Associations of Cholesteryl Ester Transfer Protein TaqIB Polymorphism with the Composite Ischemic Cardiovascular Disease Risk and HDL-C Concentrations: A Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Eligibility Criteria
2.3. Exclusion Criteria
2.4. Data Extraction
2.5. Quality Assessment for Individual Studies
2.6. Data Analysis
3. Results
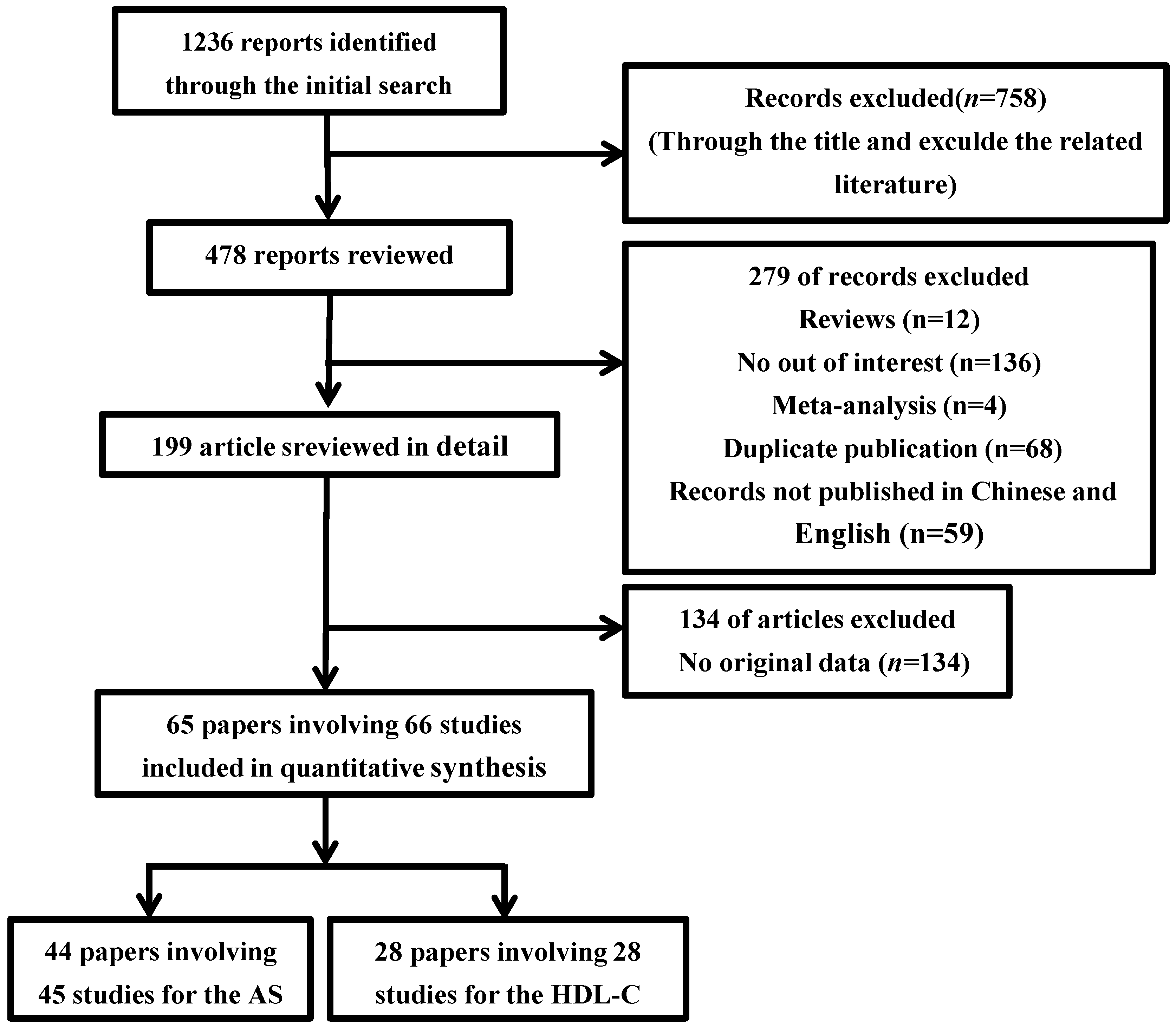
3.1. Selection and Characteristics of Studies
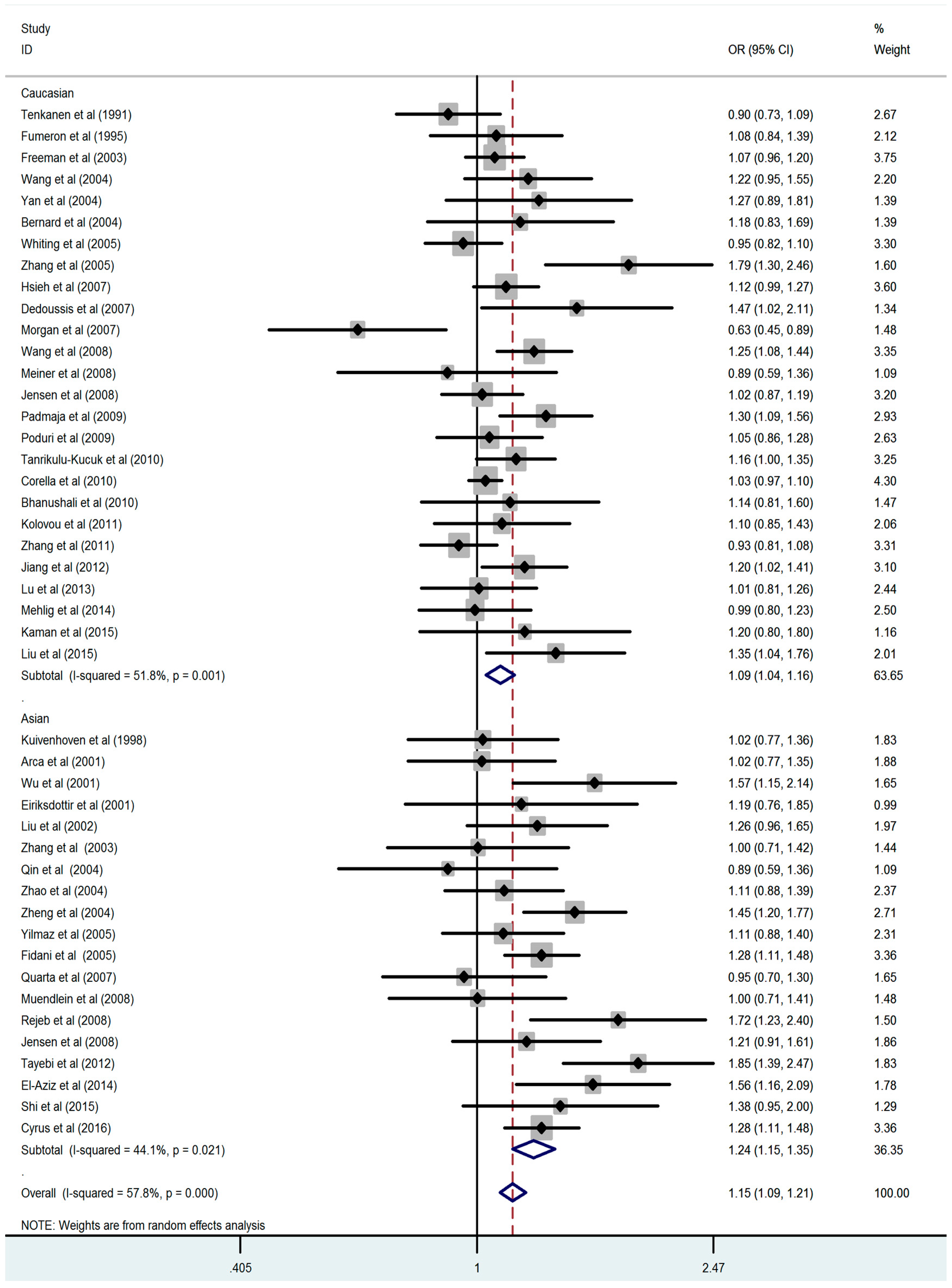
3.2. Association between the CETP TaqIB Polymorphism and the Composite Ischemic CVD Risk
3.3. Association between the CETP TaqIB Polymorphism and HDL-C Concentrations
3.4. Sensitivity Analysis
3.5. Heterogeneity Analysis
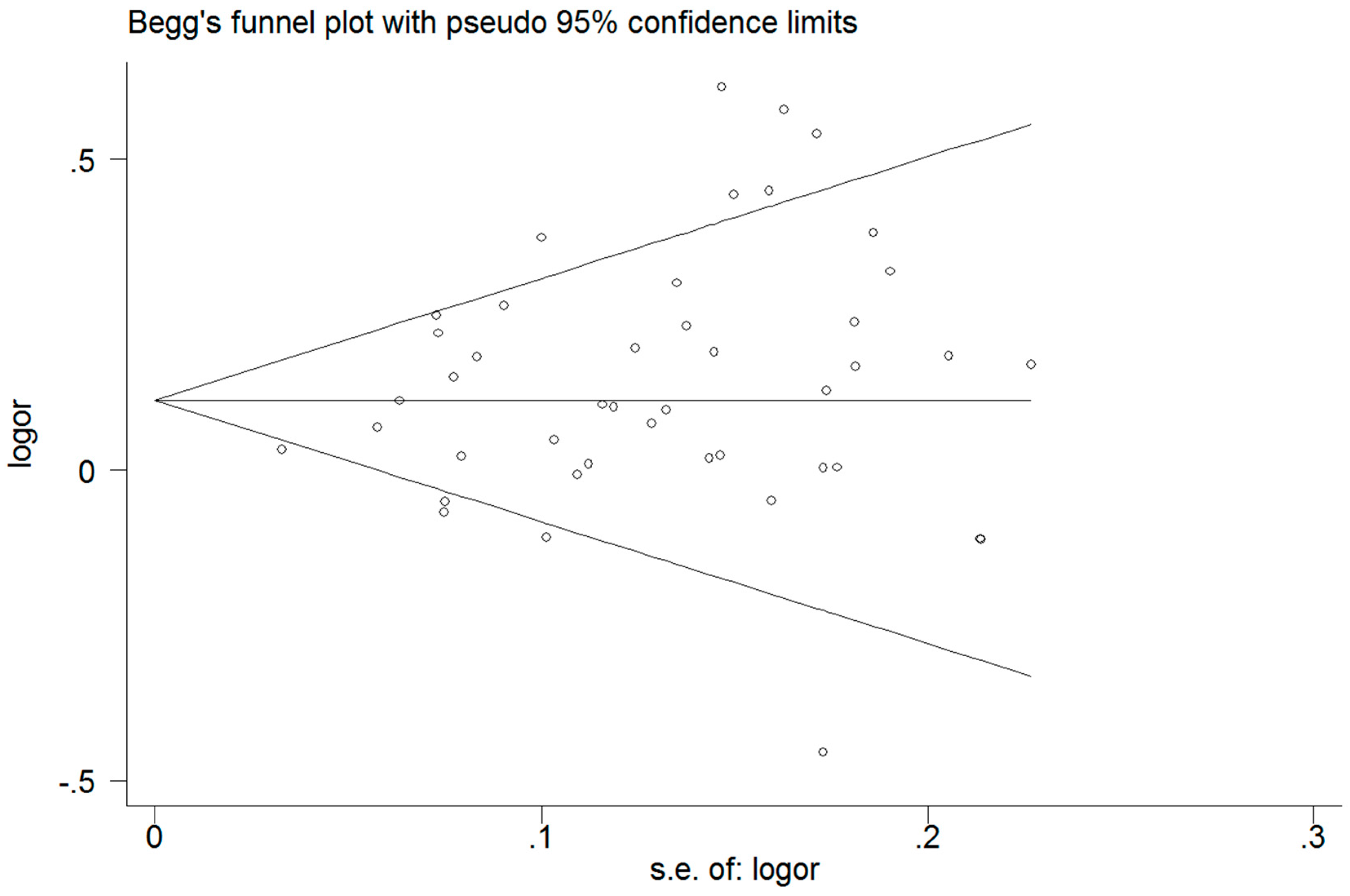
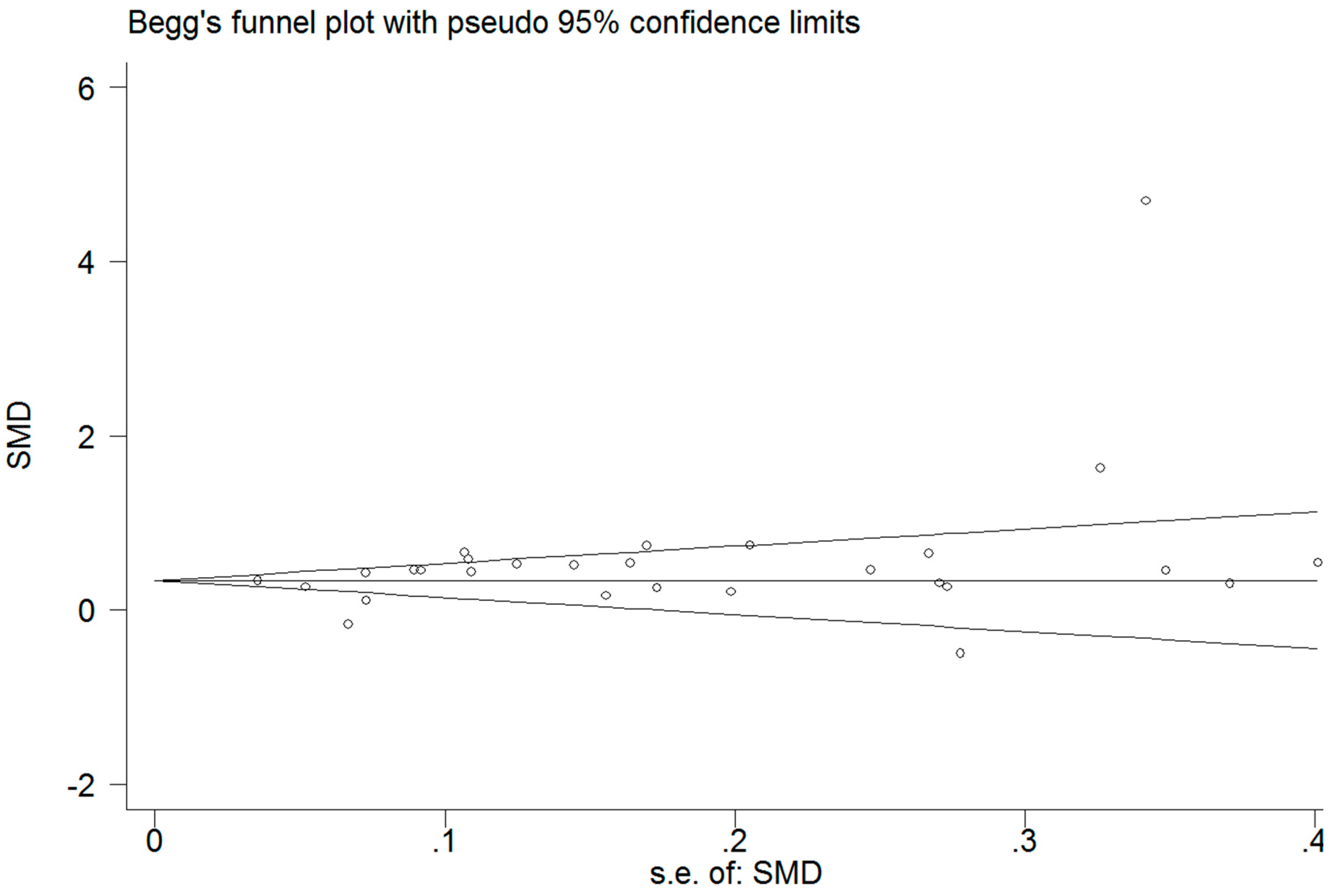
3.6. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Costopoulos, C.; Niespialowska-Steuden, M.; Kukreja, N.; Gorog, D.A. Novel oral anticoagulants in acute coronary syndrome. Int. J. Cardiol. 2013, 167, 2449–2455. [Google Scholar] [CrossRef] [PubMed]
- Goldbourt, U.; Yaari, S.; Medalie, J.H. Isolated low HDL cholesterol as a risk factor for coronary heart disease mortality. A 21-year follow-up of 8000 men. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Gotto, A.M.; Brinton, E.A. Assessing low levels of high-density lipoprotein cholesterol as a risk factor in coronary heart disease: A working group report and update. J. Am. Coll. Cardiol. 2004, 43, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.K.; Kaul, S.; Nilsson, J.; Cercek, B. Exploiting the vascular protective effects of high-density lipoprotein and its apolipoproteins: An idea whose time for testing is coming, part I. Circulation 2001, 104, 2376–2383. [Google Scholar] [CrossRef] [PubMed]
- Foitzik, S.; Kureck, I.M.; Rüger, M.H.; Metzler, D. The role of plasma lipid transfer proteins in lipoprotein metabolism and atherogenesis. J. Lipid Res. 2009, 50, S201–S206. [Google Scholar]
- Tall, A.R. Functions of cholesterol ester transfer protein and relationship to coronary artery disease risk. J. Clin. Lipid. 2010, 4, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Talmud, P.J.; Hawe, E.; Robertson, K.; Miller, G.J.; Miller, N.E.; Humphries, S.E. Genetic and environmental determinants of plasma high density lipoprotein cholesterol and apolipoprotein AI concentrations in healthy middle-aged men. Ann. Hum. Genet. 2002, 66, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Ordovas, J.M. Genetic polymorphisms and activity of cholesterol ester transfer protein (CETP): Should we be measuring them? Clin. Chem. Lab. Med. 2000, 38, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Boekholdt, S.M.; Sacks, F.M.; Jukema, J.W.; Shepherd, J.; Freeman, D.J.; Mcmahon, A.D.; Cambien, F.; Nicaud, V.; De Grooth, G.J.; Talmud, P.J. Cholesteryl ester transfer protein TaqIB variant, high-density lipoprotein cholesterol levels, cardiovascular risk, and efficacy of pravastatin treatment: Individual patient meta-analysis of 13,677 subjects. Circulation 2005, 111, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Wu, X.Y.; Xu, J.; Qian, Y.; Zhou, C.W.; Wang, B. Apo A5 −1131T/C, FgB −455G/A, −148C/T, and CETP TaqIB gene polymorphisms and coronary artery disease in the Chinese population: A meta-analysis of 15,055 subjects. Mol. Biol. Rep. 2013, 40, 1997–2014. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, S.B.; Wang, L.J.; Lei, M.M.; Wang, Y.; Miao, C.; Jin, Y.Z. Seven functional polymorphisms in the CETP gene and myocardial infarction risk: A meta-analysis and meta-regression. PLoS ONE 2014, 9, e88118. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Zhou, Z.W.; Fang, B.J.; Zhao, C.G.; Zhou, D. Meta-analysis of cholesteryl ester transfer protein TaqIB polymorphism and risk of myocardial infarction. Medicine 2014, 93, e160. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. J. Am. Med. Assoc. 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Int. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; O’Connell, D.; Peterson, J.; Welch, V.; Shea, B.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta- analyses. Available online: www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 20 Ocotber 2011).
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berkey, C.S.; Hoaglin, D.C.; Mosteller, F.; Colditz, G.A. A random-effects regression model for meta-analysis. Stat. Med. 1995, 14, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Mantel, N.; Haenszel, W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 1959, 22, 639–640. [Google Scholar]
