The Food Contaminants Pyrrolizidine Alkaloids Disturb Bile Acid Homeostasis Structure-Dependently in the Human Hepatoma Cell Line HepaRG
Abstract
1. Introduction
2. Materials and Methods
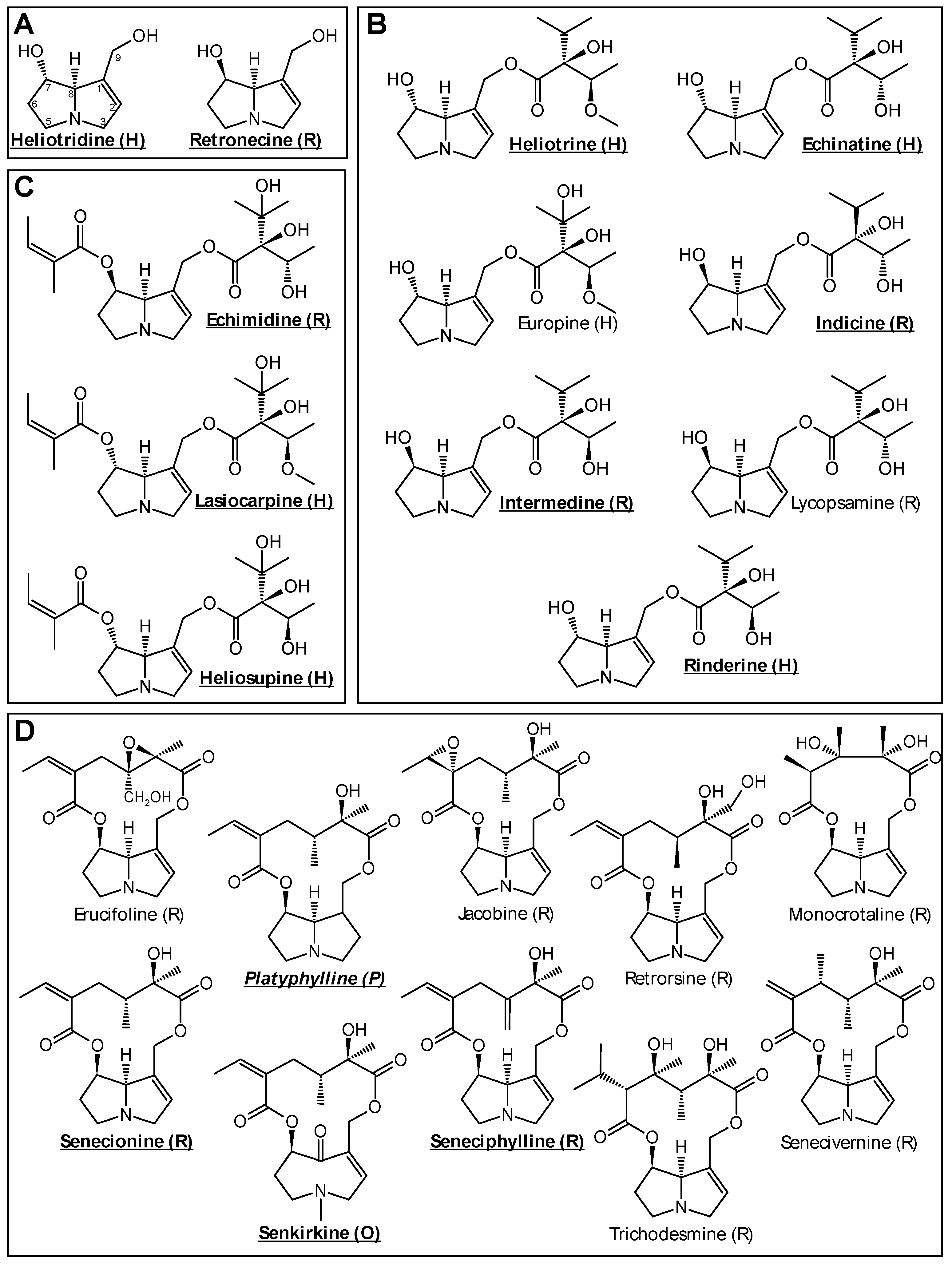
2.1. Chemicals
2.2. HepaRG Cell Culture
2.3. Cell Viability Assay
2.4. Isolation of Total RNA and Quantitative Real-Time Polymerase Chain Reaction (qPCR)
- initial denaturation (15 min at 95 °C)
- 40 cycles of denaturation (30 s at 95 °C) and annealing/elongation (1 min at 60 °C)
- final elongation (10 min at 60 °C)
- dissociation curve
2.5. Bile Acid Quantification
2.6. Statistical Analysis
3. Results
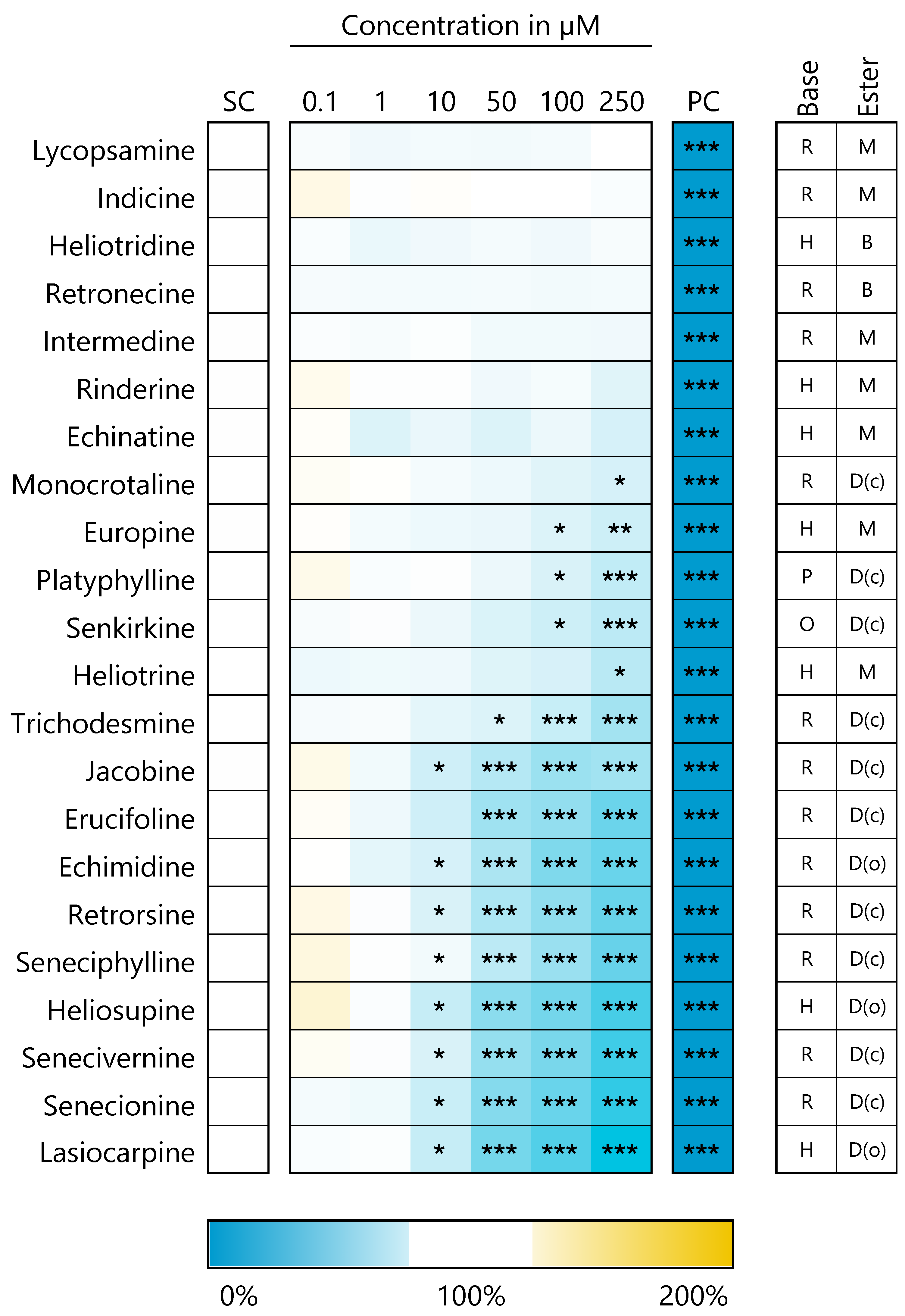
3.1. PA-Induced Cytotoxic Effects in HepaRG Cells
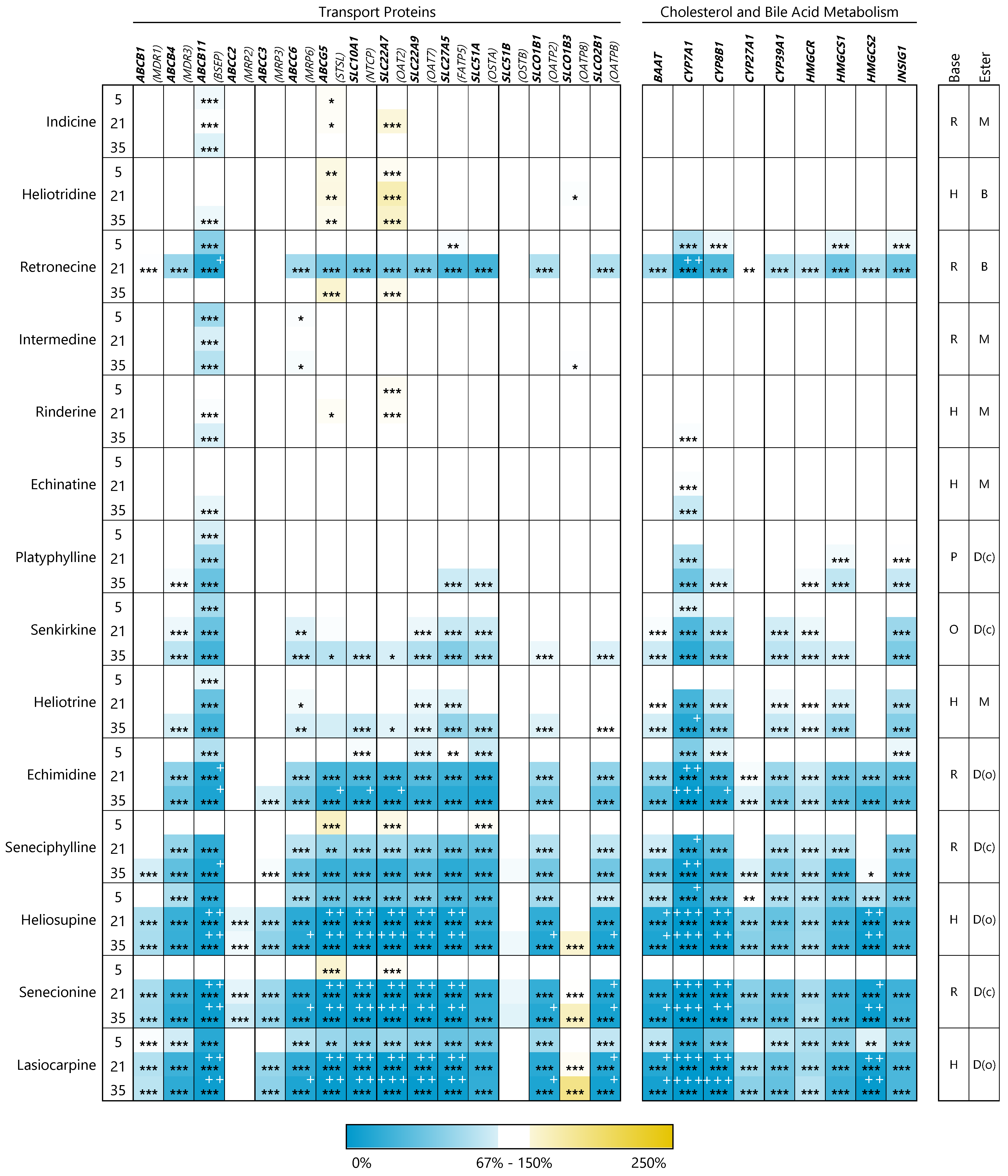
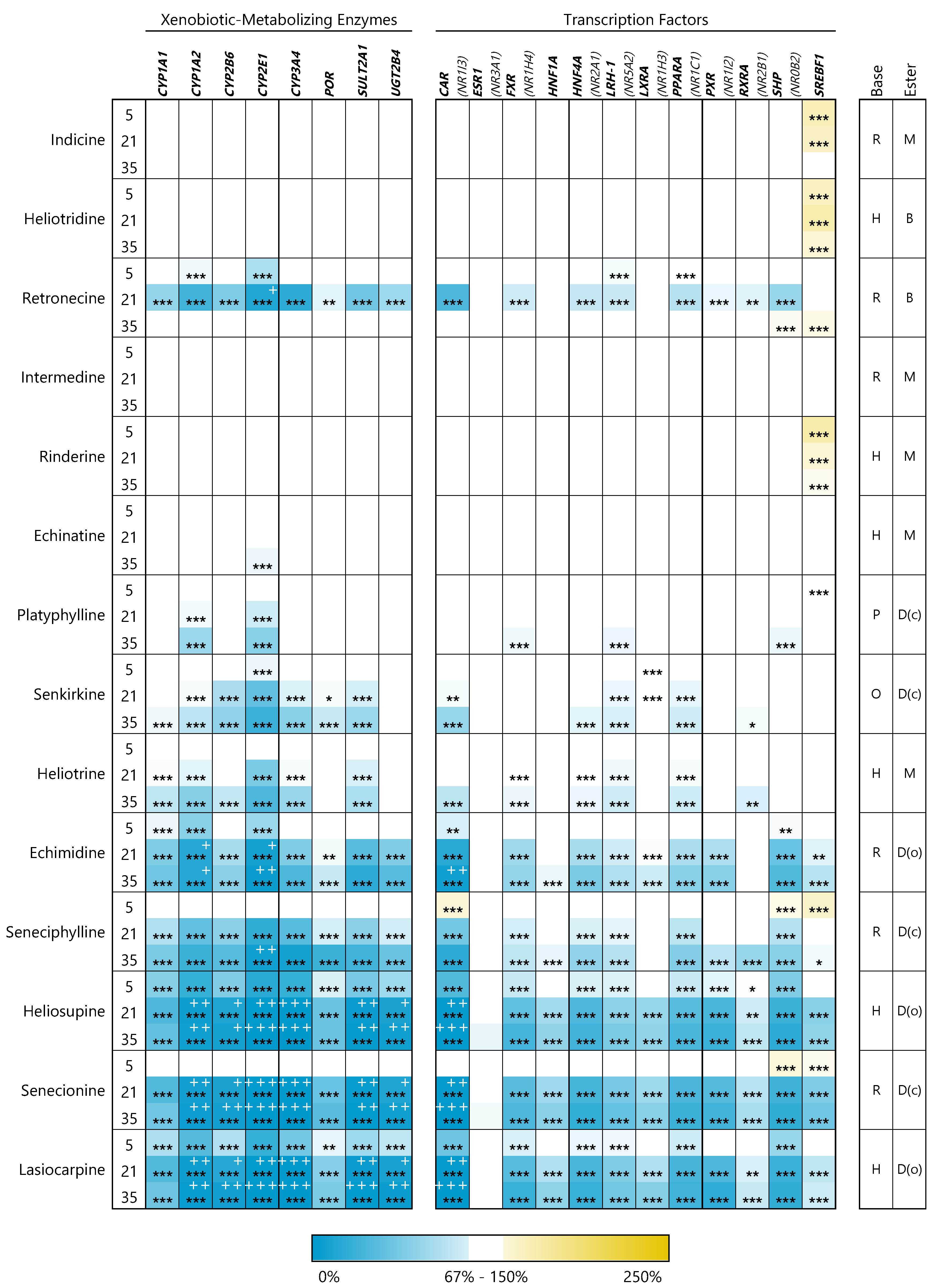
3.2. PAs Affect Structure-Dependently Expression of Genes Involved in Bile Acid Homeostasis
3.2.1. Transport Proteins
3.2.2. Enzymes of Cholesterol and Bile Acid Metabolism
3.2.3. Xenobiotic-Metabolizing Enzymes
3.2.4. Transcription Factors
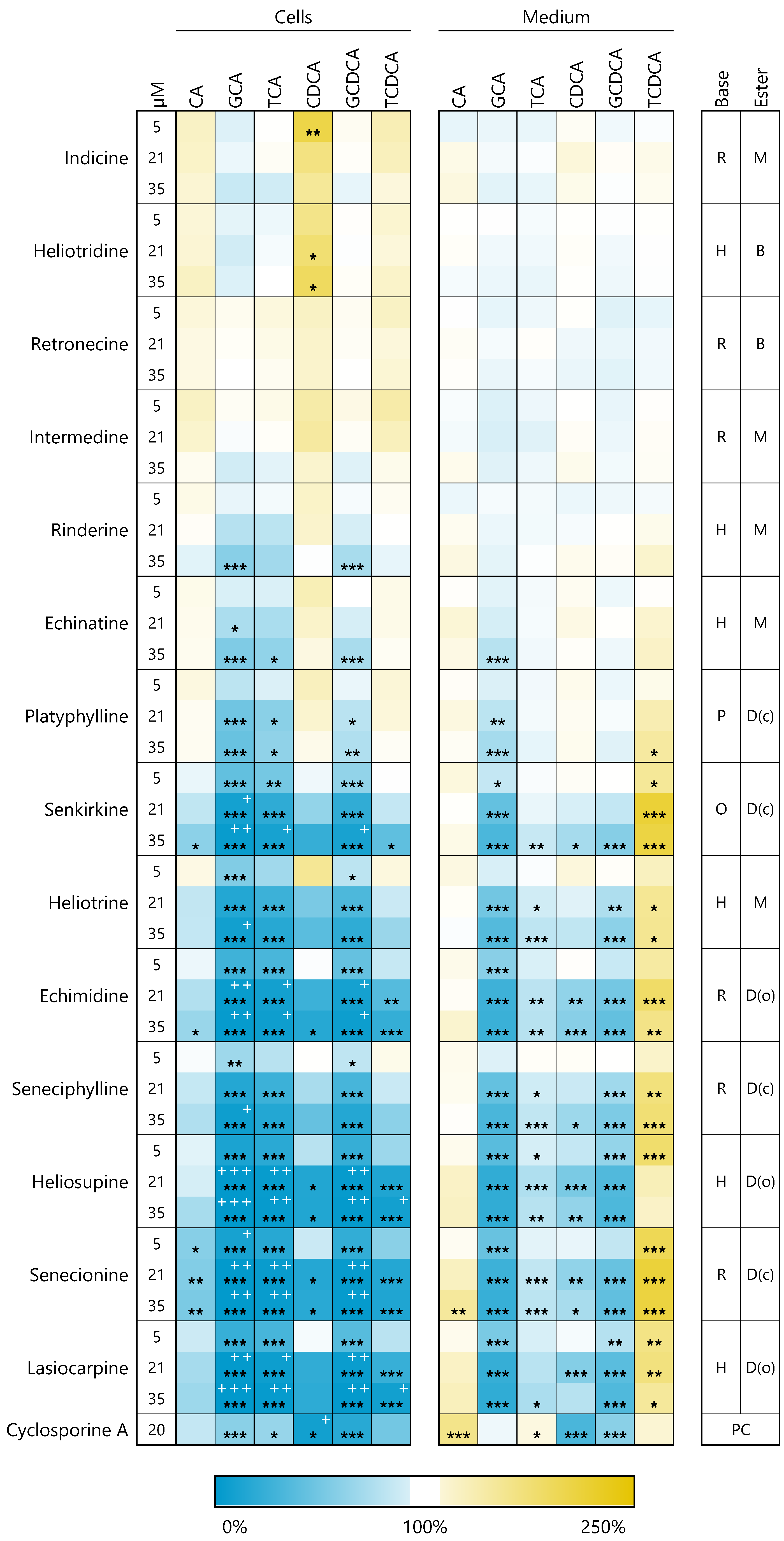
3.3. PAs Disturb Bile Acid Homeostasis Intra- and Extracellular
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACN | Acetonitrile |
| BA | Bile acids |
| CA | Cholic acid |
| CDCA | Chenodeoxycholic acid |
| CYP | Cytochrome P450 |
| DHP | Dehydropyrrolizidine alkaloid |
| DMSO | Dimethyl sulfoxide |
| FBS | Fetal bovine serum |
| GCA | Glycocholic acid |
| GCDCAHSOS | Glycochenodeoxycholic acidHepatic sinusoidal obstruction syndrome |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromid |
| PA(s) | Pyrrolizidine alkaloid(s) |
| PBS | Phosphate-buffered saline |
| qPCR | quantitative Polymerase chain reaction |
| SDS | Sodium dodecyl sulfate |
| TCA | Taurocholic acid |
| TCDCA | Taurochenodeoxycholic acid |
References
- BfR. Pyrrolizidine alkaloids in herbal teas and teas. BfR Opin. 2013, 2013, 1–31. [Google Scholar]
- Edgar, J.A.; Roeder, E.; Molyneux, R.J. Honey from plants containing pyrrolizidine alkaloids: A potential threat to health. J. Agric. Food Chem. 2002, 50, 2719–2730. [Google Scholar] [CrossRef] [PubMed]
