COVID-19 Medical and Pharmacological Management in the European Countries Compared to Italy: An Overview
Abstract
:1. Introduction
2. Facing the Pandemic Emergency in the European Union (EU)
2.1. The Case of Paracetamol and the Concept of Wait and Watch (Monitoring) in Italy
2.2. Managing the COVID-19 Pandemic Emergency in the Main European Countries: A Background
2.3. Managing the COVID-19 Pandemic Emergency in the Main European Countries: Portugal and Spain
2.4. Managing the COVID-19 Pandemic Emergency in the Main European Countries: People Recurring to Hospitals (Emergency Units) as the Only Alternative
3. Before Going to Hospitals: How Can Positive Patients, Residents in the European Countries, Be Treated? The Concerning Issue of Post-Mortem Data
Paucity in Post-Mortem Investigations and Their Scarcity in the COVID-19 Pharmacology Approach: A Cause of Concern
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- COVID-19 Excess Mortality Collaborators. Estimating excess mortality due to the COVID-19 pandemic: A systematic analysis of COVID-19-related mortality, 2020-21. Lancet 2022, 10. [Google Scholar] [CrossRef]
- Tyrovolas, S.; Tsiampalis, T.; Morena, M.; Leung, A.Y.M.; Faka, A.; Chalkias, C.; Tsiodras, S.; Panagiotakos, D. COVID-19 Mortality in Europe, by Latitude and Obesity Status: A Geo-Spatial Analysis in 40 Countries. Nutrients 2022, 14, 471. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, C.; Egrot, M.; Desclaux, A.; Sams, K. CoMeSCov. Recognising Italy’s mistakes in the public health response to COVID-19. Lancet 2022, 399, 357–358. [Google Scholar] [CrossRef]
- Dorrucci, M.; Minelli, G.; Boros, S.; Manno, V.; Prati, S.; Battaglini, M.; Corsetti, G.; Andrianou, X.; Riccardo, F.; Fabiani, M.; et al. Excess Mortality in Italy During the COVID-19 Pandemic: Assessing the Differences Between the First and the Second Wave, Year 2020. Front. Public Health 2021, 9, 669209. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Cipriani, F.; Romeo, G.; Balzi, D. Analysis of the excess mortality and factors associated with deaths from COVID-19 versus other causes in Central Tuscany (Italy) in 2020. Epidemiol. Prev. 2021, 45, 496–503. [Google Scholar]
- Odone, A.; Delmonte, D.; Gaetti, G.; Signorelli, C. Doubled mortality rate during the COVID-19 pandemic in Italy: Quantifying what is not captured by surveillance. Public Health 2021, 190, 108–115. [Google Scholar] [CrossRef]
- Blangiardo, M.; Cameletti, M.; Pirani, M.; Corsetti, G.; Battaglini, M.; Baio, G. Estimating weekly excess mortality at sub-national level in Italy during the COVID-19 pandemic. PLoS ONE 2020, 15, e0240286. [Google Scholar] [CrossRef]
- Sandrini, M.; Andreano, A.; Murtas, R.; Tunesi, S.; Riussi, A.; Guido, D.; Greco, M.T.; Gattoni, M.E.; Gervasi, F.; Consolazio, D.; et al. Assessment of the Overall Mortality during the COVID-19 Outbreak in the Provinces of Milan and Lodi (Lombardy Region, Northern Italy). Epidemiol. Prev. 2020, 44, 244–251. [Google Scholar]
- Amore, S.; Puppo, E.; Melara, J.; Terracciano, E.; Gentili, S.; Liotta, G. Impact of COVID-19 on older adults and role of long-term care facilities during early stages of epidemic in Italy. Sci. Rep. 2021, 11, 12530. [Google Scholar] [CrossRef]
- de Leo, D.; Trabucchi, M. COVID-19 and the Fears of Italian Senior Citizens. Int. J. Environ. Res. Public Health 2020, 17, 3572. [Google Scholar] [CrossRef]
- Pandolfi, S.; Simonetti, V.; Ricevuti, G.; Chirumbolo, S. Paracetamol in the home treatment of early COVID-19 symptoms: A possible foe rather than a friend for elderly patients? J. Med. Virol. 2021, 93, 5704–5706, Erratum in J. Med. Virol. 2022, Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, S.; Chirumbolo, S.; Ricevuti, G.; Valdenassi, L.; Bjørklund, G.; Lysiuk, R.; Doşa, M.D.; Lenchyk, L.; Fazio, S. Home pharmacological therapy in early COVID-19 to prevent hospitalization and reduce mortality: Time for a suitable proposal. Basic Clin. Pharmacol. Toxicol. 2021, 130, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Suter, F.; Consolaro, E.; Pedroni, S.; Moroni, C.; Pastò, E.; Paganini, M.V.; Pravettoni, G.; Cantarelli, U.; Rubis, N.; Perico, N.; et al. A simple, home-therapy algorithm to prevent hospitalisation for COVID-19 patients: A retrospective observational matched-cohort study. EClinicalMedicine 2021, 37, 100941. [Google Scholar] [CrossRef] [PubMed]