- Dersimonian, R.; Kacker, R. Random-effects model for meta-analysis of clinical trials: An update. Contem. Clin. Trials 2007, 28, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Dersimonian, R.; Laird, N. Meta-analysis in clinical trials. Controll. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Galbraith, R.F. Graphical display of estimates having differing standard errors. Technometrics 1988, 30, 271–281. [Google Scholar] [CrossRef]
- Tanrikulu-Kucuk, S.; Ademoglu, E.; Gurdol, F.; Bilge, A.; Mutlu-Turkoglu, U.; Nisanci, Y. Cholesteryl ester transfer protein Taq1B polymorphism in an angiographically assessed Turkish population: No effects on coronary artery disease risk. Genet. Test. Mol. Biol. 2010, 14, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Kaman, D.; N, İ.; N, İ.; Akbulut, M. TaqIB and severity of coronary artery disease in the Turkish population: A pilot study. Bosn. J. Basic Med. Sci. 2015, 15, 5344–5346. [Google Scholar] [CrossRef] [PubMed]
- Kolovou, G.; Vasiliadis, I.; Kolovou, V.; Karakosta, A.; Mavrogeni, S.; Papadopoulou, E.; Papamentzelopoulos, S.; Giannakopoulou, V.; Marvaki, A.; Degiannis, D. The role of common variants of the cholesteryl ester transfer protein gene in left main coronary artery disease. Lipid. Health Dis. 2011, 10, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liu, Y.J.; He, L. Studies on the correlation between CETP TaqIB gene polymorphism and blood lipid and coronary heart disease. J. Tianjin Med. Univ. 2015, 2, 117–120. [Google Scholar]
- Muendlein, A.; Saely, C.H.; Marte, T.; Schmid, F.; Koch, L.; Rein, P.; Langer, P.; Aczel, S.; Drexel, H. Synergistic effects of the apolipoprotein E ɛ3/ɛ2/ɛ4, the cholesteryl ester transfer protein TaqIB, and the apolipoprotein C3 −482 C > T polymorphisms on their association with coronary artery disease. Atherosclerosis 2008, 199, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Padmaja, N.; Kumar, R.M.; Balachander, J.; Adithan, C. Cholesteryl ester transfer protein TaqIB, -629C>A and I405V polymorphisms and risk of coronary heart disease in an Indian population. Clin. Chim. Acta Int. J. Clin. Chem. 2009, 402, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Zhao, B.R.; Geng, J.; Li, Y.L.; Cui, R.Z. The association of the Hpoprotein Hpase $447X and cholesteryl ester transfer protein TaqlB polymorphism with coronary heart disease. Chin. J. Cardiol. 2004, 32, 522–525. [Google Scholar]
- Rejeb, J.; Omezzine, A.; Rebhi, L.; Naffeti, I.; Kchok, K.; Belkahla, R.; Hadjmbarek, I.B.; Rejeb, N.B.; Nabli, N.; Boujelbene, A. Association of the cholesteryl ester transfer protein Taq1 B2B2 genotype with higher high-density lipoprotein cholesterol concentrations and lower risk of coronary artery disease in a Tunisian population. Arch. Cardiovsc. Dis. 2008, 101, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.X.; Bian, C.H.; Liu, R.; Liu, Y.J.; Zhao, Y.Q. The correlation study between CETP-TaqIB gene polymorphism and coronary heart disease. Chin. J. Care. Med. 2015, 35, 337–341. [Google Scholar]
- Wang, W.; Zhou, X.; Liu, F.; Hu, H.N.; Han, D.F. Association of the TaqIB polymorphism and D442G mutation of cholesteryl ester transfer protein gene with coronary heart disease. Chin. J. Cardiol. 2004, 32, 981–985. [Google Scholar]
- Wu, J.H.; Lee, Y.T.; Hsu, H.C.; Hsieh, L.L. Influence of CETP gene variation on plasma lipid levels and coronary heart disease: A survey in Taiwan. Atherosclerosis 2001, 159, 451–458. [Google Scholar] [CrossRef]
- Yan, S.K.; Zhu, Y.L.; Cheng, S.; Song, Y.H.; Yan, X.W. Relationship between coronary heart disease and TaqIB & MspI polymorphisms of cholesteryl ester transfer protein gene in Han nationality. Chin. J. Lab. Med. 2004, 27, 671–675. [Google Scholar]
- Zhang, Y.J.; Geng, J.; Qin, Q.; Mao, Y.M.; Cui, R.Z. The effects of TaqIB polymorphism of cholesteryl ester transfer proteinon coronary heart disease. Tianjin Med. J. 2003, 31, 758–760. [Google Scholar]
- Zhao, S.P.; Li, H.; Xiao, Z.J.; Nie, S. The effect of TaqIB polymorphism of cholesteryl ester transfer protein gene on the lipoprotein level. Chin. J. Cardiol. 2004, 32, 816–818. [Google Scholar]
- Zheng, K.Q.; Zhang, S.Z.; He, Y.; Zhang, L.; Zhang, K.L. Association between cholesteryl ester transfer protein gene polymorphisms and variations in lipid levels in patients with coronary heart disease. Chin. Med. J. 2004, 117, 1288–1292. [Google Scholar] [PubMed]
