Personalized Type 2 Diabetes Management Using a Mobile Application Integrated with Electronic Medical Records: An Ongoing Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Intervention
2.3. Eligibility
2.4. Primary and Secondary Outcomes
2.5. Measurements
2.6. Sample Size Calculations
2.7. Randomization
2.8. Statistical Analysis
2.9. Ethics
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Salpea, P.; Karuranga, S.; Petersohn, I.; Malanda, B.; Gregg, E.W.; Unwin, N.; Wild, S.H.; Williams, R. Mortality attributable to diabetes in 20–79 years old adults, 2019 estimates: Results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Res. Clin. Pract. 2020, 162, 108086. [Google Scholar] [CrossRef]
- American Diabetes Association. Implications of the United Kingdom prospective diabetes study. Diabetes Care 2002, 25, s28–s32. [Google Scholar] [CrossRef]
- Kim, B.Y.; Won, J.C.; Lee, J.H.; Kim, H.S.; Park, J.H.; Ha, K.H.; Won, K.C.; Kim, D.J.; Park, K.S. Diabetes fact sheets in Korea, 2018: An appraisal of current status. Diabetes Metab. J. 2019, 43, 487–494. [Google Scholar] [CrossRef]
- Saydah, S.H.; Fradkin, J.; Cowie, C.C. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA 2004, 291, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Nundy, S.; Dick, J.J.; Solomon, M.C.; Peek, M.E. Developing a behavioral model for mobile phone-based diabetes interventions. Patient Educ. Couns. 2013, 90, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Carter, B.; Hewitt, J.; Francisa, T.; Mayor, S. Do mobile phone applications improve glycemic control (hba1c) in the self-management of diabetes? A systematic review, meta-analysis, and grade of 14 randomized trials. Diabetes Care 2016, 39, 2089–2095. [Google Scholar] [CrossRef]
- Hou, C.; Xu, Q.; Diao, S.; Hewitt, J.; Li, J.; Carter, B. Mobile phone applications and self-management of diabetes: A systematic review with meta-analysis, meta-regression of 21 randomized trials and grade. Diabetes Obes. Metab. 2018, 20, 2009–2013. [Google Scholar] [CrossRef]
- Cui, M.; Wu, X.; Mao, J.; Wang, X.; Nie, M. T2dm self-management via smartphone applications: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0166718. [Google Scholar] [CrossRef]
- Shan, R.; Sarkar, S.; Martin, S.S. Digital health technology and mobile devices for the management of diabetes mellitus: State of the art. Diabetologia 2019, 62, 877–887. [Google Scholar] [CrossRef]
- Quinn, C.C.; Gruber-Baldini, A.L.; Shardell, M.; Weed, K.; Clough, S.S.; Peeples, M.; Terrin, M.; Bronich-Hall, L.; Barr, E.; Lender, D. Mobile diabetes intervention study: Testing a personalized treatment/behavioral communication intervention for blood glucose control. Contemp. Clin. Trials 2009, 30, 334–346. [Google Scholar] [CrossRef]
- Kirwan, M.; Vandelanotte, C.; Fenning, A.; Duncan, M.J. Diabetes self-management smartphone application for adults with type 1 diabetes: Randomized controlled trial. J. Med. Internet Res. 2013, 15, e235. [Google Scholar] [CrossRef]
- Rossi, M.C.; Nicolucci, A.; Di Bartolo, P.; Bruttomesso, D.; Girelli, A.; Ampudia, F.J.; Kerr, D.; Ceriello, A.; Mayor Cde, L.; Pellegrini, F.; et al. Diabetes interactive diary: A new telemedicine system enabling flexible diet and insulin therapy while improving quality of life: An open-label, international, multicenter, randomized study. Diabetes Care 2010, 33, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Ko, S.H.; Kim, B.Y.; Kang, E.S.; Noh, J.; Kim, S.K.; Park, S.O.; Hur, K.Y.; Chon, S.; Moon, M.K.; et al. 2019 clinical practice guidelines for type 2 diabetes mellitus in korea. Diabetes Metab. J. 2019, 43, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Google Developers. Google Developers Web Site. Available online: https://developers.google.com/fit/overview/ (accessed on 4 November 2020).
