Protocol Report on the Transcranial Photobiomodulation for Alzheimer’s Disease (TRAP-AD) Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Objectives
- Aim #1: To assess the efficacy of t-PBM for cognitive deficits in amnestic MCI (aMCI) and mild dementia due to AD. Participants will be randomized to t-PBM or sham and their cognitive functions will be monitored with a change in the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS-Update) [64] Total Scale score after 8 weeks of treatment;
- Aim #2: To evaluate the safety, tolerability, and feasibility of t-PBM treatment in patients with aMCI and mild dementia due to AD. We will monitor changes in treatment emergent adverse events by using Systematic Assessment for Treatment Emergent Events (SAFTEE) [65], as well as scales that assess suicidal ideation and sleep quality;
- Aim #3: To explore brain mechanisms of t-PBM in aMCI and mild dementia due to AD. We will assess possible mediators of the treatment response including baseline tau burden (measured using Positron Emitting Topography [PET] with a 18F MK-6240 tracer), changes in measures of mitochondrial function (assessed using Phosphorus Magnetic Resonance Spectroscopic Imaging [31P-MRSI]), and blood flow in the prefrontal cortex (PFC) (measured with a Blood-Oxygen-Level-Dependent [BOLD] signal change using functional Magnetic Resonance Imaging [fMRI]).
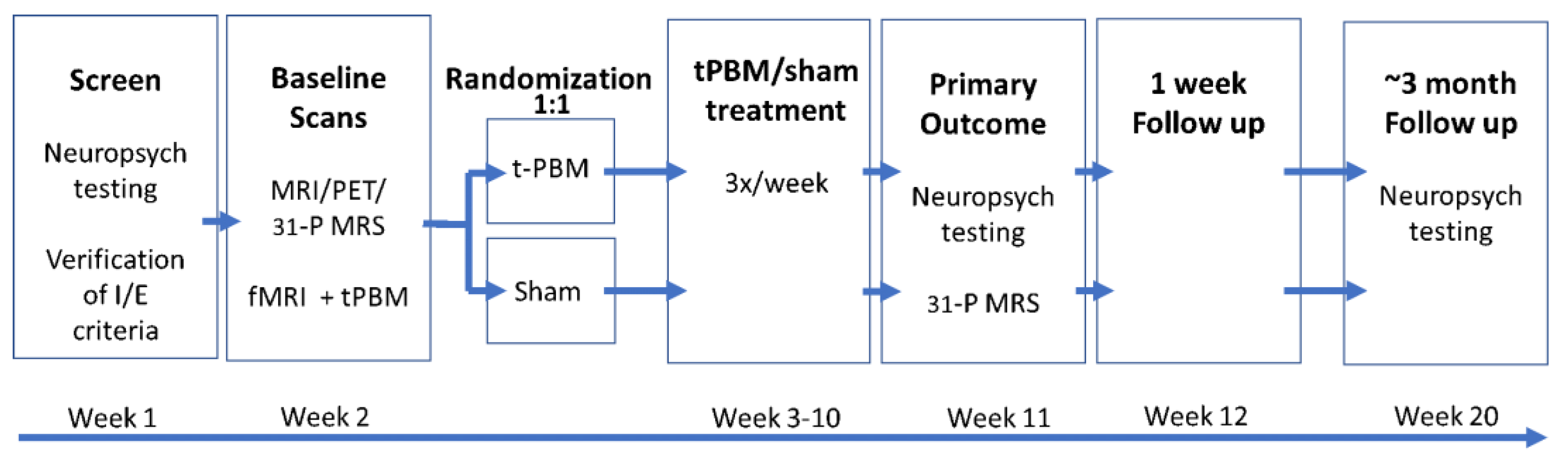
2.2. Study Design
2.3. Study Participants
2.4. Screening Procedures
2.5. Baseline Data
2.6. Randomization
2.7. t-PBM Treatment Phase
2.8. Post-Study Data
2.9. Data Management and Analysis
2.10. Demographic Data on Existing Participants
3. Discussion
4. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- 2021 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2021, 17, 327–406. [CrossRef] [PubMed]
- Wong, W. Economic burden of Alzheimer disease and managed care considerations. Am. J. Manag. Care 2020, 26, S177–S183. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Hampel, H.; Feldman, H.H.; Scheltens, P.; Aisen, P.; Andrieu, S.; Bakardjian, H.; Benali, H.; Bertram, L.; Blennow, K.; et al. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimer’s Dement. 2016, 12, 292–323. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C. Mild cognitive impairment and preclinical Alzheimer’s disease. Geriatrics 2005, 9–14, 16025770. [Google Scholar]
- Ricciarelli, R.; Fedele, E. The amyloid cascade hypothesis in Alzheimer’s disease: It’s time to change our mind. Curr. Neuropharmacol. 2017, 15, 926–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kametani, F.; Hasegawa, H. Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer’s disease. Front. Neurosci. 2018, 12, 25. [Google Scholar] [CrossRef] [Green Version]
- Nelson, P.T.; Braak, H.; Markesbery, W.R. Neuropathology and cognitive impairment in Alzheimer disease: A complex but coherent relationship. J. Neuropathol. Exp. Neurol. 2009, 68, 1–14. [Google Scholar] [CrossRef]
- Iqbal, K.; Liu, F.; Gong, C.X.; Grundke-Iqbal, I. Tau in Alzheimer disease and related tauopathies. Curr. Alzheimer Res. 2010, 7, 656–664. [Google Scholar] [CrossRef] [Green Version]
- Swerdlow, R.H.; Burns, J.M.; Khan, S.M. The Alzheimer’s disease mitochondrial cascade hypothesis: Progress and perspectives. Biochim. Et Biophys. Acta 2014, 1842, 1219–1231. [Google Scholar] [CrossRef] [Green Version]
