandura, A. Self-Efficacy: The Exercise of Control; W H Freeman: New York, NY, USA, 1997; ISBN 9780716728504. [Google Scholar]
- Gwaltney, C.J.; Metrik, J.; Kahler, C.W.; Shiffman, S. Self-efficacy and smoking cessation: A meta-analysis. Psychol. Addict. Behav. 2009, 23, 56–66. [Google Scholar] [CrossRef]
- Abroms, L.C.; Windsor, R.; Simons-Morton, B. Getting young adults to quit smoking: A formative evaluation of the X-Pack Program. Nicotine Tob. Res. 2008, 10, 27–33. [Google Scholar] [CrossRef] [PubMed]
- An, L.C.; Klatt, C.; Perry, C.L.; Lein, E.B.; Hennrikus, D.J.; Pallonen, U.E.; Bliss, R.L.; Lando, H.A.; Farley, D.M.; Ahluwalia, J.S.; et al. The RealU online cessation intervention for college smokers: A randomized controlled trial. Prev. Med. 2008, 47, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Portney, L.G.; Watkins, M.P. Chapter 9 Experimental Control. In Foundations of Clinical Research: Applications to Practice, 2nd ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2000; pp. 153–176. [Google Scholar]
- Bedfont Scientific Limited. Smokerlyzer Range for Use with piCO, piCObaby and Micro+ User Manual. Available online: https://www.bedfont.com/documents/2910-LAB679%20Smokerlyzer%20Manual%20-%20Issue%204.pdf (accessed on 4 April 2021).
- Hughes, J.R.; Carpenter, M.J.; Naud, S. Do point prevalence and prolonged abstinence measures produce similar results in smoking cessation studies? A systematic review. Nicotine Tob. Res. 2010, 12, 756–762. [Google Scholar] [CrossRef]
- Borland, R.; Yong, H.H.; O’Connor, R.J.; Hyland, A.; Thompson, M.E. The reliability and predictive validity of the Heaviness of Smoking Index and its two components: Findings from the International Tobacco Control Four Country study. Nicotine Tob. Res. 2010, 12 (Suppl. S1), S45–S50. [Google Scholar] [CrossRef] [PubMed]
- Müssener, U.; Bendtsen, M.; Karlsson, N.; White, I.R.; McCambridge, J.; Bendtsen, P. Effectiveness of Short Message Service Text-Based Smoking Cessation Intervention Among University Students: A Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 321–328. [Google Scholar] [CrossRef]
- Matkin, W.; Ordóñez-Mena, J.M.; Hartmann-Boyce, J. Telephone counselling for smoking cessation. Cochrane Database Syst. Rev. 2019, 5, CD002850. [Google Scholar] [CrossRef]
- Barnett, A.; Ding, H.; Hay, K.E.; Yang, I.A.; Bowman, R.V.; Fong, K.M.; Marshall, H.M. The effectiveness of smartphone applications to aid smoking cessation: A meta-analysis. Clin. eHealth 2020, 3, 69–81. [Google Scholar] [CrossRef]
| Characteristic | Quit with US (n = 137), n (%) | Control (n = 136), n (%) | Total (n = 273), n (%) | p-Value |
|---|---|---|---|---|
| Sex | ||||
| Male | 80 (58.4) | 84 (61.8) | 164 (60.1) | 0.622 |
| Female | 57 (41.6) | 52 (38.2) | 109 (39.9) | |
| Age, mean (SD), years | 21.20 (1.63) | 20.93 (1.60) | 21.06 (1.62) | 0.179 |
| Age at cigarette initiation, mean (SD), years | 17.14 (2.29) | 16.93 (2.56) | 17.04 (2.43) | 0.486 |
| Cigarette smoking frequency | ||||
| Daily smokers | 75 (54.7) | 83 (61.0) | 158 (57.9) | 0.327 |
| Nondaily smokers | 62 (45.3) | 53 (39.0) | 115 (42.1) | |
| Daily cigarette consumption | ||||