- Kakar, F.; Akbarian, Z.; Leslie, T.; Mustafa, M.L.; Watson, J.; van Egmond, H.P.; Omar, M.F.; Mofleh, J. An outbreak of hepatic veno-occlusive disease in Western afghanistan associated with exposure to wheat flour contaminated with pyrrolizidine alkaloids. J. Toxicol. 2010, 2010, 313280. [Google Scholar] [CrossRef] [PubMed]
- Bodi, D.; Ronczka, S.; Gottschalk, C.; Behr, N.; Skibba, A.; Wagner, M.; Lahrssen-Wiederholt, M.; Preiss-Weigert, A.; These, A. Determination of pyrrolizidine alkaloids in tea, herbal drugs and honey. Food Addit. Contam. Part A 2014, 31, 1886–1895. [Google Scholar] [CrossRef]
- Mulder, P.P.J.; Lopez, P.; Castellari, M.; Bodi, D.; Ronczka, S.; Preiss-Weigert, A.; These, A. Occurrence of pyrrolizidine alkaloids in animal- and plant-derived food: Results of a survey across Europe. Food Addit. Contam. Part A 2018, 35, 118–133. [Google Scholar] [CrossRef]
- Chen, L.; Mulder, P.P.J.; Peijnenburg, A.; Rietjens, I. Risk assessment of intake of pyrrolizidine alkaloids from herbal teas and medicines following realistic exposure scenarios. Food Chem. Toxicol. 2019, 130, 142–153. [Google Scholar] [CrossRef] [PubMed]
- BfR. Updated risk assessment on levels of 1,2-unsaturated pyrrolizidine alkaloids (PAs) in foods. BfR Opin. 2020, 2020, 1–62. [Google Scholar] [CrossRef]
- Roeder, E. Medicinal plants in Europe containing pyrrolizidine alkaloids. Pharmazie 1995, 50, 83–98. [Google Scholar] [PubMed]
- Roeder, E. Medicinal plants in China containing pyrrolizidine alkaloids. Pharmazie 2000, 55, 711–726. [Google Scholar] [PubMed]
- Steenkamp, V.; Stewart, M.J.; van der Merwe, S.; Zuckerman, M.; Crowther, N.J. The effect of Senecio latifolius a plant used as a South African traditional medicine, on a human hepatoma cell line. J. Ethnopharmacol. 2001, 78, 51–58. [Google Scholar] [CrossRef]
- Allgaier, C.; Franz, S. Risk assessment on the use of herbal medicinal products containing pyrrolizidine alkaloids. Regul. Toxicol. Pharmacol. 2015, 73, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Kirk, H.; Vrieling, K.; Van Der Meijden, E.; Klinkhamer, P.G.L. Species by Environment Interactions Affect Pyrrolizidine Alkaloid Expression in Senecio jacobaea, Senecio aquaticus, and Their Hybrids. J. Chem. Ecol. 2010, 36, 378–387. [Google Scholar] [CrossRef] [PubMed]