- Consolaro, E.; Suter, F.; Rubis, N.; Nurse, R.; Pedroni, S.; Moroni, C.; Pastò, E.; Paganini, M.V.; Pravettoni, G.; Cantarelli, U.; et al. A home-treatment algorithmbased on anti-inflammatory drugs to prevcent hospitalization of patients with early COVID-19. A matched-cohort study (COVER 2). MedRXiv 2021. [Google Scholar] [CrossRef]
- Sestili, P.; Fimognari, C. Paracetamol-Induced Glutathione Consumption: Is There a Link With Severe COVID-19 Illness? Front. Pharmacol. 2020, 11, 579944. [Google Scholar] [CrossRef]
- Larsen, J.R.; Martin, M.R.; Martin, J.D.; Kuhn, P.; Hicks, J.B. Modeling the Onset of Symptoms of COVID-19. Front. Public Health 2020, 8, 473. [Google Scholar] [CrossRef]
- Casey, D.; Geulayov, G.; Bale, E.; Brand, F.; Clements, C.; Kapur, N.; Ness, J.; Patel, A.; Waters, K.; Hawton, K. Paracetamol self-poisoning: Epidemiological study of trends and patient characteristics from the multicentre study of self-harm in England. J. Affect. Disord. 2020, 276, 699–706. [Google Scholar] [CrossRef]
- Hawton, K.; Bergen, H.; Simkin, S.; Arensman, E.; Corcoran, P.; Cooper, J.; Waters, K.; Gunnell, D.; Kapur, N. Impact of different pack sizes of paracetamol in the United Kingdom and Ireland on intentional overdoses: A comparative study. BMC Public Health 2011, 11, 460. [Google Scholar] [CrossRef]
- Simkin, S.; Hawton, K.; Kapur, N.; Gunnell, D. What can be done to reduce mortality from paracetamol overdoses? A patient interview study. QJM 2012, 105, 41–51. [Google Scholar] [CrossRef]
- Morthorst, B.R.; Erlangsen, A.; Nordentoft, M.; Hawton, K.; Hoegberg, L.C.G.; Dalhoff, K.P. Availability of Paracetamol Sold Over the Counter in Europe: A Descriptive Cross-Sectional International Survey of Pack Size Restriction. Basic Clin. Pharm. Toxicol. 2018, 122, 643–649. [Google Scholar] [CrossRef] [Green Version]
- Schneider, A.; Kirsten, H.; Lordick, F.; Lordick, F.; Lübbert, C.; von Braun, A. COVID-19 in outpatients-Is fever a useful indicator for SARS-CoV-2 infection? PLoS ONE 2021, 16, e0246312. [Google Scholar]
- Ws Chew, N.; Nicholas Ngiam, J.; Meng Tham, S.; Yu Lim, Z.; Yi-Wei Li, T.; Cen, S.; Soo Yap, E.; Anantharajah, T.P.; Santosa, A.; Brenda Cross, G.; et al. Fever as a predictor of adverse outcomes in COVID-19. QJM 2021, 20, 1043–1050. [Google Scholar]
- Romano, S.; Galante, H.; Figueira, D.; Mendes, Z.; Rodrigues, A.T. Time-trend analysis of medicine sales and shortages during COVID-19 outbreak: Data from community pharmacies. Res. Soc. Adm. Pharm. 2021, 17, 1876–1881. [Google Scholar] [CrossRef]
- Fang, L.; Karakiulakis, G.; Roth, M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020, 8, e21. [Google Scholar] [CrossRef]
- Day, M. COVID-19: Ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ 2020, 368, m1086. [Google Scholar] [CrossRef] [Green Version]
- Kazama, I.; Senzaki, M. Does immunosuppressive property of non-steroidal anti-inflammatory drugs (NSAIDs) reduce COVID-19 vaccine-induced systemic side effects? Drug Discov. Ther. 2021, 15, 278–280. [Google Scholar] [CrossRef]
- Drake, T.M.; Fairfield, C.J.; Pius, R.; Knight, S.R.; Norman, L.; Girvan, M.; Hardwick, H.E.; Docherty, A.B.; Thwaites, R.S.; Openshaw, P.J.M.; et al. Non-steroidal anti-inflammatory drug use and outcomes of COVID-19 in the ISARIC Clinical Characterisation Protocol UK cohort: A matched, prospective cohort study. Lancet Rheumatol. 2021, 3, e498–e506. [Google Scholar] [CrossRef]
- Onuora, S. NSAIDs not linked to worse COVID-19 outcomes. Nat. Rev. Rheumatol. 2021, 17, 378. [Google Scholar] [CrossRef]
- Lund, L.C.; Kristensen, K.B.; Reilev, M.; Christensen, S.; Thomsen, R.W.; Christiansen, C.F.; Støvring, H.; Johansen, N.B.; Brun, N.C.; Hallas, J.; et al. Adverse outcomes and mortality in users of non-steroidal anti-inflammatory drugs who tested positive for SARS-CoV-2: A Danish nationwide cohort study. PLoS Med. 2020, 17, e1003308. [Google Scholar] [CrossRef]