- Cleland, C.L.; Hunter, R.F.; Kee, F.; Cupples, M.E.; Sallis, J.F.; Tully, M.A. Validity of the global physical activity questionnaire (gpaq) in assessing levels and change in moderate-vigorous physical activity and sedentary behaviour. BMC Public Health 2014, 14, 1255. [Google Scholar] [CrossRef]
- Lee, J.; Lee, C.; Min, J.; Kang, D.W.; Kim, J.Y.; Yang, H.I.; Park, J.; Lee, M.K.; Lee, M.Y.; Park, I.; et al. Development of the Korean global physical activity questionnaire: Reliability and validity study. Glob. Health Promot. 2020, 27, 44–55. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Levy, J.C.; Matthews, D.R.; Hermans, M.P. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998, 21, 2191–2192. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Trials Unit. HOMA2 Calculator. Available online: https://www.dtu.ox.ac.uk/homacalculator/ (accessed on 4 November 2020).
- Song, Y.S.; Hwang, Y.C.; Ahn, H.Y.; Park, C.Y. Comparison of the usefulness of the updated homeostasis model assessment (HOMA2) with the original homa1 in the prediction of type 2 diabetes mellitus in Koreans. Diabetes Metab. J. 2016, 40, 318–325. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 6. Glycemic Targets: Standards of medical care in diabetes-2021. Diabetes Care 2021, 44, S73–S84. [Google Scholar] [CrossRef]
- Workgroup on Hypoglycemia, American Diabetes Association. Defining and reporting hypoglycemia in diabetes: A report from the american diabetes association workgroup on hypoglycemia. Diabetes Care 2005, 28, 1245–1249. [Google Scholar] [CrossRef]
- Rabin, R.; De Charro, F. Eq-5d: A measure of health status from the euroqol group. Ann. Med. 2001, 33, 337–343. [Google Scholar] [CrossRef]
- Kim, S.H.; Ahn, J.; Ock, M.; Shin, S.; Park, J.; Luo, N.; Jo, M.W. The eq-5d-5l valuation study in korea. Qual. Life Res. 2016, 25, 1845–1852. [Google Scholar] [CrossRef] [PubMed]
- Quinn, C.C.; Shardell, M.D.; Terrin, M.L.; Barr, E.A.; Ballew, S.H.; Gruber-Baldini, A.L. Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes Care 2011, 34, 1934–1942. [Google Scholar] [CrossRef] [PubMed]
- Solomon, C.G. Reducing cardiovascular risk in type 2 diabetes. N. Engl. J. Med. 2003, 348, 457–459. [Google Scholar] [CrossRef] [PubMed]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar]
- American Diabetes Association. 5. Facilitating behavior change and well-being to improve health outcomes: Standards of medical care in diabetes-2020. Diabetes Care 2020, 43, S48–S65. [Google Scholar] [CrossRef] [PubMed]
- Parchman, M.L.; Romero, R.L.; Pugh, J.A. Encounters by patients with type 2 diabetes--complex and demanding: An observational study. Ann. Fam. Med. 2006, 4, 40–45. [Google Scholar] [CrossRef]
- Bindman, A.B.; Forrest, C.B.; Britt, H.; Crampton, P.; Majeed, A. Diagnostic scope of and exposure to primary care physicians in australia, new zealand, and the united states: Cross sectional analysis of results from three national surveys. BMJ 2007, 334, 1261. [Google Scholar] [CrossRef]
- Renders, C.M.; Valk, G.D.; Griffin, S.; Wagner, E.H.; Eijk, J.T.; Assendelft, W.J. Interventions to improve the management of diabetes mellitus in primary care, outpatient and community settings. Cochrane Database Syst. Rev. 2001, 2000, Cd001481. [Google Scholar] [CrossRef]
- El-Gayar, O.; Timsina, P.; Nawar, N.; Eid, W. Mobile applications for diabetes self-management: Status and potential. J. Diabetes Sci. Technol. 2013, 7, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Orsama, A.L.; Lähteenmäki, J.; Harno, K.; Kulju, M.; Wintergerst, E.; Schachner, H.; Stenger, P.; Leppänen, J.; Kaijanranta, H.; Salaspuro, V.; et al. Active assistance technology reduces glycosylated hemoglobin and weight in individuals with type 2 diabetes: Results of a theory-based randomized trial. Diabetes Technol. Ther. 2013, 15, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Statista. Number of Mobile Phone Users Worldwide from 2015 to 2020. Available online: https://www.statista.com/statistics/274774/forecast-of-mobile-phone-users-worldwide/ (accessed on 4 November 2020).