- EFSA. CONTAM Panel. Risks for human health related to the presence of pyrrolizidine alkaloids in honey, tea, herbal infusions and food supplements. EFSA J. 2017, 15, 4908. [Google Scholar]
- Wiedenfeld, H.; Edgar, J. Toxicity of pyrrolizidine alkaloids to humans and ruminants. Phytochem. Rev. 2011, 10, 137–151. [Google Scholar] [CrossRef]
- Chen, Z.; Huo, J.R. Hepatic veno-occlusive disease associated with toxicity of pyrrolizidine alkaloids in herbal preparations. Neth. J. Med. 2010, 68, 252–260. [Google Scholar]
- Reimann, A.; Nurhayati, N.; Backenkohler, A.; Ober, D. Repeated evolution of the pyrrolizidine alkaloid-mediated defense system in separate angiosperm lineages. Plant Cell 2004, 16, 2772–2784. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, P.; Dillon, J.-C.; Moren, A.; Talbak, S.; Barakaev, S. Heliotrope poisoning in Tadjikistan. Lancet 1993, 341, 1663. [Google Scholar] [CrossRef]
- Stegelmeier, B.L.; Edgar, J.A.; Colegate, S.M.; Gardner, D.R.; Schoch, T.K.; Coulombe, R.A.; Molyneux, R.J. Pyrrolizidine alkaloid plants, metabolism and toxicity. J. Nat. Toxins 1999, 8, 95–116. [Google Scholar]
- Wiedenfeld, H.; Röder, E.; Bourauel, T.; Edgar, J.A.; Alkaloids, P. Pyrrolizidine Alkaloids-Structure and Toxicity; Bonn University Press: Bonn, Germany, 2008. [Google Scholar]
- Fu, P.P.; Xia, Q.; Lin, G.; Chou, M.W. Pyrrolizidine alkaloids—Genotoxicity, metabolism enzymes, metabolic activation, and mechanisms. Drug Metab. Rev. 2004, 36, 1–55. [Google Scholar] [CrossRef]
- Chen, T.; Mei, N.; Fu, P.P. Genotoxicity of pyrrolizidine alkaloids. J. Appl. Toxicol. 2010, 30, 183–196. [Google Scholar] [CrossRef]
- Ruan, J.; Yang, M.; Fu, P.; Ye, Y.; Lin, G. Metabolic activation of pyrrolizidine alkaloids: Insights into the structural and enzymatic basis. Chem. Res. Toxicol. 2014, 27, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Liao, C.; Ye, Y.; Lin, G. Lack of metabolic activation and predominant formation of an excreted metabolite of nontoxic platynecine-type pyrrolizidine alkaloids. Chem. Res. Toxicol. 2014, 27, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Mattocks, A.R.; White, I.N. Pyrrolic metabolites from non-toxic pyrrolizidine alkaloids. Nat. New Biol. 1971, 231, 114–115. [Google Scholar] [CrossRef]