- Estella, Á.; Garnacho-Montero, J. Del empirismo a la evidencia científica en el tratamiento con antivíricos en los casos graves de infección por coronavirus en tiempos de epidemia [From empiricism to scientific evidence in antiviral treatment in severe cases of coronavirus infection in times of epidemic]. Med. Intensiva 2020, 44, 509–512. [Google Scholar]
- Kupferschmidt, K.; Cohen, J. Race to find COVID-19 treatments accelerates. Science 2020, 367, 1412–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marto, N.; Monteiro, E.C. Medicines for the Treatment Of COVID-19: Awaiting the Evidence. Acta Med. Port. 2020, 33, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Fortaleza, C.M.C.B. Evidence, rationality, and ignorance: Agnotological issues in COVID-19 science. Rev. Soc. Bras. Med. Trop. 2020, 53, e20200475. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.M.; Monogue, M.L.; Jodlowski, T.Z.; Cutrell, J.B. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 323, 1824–1836. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, A.; Vecchié, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; Van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M.; et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021, 21, 319–329. [Google Scholar] [CrossRef]
- Parthasarathy, U.; Martinelli, R.; Vollmann, E.H.; Best, K.; Therien, A.G. The impact of DAMP-mediated inflammation in severe COVID-19 and related disorders. Biochem. Pharmacol. 2021, 195, 114847. [Google Scholar] [CrossRef]
- Romay-Barja, M.; Pascual-Carrasco, M.; De Tena-Dávila, M.J.; Falcón, M.; Rodriguez-Blazquez, C.; Forjaz, M.J.; Ayala, A.; Molina-de la Fuente, I.; Burgos, A.; Muñoz, A.; et al. How patients with COVID-19 managed the disease at home during the first wave in Spain: A cross-sectional study. BMJ Open 2021, 11, e048702. [Google Scholar] [CrossRef]
- Galluccio, F.; Ergonenc, T.; Garcia Martos, A.; Allam, A.E.; Pérez-Herrero, M.; Aguilar, R.; Emmi, G.; Spinicci, M.; Terrancle Juan, I.; Fajardo-Pérez, M. Treatment algorithm for COVID-19: A multidisciplinary point of view. Clin. Rheumatol. 2020, 39, 2077–2084. [Google Scholar] [CrossRef]
- Pandolfi, S.; Chirumbolo, S. Home therapy of COVID-19 at the earliest may greatly prevent hospitalization. Basic Clin. Pharmacol. Toxicol. 2021, 129, 395–396. [Google Scholar] [CrossRef]
- Vetrugno, G.; Laurenti, P.; Franceschi, F.; Foti, F.; D’Ambrosio, F.; Cicconi, M.; LAMilia, D.I.; Di Pumpo, M.; Carini, E.; Pascucci, D.; et al. Gemelli decision tree Algorithm to Predict the need for home monitoring or hospitalization of confirmed and unconfirmed COVID-19 patients (GAP-Covid19): Preliminary results from a retrospective cohort study. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2785–2794. [Google Scholar]
- Mauro, V.; Lorenzo, M.; Paolo, C.; Sergio, H. Treat all COVID 19-positive patients, but do not forget those negative with chronic diseases. Intern. Emerg. Med. 2020, 15, 787–790, Erratum in Intern. Emerg. Med. 2021, 16, 249. [Google Scholar] [CrossRef] [PubMed]
- Zach, H.; Hanová, M.; Letkovičová, M. Distribution of COVID-19 cases and deaths in Europe during the first 12 peak weeks of outbreak. Cent. Eur. J. Public Health 2021, 29, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Madan, M.; Mohan, A.; Madan, K.; Hadda, V.; Tiwari, P.; Guleria, R.; Mittal, S. Timing of Anti-viral therapy in COVID-19: Key to success. Adv. Respir. Med. 2021, 89, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Sundararaj Stanleyraj, J.; Sethuraman, N.; Gupta, R.; Thiruvoth, S.; Gupta, M.; Ryo, A. Treating COVID-19: Are we missing out the window of opportunity? J. Antimicrob. Chemother. 2021, 76, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Fazio, S.; Bellavite, P.; Zanolin, E.; McCullough, P.A.; Pandolfi, S.; Affuso, F. Retrospective Study of Outcomes and Hospitalization Rates of Patients in Italy with a Confirmed Diagnosis of Early COVID-19 and Treated at Home Within 3 Days or After 3 Days of Symptom Onset with Prescribed and Non-Prescribed Treatments Between November 2020 and August 2021. Med. Sci. Monit. 2021, 28, 935379. [Google Scholar] [CrossRef]