| ≤10 | 123 (89.8) | 121 (89.0) | 244 (89.4) | 0.768 |
| 11–20 | 13 (9.5) | 15 (11.0) | 28 (10.3) | |
| 21–30 | 1 (0.7) | 0 | 1 (0.4) | |
| mean (SD) | 5.74 (4.93) | 5.87 (4.63) | 5.80 (4.77) | 0.822 |
| Time to first cigarette after waking up | ||||
| ≤5 min | 18 (13.1) | 15 (11.0) | 33 (12.1) | 0.336 |
| 6–30 min | 14 (10.2) | 24 (17.6) | 38 (13.9) | |
| 31–60 min | 22 (16.1) | 18 (13.2) | 40 (14.6) | |
| >60 min | 83 (60.6) | 79 (58.1) | 162 (59.3) | |
| HSI score 1 | ||||
| 0–2 (low nicotine dependence) | 117 (85.4) | 118 (86.8) | 235 (86.1) | 1.000 |
| 3–4 (moderate nicotine dependence) | 19 (13.9) | 18 (13.2) | 37 (13.6) | |
| 5–6 (high nicotine dependence) | 1 (0.7) | 0 | 1 (0.4) | |
| mean (SD) | 0.87 (1.22) | 0.93 (1.22) | 0.90 (1.22) | 0.695 |
| Past year cigarette quit attempt | ||||
| Yes | 102 (74.4) | 96 (70.6) | 198 (72.5) | 0.500 |
| No | 35 (25.6) | 40 (29.4) | 75 (27.5) | |
| Cigarette quit intention | ||||
| Ready to quit smoking | 35 (25.6) | 32 (23.5) | 67 (24.5) | 0.779 |
| In the next 30 days | 102 (74.4) | 104 (76.5) | 206 (75.5) | |
| Smartphone operating system | ||||
| iOS | 68 (49.6) | 74 (54.4) | 142 (52.0) | 0.468 |
| Android | 69 (50.4) | 62 (45.6) | 131 (48.0) | |
| Smartphone use frequency per day | ||||
| ≤10 times | 13 (9.5) | 18 (13.2) | 31 (11.4) | 0.456 |
| 11–20 times | 36 (26.3) | 42 (30.9) | 78 (28.6) | |
| 21–30 times | 45 (32.8) | 43 (31.6) | 88 (32.2) | |
| ≥31 times | 43 (31.4) | 33 (24.3) | 76 (27.8) | |
| Smartphone use period per time | ||||
| ≤15 min | 21 (15.3) | 27 (19.8) | 48 (17.6) | 0.541 |
| 16–30 min | 45 (32.8) | 52 (38.2) | 97 (35.5) | |
| 31–45 min | 22 (16.1) | 20 (14.7) | 42 (15.4) | |
| 46–60 min | 26 (19.0) | 21 (15.4) | 47 (17.2) | |
| ≥61 min | 23 (16.8) | 16 (11.8) | 39 (14.3) | |
| Previous use of smartphone app for smoking cessation | ||||
| Yes | 0 (0) | 0 (0) | 0 (0) | - |
| No | 137 (100.0) | 136 (100.0) | 273 (100.0) | |
| Exhaled CO concentration, mean (SD), ppm | 9.34 (4.79) | 9.48 (4.33) | 9.41 (4.56) | 0.787 |
| Scores on knowledge and attitudes | ||||
| Knowledge of smoking and smoking cessation, 2 mean (SD) | 12.82 (1.48) | 12.60 (1.56) | 12.71 (1.52) | 0.228 |
| Attitudes toward smoking and smoking cessation, 3 mean (SD) | 39.74 (2.99) | 39.18 (3.28) | 39.46 (3.14) | 0.141 |
| Exhaled CO Concentration Level Verified 7-Day Point Prevalence Abstinence 1 | Quit with US, n (%) | Control, n (%) | RR (95% CI) | p-Value |
|---|---|---|---|---|
| Intention-to-treat analysis | (n = 137) | (n = 136) | ||
| Abstainers | 80 (58.4) | 42 (30.9) | 1.89 (1.42 to 2.52) | <0.001 |
| Nonabstainers | 57 (41.6) | 94 (69.1) | 1.00 | |
| Per protocol analysis | (n = 119) | (n = 119) | ||
| Abstainers | 80 (67.2) | 42 (35.3) | 1.90 (1.45 to 2.50) | <0.001 |
| Nonabstainers | 39 (32.8) | 77 (64.7) | 1.00 |
| Outcome Variable | Quit with US (n = 119), Mean (SD) | Control (n = 119), Mean (SD) | p-Value 1 | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | Change (Follow-Up -Baseline) | Baseline | Follow-Up | Change (Follow-Up -Baseline) | ||
| Smoking behaviors 2 | |||||||