- Gluck, J.; Waizenegger, J.; Braeuning, A.; Hessel-Pras, S. Pyrrolizidine Alkaloids Induce Cell Death in Human HepaRG Cells in a Structure-Dependent Manner. Int. J. Mol. Sci. 2020, 22, 202. [Google Scholar] [CrossRef] [PubMed]
- Waizenegger, J.; Braeuning, A.; Templin, M.; Lampen, A.; Hessel-Pras, S. Structure-dependent induction of apoptosis by hepatotoxic pyrrolizidine alkaloids in the human hepatoma cell line HepaRG: Single versus repeated exposure. Food Chem. Toxicol. 2018, 114, 215–226. [Google Scholar] [CrossRef]
- Ebmeyer, J.; Rasinger, J.D.; Hengstler, J.G.; Schaudien, D.; Creutzenberg, O.; Lampen, A.; Braeuning, A.; Hessel-Pras, S. Hepatotoxic pyrrolizidine alkaloids induce DNA damage response in rat liver in a 28-day feeding study. Arch. Toxicol. 2020, 94, 1739–1751. [Google Scholar] [CrossRef]
- Ebmeyer, J.; Franz, L.; Lim, R.; Niemann, B.; Glatt, H.; Braeuning, A.; Lampen, A.; Hessel-Pras, S. Sensitization of Human Liver Cells Toward Fas-Mediated Apoptosis by the Metabolically Activated Pyrrolizidine Alkaloid Lasiocarpine. Mol. Nutr. Food Res. 2019, 63, e1801206. [Google Scholar] [CrossRef] [PubMed]
- Ebmeyer, J.; Behrend, J.; Lorenz, M.; Gunther, G.; Reif, R.; Hengstler, J.G.; Braeuning, A.; Lampen, A.; Hessel-Pras, S. Pyrrolizidine alkaloid-induced alterations of prostanoid synthesis in human endothelial cells. Chem. Biol. Interact. 2019, 298, 104–111. [Google Scholar] [CrossRef]
- Luckert, C.; Hessel, S.; Lenze, D.; Lampen, A. Disturbance of gene expression in primary human hepatocytes by hepatotoxic pyrrolizidine alkaloids: A whole genome transcriptome analysis. Toxicol. In Vitro 2015, 29, 1669–1682. [Google Scholar] [CrossRef]
- Yang, X.Q.; Ye, J.; Li, X.; Li, Q.; Song, Y.H. Pyrrolizidine alkaloids-induced hepatic sinusoidal obstruction syndrome: Pathogenesis, clinical manifestations, diagnosis, treatment, and outcomes. World J. Gastroenterol. 2019, 25, 3753–3763. [Google Scholar] [CrossRef]
- Marksteiner, J.; Blasko, I.; Kemmler, G.; Koal, T.; Humpel, C. Bile acid quantification of 20 plasma metabolites identifies lithocholic acid as a putative biomarker in Alzheimer’s disease. Metabolomics 2018, 14, 1–10. [Google Scholar] [CrossRef]