- Arca, M.; Montali, A.; Ombres, D.; Battiloro, E.; Campagna, F.; Ricci, G.; Verna, R. Lack of association of the common TaqIB polymorphism in the cholesteryl ester transfer protein gene with angiographically assessed coronary atherosclerosis. Clin. Genet. 2001, 60, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Corella, D.; Carrasco, P.; Amiano, P.; Arriola, L.; Chirlaque, M.D.; Huerta, J.M.; Martínez, C.; Martinez-Camblor, P.; Molina, E.; Navarro, C. Common cholesteryl ester transfer protein gene variation related to high-density lipoprotein cholesterol is not associated with decreased coronary heart disease risk after a 10-year follow-up in a Mediterranean cohort: Modulation by alcohol consumption. Atherosclerosis 2010, 211, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Eiriksdottir, G.; Bolla, M.K.; Thorsson, B.; Sigurdsson, G.; Humphries, S.E.; Gudnason, V. The −629C>A polymorphism in the CETP gene does not explain the association of TaqIB polymorphism with risk and age of myocardial infarction in Icelandic men. Atherosclerosis 2001, 159, 187–192. [Google Scholar] [CrossRef]
- Fumeron, F.; Betoulle, D.; Luc, G.; Behague, I.; Ricard, S.; Poirier, O.; Jemaa, R.; Evans, A.; Arveiler, D.; Marques-Vidal, P. Alcohol intake modulates the effect of a polymorphism of the cholesteryl ester transfer protein gene on plasma high density lipoprotein and the risk of myocardial infarction. J. Clin. Investig. 1995, 96, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, H.; İsbir, T.; Agachan, B.; Karaali, Z.E. Effects of cholesterol ester transfer protein Taq1B gene polymorphism on serum lipoprotein levels in Turkish coronary artery disease patients. Cell Biochem. Funct. 2005, 23, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Bernard, K.; Alison, P.; Sarah, P.; Sarah, C.; Linda, Y.; John, D.; Colin, M.K.; Marc, D.; Mark, L.; Richard, P. Lipid-related genes and myocardial infarction in 4685 cases and 3460 controls: Discrepancies between genotype, blood lipid concentrations, and coronary disease risk. Int. J. Epidemiol. 2004, 33, 1002–1013. [Google Scholar]
- Kuivenhoven, J.A.; Jukema, J.W.; Zwinderman, A.H.; Knijff, P.D.; McPherson, R.; Bruschke, A.V.G.; Lie, K.I.; Kastelein, J.J.P. The role of a common variant of the cholesteryl ester transfer protein gene in the progression of coronary atherosclerosis. N. Engl. J. Med. 1998, 338, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Schmitz, C.; Stampfer, M.J.; Sacks, F.; Hennekens, C.H.; Lindpaintner, K.; Ridker, P.M. A prospective study of TaqIB polymorphism in the gene coding for cholesteryl ester transfer protein and risk of myocardial infarction in middle-aged men. Atherosclerosis 2002, 161, 469–474. [Google Scholar] [CrossRef]
- Lu, Y.; Tayebi, N.; Li, H.; Saha, N.; Yang, H.; Heng, C.K. Association of CETP Taq1B and -629C > A polymorphisms with coronary artery disease and lipid levels in the multi-ethnic Singaporean population. Lipid. Health Dis. 2013, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mehlig, K.; Strandhagen, E.; Svensson, P.A.; Rosengren, A.; Torén, K.; Thelle, D.S.; Lissner, L. CETP TaqIB genotype modifies the association between alcohol and coronary heart disease: The INTERGENE case-control study. Alcohol 2014, 48, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Meiner, V.; Friedlander, Y.; Milo, H.; Sharon, N.; Ben-Avi, L.; Shpitzen, S.; Leitersdorf, E.; Siscovick, D.S.; Schwartz, S.M. Cholesteryl ester transfer protein (CETP) genetic variation and early onset of non-fatal myocardial infarction. Ann. Hum. Genet. 2008, 72, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Poduri, A.; Khullar, M.; Bahl, A.; Sharma, Y.P.; Talwar, K.K.A. Combination of proatherogenic single-nucleotide polymorphisms is associated with increased risk of coronary artery disease and myocardial infarction in Asian Indians. DNA Cell Biol. 2009, 28, 451–460. [Google Scholar] [CrossRef] [PubMed]
- El-Aziz, T.A.A.; Mohamed, R.H.; Hagrass, H.A. Increased risk of premature coronary artery disease in Egyptians with ABCA1 (R219K), CETP (TaqIB), and LCAT (4886C/T) genes polymorphism. J. Clin. Lipid. 2014, 8, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Tenkanen, H.; Koskinen, P.; Kontula, K.; Aalto-Setälä, K.; Mänttäri, M.; Manninen, V.; Runeberg, S.L.; Taskinen, M.R.; Ehnholm, C. Polymorphisms of the gene encoding cholesterol ester transfer protein and serum lipoprotein levels in subjects with and without coronary heart disease. Hum. Genet. 1991, 87, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Cui, H.B.; Wang, D.Q.; Chen, X.M.; Zhang, H.K.; Cui, C.C.; Chen, X.Y.; Liu, X.H.; Zhang, Z.; Bai, F.; et al. Geographical characteristics of single nucleotide polymorphism of candidate genes associated with coronary artery disease in Chinese Hart population. China J. Cardiol. 2008, 36, 24–29. [Google Scholar]