- Arambepola, C.; Ricci-Cabello, I.; Manikavasagam, P.; Roberts, N.; French, D.P.; Farmer, A. The impact of automated brief messages promoting lifestyle changes delivered via mobile devices to people with type 2 diabetes: A systematic literature review and meta-analysis of controlled trials. J. Med. Internet Res. 2016, 18, e86. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, G.; Hijazi, A.; Haghparast-Bidgoli, H. Cost and cost-effectiveness of mhealth interventions for the prevention and control of type 2 diabetes mellitus: A systematic review. Diabetes Res. Clin. Pract. 2020, 162, 108084. [Google Scholar] [CrossRef] [PubMed]
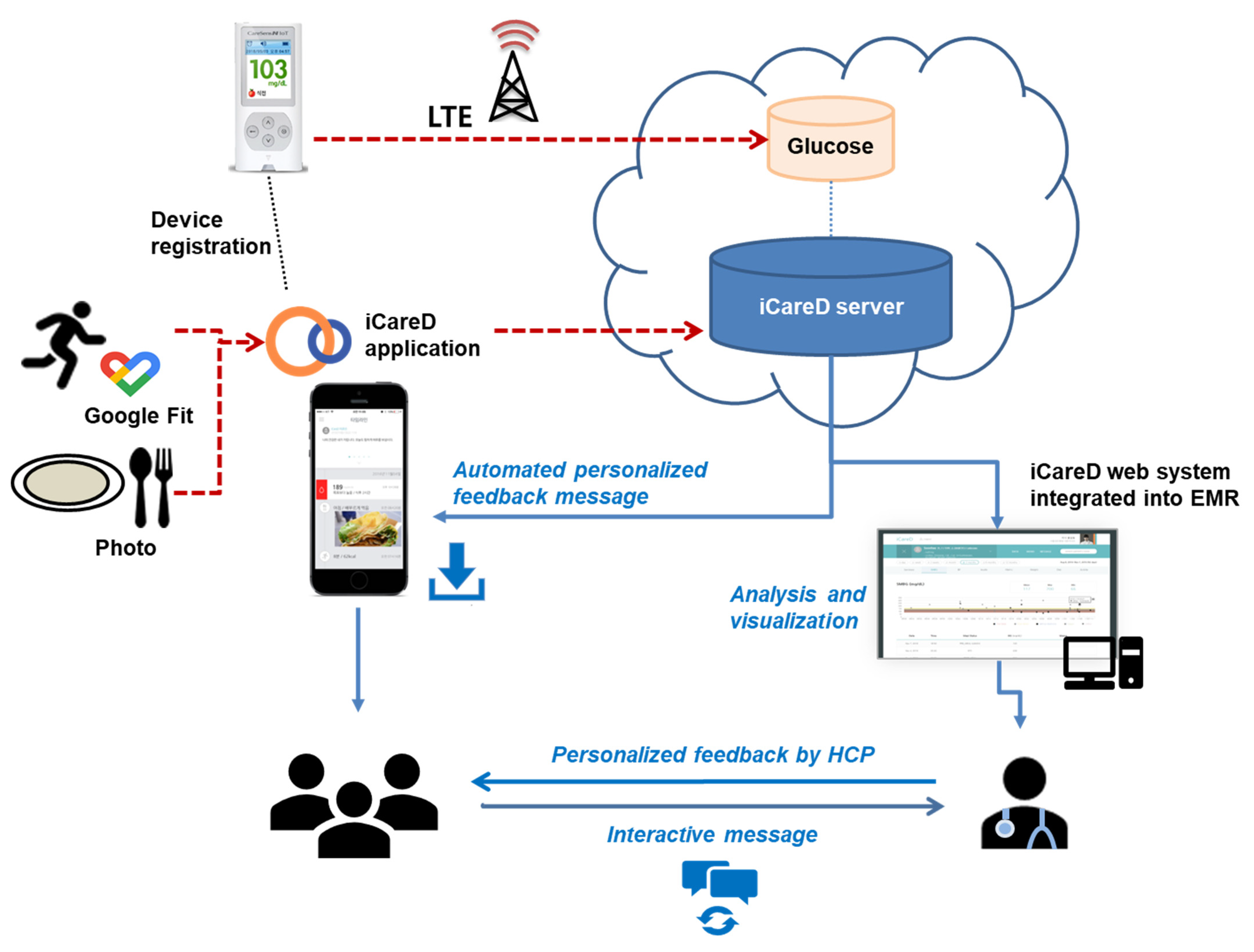

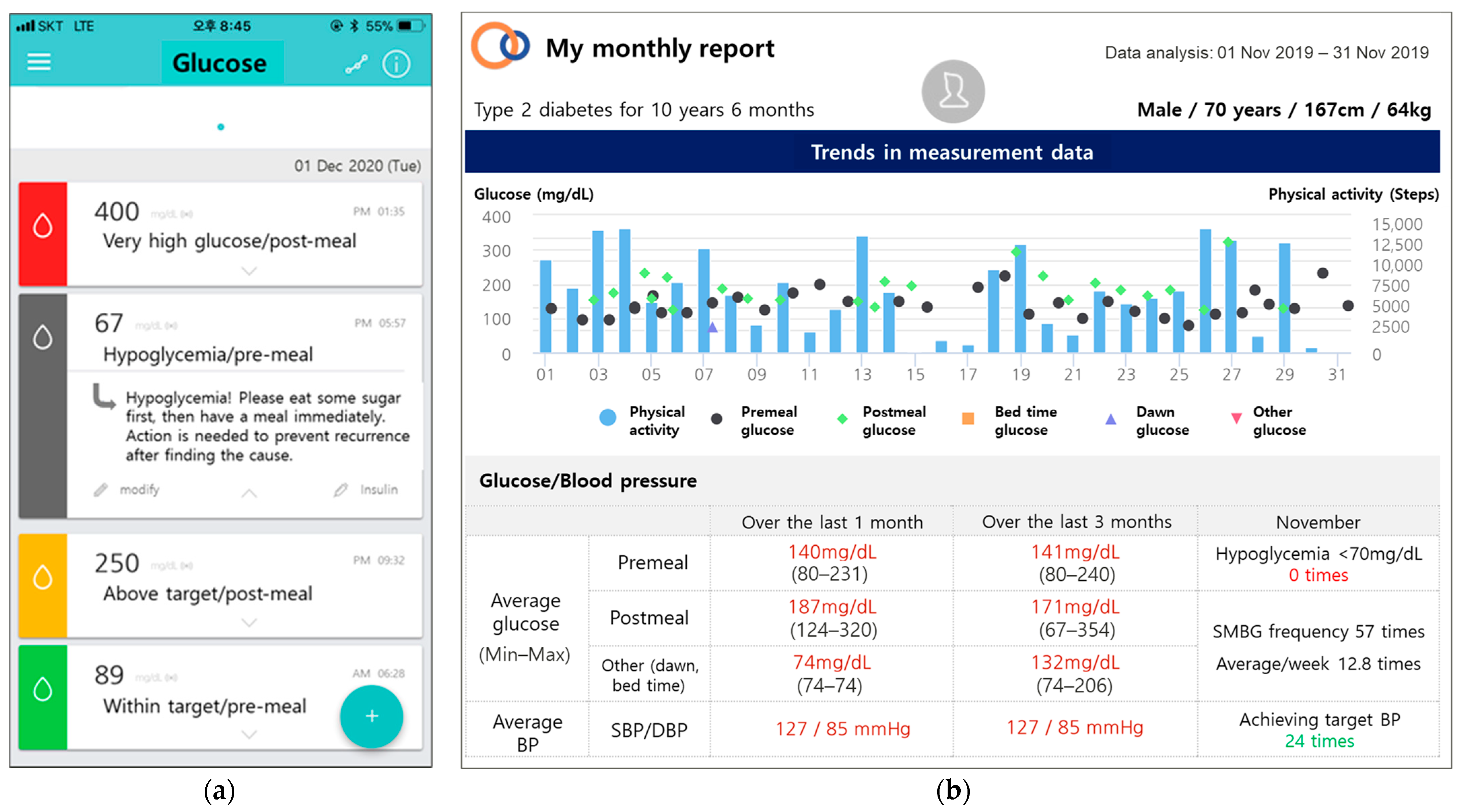
| Components | Control | Intervention | |
|---|---|---|---|
| Group 1 | Group 2 | Group 3 | |
| Education | Comprehensive management of diabetes, including self-care | Comprehensive management of diabetes, including self-care | Comprehensive management of diabetes, including self-care |
| Instruction | Conduct and record SMBG four times/day | Conduct and record SMBG four times/day, upload diet photos | Conduct and record SMBG four times/day, upload diet photos |
| Monitoring | SMBG and lifestyle * questionnaire, laboratory data | SMBG and lifestyle * log in the web-based system, individualized monthly reports about comprehensive management, laboratory data | SMBG and lifestyle * log in the web-based system, individualized monthly reports about comprehensive management, laboratory data |