- Feuillet, V.; Canard, B.; Trautmann, A. Combining Antivirals and Immunomodulators to Fight COVID-19. Trends Immunol. 2021, 42, 31–44. [Google Scholar] [CrossRef]
- de Girolamo, L.; Peretti, G.M.; Maffulli, N.; Brini, A.T. COVID-19-The real role of NSAIDs in Italy. J. Orthop. Surg. Res. 2020, 15, 165. [Google Scholar] [CrossRef]
- AIFA. The Medicines Utilisation Monitoring Centre. In National Report on Medicines use in Italy. Year 2019; Italian Medicines Agency: Rome, Italy, 2020; p. 576. [Google Scholar]
- Laurent, S.; Agabiti-Rosei, C.; Bruno, R.M.; Rizzoni, D. Microcirculation and Macrocirculation in Hypertension: A Dangerous Cross-Link? Hypertension 2022, 79, 479–490. [Google Scholar] [CrossRef]
- Strain, W.D.; Paldánius, P.M. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc. Diabetol. 2018, 17, 57, Erratum in Cardiovasc. Diabetol. 2021, 20, 120. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Villegas, A.; Bautista-Mesa, R.J.; Baena-Lopez, M.A.; Garzon-Miralles, A.; Castellano-Ortega, M.A.; Leal-Costa, C.; Peiro, S. Impact of the COVID-19 Pandemic on Healthcare Activity in the Regional Hospitals of Andalusia (Spain). J. Clin. Med. 2022, 11, 363. [Google Scholar] [CrossRef]
- Elke, B.; Juliane, W.; Helene, E.; Ulrike, N.; Dimitra, P.; Christoph, R.; Tanja, R.; Reinhard, B. A country-level analysis comparing hospital capacity and utilisation during the first COVID-19 wave across Europe. Health Policy 2021, S0168-8510(21)00290-6, 9. [Google Scholar]
- Ballesteros Sanz, M.Á.; Hernández-Tejedor, A.; Estella, Á.; Jiménez Rivera, J.J.; González de Molina Ortiz, F.J.; Sandiumenge Camps, A.; de Haro, C.; Aguilar Alonso, E.; Bordejé Laguna, L.; González de Molina Ortiz, F.J.; et al. Recommendations of the Working Groups from the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC) for the management of adult critically ill patients in the coronavirus disease (COVID-19). Med. Intensiva 2020, 44, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Belmonte, L.M.; López-Carmona, M.D.; Quevedo-Marín, J.L.; Ricci, M.; Martín-Carmona, J.; Sanz-Cánovas, J.; López-Sampalo, A.; Martín-Escalante, M.D.; Bernal-López, M.R.; Gómez-Huelgas, R. Differences between Clinical Protocols for the Treatment of Coronavirus Disease 2019 (COVID-19) in Andalusia, Spain. Int. J. Environ. Res. Public Health 2020, 17, 6845. [Google Scholar] [CrossRef] [PubMed]
- Mitjà, O.; Corbacho-Monné, M.; Ubals, M.; Tebé, C.; Peñafiel, J.; Tobias, A.; Ballana, E.; Alemany, A.; Riera-Martí, N.; Pérez, C.A.; et al. Hydroxychloroquine for Early Treatment of Adults With Mild Coronavirus Disease 2019: A Randomized, Controlled Trial. Clin. Infect. Dis. 2021, 73, e4073–e4081. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M. El sector público y el sector privado de la sanidad: ¿estabilidad o cambio? [The public and the private healthcare sector in Spain: Stability or change?]. Gac. Sanit. 2019, 33, 499–501. [Google Scholar] [CrossRef]
- Ferre, F.; de Belvis, A.G.; Valerio, L.; Longhi, S.; Lazzari, A.; Fattore, G.; Ricciardi, W.; Maresso, A. Italy: Health system review. Health Syst. Transit. 2014, 16, 1–168. [Google Scholar]
- de Almeida Simoes, J.; Augusto, G.F.; Fronteira, I.; Hernandez-Quevedo, C. Portugal: Health System Review. Health Syst. Transit. 2017, 19, 1–184. [Google Scholar]
- Sirven, N.; Rapp, T. The Dynamics of Hospital Use among Older People Evidence for Europe Using SHARE Data. Health Serv. Res. 2017, 52, 1168–1184. [Google Scholar] [CrossRef]
- Pifarré IArolas, H.; Acosta, E.; López-Casasnovas, G.; Lo, A.; Nicodemo, C.; Riffe, T.; Myrskylä, M. Years of life lost to COVID-19 in 81 countries. Sci. Rep. 2021, 11, 3504. [Google Scholar] [CrossRef]
- Islam, N.; Jdanov, D.A.; Shkolnikov, V.M.; Khunti, K.; Kawachi, I.; White, M.; Lewington, S.; Lacey, B. Effects of covid-19 pandemic on life expectancy and premature mortality in 2020: Time series analysis in 37 countries. BMJ 2021, 375, e066768. [Google Scholar] [CrossRef]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef] [Green Version]
