Effects of Resistance Training on C-Reactive Protein and Inflammatory Cytokines in Elderly Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Literature Search
2.3. Data Extraction
2.4. Data Analysis
2.5. Methodological Quality
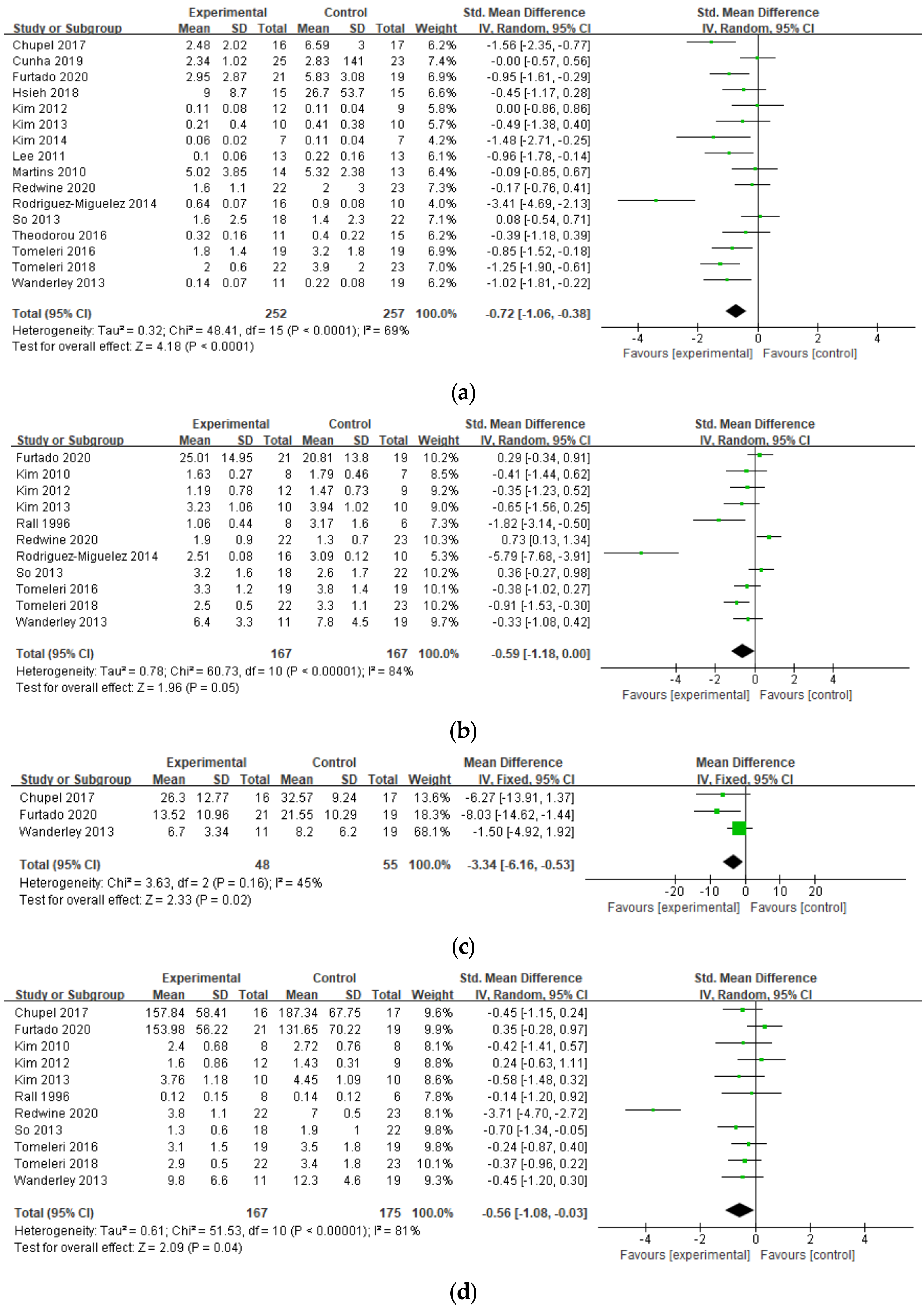
3. Results
3.1. Literature Search
3.2. Study Characteristics
3.3. RT Effects
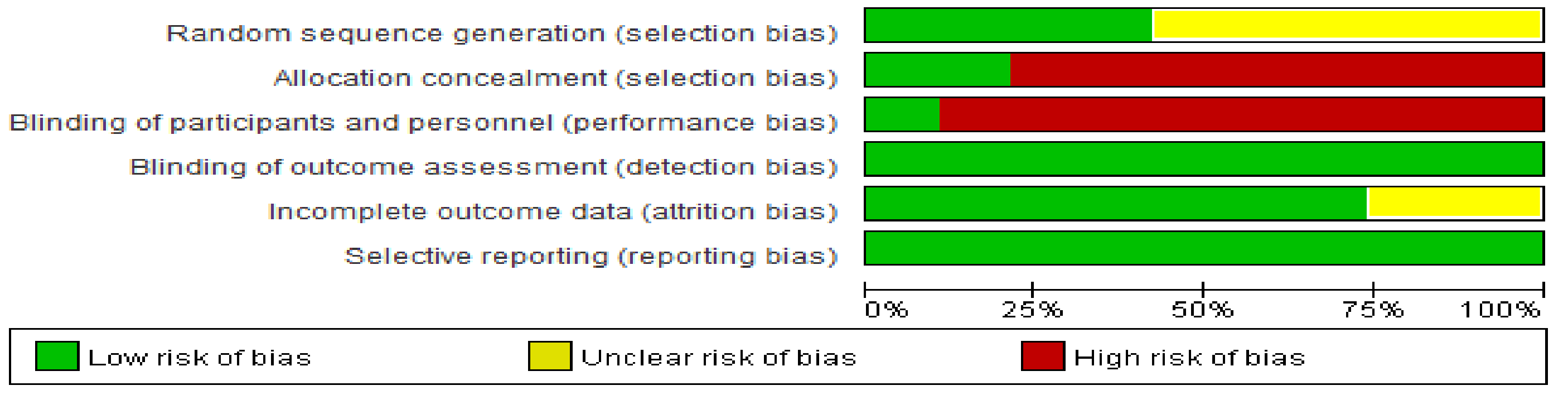
3.4. Methodological Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korea Disease Control and Prevention Agency. Current Status of Overseas Outbreaks of Coronavirus Infectious Diseases. Available online: http://ncov.mohw.go.kr/bdBoardList_Real.do?brdId=1&brdGubun=14&ncvContSeq=&contSeq=&board_id=&gubun= (accessed on 24 November 2021).
- Levin, A.T.; Hanage, W.P.; Owusu-Boaitey, N.; Cochran, K.B.; Walsh, S.P.; Meyerowitz-Katz, G. Assessing the age specificity of infection fatality rates for COVID-19: Systematic review, meta-analysis, and public policy implications. Eur. J. Epidemiol. 2020, 35, 1123–1138. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, J.N.; Gilroy, D.W. Resolution of inflammation: A new therapeutic frontier. Nat. Rev. Drug Discov. 2016, 15, 551–567. [Google Scholar] [CrossRef] [PubMed]
- Straub, R.H. The brain and immune system prompt energy shortage in chronic inflammation and ageing. Nat. Rev. Rheumatol. 2017, 13, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Chang, J.; Lartigue, L.; Bolen, C.R.; Haddad, F.; Gaudilliere, B.; Ganio, E.A.; Fragiadakis, G.K.; Spitzer, M.H.; Douchet, I.; et al. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat. Med. 2017, 23, 174–184. [Google Scholar] [CrossRef]
- Kotas, M.E.; Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 2015, 160, 816–827. [Google Scholar] [CrossRef] [Green Version]
- Pietrobon, A.J.; Teixeira, F.M.E.; Sato, M.N. Immunosenescence and Inflammaging: Risk factors of severe COVID-19 in older people. Front. Immunol. 2020, 11, 579220. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Calcinotto, A.; Kohli, J.; Zagato, E.; Pellegrini, L.; Demaria, M.; Alimonti, A. Cellular senescence: Aging, cancer, and injury. Physiol. Rev. 2019, 99, 1047–1078. [Google Scholar] [CrossRef]
- Byrd, D.A.; Judd, S.E.; Flanders, W.D.; Hartman, T.J.; Fedirko, V.; Bostick, R.M. Development and validation of novel dietary and lifestyle inflammation scores. J. Nutr. 2019, 149, 2206–2218. [Google Scholar] [CrossRef]
- De Rosa, V.; La Cava, A.; Matarese, G. Metabolic pressure and the breach of immunological self-tolerance. Nat. Immunol. 2017, 18, 1190–1196. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Margină, D.; Ungurianu, A.; Purdel, C.; Tsoukalas, D.; Sarandi, E.; Thanasoula, M.; Tekos, F.; Mesnage, R.; Kouretas, D.; Tsatsakis, A. chronic inflammation in the context of everyday life: Dietary changes as mitigating factors. Int. J. Environ. Res. Public Health 2020, 17, 4135. [Google Scholar] [CrossRef]