- Swerdlow, R.H.; Khan, S.M. A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Med. Hypotheses 2004, 63, 8–20. [Google Scholar] [CrossRef]
- Blonz, E.R. Alzheimer’s disease as the product of a progressive energy deficiency syndrome in the central nervous system: The neuroenergetic hypothesis. J. Alzheimer’s Dis. 2017, 60, 1223–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosconi, L. Glucose metabolism in normal aging and Alzheimer’s disease: Methodological and physiological considerations for PET studies. Clin. Transl. Imaging 2013, 1, 217–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanzillotta, C.; Di Domenico, F.; Perluigi, M.; Butterfield, D.A. Targeting mitochondria in Alzheimer disease: Rationale and perspectives. CNS Drugs 2019, 33, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K.; Clemons, T.E.; McBee, W.L.; Lindblad, A.S. Impact of antioxidants, zinc, and copper on cognition in the elderly: A randomized, controlled trial. Neurology 2004, 63, 1705–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sano, M.; Ernesto, C.; Thomas, R.G.; Klauber, M.R.; Schafer, K.; Grundman, M.; Woodbury, P.; Growdon, J.; Cotman, C.W.; Pfeiffer, E.; et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. N. Engl. J. Med. 1997, 336, 1216–1222. Available online: https://www.nejm.org/doi/full/10.1056/NEJM199704243361704 (accessed on 7 June 2023). [CrossRef] [PubMed] [Green Version]
- Morris, M.C.; Beckett, L.A.; Scherr, P.A.; Hebert, L.; Bennett, D.A.; Field, T.S.; Evans, D.A. Vitamin E and vitamin C supplement use and risk of incident Alzheimer disease. Alzheimer Dis. Assoc. Disord. 1998, 12, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Luchsinger, J.A.; Tang, M.; Shea, S.; Mayeux, R. Antioxidant vitamin intake and risk of Alzheimer disease. Arch. Neurol. 2003, 60, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Evans, D.A.; Tangney, C.C.; Bienias, J.; Wilson, R.S.; Aggarwal, N.T.; Scherr, P.A. Relation of the tocopherol forms to incident Alzheimer disease and to cognitive change. Am. J. Clin. Nutr. 2005, 81, 508–514. [Google Scholar] [CrossRef] [Green Version]
- Terada, A.; Yoshida, M.; Seko, Y.; Kobayashi, T.; Yoshida, K.; Nakada, M.; Nakada, K.; Echizen, H.; Ogata, H.; Rikihisa, T. Active oxygen species generation and cellular damage by additives of parenteral preparations: Selenium and sulfhydryl compounds. Nutrition 1999, 15, 651–655. [Google Scholar] [CrossRef]
- Johri, A. Disentangling mitochondria in Alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 11520. [Google Scholar] [CrossRef]
- Spera, V.; Sitnikova, T.; Ward, M.J.; Farzam, P.; Hughes, J.; Gazecki, S.; Bui, E.; Maiello, M.; De Taboada, L.; Hamblin, M.R.; et al. Pilot study on dose-dependent effects of transcranial photobiomodulation on brain electrical oscillations: A potential therapeutic target in alzheimer’s disease. J. Alzheimer’s Dis. 2021, 83, 1481–1498. [Google Scholar] [CrossRef]
- Henderson, T.A.; Morries, L.D. Near-infrared photonic energy penetration: Can infrared phototherapy effectively reach the human brain? Neuropsychiatr. Dis. Treat. 2015, 11, 2191–2208. [Google Scholar] [CrossRef] [Green Version]