| Intervention | Regular care only | Regular care with mobile diabetes management | Regular care with mobile diabetes management |
| Feedback from HCPs | During the visits (every 13 weeks) | During the visits (every 13 weeks) | During the visits (every 13 weeks) and between the visits (every two weeks) through the mobile application |
| Immediate intervention between visits | Not possible | Not possible | Possible |
| Interactive patient–physician communication between visits | No | No | Yes |
| Level | Assessment | Color Code | Glucose Level (mg/dL) | ||||
|---|---|---|---|---|---|---|---|
| Preprandial | Postprandial | Bedtime | Dawn | Other | |||
| 1 | Severe hypoglycemia | Black ■ | <50 | <50 | <50 | <50 | <50 |
| 2 | Hypoglycemia | Dark grey ■ | 50–69 | 50–69 | 50–69 | 50–69 | 50–69 |
| 3 | Potential hypoglycemia | Grey ■ | 70–79 | 70–89 | 70–89 | 70–79 | 70–79 |
| 4 | Within the target | Green ■ | 80–130 | 90–180 | 90–139 | 80–130 | 80–130 |
| 5 | Above the target | Yellow ■ | 131–179 | 181–249 | 140–249 | 131–179 | 131–179 |
| 6 | High | Orange ■ | 180–249 | 250–349 | 250–349 | 180–249 | 180–249 |