- Franceschi, C.; Salvioli, S.; Garagnani, P.; de Eguileor, M.; Monti, D.; Capri, M. Immunobiography and the heterogeneity of immune responses in the elderly: A focus on inflammaging and trained immunity. Front. Immunol. 2017, 8, 982. [Google Scholar] [CrossRef]
- Fuentes, E.; Fuentes, M.; Alarcón, M.; Palomo, I. Immune system dysfunction in the elderly. An. Acad. Bras. Cienc. 2017, 89, 285–299. [Google Scholar] [CrossRef] [Green Version]
- Fulop, T.; Witkowski, J.M.; Olivieri, F.; Larbi, A. The integration of inflammaging in age-related diseases. Semin Immunol. 2018, 40, 17–35. [Google Scholar] [CrossRef]
- Li, T.; Huang, Y.; Cai, W.; Chen, X.; Men, X.; Lu, T.; Wu, A.; Lu, Z. Age-related cerebral small vessel disease and inflammaging. Cell Death Dis. 2020, 11, 932. [Google Scholar] [CrossRef]
- Mészáros, Á.; Molnár, K.; Nógrádi, B.; Hernádi, Z.; Nyúl-Tóth, Á.; Wilhelm, I.; Krizbai, I.A. Neurovascular Inflammaging in Health and Disease. Cells 2020, 9, E1614. [Google Scholar] [CrossRef]
- Peluso, I.; Palmery, M. The relationship between body weight and inflammation: Lesson from anti-TNF-α antibody therapy. Hum. Immunol. 2016, 77, 47–53. [Google Scholar] [CrossRef]
- Krüger, K.; Mooren, F.C.; Pilat, C. The immunomodulatory effects of physical activity. Curr. Pharm. Des. 2016, 22, 3730–3748. [Google Scholar] [CrossRef]
- Liberman, K.; Forti, L.N.; Beyer, I.; Bautmans, I. The effects of exercise on muscle strength, body composition, physical functioning and the inflammatory profile of older adults: A systematic review. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 30–53. [Google Scholar] [CrossRef]
- de Labra, C.; Guimaraes-Pinheiro, C.; Maseda, A.; Lorenzo, T.; Millán-Calenti, J.C. Effects of physical exercise interventions in frail older adults: A systematic review of randomized controlled trials. BMC Geriatr. 2015, 15, 154. [Google Scholar] [CrossRef] [Green Version]
- Lopez, P.; Pinto, R.S.; Radaelli, R.; Rech, A.; Grazioli, R.; Izquierdo, M.; Cadore, E.L. Benefits of resistance training in physically frail elderly: A systematic review. Aging Clin. Exp. Res. 2018, 30, 889–899. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Ratamess, N.A. Fundamentals of resistance training: Progression and exercise prescription. Med. Sci. Sports Exerc. 2004, 36, 674–688. [Google Scholar] [CrossRef]