- Jagdeo, J.R.; Adams, L.E.; Brody, N.I.; Siegel, D.M. Transcranial red and near infrared light transmission in a cadaveric model. PLoS ONE 2012, 7, e47460. [Google Scholar] [CrossRef]
- Tedford, C.E.; DeLapp, S.; Jacques, S.; Anders, J. Quantitative analysis of transcranial and intraparenchymal light penetration in human cadaver brain tissue. Lasers Surg. Med. 2015, 47, 312–322. Available online: https://onlinelibrary.wiley.com/doi/pdf/10.1002/lsm.22343 (accessed on 6 July 2023). [CrossRef]
- Mochizuki-Oda, N.; Kataoka, Y.; Cui, Y.; Yamada, H.; Heya, M.; Awazu, K. Effects of near-infra-red laser irradiation on adenosine triphosphate and adenosine diphosphate contents of rat brain tissue. Neurosci. Lett. 2002, 323, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.Y.; Carroll, J.D.; Hamblin, M.R. The nuts and bolts of low-level laser (light) therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oron, U.; Ilic, S.; De Taboada, L.; Streeter, J. Ga-As (808 nm) laser irradiation enhances ATP production in human neuronal cells in culture. Photomed. Laser Surg. 2007, 25, 180–182. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Naim, J.O.; McGowan, M.; Ippolito, K.; Lanzafame, R.J. Photomodulation of oxidative metabolism and electron chain enzymes in rat liver mitochondria. Photochem. Photobiol. 1997, 66, 866–871. [Google Scholar] [CrossRef]
- Mosconi, L.; Mistur, R.; Switalski, R.; Tsui, W.H.; Glodzik, L.; Li, Y.; Pirraglia, E.; De Santi, S.; Reisberg, B.; Wisniewski, T.; et al. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 811–822. [Google Scholar] [CrossRef] [Green Version]
- Mosconi, L.; Tsui, W.H.; Herholz, K.; Pupi, A.; Drzezga, A.; Lucignani, G.; Reiman, E.M.; Holthoff, V.; Kalbe, E.; Sorbi, S.; et al. Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer’s disease, and other dementias. J. Nucl. Med. 2008, 49, 390–398. [Google Scholar] [CrossRef] [Green Version]
- Flannery, P.J.; Trushina, E. Mitochondrial dynamics and transport in Alzheimer’s disease. Mol. Cell. Neurosci. 2019, 98, 109–120. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, L.F.; Hamblin, M.R. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 7000417. [Google Scholar] [CrossRef] [Green Version]
- Hipskind, S.G.; Grover, F.L., Jr.; Fort, T.R.; Helffenstein, D.; Burke, T.J.; Quint, S.A.; Bussiere, G.; Stone, M.; Hurtado, T. Pulsed transcranial red/near-infrared light therapy using light-emitting diodes improves cerebral blood flow and cognitive function in veterans with chronic traumatic brain injury: A case series. Photomed. Laser Surg. 2018, 37, 77–84. [Google Scholar] [CrossRef]
- Greco, M.; Guida, G.; Perlino, E.; Marra, E.; Quagliariello, E. Increase in RNA and protein synthesis by mitochondria irradiated with helium-neon laser. Biochem. Biophys. Res. Commun. 1989, 163, 1428–1434. [Google Scholar] [CrossRef]