| 7 | Very high | Red ■ | ≥250 | ≥350 | ≥350 | ≥250 | ≥250 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.-Y.; Yun, J.-S.; Cha, S.-A.; Lim, S.-Y.; Lee, J.-H.; Ahn, Y.-B.; Yoon, K.-H.; Ko, S.-H. Personalized Type 2 Diabetes Management Using a Mobile Application Integrated with Electronic Medical Records: An Ongoing Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 5300. https://doi.org/10.3390/ijerph18105300
Lee E-Y, Yun J-S, Cha S-A, Lim S-Y, Lee J-H, Ahn Y-B, Yoon K-H, Ko S-H. Personalized Type 2 Diabetes Management Using a Mobile Application Integrated with Electronic Medical Records: An Ongoing Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2021; 18(10):5300. https://doi.org/10.3390/ijerph18105300
Chicago/Turabian StyleLee, Eun-Young, Jae-Seung Yun, Seon-Ah Cha, Sun-Young Lim, Jin-Hee Lee, Yu-Bae Ahn, Kun-Ho Yoon, and Seung-Hyun Ko. 2021. "Personalized Type 2 Diabetes Management Using a Mobile Application Integrated with Electronic Medical Records: An Ongoing Randomized Controlled Trial" International Journal of Environmental Research and Public Health 18, no. 10: 5300. https://doi.org/10.3390/ijerph18105300
APA StyleLee, E.-Y., Yun, J.-S., Cha, S.-A., Lim, S.-Y., Lee, J.-H., Ahn, Y.-B., Yoon, K.-H., & Ko, S.-H. (2021). Personalized Type 2 Diabetes Management Using a Mobile Application Integrated with Electronic Medical Records: An Ongoing Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 18(10), 5300. https://doi.org/10.3390/ijerph18105300