- Whiting, B.M.; Anderson, J.L.; Muhlestein, J.B.; Horne, B.D.; Bair, T.L.; Pearson, R.R.; Carlquist, J.F. Candidate gene susceptibility variants predict intermediate end points but not angiographic coronary artery disease. Am. Heart J. 2005, 150, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.B.; Jiang, Z.W.; Sun, B.G.; Lu, Y.S.; Wen, Q.Z.; Zhuang, W.Y.; Wang, F. Relationship of Taq IB polymorphism in the cholesteryl ester transfer protein gene to coronary artery disease. Chin. J. Arterioscler. 2005, 13, 88–90. [Google Scholar]
- Zhang, Y.; Xi, J.L.; Yun, M.L.; Zhang, Y.X.; Zhou, D.F. Study on the relationship between CETP polymorphism and plasma lipid in patient with CHD of Han ethnic in Hannan. Mod. Prev. Med. 2011, 38, 691–693. [Google Scholar]
- Freeman, D.J.; Samani, N.J.; Wilson, V.; McMahon, A.D.; Braund, P.S.; Cheng, S.; Caslake, M.J.; Packard, C.J.; Gaffney, D. A polymorphism of the cholesteryl ester transfer protein gene predicts cardiovascular events in non-smokers in the west of scotland coronary prevention study. Eur. Heart J. 2003, 24, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Dedoussis, G.V.; Panagiotakos, D.B.; Louizou, E.; Mantoglou, I.; Chrysohoou, C.; Lamnisou, K.; Pitsavos, C.; Stefanadis, C. Cholesteryl ester-transfer protein (CETP) polymorphism and the association of acute coronary syndromes by obesity status in Greek subjects: The CARDIO2000-GENE study. Hum. Hered. 2007, 63, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.M.; Krumholz, H.M.; Lifton, R.P.; Spertus, J.A. Nonvalidation of reported genetic risk factors for acute coronary syndrome in a large-scale replication study. J. Am. Med. Assoc. 2007, 297, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.C.; Tien, K.J.; Chang, S.J.; Lo, C.S.; Hsin, S.C.; Hsiao, J.Y.; Hsu, S.C.; Liang, H.T.; Chen, H.C.; Shin, S.J. Cholesteryl ester transfer protein B1B1 genotype as a predictor of coronary artery disease in Taiwanese with type 2 diabetes mellitus. Metab. Clin. Exp. 2007, 56, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Cyrus, C.; Vatte, C.; Al-Nafie, A.; Chathoth, S.; Al-Ali, R.; Al-Shehri, A.; Akhtar, M.S.; Almansori, M.; Al-Muhanna, F.; Keating, B. The impact of common polymorphisms inCETPandABCA1genes with the risk of coronary artery disease in Saudi Arabians. Hum. Genom. 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Tayebi, N. Association of CETP and ADTRP Genes with Coronary Artery Disease in Multi-Ethnic Singaporean Population. Master’s Thesis, National University of Singapore, Singapore, 2012. [Google Scholar]
- Jensen, M.K.; Mukamal, K.J.; Kim, O.; Rimm, E.B. Alcohol consumption, TaqIB polymorphism of cholesteryl ester transfer protein, high-density lipoprotein cholesterol, and risk of coronary heart disease in men and women. Eur. Heart J. 2008, 29, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Fidani, L.; Hatzitolios, A.I.; Goulas, A.; Savopoulos, C.; Basayannis, C.; Kotsis, A. Cholesteryl ester transfer protein TaqIB and lipoprotein lipase Ser447Ter gene polymorphisms are not associated with ischaemic stroke in Greek patients. Neurosci. Lett. 2005, 384, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Quarta, G.; Stanzione, R.; Evangelista, A.; Zanda, B.; Sciarretta, S.; Angelantonio, E.D.; Marchitti, S.; Murro, D.D.; Volpe, M.; Rubattu, S. A protective role of a cholesteryl ester transfer protein gene variant towards ischaemic stroke in Sardinians. J. Int. Med. 2007, 262, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Q.; Wang, F.; Zhou, L.F.; Chen, C.G.; Qi, J.Y. Relationship of cholesteryl ester transfer protein TAQIB polymorphisms with cerebral infraction. J. Med. Res. 2012, 41, 89–92. [Google Scholar]
- Bhanushali, A.A.; Das, B.R. Genetic variants at the APOE, lipoprotein lipase (LpL), cholesteryl ester transfer protein (CETP), and endothelial nitric oxide (eNOS) genes and coronary artery disease (CAD): CETP Taq1 B2B2 associates with lower risk of CAD in Asian Indians. J.Commun. Genet. 2010, 1, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Gudnason, V.; Kakko, S.; Nicaud, V.; Savolainen, M.; Kesäniemi, Y.; Tahvanainen, E.; Humphries, S. Cholesteryl ester transfer protein gene effect on CETP activity and plasma high-density lipoprotein in European populations. Eur. J. Clin. Investig. 1999, 29, 116–128. [Google Scholar] [CrossRef]