- Macêdo Santiago, L.Â.; Neto, L.G.L.; Borges Pereira, G.; Leite, R.D.; Mostarda, C.T.; de Oliveira Brito Monzani, J.; Sousa, W.R.; Rodrigues Pinheiro, A.J.M.; Navarro, F. Effects of resistance training on immunoinflammatory response, TNF-alpha gene expression, and body composition in elderly women. J. Aging Res. 2018, 2018, 1467025. [Google Scholar] [CrossRef] [Green Version]
- Furtado, G.E.; Chupel, M.U.; Minuzzi, L.G.; Rama, L.; Colado, J.C.; Hogervorst, E.; Ferreira, J.P.; Teixeira, A.M. The mediating effect of different exercise programs on the immune profile of frail older women with cognitive impairment. Curr. Pharm. Des. 2020, 26, 906–915. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Science, 2nd ed.; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1998. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med. Sci. Sports Exerc. 2009, 41, 687–708. [Google Scholar] [CrossRef]
- Chupel, M.U.; Direito, F.; Furtado, G.E.; Minuzzi, L.G.; Pedrosa, F.M.; Colado, J.C.; Ferreira, J.P.; Filaire, E.; Teixeira, A.M. Strength training decreases inflammation and increases cognition and physical fitness in older women with cognitive impairment. Front. Physiol. 2017, 8, 377. [Google Scholar] [CrossRef] [Green Version]
- Cunha, P.M.; Ribeiro, A.S.; Nunes, J.P.; Tomeleri, C.M.; Nascimento, M.A.; Moraes, G.K.; Sugihara, P., Jr.; Barbosa, D.S.; Venturini, D.; Cyrino, E.S. Resistance training performed with single-set is sufficient to reduce cardiovascular risk factors in untrained older women: The randomized clinical trial. Active aging longitudinal study. Arch. Gerontol. Geriatr. 2019, 81, 171–175. [Google Scholar] [CrossRef]
- Hsieh, P.L.; Tseng, C.H.; Tseng, Y.J.; Yang, W.S. Resistance training improves muscle function and cardiometabolic risks but not quality of life in older people with type 2 diabetes mellitus: A randomized controlled trial. J. Geriatr. Phys. Ther. 2018, 41, 65–76. [Google Scholar] [CrossRef]
- Kim, C.H.; Lee, J.W.; Park, H.Y. The effect of yoga exercise and band exercise erformance in elderly women on the inflammatory makers and ageing hormone. KSW 2014, 9, 209–220. [Google Scholar]
- Kim, K.T.; Cho, J.H. The effect of elastic band and aerobic exercise on fitness, blood lipids, and vascular inflammatory markers in elderly women. A. J. Kinesiol. 2013, 15, 129–138. [Google Scholar]
- Kim, N.R.; Park, H.Y. Change of cellular immunity substance by exercise types in elderly women. KJGD 2010, 18, 163–170. [Google Scholar]
- Kim, Y.Y.; Lim, S.; Choi, S.M.; Lee, M.G. Effects of 12 weeks of aerobic and resistance training on abdominal fat, physical fitness, adipokines, and inflammatory markers in female elderly patients with type 2 diabetes. KJSS 2012, 23, 489–501. [Google Scholar]
- Lee, J.W. The Effect of aerobic exercise and type of band exercise on inflammatory markers of elderly women patients with metabolic syndrome. KJSS 2011, 20, 1041–1053. [Google Scholar]