- Tonnies, E.; Trushina, E. Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhao, B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxidative Med. Cell. Longev. 2013, 2013, 316523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.K.; Mishra, G.; Maurya, A.; Awasthi, R.; Kumari, K.; Thakur, A.; Rai, A.; Rai, G.K.; Sharma, B.; Kulkarni, G.T.; et al. Role of TREM2 in Alzheimer’s disease and its consequences on beta-amyloid, tau and neurofibrillary tangles. Curr. Alzheimer Res. 2019, 16, 1216–1229. [Google Scholar] [CrossRef] [PubMed]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimer’s Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-derived neurotrophic factor: A key molecule for memory in the healthy and the pathological brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Kazim, S.F.; Iqbal, K. Neurotrophic factor small-molecule mimetics mediated neuroregeneration and synaptic repair: Emerging therapeutic modality for Alzheimer’s disease. Mol. Neurodegener. 2016, 11, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scharfman, H.E.; Chao, M.V. The entorhinal cortex and neurotrophin signaling in Alzheimer’s disease and other disorders. Cogn. Neurosci. 2013, 4, 123–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzi, C.F.; Mauriz, J.L.; Freitas Correa, D.S.; Moreira, A.J.; Zettler, C.G.; Filippin, L.I.; Marroni, N.P.; Gonzalez-Gallego, J. Effects of low-level laser therapy (LLLT) on the nuclear factor (NF)-kappaB signaling pathway in traumatized muscle. Lasers Surg. Med. 2006, 38, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Chludzinska, L.; Ananicz, E.; Jaroslawska, A.; Komorowska, M. Near-infrared radiation protects the red cell membrane against oxidation. Blood Cells Mol. Dis. 2005, 35, 74–79. [Google Scholar] [CrossRef]
- Araki, H.; Imaoka, A.; Kuboyama, N.; Abiko, Y. Reduction of interleukin-6 expression in human synoviocytes and rheumatoid arthritis rat joints by linear polarized near infrared light (Superlizer) irradiation. Laser Ther. 2011, 20, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Yamaura, M.; Yao, M.; Yaroslavsky, I.; Cohen, R.; Smotrich, M.; Kochevar, I.E. Low level light effects on inflammatory cytokine production by rheumatoid arthritis synoviocytes. Lasers Surg. Med. 2009, 41, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Anders, J.J. The potential of light therapy for central nervous system injury and disease. Photomed. Laser Surg. 2009, 27, 379–380. [Google Scholar] [CrossRef] [Green Version]
- Wong-Riley, M.T.T.; Liang, H.L.; Eells, J.T.; Chance, B.; Henry, M.M.; Buchmann, E.; Kane, M.; Whelan, H.T. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: Role of cytochrome c oxidase. J. Biol. Chem. 2005, 280, 4761–4771. [Google Scholar] [CrossRef] [Green Version]
- Giuliani, A.; Lorenzini, L.; Gallamini, M.; Massella, A.; Giardino, L.; Calza, L. Low infra red laser light irradiation on cultured neural cells: Effects on mitochondria and cell viability after oxidative stress. BMC Complement. Altern. Med. 2009, 9, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas, J.C.; Lee, J.; John, J.M.; Gonzalez-Lima, F. Neuroprotective effects of near-infrared light in an in vivo model of mitochondrial optic neuropathy. J. Neurosci. Off J. Soc. Neurosci. 2008, 28, 13511–13521. [Google Scholar] [CrossRef] [Green Version]
- Ando, T.; Xuan, W.; Xu, T.; Dai, T.; Sharma, S.K.; Kharkwal, G.B.; Huang, Y.; Wu, Q.; Whalen, M.J.; Sato, S.; et al. Comparison of therapeutic effects between pulsed and continuous wave 810-nm wavelength laser irradiation for traumatic brain injury in mice. PLoS ONE 2011, 6, e26212. [Google Scholar] [CrossRef] [Green Version]