- Goto, A.; Sasai, K.; Suzuki, S.; Fukutomi, T.; Ito, S.; Matsushita, T.; Okamoto, M.; Suzuki, T.; Itoh, M.; Okumura-Noji, K. Cholesteryl ester transfer protein and atherosclerosis in Japanese subjects: A study based on coronary angiography. Atherosclerosis 2001, 159, 153–163. [Google Scholar] [CrossRef]
- Goff, W.L.; Guerin, M.; Nicaud, V.; Dachet, C.; Luc, G.; Arveiler, D.; Ruidavets, J.B.; Evans, A.; Kee, F.; Morrison, C. A novel cholesteryl ester transfer protein promoter polymorphism (−971G/A) associated with plasma high-density lipoprotein cholesterol levels. Atherosclerosis 2002, 161, 269–279. [Google Scholar] [CrossRef]
- Katsunori, I.; Hiroshi, M.; Tamio, T.; Nobuhiro, Y.; Shinichi, O.; Jun, S.; Kouki, T.; Yasushi, S. Association of cholesteryl ester transfer protein activity and TaqIB polymorphism with lipoprotein variations in Japanese subjects. Metab. Clin. Exp. 2003, 52, 1564–1570. [Google Scholar]
- Weitgasser, R.; Galvan, G.; Malaimare, L.; Derflinger, I.; Hedegger, M.; Lang, J.; Iglseder, B.; Ladurner, G.; Paulweber, B. Cholesteryl ester transfer protein TaqIB polymorphism and its relation to parameters of the insulin resistance syndrome in an Austrian cohort. Biomed. Pharmacother. 2004, 58, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Song, X.X.; Zhang, M.; Qi, W.H.; Zhang, X.W. Association between insulin resistance and cholesteryl ester transfer protein gene polymorphism in type 2 diabetesmellitus. Chin. J. Med. Genet. 2005, 22, 298–301. [Google Scholar]
- Huang, Z.Y.; Guo, H.W.; Xu, Z.H.; Xue, K.; Zhou, X. Association of gene polymorphism at cholesterol ester transfer protein locus with obesity and response to dietary intervention in obesity. J. Hyg. Res. 2006, 35, 447–450. [Google Scholar]
- Zhang, Q.; Deng, B.; Li, P.L.; Yan, Y.; Wang, X.Y. Perioperative insulin resistance and TaqIB polymorphisms of cholesteryle ester transfer protein(CETP) in patients with essential hypertension. J. Clin. Anesthesiol. 2007, 23, 973–976. [Google Scholar]
- Cui, Y.L.; Zhao, X.L.; Wu, F.; Guo, S.J.; Li, J.J. Relationship between TaqIB genetic polymorphism at cholesterol ester transfer protein locus and the efficacy ofpolicosanol. Chin. J. New Drugs 2007, 16, 240–243. [Google Scholar]
- Meena, K.; Misra, A.; Pandey, R.M.; Luthra, K. CETP TaqIB polymorphisms and CETP activity in normolipidemic healthy northern Indians. Diabet. Metab. Syndr. Clin. Res. Rev. 2007, 1, 239–244. [Google Scholar] [CrossRef]
- Zhang, P.H.; Li, G.M.; Wang, Y.; Zheng, K.Q. TaqIB polymorphism in CETP gene in 140 hyperlipemia patients and lipid regulating effects of statins. J. Guangdong Med. Coll. 2008, 26, 492–495. [Google Scholar]
- Wang, Y.Z.; Yan, S.K.; Song, Y.H. Relationship of cholesterol ester transfer protein TaqIB gene polymorphism with type2 diabetes mellitus in Chinese Han nationality patients. Chin. J. Clin. Lab. Sci. 2008, 4, 297–300. [Google Scholar]
- Qiu, H.; Zhang, Q.; Deng, B.; Jiang, B.H.; Jin, H.G. The association study of cerebrocardiac vascular diseasew ith TaqIB gene polymorphisms of cholesteryle ester transfer protein in the patients with prmiary hypertension. Chin. J. Clin. Med. 2009, 16, 341–343. [Google Scholar]
- Tao, X.M.; Li, G.J.; Hou, S.Q.; Xiao, Z.S.; Tong, W.J.; Liu, Y.Y.; Gang, W.U.; Zhang, Y.H.; Qiu, C.C. Association of cholesteryl ester transfer protein gene TaqIB polymorphism with essential hypertension in Chinese mongolian population. Basic Clin. Med. 2010, 30, 677–682. [Google Scholar]
- Kappelle, P.J.W.H.; Gansevoort, R.T.; Hillege, H.J.; Wolffenbuttel, B.H.R.; Dullaart, R.P.F. Common variation in Cholesteryl Ester Transfer Protein : Relationship of first major adverse cardiovascular events with the apolipoprotein B/apolipoprotein A-I ratio and the total cholesterol/high-density lipoprotein cholesterol ratio. J. Clin. Lipid. 2013, 7, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, L.; Xie, N.Z.; Deng, B.; Lv, L.X.; Zheng, L.Q. Relationship between the cholesterol ester transfer protein TaqIB polymorphism and the lipid-lowering effect of atorvastatin in patients with coronary atherosclerotic heart disease. Genet. Mol. Res. 2014, 13, 2140–2148. [Google Scholar] [CrossRef] [PubMed]
- Galati, F.; Colonna, P.; Galati, A.; Ciardiello, C.; Bozzetti, M.P.; Massari, S. CETP TaqIB polymorphism, serum lipid levels and risk of atrial fibrillation: A case-control study. J. Atr. Fibrillation 2014, 6, 24–29. [Google Scholar]