- Dawson, P.A. Chapter 12—Bile Acid Metabolism. In Biochemistry of Lipids, Lipoproteins and Membranes, 6th ed.; Ridgway, N.D., McLeod, R.S., Eds.; Elsevier: Boston, MA, USA, 2016; pp. 359–389. [Google Scholar] [CrossRef]
- Dawson, P.A. Chapter 53—Bile Formation and the Enterohepatic Circulation. In Physiology of the Gastrointestinal Tract, 5th ed.; Johnson, L.R., Ghishan, F.K., Kaunitz, J.D., Merchant, J.L., Said, H.M., Wood, J.D., Eds.; Academic Press: Boston, MA, USA, 2012; pp. 1461–1484. [Google Scholar] [CrossRef]
- García-Cañaveras, J.C.; Donato, M.T.; Castell, J.V.; Lahoz, A. Targeted profiling of circulating and hepatic bile acids in human, mouse, and rat using a UPLC-MRM-MS-validated method. J. Lipid Res. 2012, 53, 2231–2241. [Google Scholar] [CrossRef]
- Hofmann, A.F.; Hagey, L.R. Bile Acids: Chemistry, Pathochemistry, Biology, Pathobiology, and Therapeutics. Cell. Mol. Life Sci. 2008, 65, 2461–2483. [Google Scholar] [CrossRef]
- Trauner, M.; Meier, P.J.; Boyer, J.L. Molecular Pathogenesis of Cholestasis. N. Engl. J. Med. 1998, 339, 1217–1227. [Google Scholar] [CrossRef]
- Anthérieu, S.; Azzi, P.B.-E.; Dumont, J.; Abdel-Razzak, Z.; Guguen-Guillouzo, C.; Fromenty, B.; Robin, M.-A.; Guillouzo, A. Oxidative stress plays a major role in chlorpromazine-induced cholestasis in human HepaRG cells. Hepatology 2013, 57, 1518–1529. [Google Scholar] [CrossRef]
- Sharanek, A.; Burban, A.; Humbert, L.; Bachour-El Azzi, P.; Felix-Gomes, N.; Rainteau, D.; Guillouzo, A. Cellular Accumulation and Toxic Effects of Bile Acids in Cyclosporine A-Treated HepaRG Hepatocytes. Toxicol. Sci. 2015, 147, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Waizenegger, J.; Gluck, J.; Henricsson, M.; Luckert, C.; Braeuning, A.; Hessel-Pras, S. Pyrrolizidine Alkaloids Disturb Bile Acid Homeostasis in the Human Hepatoma Cell Line HepaRG. Foods 2021, 10, 161. [Google Scholar] [CrossRef]
- Hessel-Pras, S.; Braeuning, A.; Guenther, G.; Adawy, A.; Enge, A.M.; Ebmeyer, J.; Henderson, C.J.; Hengstler, J.G.; Lampen, A.; Reif, R. The pyrrolizidine alkaloid senecionine induces CYP-dependent destruction of sinusoidal endothelial cells and cholestasis in mice. Arch. Toxicol. 