| Daily cigarette consumption | 5.71 (4.77) | 1.21 (2.38) | −4.50 (3.74) | 5.94 (4.54) | 2.66 (3.20) | −3.28 (3.50) | 0.010 |
| HSI score 3 | 0.86 (1.20) | 0.13 (0.53) | −0.73 (1.12) | 0.92 (1.22) | 0.34 (0.76) | −0.59 (0.94) | 0.288 |
| Smoking behaviors of nonabstainers | (n = 39) | (n = 77) | |||||
| Daily cigarette consumption | 9.20 (5.61) | 3.69 (2.88) | −5.51 (4.60) | 7.08 (4.67) | 4.10 (3.15) | −2.97 (3.49) | 0.004 |
| HSI score 3 | 1.56 (1.33) | 0.38 (0.88) | −1.18 (1.27) | 1.22 (1.27) | 0.52 (0.90) | −0.70 (0.95) | 0.043 |
| Exhaled CO concentration level(ppm) | 9.43 (4.85) | 5.82 (3.78) | −3.60 (3.56) | 9.48 (4.34) | 7.04 (3.98) | −2.44 (3.83) | 0.016 |
| Scores on knowledge and attitudes | |||||||
| Knowledge of smoking and smoking cessation 4 | 12.86 (1.42) | 13.94 (1.10) | 1.08 (1.38) | 12.63 (1.53) | 13.22 (1.38) | 0.59 (1.40) | 0.006 |
| Attitudes toward smoking and smoking cessation 5 | 39.76 (3.02) | 41.92 (2.54) | 2.16 (2.57) | 39.34 (3.35) | 40.34 (2.84) | 0.99 (2.80) | 0.001 |
| Variable | Mean (SD) |
|---|---|
| Frequency of using the smartphone app per day, n (%) | |
| 1 time | 87 (73.1) |
| ≥2 times | 32 (26.9) |
| Period of using the smartphone app per time, n (%) | |
| ≤5 min | 66 (55.5) |
| 6–10 min | 49 (41.2) |
| 11–15 min | 4 (3.4) |
| Perceived usefulness of each main page 1 | |
| Suggested by US | 3.91 (0.89) |
| Talk with US | 3.40 (1.01) |
| Quit with US | 4.17 (0.83) |
| Let US Help | 3.61 (0.99) |
| Success of US | 4.10 (0.97) |
| Satisfaction with the overall design 1 | 4.06 (0.82) |
| Satisfaction with the overall content 1 | 4.16 (0.80) |
| Confidence in the overall use 1 | 4.33 (0.74) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chulasai, P.; Chinwong, D.; Vientong, P.; Lertsinudom, S.; Kanjanarat, P.; Hall, J.J.; Chinwong, S. Smartphone Application for Smoking Cessation (Quit with US): A Randomized Controlled Trial among Young Adult Light Smokers in Thailand. Int. J. Environ. Res. Public Health 2022, 19, 8265. https://doi.org/10.3390/ijerph19148265
Chulasai P, Chinwong D, Vientong P, Lertsinudom S, Kanjanarat P, Hall JJ, Chinwong S. Smartphone Application for Smoking Cessation (Quit with US): A Randomized Controlled Trial among Young Adult Light Smokers in Thailand. International Journal of Environmental Research and Public Health. 2022; 19(14):8265. https://doi.org/10.3390/ijerph19148265
Chicago/Turabian StyleChulasai, Phantara, Dujrudee Chinwong, Purida Vientong, Sunee Lertsinudom, Penkarn Kanjanarat, John J. Hall, and Surarong Chinwong. 2022. "Smartphone Application for Smoking Cessation (Quit with US): A Randomized Controlled Trial among Young Adult Light Smokers in Thailand" International Journal of Environmental Research and Public Health 19, no. 14: 8265. https://doi.org/10.3390/ijerph19148265
APA StyleChulasai, P., Chinwong, D., Vientong, P., Lertsinudom, S., Kanjanarat, P., Hall, J. J., & Chinwong, S. (2022). Smartphone Application for Smoking Cessation (Quit with US): A Randomized Controlled Trial among Young Adult Light Smokers in Thailand. International Journal of Environmental Research and Public Health, 19(14), 8265. https://doi.org/10.3390/ijerph19148265