- Zhai, X.H.; Hu, Y.; Xu, C.Y.; Li, Y.C.; Zhang, Z.Y. Correlation research and molecular regulation mechanism of cholesteryl ester transfer protein gene rs708272 polymorphism with exercise induced serum lipid changes in obese adolescent. J. Wuhan Inst. Phys. Educ. 2015, 49, 84–89. [Google Scholar]
- Jeenduang, N.; Porntadavity, S.; Nuinoon, M.; Horpet, D.; Thepkwan, N.; Thaworn, P.; Theanmontri, S. Studies of the CETP TaqIB and ApoE polymorphisms in Southern Thai subjects with the metabolic syndrome. Biochem. Genet. 2015, 53, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Keene, D.; Price, C.; Shunshin, M.J.; Francis, D.P. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: Meta-analysis of randomised controlled trials including 117,411 patients. Br. Med. J. 2014, 349, g4379. [Google Scholar] [CrossRef] [PubMed]

| First Author | Year | Country | Ethnicity | Disease | Source of Controls | Study Type | Size (Case/Control) | MAF | HWE | Genotypes Distribution (Case/Control) | Score | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B1B1 | B1B2 | B2B2 | |||||||||||
| Tenkanen et al. [51] | 1991 | Finland | Caucasian | MI | PB | CS | 72/115 | 0.44 | Yes | 19/33 | 40/65 | 13/17 | 8 |
| Fumeron et al. [41] | 1995 | France | Caucasian | MI | PB | CCS | 608/724 | 0.40 | Yes | 209/258 | 312/346 | 87/120 | 8 |
| Kuivenhoven et al. [44] | 1998 | The Netherlands | Caucasian | CAD | PB | CS | 380/427 | 0.41 | Yes | 129/152 | 183/214 | 68/61 | 7 |
| Wu et al. [33] | 2001 | China | Asian | MI | HB | CCS | 149/274 | 0.46 | Yes | 45/63 | 79/159 | 25/52 | 8 |
| Arca et al. [38] | 2001 | Italy | Caucasian | CAD | PB | CCS | 408/180 | 0.41 | Yes | 153/67 | 187/77 | 68/36 | 8 |
| Eiriksdottir et al. [40] | 2001 | Iceland | Caucasian | MI | PB | CS | 378/745 | 0.45 | No | 128/194 | 191/396 | 59/155 | 8 |
| Liu et al. [45] | 2002 | USA | Caucasian | MI | PB | CS | 384/384 | 0.43 | Yes | 125/122 | 196/193 | 63/69 | 8 |
| Freeman et al. [56] | 2003 | UK | Caucasian | MI | PB | CS | 499/1105 | 0.50 | Yes | 164/239 | 259/541 | 76/225 | 8 |
| Zhang et al. [35] | 2003 | China | Asian | CAD | HB | CCS | 234/164 | 0.41 | No | 76/49 | 126/95 | 32/20 | 6 |
| Qin et al. [29] | 2004 | China | Asian | CAD | HB | CCS | 249/167 | 0.41 | Yes | 81/49 | 131/97 | 37/21 | 6 |
| Wang et al. [32] | 2004 | China | Asian | CAD | HB | CCS | 128/247 | 0.42 | Yes | 50/72 | 66/123 | 12/52 | 6 |
| Yan et al. [34] | 2004 | China | Asian | CAD | HB | CCS | 106/64 | 0.41 | Yes | 41/19 | 46/34 | 19/11 | 6 |
| Zhao et al. [36] | 2004 | China | Asian | CAD | HB | CCS | 238/203 | 0.41 | No | 95/60 | 105/109 | 38/34 | 6 |
| Zheng et al. [37] | 2004 | China | Asian | CAD | HB | CCS | 203/100 | 0.39 | Yes | 66/33 | 114/55 | 23/12 | 6 |
| Bernard et al. [43] | 2004 | UK | Caucasian | MI | PB | CCS | 4442/3273 | 0.43 | No | 1477/1100 | 2175/1527 | 790/646 | 8 |
| Yilmaz et al. [42] | 2005 | Turkey | Caucasian | MI | PB | CCS | 173/111 | 0.42 | No | 66/39 | 72/46 | 35/26 | 6 |
| Fidani et al. [63] | 2005 | Greek | Caucasian | IS | HB | CCS | 96/100 | 0.41 | Yes | 35/34 | 47/45 | 14/21 | 6 |
| Whiting et al. [53] | 2005 | USA | Caucasian | CAD | PB | CS | 2392/827 | 0.42 | Yes | 792/279 | 1200/377 | 400/171 | 8 |
| Zhang et al. [54] | 2005 | China | Asian | CAD | PB | CCS | 88/94 | 0.41 | Yes | 31/32 | 40/50 | 17/12 | 6 |
| Dedoussis et al. [57] | 2007 | Greece | Caucasian | MI | HB | CCS | 237/237 | 0.41 | Yes | 83/78 | 121/120 | 33/39 | 7 |
| Morgan et al. [58] | 2007 | USA | Caucasian | MI | HB | CCS | 805/656 | 0.44 | No | 250/224 | 387/297 | 168/135 | 6 |
| Hsieh et al. [59] | 2007 | China | Asian | CAD | HB | CCS | 101/264 | 0.31 | Yes | 19/23 | 47/111 | 35/130 | 5 |
| Quarta et al. [64] | 2007 | Italy | Caucasian | IS | HB | CCS | 215/236 | 0.43 | Yes | 79/73 | 105/108 | 31/55 | 6 |
| Muendlein et al. [27] | 2008 | Austria | Caucasian | CAD | HB | CS | 332/225 | 0.40 | Yes | 125/71 | 162/116 | 45/38 | 8 |
| Rejeb et al. [30] | 2008 | Tunisian | Caucasian | CAD | HB | CS | 212/104 | 0.41 | Yes | 104/45 | 93/47 | 15/12 | 8 |
| Meiner et al. [48] | 2008 | USA | Caucasian | MI | PB | CCS | 550/620 | 0.45 | Yes | 173/166 | 282/320 | 95/134 | 6 |
| Wang et al. [52] | 2008 | China | Asian | CAD | PB | CCS | 317/298 | 0.41 | Yes | 117/99 | 148/146 | 52/53 | 6 |
| Jensen et al. [62] a | 2008 | USA | Caucasian | MI | PB | CS | 247/486 | 0.42 | Yes | 84/166 | 120/235 | 42/85 | 8 |
| Jensen et al. [62] b | 2008 | USA | Caucasian | MI | PB | CS | 259/513 | 0.41 | Yes | 89/180 | 126/244 | 44/89 | 8 |