- Martins, R.A.; Neves, A.P.; Coelho-Silva, M.J.; Veríssimo, M.T.; Teixeira, A.M. The effect of aerobic versus strength-based training on high-sensitivity C-reactive protein in older adults. Eur. J. Appl. Physiol. 2010, 110, 161–169. [Google Scholar] [CrossRef]
- Rall, L.C.; Roubenoff, R.; Cannon, J.G.; Abad, L.W.; Dinarello, C.A.; Meydani, S.N. Effects of progressive resistance training on immune response in aging and chronic inflammation. Med. Sci. Sports Exerc. 1996, 28, 1356–1365. [Google Scholar] [CrossRef]
- Redwine, L.S.; Pung, M.A.; Wilson, K.; Bangen, K.J.; Delano-Wood, L.; Hurwitz, B. An exploratory randomized sub-study of light-to-moderate intensity exercise on cognitive function, depression symptoms and inflammation in older adults with heart failure. J. Psychosom. Res. 2020, 128, 109883. [Google Scholar] [CrossRef]
- Rodriguez-Miguelez, P.; Fernandez-Gonzalo, R.; Almar, M.; Mejías, Y.; Rivas, A.; de Paz, J.A.; Cuevas, M.J.; González-Gallego, J. Role of Toll-like receptor 2 and 4 signaling pathways on the inflammatory response to resistance training in elderly subjects. Age 2014, 36, 9734. [Google Scholar] [CrossRef] [Green Version]
- So, W.Y.; Song, M.; Park, Y.H.; Cho, B.L.; Lim, J.Y.; Kim, S.H.; Song, W. Body composition, fitness level, anabolic hormones, and inflammatory cytokines in the elderly: A randomized controlled trial. Aging Clin. Exp. Res. 2013, 25, 167–174. [Google Scholar] [CrossRef]
- Theodorou, A.A.; Panayiotou, G.; Volaklis, K.A.; Douda, H.T.; Paschalis, V.; Nikolaidis, M.G.; Smilios, I.; Toubekis, A.; Kyprianou, D.; Papadopoulos, I.; et al. Aerobic, resistance and combined training and detraining on body composition, muscle strength, lipid profile and inflammation in coronary artery disease patients. Res. Sports Med. 2016, 24, 171–184. [Google Scholar] [CrossRef]
- Tomeleri, C.M.; Ribeiro, A.S.; Souza, M.F.; Schiavoni, D.; Schoenfeld, B.J.; Venturini, D.; Barbosa, D.S.; Landucci, K.; Sardinha, L.B.; Cyrino, E.S. Resistance training improves inflammatory level, lipid and glycemic profiles in obese older women: A randomized controlled trial. Exp. Gerontol. 2016, 84, 80–87. [Google Scholar] [CrossRef]
- Tomeleri, C.M.; Souza, M.F.; Burini, R.C.; Cavaglieri, C.R.; Ribeiro, A.S.; Antunes, M.; Nunes, J.P.; Venturini, D.; Barbosa, D.S.; Sardinha, L.B.; et al. Resistance training reduces metabolic syndrome and inflammatory markers in older women: A randomized controlled trial. J. Diabetes 2018, 10, 328–337. [Google Scholar] [CrossRef]