- Oron, A.; Oron, U.; Streeter, J.; De Taboada, L.; Alexandrovich, A.; Trembovler, V.; Shohami, E. Low-level laser therapy applied transcranially to mice following traumatic brain injury significantly reduces long-term neurological deficits. J. Neurotrauma 2007, 24, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xuan, W.; Ando, T.; Xu, T.; Huang, L.; Huang, Y.; Dai, T.; Dhital, S.; Sharma, S.K.; Whalen, M.J.; et al. Low-level laser therapy for closed-head traumatic brain injury in mice: Effect of different wavelengths. Lasers Surg. Med. 2012, 44, 218–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuan, W.; Vatansever, F.; Huang, L.; Wu, Q.; Xuan, Y.; Dai, T.; Ando, T.; Xu, T.; Huang, Y.; Hamblin, M.R. Transcranial low-level laser therapy improves neurological performance in traumatic brain injury in mice: Effect of treatment repetition regimen. PLoS ONE 2013, 8, e53454. [Google Scholar] [CrossRef] [Green Version]
- Xuan, W.; Agrawal, T.; Huang, L.; Gupta, G.K.; Hamblin, M.R. Low-level laser therapy for traumatic brain injury in mice increases brain derived neurotrophic factor (BDNF) and synaptogenesis. J. Biophotonics 2015, 8, 502–511. [Google Scholar] [CrossRef]
- Farfara, D.; Tuby, H.; Trudler, D.; Doron-Mandel, E.; Maltz, L.; Vassar, R.J.; Frenkel, D.; Oron, U. Low-level laser therapy ameliorates disease progression in a mouse model of Alzheimer’s disease. J. Mol. Neurosci. 2015, 55, 430–436. [Google Scholar] [CrossRef]
- Grillo, S.L.; Duggett, N.A.; Ennaceur, A.; Chazot, P.L. Non-invasive infra-red therapy (1072 nm) reduces beta-amyloid protein levels in the brain of an Alzheimer’s disease mouse model, TASTPM. J. Photochem. Photobiol. B Biol. 2013, 123, 13–22. [Google Scholar] [CrossRef]
- De Taboada, L.; Yu, J.; El-Amouri, S.; Gattoni-Celli, S.; Richieri, S.; McCarthy, T.; Streeter, J.; Kindy, M.S. Transcranial laser therapy attenuates amyloid-beta peptide neuropathology in amyloid-beta protein precursor transgenic mice. J. Alzheimer’s Dis. 2011, 23, 521–535. [Google Scholar] [CrossRef]
- Purushothuman, S.; Johnstone, D.M.; Nandasena, C.; van Eersel, J.; Ittner, L.M.; Mitrofanis, J.; Stone, J. Near infrared light mitigates cerebellar pathology in transgenic mouse models of dementia. Neurosci. Lett. 2015, 591, 155–159. [Google Scholar] [CrossRef]
- Hacke, W.; Schellinger, P.D.; Albers, G.W.; Bornstein, N.M.; Dahlof, B.L.; Fulton, R.; Kasner, S.E.; Shuaib, A.; Richieri, S.P.; Dilly, S.G.; et al. Transcranial laser therapy in acute stroke treatment: Results of neurothera effectiveness and safety trial 3, a phase III clinical end point device trial. Stroke 2014, 45, 3187–3193. [Google Scholar] [CrossRef] [Green Version]
- Lampl, Y.; Zivin, J.A.; Fisher, M.; Lew, R.; Welin, L.; Dahlof, B.; Borenstein, P.; Andersson, B.; Perez, J.; Caparo, C.; et al. Infrared laser therapy for ischemic stroke: A new treatment strategy: Results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1). Stroke 2007, 38, 1843–1849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassano, P.; Norton, R.J.; Caldieraro, M.A.; Vahedifard, F.; Vizcaino, F.; McEachern, K.M.; Iosifescu, D.V. Tolerability and safety of transcranial photobiomodulation for mood and anxiety disorders. Photonics 2022, 9, 507. [Google Scholar] [CrossRef]
