Subcutaneous Panniculitis-like T-Cell Lymphoma: Diagnostic Challenge and Successful Multimodal Management with Integra® Dermal Matrix—Case Report and Review of the Literature
Abstract
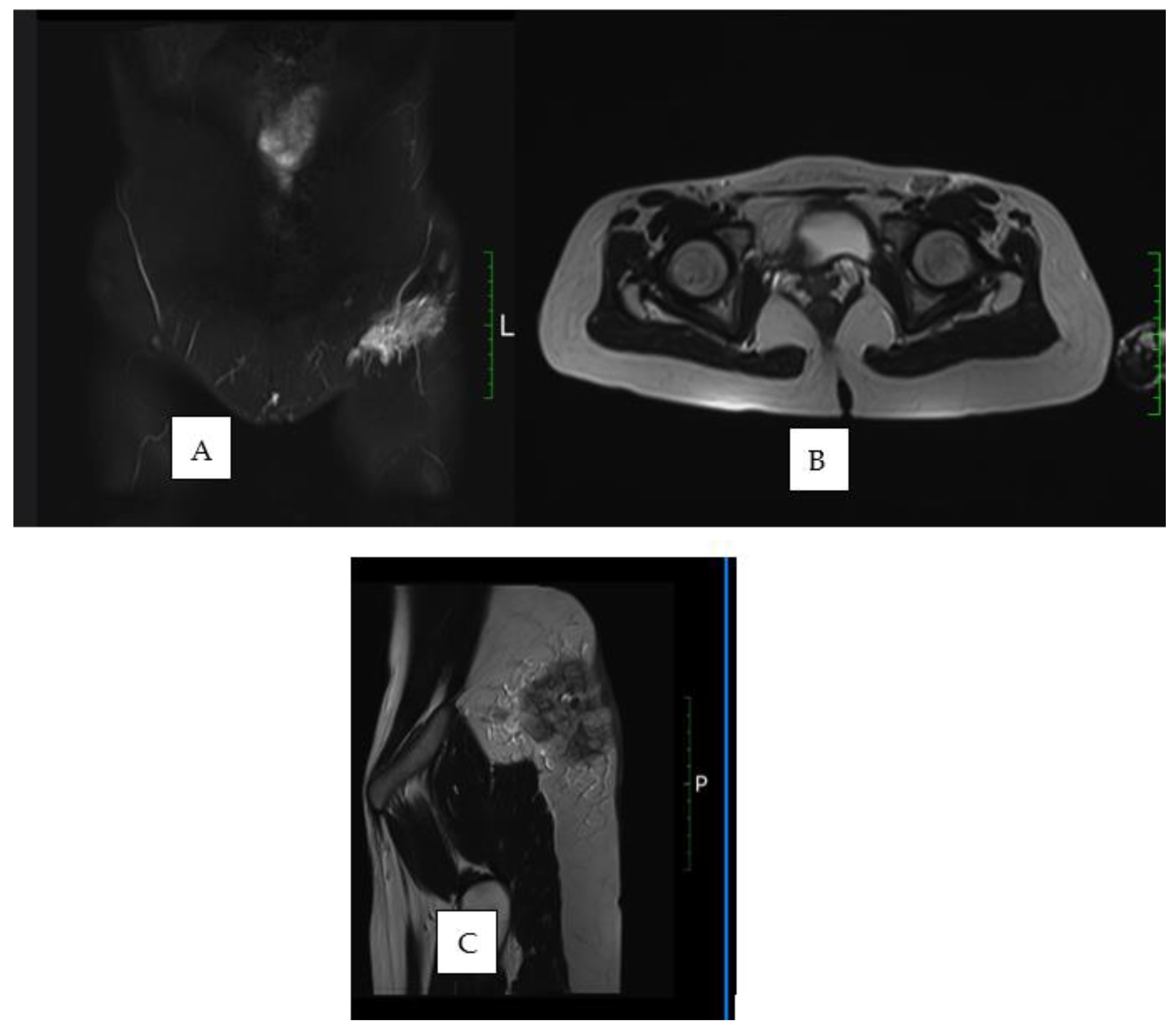
1. Introduction
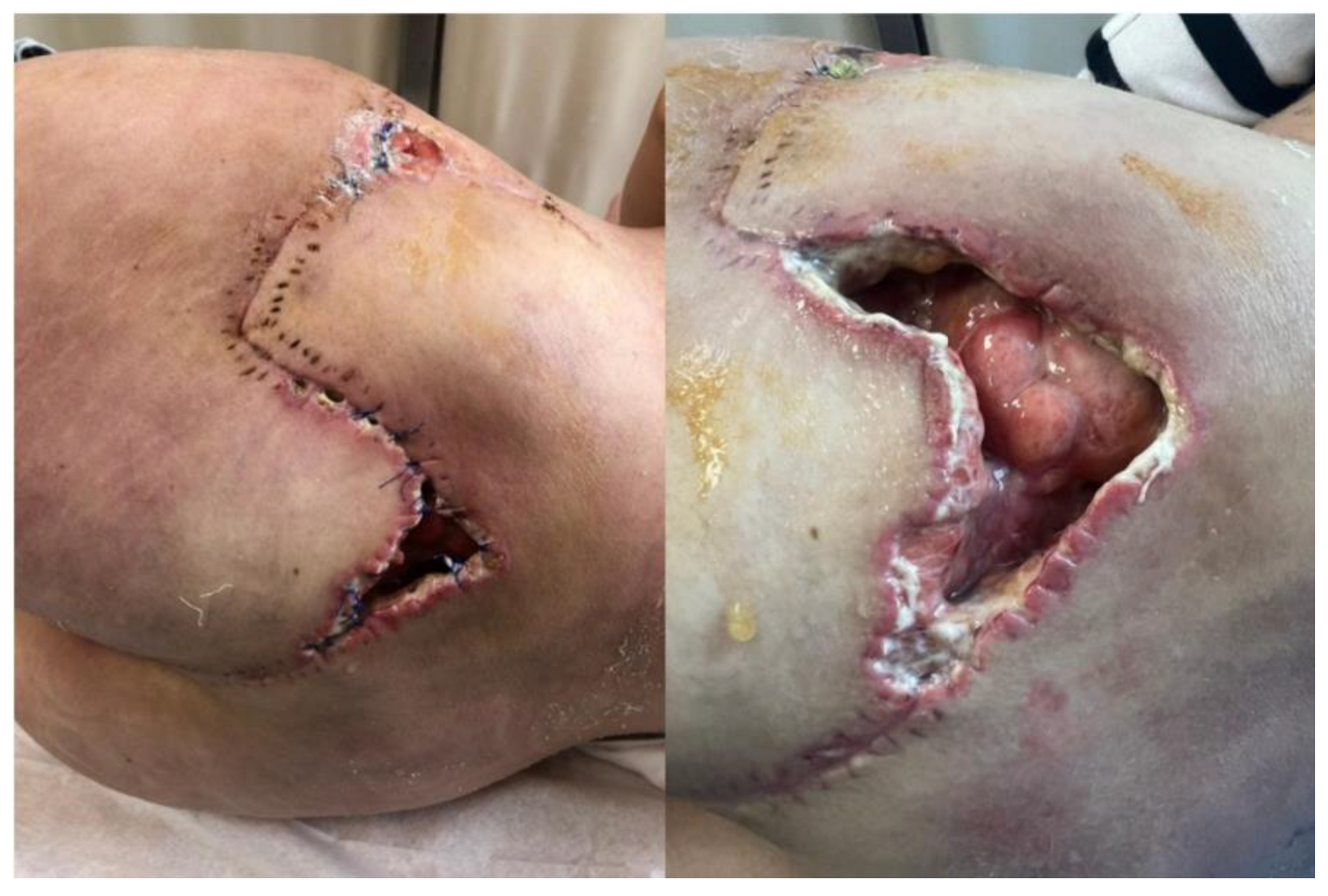