- Ilinca, S.; Calciolari, S. The patterns of health care utilization by elderly Europeans: Frailty and its implications for health systems. Health Serv. Res. 2015, 50, 305–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohn, J.L.; Liu, J.S. The dynamics of medical care use in the British household panel survey. Health Econ. 2013, 22, 687–710. [Google Scholar] [CrossRef] [PubMed]
- Mazzuco, S.; Campostrini, S. Life expectancy drop in 2020. Estimates based on Human Mortality Database. PLoS ONE 2022, 17, e0262846. [Google Scholar] [CrossRef] [PubMed]
- Darmon, N.; Khlat, M. An overview of the health status of migrants in France, in relation to their dietary practices. Public Health Nutr. 2001, 4, 163–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khlat, M.; Legleye, S.; Bricard, D. Gender Patterns in Immigrants’ Health Profiles in France: Tobacco, Alcohol, Obesity and Self-Reported Health. Int. J. Environ. Res. Public Health 2020, 17, 8759. [Google Scholar] [CrossRef] [PubMed]
- Benoît, C.; Coron, G. Private Health Insurance in France: Between Europeanization and Collectivization. Milbank Q. 2019, 97, 1108–1150. [Google Scholar] [CrossRef]
- Leonardi, G.; Colonnello, V.; Farinelli, M.; Bertoletti, E.; Russo, P.M. One size does not fit all: Individual differences in attachment style and fear of COVID-19 in hospitalized elderly patients. Psychogeriatrics 2021, 21, 848–849. [Google Scholar] [CrossRef]
- Wastesson, J.W.; Martikainen, J.E.; Zoëga, H.; Schmidt, M.; Karlstad, Ø.; Pottegård, A. Trends in Use of Paracetamol in the Nordic Countries. Basic Clin. Pharm. Toxicol. 2018, 123, 301–307. [Google Scholar] [CrossRef]
- Gedeborg, R.; Svennblad, B.; Holm, L.; Sjögren, H.; Bardage, C.; Personne, M.; Sjöberg, G.; Feltelius, N.; Zethelius, B. Increased availability of paracetamol in Sweden and incidence of paracetamol poisoning: Using laboratory data to increase validity of a population-based registry study. Pharmacoepidemiol. Drug Saf. 2017, 26, 518–527. [Google Scholar] [CrossRef]
- Hawkins, L.C.; Edwards, J.N.; Dargan, P.I. Impact of restricting paracetamol pack sizes on paracetamol poisoning in the United Kingdom: A review of the literature. Drug Saf. 2007, 30, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Bateman, D.N.; Gorman, D.R.; Bain, M.; Inglis, J.H.; House, F.R.; Murphy, D. Legislation restricting paracetamol sales and patterns of self-harm and death from paracetamol-containing preparations in Scotland. Br. J. Clin. Pharmacol. 2006, 62, 573–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, O.W.; Griffiths, C.; Majeed, A. Interrupted time-series analysis of regulations to reduce paracetamol (acetaminophen) poisoning. PLoS Med. 2007, 4, e105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudec, R.; Božeková, L.; Tisoňová, J. Consumption of three most widely used analgesics in six European countries. J. Clin. Pharm. Ther. 2012, 37, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S.; Simonetti, V.; Franzini, M.; Valdenassi, L.; Bertossi, D.; Pandolfi, S. Estimating coronavirus disease 2019 (COVID-19)-caused deaths in hospitals and healthcare units: Do hospital-acquired infections play a role? Comments with a proposal. Infect. Control. Hosp. Epidemiol. 2021, 19, 1–2. [Google Scholar] [CrossRef]
- Romanova, E.S.; Vasilyev, V.V.; Startseva, G.; Karev, V.; Rybakova, M.G.; Platonov, P.G. Cause of death based on systematic post-mortem studies in patients with positive SARS-CoV-2 tissue PCR during the COVID-19 pandemic. J. Intern. Med. 2021, 290, 655–665. [Google Scholar] [CrossRef]
- Grosse, C.; Grosse, A.; Salzer, H.J.F.; Dünser, M.W.; Motz, R.; Langer, R. Analysis of cardiopulmonary findings in COVID-19 fatalities: High incidence of pulmonary artery thrombi and acute suppurative bronchopneumonia. Cardiovasc. Pathol. 2020, 49, 107263. [Google Scholar] [CrossRef]
- Edler, C.; Schröder, A.S.; Aepfelbacher, M.; Fitzek, A.; Heinemann, A.; Heinrich, F.; Klein, A.; Langenwalder, F.; Lütgehetmann, M.; Meißner, K.; et al. Dying with SARS-CoV-2 infection-an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int. J. Legal Med. 2020, 134, 1275–1284. [Google Scholar] [CrossRef]