- Wanderley, F.A.; Moreira, A.; Sokhatska, O.; Palmares, C.; Moreira, P.; Sandercock, G.; Oliveira, J.; Carvalho, J. Differential responses of adiposity, inflammation and autonomic function to aerobic versus resistance training in older adults. Exp. Gerontol. 2013, 48, 326–333. [Google Scholar] [CrossRef]
- Sardeli, A.V.; Tomeleri, C.M.; Cyrino, E.S.; Fernhall, B.; Cavaglieri, C.R.; Chacon-Mikahil, M.P.T. Effect of resistance training on inflammatory markers of older adults: A meta-analysis. Exp. Gerontol. 2018, 111, 188–196. [Google Scholar] [CrossRef]
- Rose, G.L.; Mielke, G.I.; Durr, M.; Schaumberg, M.A. Effect of resistance training on chronic inflammation: A systematic review and meta-analysis. Transl. Sports Med. 2021, 4, 900–913. [Google Scholar] [CrossRef]
- Wu, Y.; Potempa, L.A.; El Kebir, D.; Filep, J.G. C-reactive protein and inflammation: Conformational changes affect function. Biol. Chem. 2015, 396, 1181–1197. [Google Scholar] [CrossRef]
- Schaap, L.A.; Pluijm, S.M.; Deeg, D.J.; Visser, M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am. J. Med. 2006, 119, 526. [Google Scholar] [CrossRef]
- Schaap, L.A.; Pluijm, S.M.; Deeg, D.J.; Harris, T.B.; Kritchevsky, S.B.; Newman, A.B.; Colbert, L.H.; Pahor, M.; Rubin, S.M.; Tylavsky, F.A.; et al. Higher inflammatory marker levels in older persons: Associations with 5-year change in muscle mass and muscle strength. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 1183–1189. [Google Scholar] [CrossRef] [Green Version]
- Goodman, M.N. Interleukin-6 induces skeletal muscle protein breakdown in rats. Proc. Soc. Exp. Biol. Med. 1994, 205, 182–185. [Google Scholar] [CrossRef]
- Fedewa, M.V.; Hathaway, E.D.; Ward-Ritacco, C.L. Effect of exercise training on C reactive protein: A systematic review and meta-analysis of randomised and non-randomised controlled trials. Br. J. Sports Med. 2017, 51, 670–676. [Google Scholar] [CrossRef]
- Rose, G.L.; Skinner, T.L.; Mielke, G.I.; Schaumberg, M.A. The effect of exercise intensity on chronic inflammation: A systematic review and meta-analysis. J. Sci. Med. Sport 2021, 24, 345–351. [Google Scholar] [CrossRef]
- Pérez Chaparro, C.G.A.; Zech, P.; Schuch, F.; Wolfarth, B.; Rapp, M.; Heiβel, A. Effects of aerobic and resistance exercise alone or combined on strength and hormone outcomes for people living with HIV. A meta-analysis. PLoS ONE 2018, 13, e0203384. [Google Scholar] [CrossRef]
- Hayashino, Y.; Jackson, J.L.; Hirata, T.; Fukumori, N.; Nakamura, F.; Fukuhara, S.; Tsujii, S.; Ishii, H. Effects of exercise on C-reactive protein, inflammatory cytokine and adipokine in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. Metabolism 2014, 63, 431–440. [Google Scholar] [CrossRef]