2020, 94, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Kanebratt, K.P.; Andersson, T.B. Evaluation of HepaRG Cells as an in Vitro Model for Human Drug Metabolism Studies. Drug Metab. Dispos. 2008, 36, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tremaroli, V.; Karlsson, F.; Werling, M.; Ståhlman, M.; Kovatcheva-Datchary, P.; Olbers, T.; Fändriks, L.; le Roux, C.W.; Nielsen, J.; Bäckhed, F. Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell. Metab. 2015, 22, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Sharanek, A.; Azzi, P.B.; Al-Attrache, H.; Savary, C.C.; Humbert, L.; Rainteau, D.; Guguen-Guillouzo, C.; Guillouzo, A. Different dose-dependent mechanisms are involved in early cyclosporine a-induced cholestatic effects in hepaRG cells. Toxicol. Sci. 2014, 141, 244–253. [Google Scholar] [CrossRef]
- Louisse, J.; Rijkers, D.; Stoopen, G.; Holleboom, W.J.; Delagrange, M.; Molthof, E.; Mulder, P.P.J.; Hoogenboom, R.; Audebert, M.; Peijnenburg, A. Determination of genotoxic potencies of pyrrolizidine alkaloids in HepaRG cells using the γH2AX assay. Food Chem. Toxicol. 2019, 131, 110532. [Google Scholar] [CrossRef] [PubMed]
- Merz, K.H.; Schrenk, D. Interim relative potency factors for the toxicological risk assessment of pyrrolizidine alkaloids in food and herbal medicines. Toxicol. Lett. 2016, 263, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Allemang, A.; Mahony, C.; Lester, C.; Pfuhler, S. Relative potency of fifteen pyrrolizidine alkaloids to induce DNA damage as measured by micronucleus induction in HepaRG human liver cells. Food Chem. Toxicol. 2018, 121, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Rutz, L.; Schrenk, D. Structure-dependent hepato-cytotoxic potencies of selected pyrrolizidine alkaloids in primary rat hepatocyte culture. Food Chem. Toxicol. 2020, 135, 110923. [Google Scholar] [CrossRef]
- Rutz, L.; Gao, L.; Kupper, J.H.; Schrenk, D. Structure-dependent genotoxic potencies of selected pyrrolizidine alkaloids in metabolically competent HepG2 cells. Arch. Toxicol. 2020. [Google Scholar] [CrossRef]
- Lester, C.; Troutman, J.; Obringer, C.; Wehmeyer, K.; Stoffolano, P.; Karb, M.; Xu, Y.; Roe, A.; Carr, G.; Blackburn, K.; et al. Intrinsic relative potency of a series of pyrrolizidine alkaloids characterized by rate and extent of metabolism. Food Chem. Toxicol. 2019, 131, 110523. [Google Scholar] [CrossRef]