- Sadegh Beigee, F.; Pourabdollah Toutkaboni, M.; Khalili, N.; Nadji, S.A.; Dorudinia, A.; Rezaei, M.; Askari, E.; Farzanegan, B.; Marjani, M.; Rafiezadeh, A. Diffuse alveolar damage and thrombotic microangiopathy are the main histopathological findings in lung tissue biopsy samples of COVID-19 patients. Pathol. Res. Pract. 2020, 216, 153228. [Google Scholar] [CrossRef]
- Dwiputra Hernugrahanto, K.; Novembri Utomo, D.; Hariman, H.; Budhiparama, N.C.; Medika Hertanto, D.; Santoso, D.; Hogendoorn, P.C.W. Thromboembolic involvement and its possible pathogenesis in COVID-19 mortality: Lesson from post-mortem reports. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1670–1679. [Google Scholar]
- Di Luise, E.; Magni, P.A. Interim recommendations for the management of forensic investigation during the COVID-19 pandemic: An Italian perspective. Sci. Justice 2021, 61, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Colleluori, G.; Graciotti, L.; Pesaresi, M.; Di Vincenzo, A.; Perugini, J.; Di Mercurio, E.; Caucci, S.; Bagnarelli, P.; Zingaretti, C.M.; Nisoli, E.; et al. Visceral fat inflammation and fat embolism are associated with lung’s lipidic hyaline membranes in subjects with COVID-19. Int. J. Obes. 2022, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mahé, I.; Caulin, C.; Bergmann, J.F. Does paracetamol potentiate the effects of oral anticoagulants?: A literature review. Drug. Saf. 2004, 27, 325–333. [Google Scholar] [PubMed]
- Mahé, I.; Bertrand, N.; Drouet, L.; Simoneau, G.; Mazoyer, E.; Bal dit Sollier, C.; Caulin, C.; Bergmann, J.F. Paracetamol: A haemorrhagic risk factor in patients on warfarin. Br. J. Clin. Pharmacol. 2005, 59, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Parra, D.; Beckey, N.P.; Stevens, G.R. The effect of acetaminophen on the international normalized ratio in patients stabilized on warfarin therapy. Pharmacotherapy 2007, 27, 675–683. [Google Scholar] [CrossRef]
- Mahé, I.; Bertrand, N.; Drouet, L.; Bal Dit Sollier, C.; Simoneau, G.; Mazoyer, E.; Caulin, C.; Bergmann, J.F. Interaction between paracetamol and warfarin in patients: A double-blind, placebo-controlled, randomized study. Haematologica 2006, 91, 1621–1627. [Google Scholar]
- Pinson, G.M.; Beall, J.W.; Kyle, J.A. A review of warfarin dosing with concurrent acetaminophen therapy. J. Pharm. Pract. 2013, 26, 518–521. [Google Scholar] [CrossRef]
- Munsterhjelm, E.; Munsterhjelm, N.M.; Niemi, T.T.; Ylikorkala, O.; Neuvonen, P.J.; Rosenberg, P.H. Dose-dependent inhibition of platelet function by acetaminophen in healthy volunteers. Anesthesiology 2005, 103, 712–717. [Google Scholar] [CrossRef]
- COVID-19 Autopsy. The first COVID-19 autopsy in Spain performed during the early stages of the pandemic. Rev. Esp Patol. 2020, 53, 182–187. [Google Scholar]
- Navarro Conde, P.; Alemany Monraval, P.; Medina Medina, C.; Jiménez Sánchez, A.; Andrés Teruel, J.C.; Ferrando Marco, J.; Puglia Santos, V.; Mayordomo Aranda, E. Autopsy findings from the first known death from Severe Acute Respiratory Syndrome SARS-CoV-2 in Spain. Rev. Esp. Patol. 2020, 53, 88–192. [Google Scholar] [CrossRef]
- Filograna, L.; Grassi, S.; Manenti, G.; Di Donna, C.; Tatulli, D.; Nardoni, F.; Masini, V.; Ausania, F.; Grassi, V.M.; Floris, R.; et al. Postmortem CT pulmonary findings in SARS-CoV-2-positive cases: Correlation with lung histopathological findings and autopsy results. Int. J. Leg. Med. 2022, 20, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Grassi, S.; Arena, V.; Cattani, P.; Dell’Aquila, M.; Liotti, F.M.; Sanguinetti, M.; Oliva, A.; GEMELLI AGAINST COVID-19 Group. SARS-CoV-2 viral load and replication in postmortem examinations. Int. J. Leg. Med. 2022, 24, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.A.; Lopez, G.; Nebuloni, M.; Ottolina, D.; Montomoli, J.; Carsana, L.; Fossali, T.; Castelli, A.; Rech, R.; Cogliati, C.; et al. Lung histopathologic clusters in severe COVID-19: A link between clinical picture and tissue damage. Crit. Care 2021, 13, 423. [Google Scholar] [CrossRef] [PubMed]