- Khalafi, M.; Malandish, A.; Rosenkranz, S.K. The impact of exercise training on inflammatory markers in postmenopausal women: A systemic review and meta-analysis. Exp. Gerontol. 2021, 150, 111398. [Google Scholar] [CrossRef]
- Idriss, H.T.; Naismith, J.H. TNF alpha and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Tzanavari, T.; Giannogonas, P.; Karalis, K.P. TNF-alpha and obesity. Curr. Dir. Autoimmun. 2010, 11, 145–156. [Google Scholar] [PubMed]
- Giannopoulou, I.; Fernhall, B.; Carhart, R.; Weinstock, R.S.; Baynard, T.; Figueroa, A.; Kanaley, J.A. Effects of diet and/or exercise on the adipocytokine and inflammatory cytokine levels of postmenopausal women with type 2 diabetes. Metabolism 2005, 54, 866–875. [Google Scholar] [CrossRef]
- Ferrucci, L.; Penninx, B.W.; Volpato, S.; Harris, T.B.; Bandeen-Roche, K.; Balfour, J.; Leveille, S.G.; Fried, L.P.; Md, J.M. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J. Am. Geriatr. Soc. 2002, 50, 1947–1954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degens, H. Age-related skeletal muscle dysfunction: Causes and mechanisms. J. Musculoskelet. Neuronal Interact. 2007, 50, 246–252. [Google Scholar]
- Petersen, A.M.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalo-Encabo, P.; Maldonado, G.; Valadés, D.; Ferragut, C.; Pérez-López, A. The role of exercise training on low-grade systemic inflammation in adults with overweight and obesity: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 13258. [Google Scholar] [CrossRef] [PubMed]
- Alizaei Yousefabadi, H.; Niyazi, A.; Alaee, S.; Fathi, M.; Mohammad Rahimi, G.R. Anti-inflammatory effects of exercise on metabolic syndrome patients: A systematic review and meta-analysis. Biol. Res. Nurs. 2021, 23, 280–292. [Google Scholar] [CrossRef]
- Tam, C.S.; Garnett, S.P.; Cowell, C.T.; Heilbronn, L.K.; Lee, J.W.; Wong, M.; Baur, L.A. IL-6, IL-8 and IL-10 levels in healthy weight and overweight children. Horm. Res. Paediatr. 2010, 73, 128–134. [Google Scholar] [CrossRef]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory effects of high and moderate intensity exercise-a systematic review. Front. Physiol. 2019, 10, 1550. [Google Scholar] [CrossRef]

| First Author and Year | Participants: Health Condition, Sample Size n (EG, CG), Mean Age, % of Females | Interventions: Method, Number of Exercises (Ex), Intensity, Number of Sets and Repetitions, Minute, Weekly Frequency, Duration | Comparisons | Outcome Measures |
|---|---|---|---|---|
| Chupel 2017 | Cognitive impairment, 33(16/17), 82.7, 100.0 | Elastic band, 8–10 ex, vigorous, 1–2 × 10–12 Rep, 30–50 min, 2–3 times/week, 28 weeks | Usual care | CRP (↓), IL-10 (↔), TNF-α (↔) |
| Cunha 2019 | Healthy 48(25/23), 70.3, 100.0 | Machines, 8 ex, moderate, 1 × 10–15 Rep, 20 min, 3 times/week, 12 weeks | Inactive | CRP (↔) |