- Saltmarche, A.E.; Naeser, M.A.; Ho, K.F.; Hamblin, M.R.; Lim, L. Significant improvement in memory and quality of life after transcranial and intranasal photobiomodulation: A randomized, controlled, single-blind pilot study with dementia. In Proceedings of the Alzheimer’s Association International Conference, Toronto, ON, Canada, 24 July 2016. [Google Scholar] [CrossRef]
- Saltmarche, A.E.; Naeser, M.A.; Ho, K.F.; Hamblin, M.R.; Lim, L. Significant improvement in cognition in mild to moderately severe dementia cases treated with transcranial plus intranasal photobiomodulation: Case series report. Photomed. Laser Surg. 2017, 35, 432–441. [Google Scholar] [CrossRef]
- Randolph, C. Repeatable Battery for the Assessment of Neuropsychological Status (RBANS); Psychological Corporation: San Antonio, TX, USA, 1998. [Google Scholar]
- Levine, J.; Schooler, N. SAFTEE (Systematic Assessment For Treatment Emergent Events). A new technique for detecting side effects in clinical trials. Clin. Neuropharmacol. 1984, 7, S460. Available online: https://journals.lww.com/clinicalneuropharm/Citation/1984/06001/SAFTEE__SYSTEMATIC_ASSESSMENT_FOR_TREATMENT.424.aspx (accessed on 8 June 2023). [CrossRef]
- Petersen, R.C. Mild cognitive impairment. Continuum 2016, 22, 404–418. [Google Scholar] [CrossRef] [Green Version]
- Morris, J.C. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 1993, 43, 2412–2414. [Google Scholar] [CrossRef]
- Sclan, S.; Reisberg, B. Functional assessment staging (FAST) in alzheimer’s disease: Reliability, validity, and ordinality. Int. Psychogeriatr. 1992, 4, 55–69. [Google Scholar] [CrossRef]
- Hsieh, S.; Schubert, S.; Hoon, C.; Mioshi, E.; Hodges, J.R. Validation of the Addenbrooke’s Cognitive Examination III in frontotemporal dementia and alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2013, 36, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Guy, W. ECDEU Assessment Manual for Psychopharmacology; US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs: Rockville, MD, USA, 1976.
- Sheehan, D.V.; Janavs, J.; Baker, R.; Sheehan, K.H.; Knapp, E.; Sheehan, M. The MINI International Neuropsychiatric Interview (Version 7.0.2) for DSM-5; Harm Research Institute, 2016. [Google Scholar]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59, 22–23. Available online: https://www.psychiatrist.com/wp-content/uploads/2021/02/15175_mini-international-neuropsychiatric-interview-mini.pdf (accessed on 8 June 2023). [PubMed]
- Posner, K.; Brent, D.; Lucas, C.; Gould, M.; Stanley, B.; Brown, G.; Fisher, P.; Zelazny, J.; Burke, A.; Oquendo, M.; et al. Columbia-Suicide Severity Rating Scale (C-SSRS); Columbia University Medical Center: New York, NY, USA, 2008; Volume 10. [Google Scholar]
- Massey, D.S.; Martin, J.A. The NIS Skin Color Scale; Office of Population Research, Princeton University: Princeton, NJ, USA, 2003. [Google Scholar]
- Sheikh, J.I.; Yesavage, J.A. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin. Gerontol. J. Aging Ment. Health 1986, 5, 165–173. [Google Scholar] [CrossRef]