- Fanni, D.; Cerrone, G.; Saba, L.; Demontis, R.; Congiu, T.; Piras, M.; Gerosa, C.; Suri, J.S.; Coni, P.; Caddori, A.; et al. Thrombotic sinusoiditis and local diffuse intrasinusoidal coagulation in the liver of subjects affected by COVID-19: The evidence from histology and scanning electron microscopy. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5904–5912. [Google Scholar]
- Congiu, T.; Demontis, R.; Cau, F.; Piras, M.; Fanni, D.; Gerosa, C.; Botta, C.; Scano, A.; Chighine, A.; Faedda, E.; et al. Scanning electron microscopy of lung disease due to COVID-19–a case report and a review of the literature. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7997–8003. [Google Scholar]
- Wang, B.; Yee Aw, T.; Stokes, K.Y. N-acetylcysteine attenuates systemic platelet activation and cerebral vessel thrombosis in diabetes. Redox Biol. 2018, 14, 218–228. [Google Scholar] [CrossRef]
- Zangrillo, A.; Gattinoni, L. Learning from mistakes during the pandemic: The Lombardy lesson. Intensive Care Med. 2020, 46, 1622–1623. [Google Scholar] [CrossRef]
- Bottari, C. Some reflections on organizational profiles in Italy in the time of COVID-19. Int. J. Risk Saf. Med. 2020, 31, 117–119. [Google Scholar] [CrossRef]

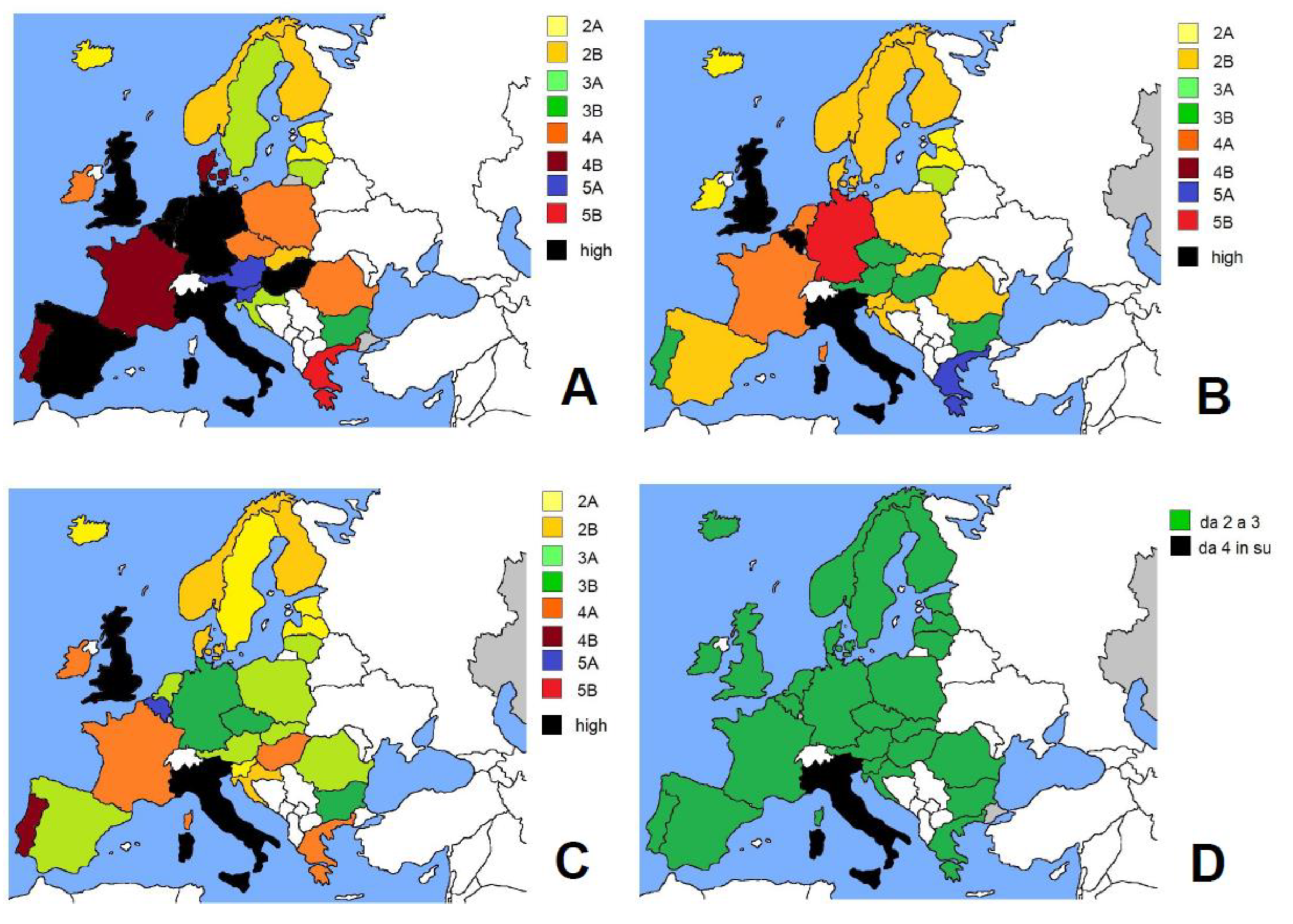
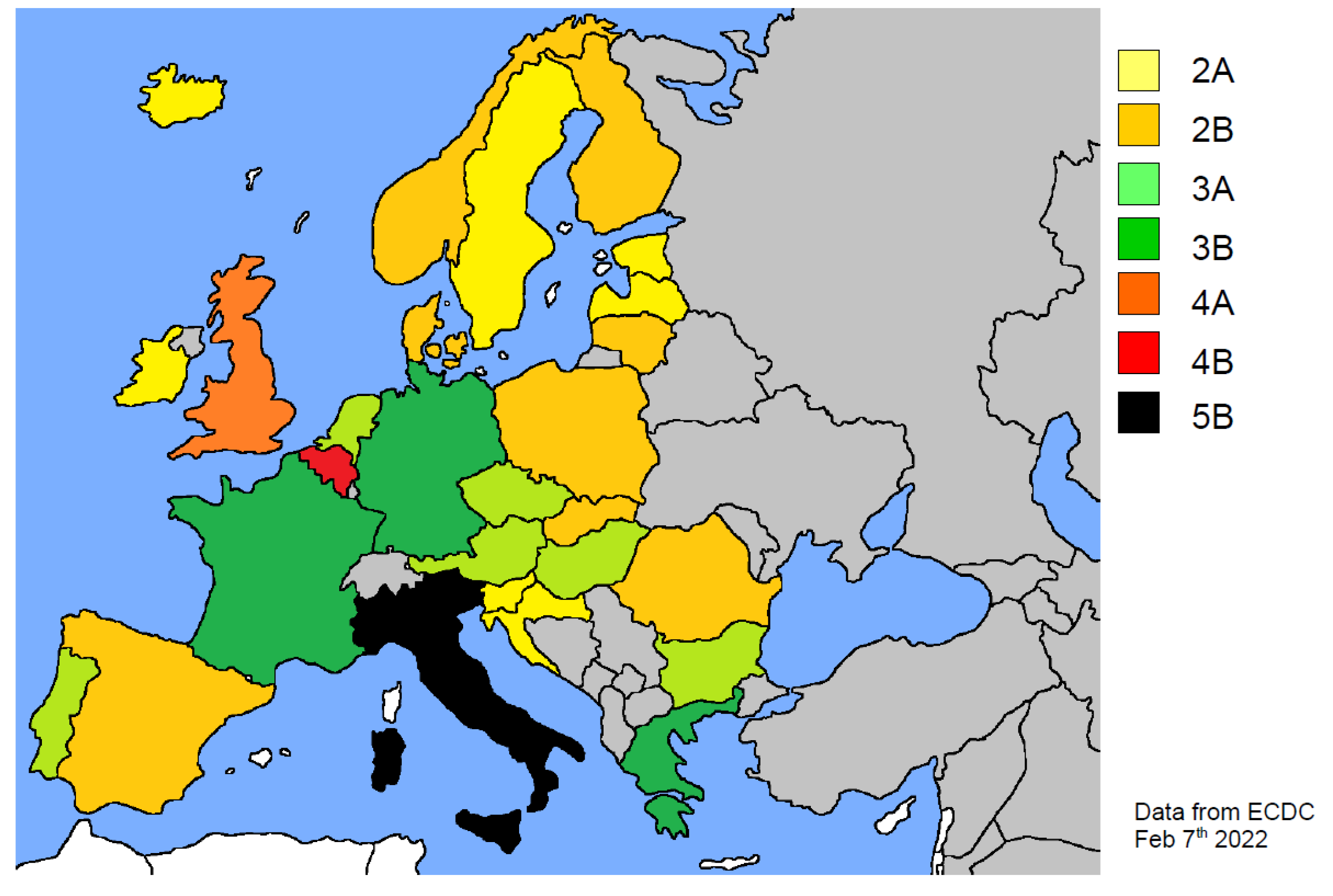
| EU Country | Population | Density (KM2) | Waves | COVID-19 Cases | Deaths | Physicians (1) | Value (2) | S CAT | |
|---|---|---|---|---|---|---|---|---|---|
| Austria | 8,917,000 | 109 | 1 2 3 4 | 17,781 365,376 228,886 1,150,187 | 734 7061 2582 2830 | 5.211 | 9.710 7.316 6.440 5.479 | 10 7 6 5 | 5A 3B 3A 2B |
| cumulative | 2,069,496 | 13,719 | 5.934 | 6 | 3A | ||||
| Belgium | 11,560,000 | 383 | 1 2 3 4 | 61,984 584,276 350,909 1,975,730 | 9736 11,265 3718 3406 | 5.956 | 66.115 13.340 10.014 6.616 | 66 13 10 6 | n.v. 6B 5A 3A |
| cumulative | 3,296,038 | 29,227 | 9.352 | 9 | 4B | ||||
| Bulgaria | 6,927,000 | 64 | 1 2 3 4 | 4989 197,915 199,829 439,652 | 223 8215 8634 12,309 | 4.207 | 7.067 6.863 6.972 5.999 | 7 7 7 3 | 3B 3B 3B 3A |
| cumulative | 995,436 | 33,770 | 6.378 | 6 | 3A | ||||
| Croatia | 4,047,000 | 73 | 1 2 3 4 | 2777 215,833 123,715 532,377 | 107 4747 2987 5187 | 3.000 | 5.812 4.605 4.762 3.711 | 6 5 5 4 | 3A 2B 2B 2A |
| cumulative | 983,780 | 14,137 | 4.049 | 4 | 2A | ||||
| Czechia | 10,700,000 | 139 | 1 2 3 4 | 12,017 918,710 676,653 1,348,532 | 347 16,197 13,507 6796 | 4.116 | 8.129 6.567 6.890 4.816 | 8 7 7 5 | 4A 3B 3B 2B |
| cumulative | 3,243,698 | 37,478 | 5.722 | 6 | 3A | ||||
| Denmark | 5,831,000 | 137 | 1 2 3 4 | 5797 170,474 82,755 1,303,294 | 214 1475 391 999 | 4.225 | 9.282 5.410 4.872 4.330 | 9 5 5 4 | 4B 2B 2B 2A |
| cumulative | 1,915,592 | 3390 | 4.467 | 5 | 2B | ||||
| Estonia | 1,331,000 | 31 | 1 2 3 4 cumulative | 1985 41,142 84,985 187,072 358,087 | 63 357 834 672 2059 | 3.463 | 4.447 3.732 3.767 3.574 3.641 | 4 4 4 4 4 | 2A 2A 2A 2A 2A |
| Finland | 5,531,000 | 18 | 1 2 3 4 | 7265 35,648 47,066 371,911 | 309 374 273 1140 | 4.640 | 5.405 4.829 4.744 4.695 | 5 5 5 5 | 2B 2B 2B 2B |
| cumulative | 514,892 | 2058 | 4.712 | 5 | 2B | ||||
| France | 67,390,000 | 119 | 1 2 3 4 | 164,787 2,633,579 2,470,210 12,127,298 | 29,823 44,099 33,497 14,241 | 6.534 | 8.687 8.526 8.148 6.673 | 9 8 8 7 | 4B 4A 4A 3B |
| cumulative | 20,758,371 | 132,506 | 7.294 | 7 | 3B | ||||
| Germany | 83,240,000 | 232 | 1 2 3 4 | 194,813 1,927,748 1,457,102 5,868,341 | 9256 60,629 21,571 25,383 | 4.300 | 15.322 11.596 7.734 5.303 | 15 11 7 5 | 7B 5B 3B 2B |
| cumulative | 11,117,599 | 118,766 | 6.778 | 7 | 3B | ||||
| Greece | 10,720,000 | 81 | 1 2 3 4 | 3409 138,482 245,349 1,284,956 | 192 5405 6299 8489 | 6.226 | 10.788 9.387 8.305 6.761 | 11 10 8 7 | 5B 5A 4A 3B |
| cumulative | 2,047,849 | 23,760 | 7.166 | 7 | 3B | ||||
| Hungary | 9,750,000 | 107 | 1 2 3 4 | 4155 341,125 436,952 730,700 | 585 11,759 17,209 11,215 | 3.408 | 18.473 7.096 7.622 5.050 | 18 7 8 5 | 9A 3B 4A 2B |
| cumulative | 1,616,846 | 41,741 | 6.170 | 6 | 3A | ||||
| Iceland | 366,425 | 3 | 1 2 3 4 | 1822 3305 604 55,834 | 10 19 1 13 | 4.142 | 4.158 4.159 4.147 4.143 | 4 4 4 4 | 2A 2A 2A 2A |