| Furtado 2020 | Cognitive impairment 40(21/19), 80.5, 100.0 | Elastic band, 8–10 ex, moderate, 2–3 × 10–15 Rep, 45 min, 2–3 times/week, 28 weeks | Usual care | CRP (↓), IL-6 (↔), IL-10 (↓), TNF-α (↔) |
| Hsieh 2018 | Type 2 diabetes 30(15/15), 71.2, 63.3 | Machines, 8 ex, vigorous, 3 × 8–12 Rep, NR, 3 times/week, 12 weeks | Usual care | CRP (↔) |
| Kim 2010 | Healthy 16(8/8), 67.1, 100.0 | Elastic band, 14 ex, moderate, 2 × 10–15 Rep, 60 min, 4 times/week, 12 weeks | Inactive | IL-6 (↔), TNF-α (↔) |
| Kim 2012 | Type 2 diabetes 21(12/9), 68.8, 100.0 | Machines, 8 ex, moderate, 2–3 × 10–15 Rep, 40–60 min, 3 times/week, 12 weeks | Usual care | CRP (↔), IL-6 (↔), TNF-α (↔) |
| Kim 2013 | Healthy 20(10/10), 66.9, 100.0 | Elastic band, 8 ex, vigorous, 3 × 10–12 Rep, 70 min, 3 times/week, 12 weeks | Inactive | CRP (↔), IL-6 (↔), TNF-α (↔) |
| Kim 2014 | Healthy 14(7/7), 71.5, 100.0 | Elastic band, 9 ex, moderate, 3 × 12 Rep, 60 min, 3 times/week, 12 weeks | Inactive | CRP (↓) |
| Lee 2011 | Metabolic syndrome 26(13/13), 68.8, 100.0 | Elastic band, 12 ex, moderate, 3 × 10–15 Rep, 50 min, 3 times/week, 12 weeks | Usual care | CRP (↓) |
| Martins 2010 | Healthy 27(14/13), 73.2, 59.3 | Callisthenic and elastic band, 8 ex, moderate, 6 × 8–12 Rep, 45 min, 3 times/week, 16 weeks | Inactive | CRP (↔) |
| Rall 1996 | Healthy 14(8/6), 69.7, 31.4 | Machines, 5 ex, vigorous, 3 × 8 Rep, 45 min, 2 times/week, 12 weeks | Inactive | IL-6 (↓), TNF-α (↔) |
| Redwine 2020 | Heart Failure 45(22/23), 66.0, 13.5 | Elastic band, NR, vigorous, NR, 60 min, 2 times/week, 16 weeks | Inactive | CRP (↔), IL-6 (↑), TNF-α (↓) |
| Rodriguez-Miguelez 2014 | Healthy 26(16/10), 69.1, 73.1 | Machines, 3 ex, vigorous, 3 × 8–12 Rep, NR, 2 times/week, 8 weeks | Inactive | CRP (↓), IL-6 (↓) |
| So 2013 | Healthy 40(18/22), 69.8, 67.5 | Elastic band, 14 ex, moderate, 2–3 × 15–25 Rep, 60 min, 3 times/week, 12 weeks | Inactive | CRP (↔), IL-6 (↔), TNF-α (↓) |
| Theodorou 2016 | Coronary artery disease 26(11/15), 62.0, 0.0 | Machines, 8 ex, moderate, 2 × 12–15 Rep, NR, 3 times/week, 32 weeks | Usual care | CRP (↔) |
| Tomeleri 2016 | Obese 38(19/19), 68.2, 100.0 | Machines, 8 ex, moderate, 3 × 10–15 Rep, 45–50 min, 3 times/week, 12 weeks | Inactive | CRP (↓), IL-6 (↔), TNF-α (↔) |
| Tomeleri 2018 | Healthy 45(22/23), 70.4, 100.0 | Machines, 8 ex, moderate, 3 × 10–15 Rep, NR, 3 times/week, 12 weeks | Inactive | CRP (↓), IL-6 (↓), TNF-α (↔) |
| Wanderley 2013 | Healthy 30(11/19), 67.6, 73.3 | Machines, 9 ex, moderate, 2 × 10–15 Rep, 50 min, 3 times/week, 32 weeks | Inactive | CRP (↓), IL-6 (↔), IL-10 (↔), TNF-α (↔) |