- Baker, A.; Simon, N.; Keshaviah, A.; Farabaugh, A.; Deckersbach, T.; Worthington, J.J.; Hoge, E.; Fava, M.; Pollack, M.P. Anxiety Symptoms Questionnaire (ASQ): Development and validation. Gen. Psychiatry 2019, 32, e100144. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, M.H.; Wisniewski, S.R.; Morris, D.W.; Fava, M.; Kurian, B.T.; Gollan, J.K.; Nierenberg, A.A.; Warden, D.; Gaynes, B.N.; Luther, J.F.; et al. Concise Associated Symptoms Tracking Scale: A brief self-report and clinician rating of symptoms associated with suicidality. J. Clin. Psychiatry 2011, 72, 765–774. [Google Scholar] [CrossRef] [Green Version]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Cella, D.; Lai, J.-S.; Nowinski, C.J.; Victorson, D.; Peterman, A.; Miller, D.; Bethoux, D.; Heinemann, A.; Rubin, S.; Cavazos, J.E.; et al. Neuro-QOL: Brief measures of health-related quality of life for clinical research in neurology. Neurology 2012, 78, 1860–1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blair, J.R.; Spreen, O. Predicting premorbid IQ: A revision of the national adult reading test. Clin. Neuropsychol. 1989, 3, 129–136. [Google Scholar] [CrossRef]
- Uttl, B. North American adult reading test: Age norms, reliability, and validity. J. Clin. Exp. Neuropsychol. 2002, 24, 1123–1137. Available online: https://doi-org.ezproxy.med.nyu.edu/10.1076/jcen.24.8.1123.8375 (accessed on 8 June 2023). [CrossRef] [PubMed]
- Salthouse, T.A.; Babcock, R.L. Decomposing adult age differences in working memory. Dev. Psychol. 1991, 27, 763–776. [Google Scholar] [CrossRef]
- Rentz, D.M.; Amariglio, R.E.; Becker, J.A.; Frey, M.; Olson, L.E.; Frishe, K.; Carmasin, J.; Maye, J.E.; Johnson, K.A.; Sperling, R.A. Face-name associative memory performance is related to amyloid burden in normal elderly. Neuropsychologia 2011, 49, 2776–2783. [Google Scholar] [CrossRef] [Green Version]
- Papp, K.V.; Amariglio, R.E.; Dekhtyar, M.; Roy, K.; Wigman, S.; Bamfo, R.; Sherman, J.; Sperling, R.A.; Rentz, D.M. Development of a psychometrically equivalent short form of the face-name associative memory exam for use along the early alzheimer’s disease trajectory. Clin. Neuropsychol. 2014, 28, 771–785. [Google Scholar] [CrossRef]
- Reitan, R.M.; Wolfson, D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation; Reitan Neuropsychology: Tucson, AZ, USA, 1985; Volume 4. [Google Scholar]
- Stroop, J.R. Studies of interference in serial verbal reactions. J. Exp. Physiol. 1935, 18, 643–662. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Adult Intelligence Scale, 4th ed.; APA PsycTests: Washington, DC, USA, 2008. [Google Scholar] [CrossRef]
- Weintraub, S.; Besser, L.; Dodge, H.H.; Teylan, M.; Ferris, S.; Goldstein, F.C.; Giordani, B.; Kramer, J.; Loewenstein, D.; Marson, D.; et al. Version 3 of the Alzheimer’s Disease Centers’ neuropsychological test battery in the Uniform Data Set (UDS). Alzheimer’s Dis. Assoc. Disord. 2018, 32, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Caldieraro, M.A.; Laufer-Silva, T.; Cassano, P. Dosimetry and clinical efficacy of transcranial photobiomodulation for major depression disorder: Could they guide dosimetry for alzheimer’s disease? J. Alzheimer’s Dis. 2021, 83, 1453–1469. [Google Scholar] [CrossRef]
- Dmochowski, G.M.; Dmochowski, J.P. Increased Blood Flow and Oxidative Metabolism in the Human Brain by Transcranial Laser Stimulation. BioRxiv. 2018. Available online: https://www.biorxiv.org/content/10.1101/459883v1.full.pdf (accessed on 8 June 2023).