| Padmaja et al. [28] | 2009 | Indian | Asian | CAD | HB | CCS | 504/338 | 0.45 | Yes | 163/86 | 264/161 | 77/91 | 6 |
| Poduri et al. [49] | 2009 | India | Asian | MI | PB | CCS | 265/150 | 0.41 | Yes | 117/3 | 107/82 | 41/35 | 6 |
| Tanrikulu-Kucuk et al. [23] | 2010 | Turkey | Caucasian | CAD | HB | CCS | 135/112 | 0.46 | No | 40/33 | 71/50 | 24/29 | 6 |
| Corella et al. [39] | 2010 | Spanish | Caucasian | CAD | PB | CS | 557/1180 | 0.47 | Yes | 224/482 | 247/537 | 86/161 | 8 |
| Bhanushali et al. [66] | 2010 | Indian | Asian | CAD | HB | CCS | 90/150 | 0.46 | Yes | 33/38 | 40/77 | 17/35 | 7 |
| Kolovou et al. [25] | 2011 | Greek | Caucasian | CAD | HB | CCS | 374/96 | 0.42 | Yes | 126/22 | 202/45 | 46/29 | 6 |
| Zhang et al. [55] | 2011 | China | Asian | CAD | PB | CCS | 334/301 | 0.34 | Yes | 172/136 | 106/120 | 56/45 | 8 |
| Jiang et al. [65] | 2012 | China | Asian | IS | HB | CCS | 220/220 | 0.29 | Yes | 130/103 | 72/86 | 18/31 | 6 |
| Tayebi et al. [61] | 2012 | Singapore | Asian | CAD | HB | CCS | 659/927 | 0.45 | Yes | 228/245 | 322/491 | 109/191 | 7 |
| Lu et al. [46] | 2013 | Singapore | Asian | CAD | PB | CCS | 659/927 | 0.45 | Yes | 228/245 | 322/491 | 109/191 | 8 |
| Mehlig et al. [47] | 2014 | Sweden | Caucasian | CAD | PB | CCS | 618/2921 | 0.43 | Yes | 209/938 | 313/1420 | 96/563 | 8 |
| El-Aziz et al. [50] | 2014 | Egypt | Caucasian | CAD | PB | CCS | 116/119 | 0.46 | Yes | 38/30 | 60/57 | 18/32 | 6 |
| Kaman et al. [24] | 2015 | Turkey | Caucasian | CAD | HB | CCS | 210/100 | 0.44 | Yes | 44/29 | 81/45 | 85/26 | 6 |
| Liu et al. [26] | 2015 | China | Asian | CAD | HB | CCS | 322/108 | 0.42 | Yes | 113/40 | 145/47 | 64/21 | 6 |
| Shi et al. [31] | 2015 | China | Asian | CAD | HB | CCS | 312/88 | 0.42 | Yes | 112/29 | 138/44 | 62/15 | 6 |
| Cyrus et al. [60] | 2016 | Saudi Arabia | Caucasian | CAD | PB | CCS | 990/618 | 0.41 | Yes | 376/183 | 454/321 | 160/114 | 6 |
| First Author | Year | Country | Ethnicity | MAF | HWE | B1B1 | B1B2 | B2B2 | Score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | n | Mean | SD | n | Mean | SD | n | |||||||
| Kuivenhoven et al. [44] | 1998 | The Netherlands | Caucasian | 0.41 | Yes | 0.88 | 0.21 | 281 | 0.93 | 0.21 | 397 | 1.01 | 0.26 | 129 | 7 |
| Gudnason et al. [67] | 1999 | Mixed | Caucasian | 0.44 | Yes | 1.13 | 0.21 | 237 | 1.19 | 0.24 | 380 | 1.27 | 0.22 | 150 | 7 |
| Eiriksdottir et al. [40] | 2001 | Iceland | Caucasian | 0.45 | Yes | 1.09 | 0.31 | 328 | 1.12 | 0.29 | 596 | 1.25 | 0.40 | 210 | 8 |
| Goto et al. [68] | 2001 | Japan | Asian | 0.43 | Yes | 1.14 | 0.28 | 37 | 1.23 | 0.37 | 47 | 1.23 | 0.33 | 22 | 6 |
| Talmud et al. [8] | 2002 | UK | Caucasian | 0.45 | Yes | 0.79 | 0.25 | 500 | 0.84 | 0.25 | 896 | 0.90 | 0.27 | 317 | 6 |
| Liu et al. [45] | 2002 | USA | Caucasian | 0.43 | Yes | 1.17 | 0.28 | 247 | 1.24 | 0.34 | 389 | 1.30 | 0.34 | 132 | 8 |
| Goff et al. [69] | 2002 | UK and France | Caucasian | 0.47 | Yes | 1.33 | 0.40 | 410 | 1.29 | 0.60 | 889 | 1.26 | 0.45 | 504 | 7 |
| Zhang et al. [35] | 2003 | China | Asian | 0.41 | No | 1.26 | 0.22 | 125 | 1.30 | 0.25 | 221 | 1.42 | 0.22 | 52 | 6 |
| Katsunori et al. [70] | 2003 | Japan | Asian | 0.4 | Yes | 1.32 | 0.46 | 217 | 1.43 | 0.57 | 279 | 1.59 | 0.62 | 95 | 7 |
| Zhao et al. [36] | 2004 | China | Asian | 0.41 | Yes | 1.19 | 0.36 | 155 | 1.27 | 0.34 | 214 | 1.38 | 0.39 | 72 | 6 |
| Weitgasser et al. [71] | 2004 | Austrian | Caucasian | 0.41 | Yes | 1.49 | 0.39 | 358 | 1.55 | 0.41 | 475 | 1.67 | 0.41 | 184 | 7 |
| Jiang et al. [72] | 2005 | China | Asian | 0.37 | No | 1.16 | 0.27 | 49 | 1.20 | 0.33 | 38 | 1.34 | 0.29 | 21 | 6 |
| Whiting et al. [53] | 2005 | USA | Caucasian | 0.42 | Yes | 0.91 | 0.33 | 1071 | 0.95 | 0.34 | 1577 | 1.00 | 0.38 | 571 | 8 |
| Huang et al. [73] | 2006 | China | Asian | 0.40 | Yes | 1.08 | 0.29 | 121 | 1.13 | 0.29 | 163 | 1.27 | 0.48 | 56 | 6 |
| Zhang et al. [74] | 2007 | China | Asian | 0.40 | No | 1.26 | 0.31 | 24 | 1.34 | 0.35 | 20 | 1.42 | 0.43 | 13 | 6 |
| Cui et al. [75] | 2007 | China | Asian | 0.46 | Yes | 1.44 | 0.32 | 17 | 1.58 | 0.46 | 24 | 1.54 | 0.36 | 13 | 6 |
| Meena et al. [76] | 2007 | Indian | Asian | 0.21 | No | 1.20 | 0.20 | 15 | 1.10 | 0.10 | 36 | 1.10 | 0.20 | 106 | 6 |
| Hsieh et al. [59] | 2007 | China | Asian | 0.31 | Yes | 43.31 | 10.63 | 42 | 43.39 | 11.09 | 158 | 46.24 | 11.83 | 165 | 5 |
| Zhang et al. [77] | 2008 | China | Asian | 0.39 | No | 1.45 | 0.31 | 46 | 1.41 | 0.23 | 78 | 2.03 | 0.47 | 16 | 6 |