| Subgroup | CRP | IL-6 | TNF-α | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect Size | 95% CI | n | I2 | Effect Size | 95% CI | n | I2 | Effect Size | 95% CI | n | I2 | |
| Age | ||||||||||||
| ≤70 years | −0.69 | −1.19, −0.20 | 9 | 73 | −0.70 | −1.43, 0.03 | 9 | 85 | −0.73 | −1.46, −0.00 | 8 | 84 |
| >70 years | −0.83 | −1.24, −0.42 | 7 | 54 | −0.31 | −1.49, 0.86 | 2 | 86 | −0.15 | −0.65, 0.34 | 3 | 46 |
| Health condition | ||||||||||||
| Healthy | −0.10 | −0.12, −0.07 | 8 | 87 | −0.57 | −0.65, −0.49 | 7 | 62 | −0.47 | −0.78, −0.16 | 6 | 36 |
| Specific condition | −0.04 | −0.08, 0.00 | 8 | 84 | 0.16 | −0.18, 0.51 | 4 | 64 | −0.73 | −1.90, 0.45 | 5 | 92 |
| Training method | ||||||||||||
| Elastic band | −0.64 | −1.07, −0.21 | 8 | 61 | 0.17 | −0.29, 0.63 | 5 | 50 | −0.89 | −1.87, 0.09 | 6 | 89 |
| Machines | −0.82 | −1.37, −0.27 | 8 | 77 | −1.28 | −2.21, −0.36 | 6 | 85 | −0.33 | −0.63, −0.04 | 5 | 0 |
| Number of exercises | ||||||||||||
| ≤8 | −0.12 | −0.15, −0.08 | 9 | 88 | −0.59 | −0.67, −0.51 | 6 | 27 | −0.04 | −0.17, 0.09 | 5 | 4 |
| >8 | −0.06 | −0.09, −0.04 | 4 | 0 | −0.20 | −0.86, 0.46 | 3 | 53 | −0.52 | −0.93, −0.12 | 3 | 0 |
| Intensity | ||||||||||||
| Moderate | −0.63 | −0.93, −0.33 | 11 | 45 | −0.23 | −0.60, 0.14 | 7 | 46 | −0.23 | −0.52, 0.06 | 7 | 15 |
| Vigorous | −1.11 | −2.02, −0.20 | 5 | 84 | −1.73 | −3.84, 0.39 | 4 | 94 | −1.21 | −2.73, 0.31 | 4 | 91 |
| Weekly frequency | ||||||||||||
| ≤2 | −0.26 | −0.32, −0.20 | 2 | 0 | −0.55 | −0.63, −0.47 | 3 | 93 | −1.93 | −5.43, 1.57 | 2 | 96 |
| >2 | −0.05 | −0.08, −0.03 | 12 | 69 | −0.37 | −0.61, −0.12 | 7 | 31 | −0.38 | −0.65, −0.10 | 7 | 0 |
| Duration (week) | ||||||||||||
| ≤8 | −0.26 | −0.32, −0.20 | 2 | 79 | −0.59 | −0.67, −0.50 | 3 | 62 | −0.24 | −0.87, 0.40 | 1 | - |
| >8 | −0.05 | −0.08, −0.03 | 14 | 78 | −0.14 | −0.36, 0.09 | 8 | 68 | −0.59 | −1.18, −0.01 | 10 | 82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-D.; Yeun, Y.-R. Effects of Resistance Training on C-Reactive Protein and Inflammatory Cytokines in Elderly Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2022, 19, 3434. https://doi.org/10.3390/ijerph19063434
Kim S-D, Yeun Y-R. Effects of Resistance Training on C-Reactive Protein and Inflammatory Cytokines in Elderly Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. International Journal of Environmental Research and Public Health. 2022; 19(6):3434. https://doi.org/10.3390/ijerph19063434
Chicago/Turabian StyleKim, Sang-Dol, and Young-Ran Yeun. 2022. "Effects of Resistance Training on C-Reactive Protein and Inflammatory Cytokines in Elderly Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" International Journal of Environmental Research and Public Health 19, no. 6: 3434. https://doi.org/10.3390/ijerph19063434