- Karu, T.I.; Pyatibrat, L.V.; Kolyakov, S.F.; Afanasyeva, N.I. Absorption measurements of a cell monolayer relevant to phototherapy: Reduction of cytochrome c oxidase under near IR radiation. J. Photochem. Photobiol. B Biol. 2005, 81, 98–106. [Google Scholar] [CrossRef]
- Heiskanen, V.; Hamblin, M.R. Photobiomodulation: Lasers vs. light emitting diodes? Photochem. Photobiol. Sci. 2018, 17, 1003–1017. [Google Scholar] [CrossRef] [Green Version]
- Hode, L. The importance of the coherency. Photomed. Laser Surg. 2005, 23, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Abritta, B.; Villarreal, M.F.; Abulafia, C.; Loewenstein, D.; Curiel Cid, R.E.; Castro, M.N.; Surace, E.; Sanchez, S.; Vigo, D.E.; Vasquez, S.; et al. Cortical thickness, brain metabolic activity, and in vivo amyloid deposition in asymptomatic, middle-aged offspring of patients with late-onset Alzheimer’s disease. J. Psychiatr. Res. 2018, 107, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Verfaillie, S.C.; Adriaanse, S.M.; Binnewijzend, M.A.; Benedictus, M.R.; Ossenkoppele, R.; Wattjes, M.P.; Pijnenburg, Y.A.L.; van der Flier, W.M.; Lammertsma, A.A.; Kuijer, J.P.A.; et al. Cerebral perfusion and glucose metabolism in Alzheimer’s disease and frontotemporal dementia: Two sides of the same coin? Eur. Radiol. 2015, 25, 3050–3059. [Google Scholar] [CrossRef] [Green Version]
- Terada, S.; Oshima, E.; Sato, S.; Ikeda, C.; Nagao, S.; Hayashi, S.; Hayashibara, C.; Yokota, O.; Uchitomi, Y. Depressive symptoms and regional cerebral blood flow in Alzheimer’s disease. Psychiatry Res. Neuroimaging 2014, 221, 86–91. [Google Scholar] [CrossRef]
- Kolahi, J.; Bang, H.; Park, J. Towards a proposal for assessment of blinding success in clinical trials: Up-to-date review. Community Dent. Oral Epidemiol. 2009, 37, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Olaithe, M.; Weinborn, M.; Lowndes, T.; Ng, A.; Hodgson, E.; Fine, L.; Parker, D.; Pushpanathan, M.; Bayliss, D.; Anderson, M.; et al. Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Normative data for older adults. Arch. Clin. Neuropsychol. 2019, 34, 1356–1366. [Google Scholar] [CrossRef]
- Benjamini, Y.B.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Baron, R.M.; Kenny, D.A. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Personal. Soc. Psychol. 1986, 51, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Kantarci, K.; Lowe, V.J.; Boeve, B.F.; Senjem, M.L.; Tosakulwong, N.; Lesnick, T.G.; Spychalla, A.J.; Gunter, J.L.; Fields, J.A.; Graff-Radford, J.; et al. AV-1451 tau and β-amyloid positron emission tomography imaging in dementia with Lewy bodies. Ann. Neurol. 2017, 81, 58–67. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Wiste, H.J.; Botha, H.; Weigand, S.D.; Therneau, T.M.; Knopman, D.S.; Graff-Radford, J.; Jones, D.T.; Ferman, T.J.; Boeve, B.F.; et al. The bivariate distribution of amyloid-β and tau: Relationship with established neurocognitive clinical syndromes. Brain 2019, 142, 3230–3242. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Wiste, H.J.; Schwarz, C.G.; Lowe, V.J.; Senjem, M.L.; Vemuri, P.; Weigand, S.D.; Therneau, T.M.; Knopman, D.S.; Gunter, J.L.; et al. Longitudinal tau PET in ageing and Alzheimer’s disease. Brain 2018, 141, 1517–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iosifescu, D.V.; Song, X.; Gersten, M.B.; Adib, A.; Cho, Y.; Collins, K.M.; Yates, K.F.; Hurtado-Puerto, A.M.; McEachern, K.M.; Osorio, R.S.; et al. Protocol Report on the Transcranial Photobiomodulation for Alzheimer’s Disease (TRAP-AD) Study. Healthcare 2023, 11, 2017. https://doi.org/10.3390/healthcare11142017
Iosifescu DV, Song X, Gersten MB, Adib A, Cho Y, Collins KM, Yates KF, Hurtado-Puerto AM, McEachern KM, Osorio RS, et al. Protocol Report on the Transcranial Photobiomodulation for Alzheimer’s Disease (TRAP-AD) Study. Healthcare. 2023; 11(14):2017. https://doi.org/10.3390/healthcare11142017
Chicago/Turabian StyleIosifescu, Dan V., Xiaotong Song, Maia B. Gersten, Arwa Adib, Yoonju Cho, Katherine M. Collins, Kathy F. Yates, Aura M. Hurtado-Puerto, Kayla M. McEachern, Ricardo S. Osorio, and et al. 2023. "Protocol Report on the Transcranial Photobiomodulation for Alzheimer’s Disease (TRAP-AD) Study" Healthcare 11, no. 14: 2017. https://doi.org/10.3390/healthcare11142017