| Wang et al. [78] | 2008 | China | Asian | 0.44 | Yes | 1.31 | 0.38 | 66 | 1.39 | 0.38 | 98 | 1.61 | 0.44 | 41 | 6 |
| Qiu et al. [79] | 2009 | China | Asian | 0.41 | Yes | 1.18 | 0.36 | 38 | 1.25 | 0.33 | 32 | 1.28 | 0.42 | 21 | 6 |
| Tao et al. [80] | 2010 | China | Asian | 0.41 | Yes | 0.95 | 0.19 | 608 | 0.96 | 0.18 | 939 | 0.97 | 0.18 | 272 | 6 |
| Kappelle et al. [81] | 2013 | The Netherlands | Caucasian | 0.42 | Yes | 1.28 | 0.37 | 2301 | 1.35 | 0.40 | 3233 | 1.41 | 0.42 | 1246 | 6 |
| Li et al. [82] | 2014 | China | Asian | 0.33 | Yes | 0.99 | 0.23 | 82 | 1.10 | 0.32 | 73 | 1.10 | 0.27 | 21 | 6 |
| Galati et al. [83] | 2014 | Italia | Caucasian | 0.42 | Yes | 1.52 | 0.45 | 73 | 1.45 | 0.30 | 106 | 1.61 | 0.42 | 39 | 7 |
| El-Aziz et al. [50] | 2014 | Egypt | Caucasian | 0.49 | Yes | 0.81 | 0.11 | 68 | 1.14 | 0.21 | 117 | 1.53 | 0.19 | 62 | 6 |
| Zhai et al. [84] | 2015 | China | Asian | 0.48 | Yes | 0.96 | 0.28 | 12 | 1.10 | 0.25 | 34 | 1.12 | 0.31 | 14 | 6 |
| Jeenduang et al. [85] | 2015 | Thailand | Asian | 0.37 | Yes | 1.34 | 0.32 | 152 | 1.35 | 0.35 | 169 | 1.39 | 0.31 | 57 | 6 |
| Position | Size (Case/Control) | Allele Model | Additive Model | Recessive Model | Dominant Model | ||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | ||
| Overall analysis | 20,866/21,298 | 1.15 (1.09–1.21) | p < 0.001 | 1.26 (1.19–1.34) | p < 0.001 | 1.13 (1.08–1.18) | p < 0.001 | 1.20 (1.14–1.27) | p < 0.001 |
| Subgroup analysis based on ethnicity | |||||||||
| Asian | 5178/5084 | 1.24 (1.15–1.35) | p < 0.001 | 1.52 (1.35–1.72) | p < 0.001 | 1.41 (1.29–1.53) | p < 0.001 | 1.28 (1.15–1.42) | p < 0.001 |
| Caucasian | 15,688/16,214 | 1.09 (1.04–1.16) | 0.001 | 1.19 (1.11–1.27) | p < 0.001 | 1.05 (1.00–1.11) | 0.041 | 1.18 (1.11–1.25) | p < 0.001 |
| Subgroup analysis based on type of diseases | |||||||||
| MI | 9067/9393 | 1.10 (1.03–1.19) | 0.009 | 1.18 (1.08–1.29) | p < 0.001 | 1.05 (0.99–1.12) | 0.104 | 1.17 (1.08–1.26) | p < 0.001 |
| IS | 531/556 | 1.39 (1.17–1.66) | p < 0.001 | 1.92 (1.33–2.77) | 0.001 | 1.40 (1.09–1.79) | p < 0.001 | 1.76 (1.25–2.47) | 0.001 |
| CAD | 11,268/11,349 | 1.15 (1.08–1.24) | p < 0.001 | 1.31 (1.21–1.43) | p < 0.001 | 1.19 (1.12–1.27) | p < 0.001 | 1.21 (1.13–1.31) | p < 0.001 |
| Subgroup analysis based on source of controls | |||||||||
| PB | 14,735/11,618 | 1.11 (1.05–1.17) | p < 0.001 | 1.21 (1.13–1.29) | p < 0.001 | 1.09 (1.04–1.15) | 0.001 | 1.17 (1.10–1.25) | p < 0.001 |
| HB | 6131/5180 | 1.20 (1.10–1.31) | p < 0.001 | 1.42 (1.26–1.59) | p < 0.001 | 1.24 (1.14–1.35) | p < 0.001 | 1.28 (1.16–1.42) | p < 0.001 |
| Subgroup analysis based on study type | |||||||||
| CCS | 15,155/15,187 | 1.14 (1.10–1.18) | p < 0.001 | 1.30 (1.21–1.39) | p < 0.001 | 1.16 (1.11–1.22) | p < 0.001 | 1.22 (1.15–1.30) | p < 0.001 |
| CS | 5711/6111 | 1.07 (1.01–1.13) | 0.023 | 1.16 (1.03–1.30) | 0.012 | 1.05 (0.97–1.14) | 0.277 | 1.15 (1.04–1.28) | 0.007 |
| Sensitivity analysis | |||||||||
| BHWE | 14,461/16,034 | 1.16 (1.09–1.23) | p < 0.001 | 1.33 (1.23–1.42) | p < 0.001 | 1.18 (1.12–1.24) | p < 0.001 | 1.24 (1.16–1.32) | p < 0.001 |
| BS | 18,902/19,454 | 1.12 (1.08–1.15) | p < 0.001 | 1.25 (1.18–1.33) | p < 0.001 | 1.13 (1.08–1.18) | p < 0.001 | 1.20 (1.14–1.27) | p < 0.001 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.-x.; Yao, M.-h.; Ding, Y.-s.; Zhang, J.-y.; Yan, Y.-z.; Liu, J.-m.; Zhang, M.; Rui, D.-s.; Niu, Q.; He, J.; et al. Associations of Cholesteryl Ester Transfer Protein TaqIB Polymorphism with the Composite Ischemic Cardiovascular Disease Risk and HDL-C Concentrations: A Meta-Analysis. Int. J. Environ. Res. Public Health 2016, 13, 882. https://doi.org/10.3390/ijerph13090882
Guo S-x, Yao M-h, Ding Y-s, Zhang J-y, Yan Y-z, Liu J-m, Zhang M, Rui D-s, Niu Q, He J, et al. Associations of Cholesteryl Ester Transfer Protein TaqIB Polymorphism with the Composite Ischemic Cardiovascular Disease Risk and HDL-C Concentrations: A Meta-Analysis. International Journal of Environmental Research and Public Health. 2016; 13(9):882. https://doi.org/10.3390/ijerph13090882
Chicago/Turabian StyleGuo, Shu-xia, Ming-hong Yao, Yu-song Ding, Jing-yu Zhang, Yi-zhong Yan, Jia-ming Liu, Mei Zhang, Dong-sheng Rui, Qiang Niu, Jia He, and et al. 2016. "Associations of Cholesteryl Ester Transfer Protein TaqIB Polymorphism with the Composite Ischemic Cardiovascular Disease Risk and HDL-C Concentrations: A Meta-Analysis" International Journal of Environmental Research and Public Health 13, no. 9: 882. https://doi.org/10.3390/ijerph13090882