| cumulative | 73,530 | 49 | 4.144 | 4 | 2A | ||||
| Ireland | 5,025,898 | 72 | 1 2 3 4 cumulative | 25,473 160,392 65,496 793,076 1,205,914 | 1736 1503 1634 887 6228 | 3.352 | 8.259 4.027 5.148 3.432 3.724 | 8 4 5 3 4 | 4A 2A 2B 1B 2A |
| Italy | 59,550,000 | 206 | 1 2 3 4 | 240,256 2,238,171 1,664,789 6,310,761 | 34.757 52,622 37,612 15,577 | 8.013 | 37.814 12.856 12.667 8.521 | 37 13 13 8 | n.v. 6B 6B 4A |
| cumulative | 11,348,701 | 147,734 | 10.695 | 11 | 5B | ||||
| Latvia | 1,902,000 | 30 | 1 2 3 4 | 1118 64,417 68,085 234,473 | 30 1158 1185 2156 | 3.302 | 4.107 3.841 3.824 3.577 | 4 4 4 4 | 2A 2A 2A 2A |
| cumulative | 450,105 | 4951 | 3.632 | 4 | 2A | ||||
| Lithuania | 2,795,000 | 43 | 1 2 3 4 | 1753 179,791 89,921 355,327 | 61 2765 1434 2877 | 5.040 | 6.536 5.701 5.726 5.388 | 6 6 6 5 | 3A 3A 3A 2B |
| cumulative | 749,616 | 7986 | 5.498 | 5 | 2B | ||||
| Netherlands | 17,440,000 | 508 | 1 2 3 4 | 50,047 857,041 671,006 2,417,928 | 6086 7574 3642 3095 | 3.707 | 65.483 8.196 6.464 4.357 | 65 8 6 4 | n.v. 4A 3A 2A |
| cumulative | 4,892,041 | 21,332 | 5.922 | 6 | 3A | ||||
| Norway | 5,379,000 | 15 | 1 2 3 4 | 8893 49,212 62,315 616,369 | 249 283 226 579 | 4.885 | 5.305 4.971 4.939 4.899 | 5 5 5 5 | 2B 2B 2B 2B |
| cumulative | 849,436 | 1466 | 4.911 | 5 | 2B | ||||
| Poland | 37,950,000 | 124 | 1 2 3 4 | 34,393 1,421,871 1,358,898 1,979,083 | 1463 34,667 36,565 29,544 | 2.379 | 7.653 5.402 5.715 4.230 | 8 5 6 4 | 4A 2B 3A 2A |
| cumulative | 5,163,780 | 106,597 | 4.939 | 5 | 2B | ||||
| Portugal | 10,310,000 | 112 | 1 2 3 4 cumulative | 42,171 644,974 128,577 1,570,523 2,915,971 | 1576 10,511 4543 1930 20222 | 5.312 | 9.497 7.137 9.269 5.450 6.089 | 9 7 9 5 6 | 4B 3B 4B 2B 3A |
| Romania | 19,290,000 | 84 | 1 2 3 4 | 26,970 601,171 348,994 982,857 | 1651 13,510 11,977 22,984 | 2.981 | 8.123 4.869 5.864 4.945 | 8 5 6 5 | 4A 2B 3A 2B |
| cumulative | 2,401,821 | 60,642 | 5.102 | 5 | 2B | ||||
| Slovakia | 5,459,000 | 114 | 1 2 3 4 | 1687 428,400 332,035 789,689 | 28 4663 7642 5201 | 3.517 | 5.409 4.758 6.141 4.268 | 5 5 6 4 | 2B 2B 3A 2A |
| cumulative | 1,587,487 | 17,850 | 4.799 | 5 | 2B | ||||
| Slovenia | 2,100,000 | 103 | 1 2 3 4 | 1612 161,054 87,215 428,317 | 111 3353 872 1305 | 3.172 | 10.264 5.316 4.202 3.486 | 10 5 4 3 | 5A 2B 2A 1B |
| cumulative | 794,443 | 5975 | 3.947 | 4 | 2A | ||||
| Spain | 47,350,000 | 91 | 1 2 3 4 | 258,900 2,067,721 817,161 5,299,211 | 29,768 31,656 15,216 8744 | 4.030 | 14.493 5.423 5.724 4.180 | 14 5 6 4 | 7A 2B 3A 2A |
| cumulative | 10,271,197 | 94,204 | 4.865 | 5 | 2B | ||||
| Sweden | 10,350,000 | 25 | 1 2 3 4 | 67,866 481,077 497,919 1,030,254 | 5475 6386 2319 1370 | 4.331 | 6.348 4.662 4.447 4.364 | 6 5 4 4 | 3A 2B 2A 2A |
| cumulative | 2,287,785 | 16,143 | 4.507 | 4 | 2A | ||||
| UK | 67,220,000 | 281 | 1 2 3 4 | 312,654 3,356,998 651,556 9,675,268 | 56,199 72,664 21,564 19,137 | 5.823 | 56.332 11.905 15.123 6.379 | 56 12 15 6 | n.v. 6A 7B 3A |
| cumulative | 17,866,632 | 158,000 | 8.083 | 8 | 4A | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandolfi, S.; Valdenassi, L.; Bjørklund, G.; Chirumbolo, S.; Lysiuk, R.; Lenchyk, L.; Doşa, M.D.; Fazio, S. COVID-19 Medical and Pharmacological Management in the European Countries Compared to Italy: An Overview. Int. J. Environ. Res. Public Health 2022, 19, 4262. https://doi.org/10.3390/ijerph19074262
Pandolfi S, Valdenassi L, Bjørklund G, Chirumbolo S, Lysiuk R, Lenchyk L, Doşa MD, Fazio S. COVID-19 Medical and Pharmacological Management in the European Countries Compared to Italy: An Overview. International Journal of Environmental Research and Public Health. 2022; 19(7):4262. https://doi.org/10.3390/ijerph19074262
Chicago/Turabian StylePandolfi, Sergio, Luigi Valdenassi, Geir Bjørklund, Salvatore Chirumbolo, Roman Lysiuk, Larysa Lenchyk, Monica Daniela Doşa, and Serafino Fazio. 2022. "COVID-19 Medical and Pharmacological Management in the European Countries Compared to Italy: An Overview" International Journal of Environmental Research and Public Health 19, no. 7: 4262. https://doi.org/10.3390/ijerph19074262