- Yan, C.C.; Huxtable, R.J. Effect of the pyrrolizidine alkaloid, monocrotaline, on bile composition of the isolated, perfused rat liver. Life Sci. 1995, 57, 617–626. [Google Scholar] [CrossRef]
- Yan, C.C.; Huxtable, R.J. The effect of the hepatotoxic pyrrolizidine alkaloid, retrorsine, on bile composition in the rat in vivo. Proc. West Pharmacol. Soc. 1996, 39, 19–22. [Google Scholar] [PubMed]
- Yan, C.C.; Huxtable, R.J. Effects of taurine and guanidinoethane sulfonate on toxicity of the pyrrolizidine alkaloid monocrotaline. Biochem. Pharmacol. 1996, 51, 321–329. [Google Scholar] [CrossRef]
- Yan, C.C.; Cooper, R.A.; Huxtable, R.J. The comparative metabolism of the four pyrrolizidine alkaloids, seneciphylline, retrorsine, monocrotaline, and trichodesmine in the isolated, perfused rat liver. Toxicol. Appl. Pharmacol. 1995, 133, 277–284. [Google Scholar] [CrossRef]
- Xiong, A.; Fang, L.; Yang, X.; Yang, F.; Qi, M.; Kang, H.; Yang, L.; Tsim, K.W.; Wang, Z. An application of target profiling analyses in the hepatotoxicity assessment of herbal medicines: Comparative characteristic fingerprint and bile acid profiling of Senecio vulgaris L. and Senecio scandens Buch.-Ham. Anal. Bioanal. Chem. 2014, 406, 7715–7727. [Google Scholar] [CrossRef] [PubMed]
- Xiong, A.; Yang, F.; Fang, L.; Yang, L.; He, Y.; Wan, Y.J.; Xu, Y.; Qi, M.; Wang, X.; Yu, K.; et al. Metabolomic and genomic evidence for compromised bile acid homeostasis by senecionine, a hepatotoxic pyrrolizidine alkaloid. Chem. Res. Toxicol. 2014, 27, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Hagenbuch, B.; Meier, P.J. Molecular cloning, chromosomal localization, and functional characterization of a human liver Na+/bile acid cotransporter. J. Clin. Invest. 1994, 93, 1326–1331. [Google Scholar] [CrossRef]
- Hagenbuch, B.; Stieger, B.; Foguet, M.; Lübbert, H.; Meier, P.J. Functional expression cloning and characterization of the hepatocyte Na+/bile acid cotransport system. Proc. Natl. Acad. Sci. USA 1991, 88, 10629–10633. [Google Scholar] [CrossRef]
- Hsiang, B.; Zhu, Y.; Wang, Z.; Wu, Y.; Sasseville, V.; Yang, W.P.; Kirchgessner, T.G. A novel human hepatic organic anion transporting polypeptide (OATP2). Identification of a liver-specific human organic anion transporting polypeptide and identification of rat and human hydroxymethylglutaryl-CoA reductase inhibitor transporters. J. Biol. Chem. 1999, 274, 37161–37168. [Google Scholar] [CrossRef]
- Meng, L.J.; Wang, P.; Wolkoff, A.W.; Kim, R.B.; Tirona, R.G.; Hofmann, A.F.; Pang, K.S. Transport of the sulfated, amidated bile acid, sulfolithocholyltaurine, into rat hepatocytes is mediated by Oatp1 and Oatp2. Hepatology 2002, 35, 1031–1040. [Google Scholar] [CrossRef]
- Hayashi, H.; Takada, T.; Suzuki, H.; Onuki, R.; Hofmann, A.F.; Sugiyama, Y. Transport by vesicles of glycine- and taurine-conjugated bile salts and taurolithocholate 3-sulfate: A comparison of human BSEP with rat Bsep. Biochim. Biophys. Acta 2005, 1738, 54–62. [Google Scholar] [CrossRef]
- Hirohashi, T.; Suzuki, H.; Takikawa, H.; Sugiyama, Y. ATP-dependent transport of bile salts by rat multidrug resistance-associated protein 3 (Mrp3). J. Biol. Chem. 2000, 275, 2905–2910. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glück, J.; Henricsson, M.; Braeuning, A.; Hessel-Pras, S. The Food Contaminants Pyrrolizidine Alkaloids Disturb Bile Acid Homeostasis Structure-Dependently in the Human Hepatoma Cell Line HepaRG. Foods 2021, 10, 1114. https://doi.org/10.3390/foods10051114
Glück J, Henricsson M, Braeuning A, Hessel-Pras S. The Food Contaminants Pyrrolizidine Alkaloids Disturb Bile Acid Homeostasis Structure-Dependently in the Human Hepatoma Cell Line HepaRG. Foods. 2021; 10(5):1114. https://doi.org/10.3390/foods10051114
Chicago/Turabian StyleGlück, Josephin, Marcus Henricsson, Albert Braeuning, and Stefanie Hessel-Pras. 2021. "The Food Contaminants Pyrrolizidine Alkaloids Disturb Bile Acid Homeostasis Structure-Dependently in the Human Hepatoma Cell Line HepaRG" Foods 10, no. 5: 1114. https://doi.org/10.3390/foods10051114
APA StyleGlück, J., Henricsson, M., Braeuning, A., & Hessel-Pras, S. (2021). The Food Contaminants Pyrrolizidine Alkaloids Disturb Bile Acid Homeostasis Structure-Dependently in the Human Hepatoma Cell Line HepaRG. Foods, 10(5), 1114. https://doi.org/10.3390/foods10051114