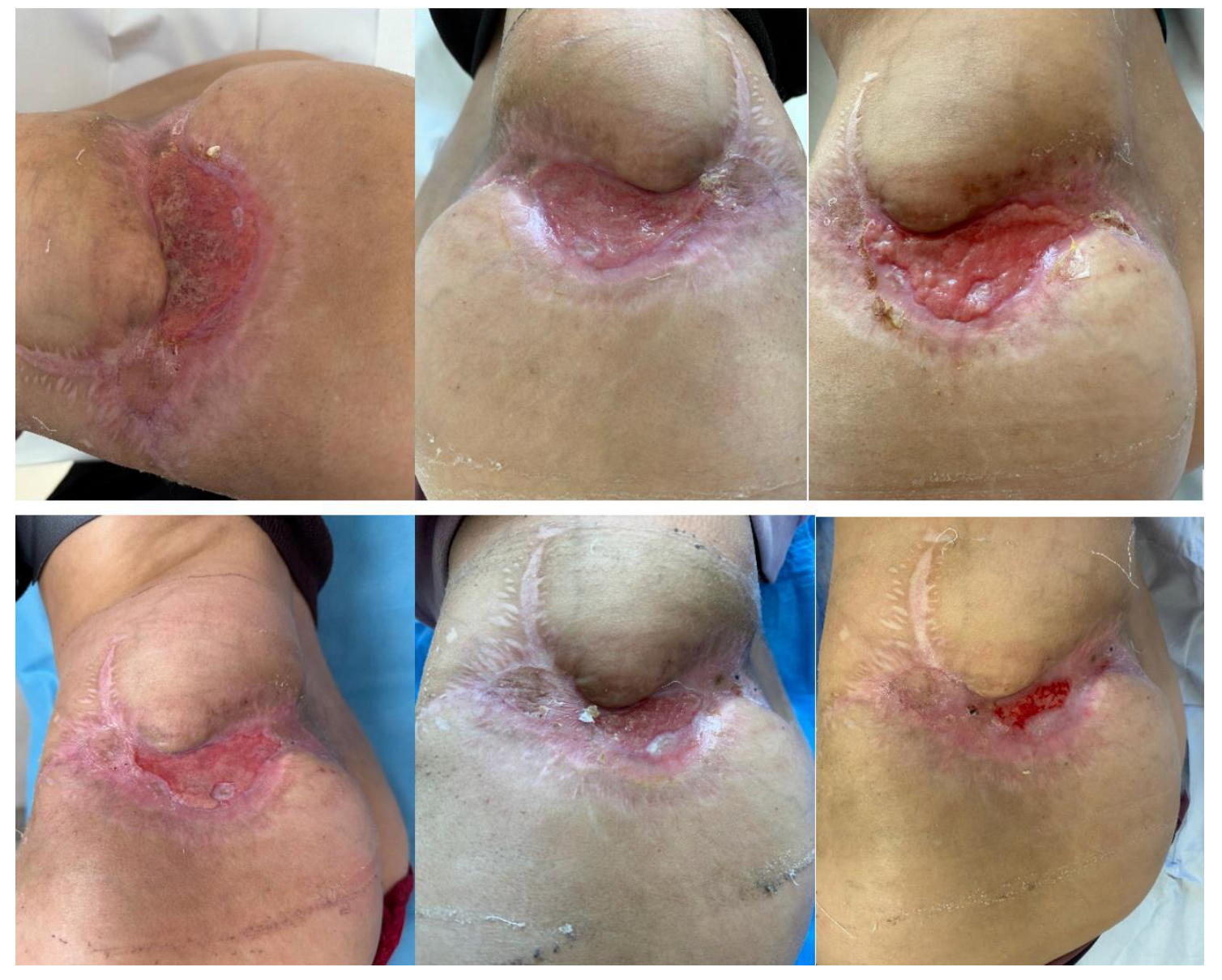
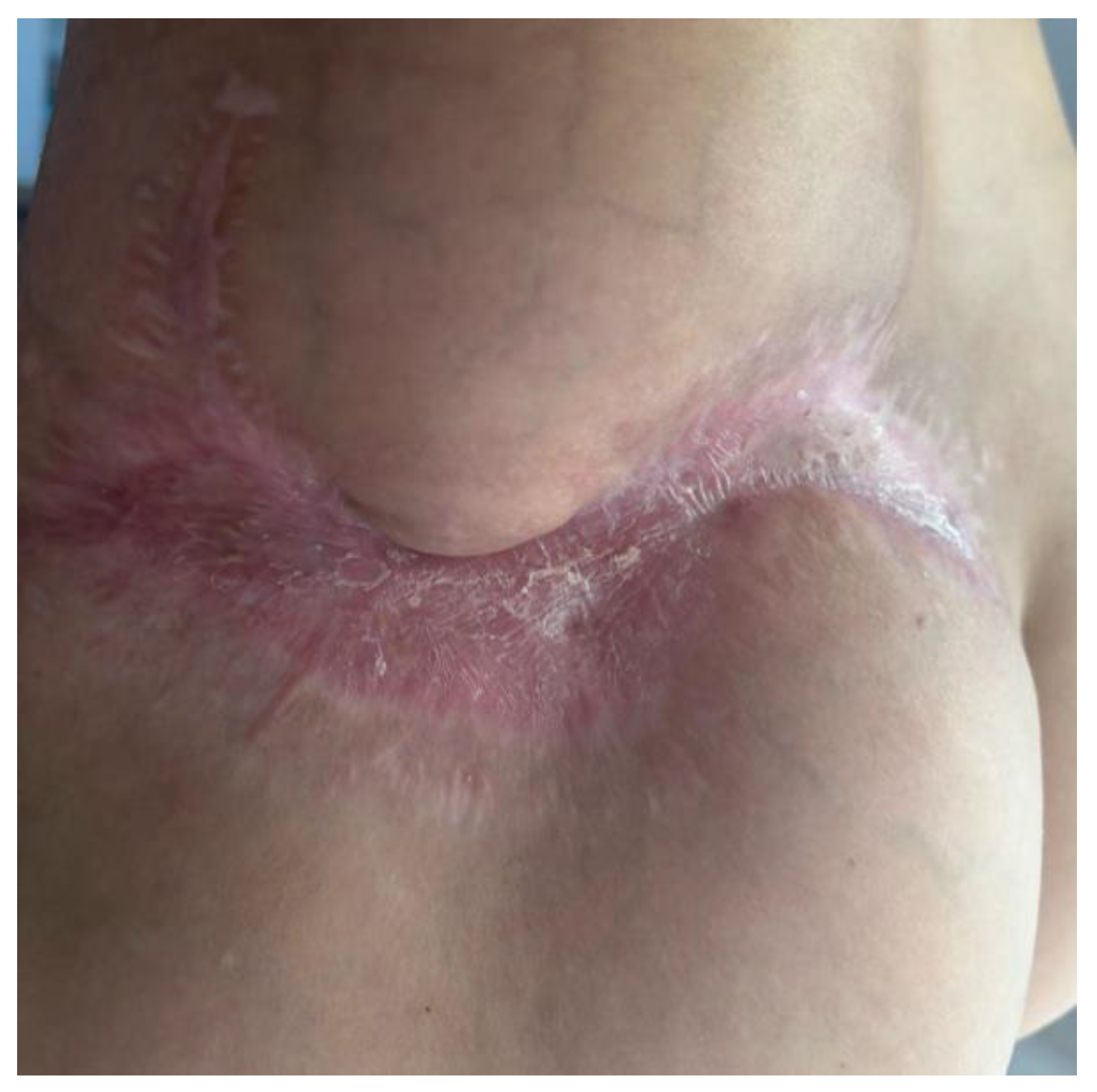
2. Materials and Methods
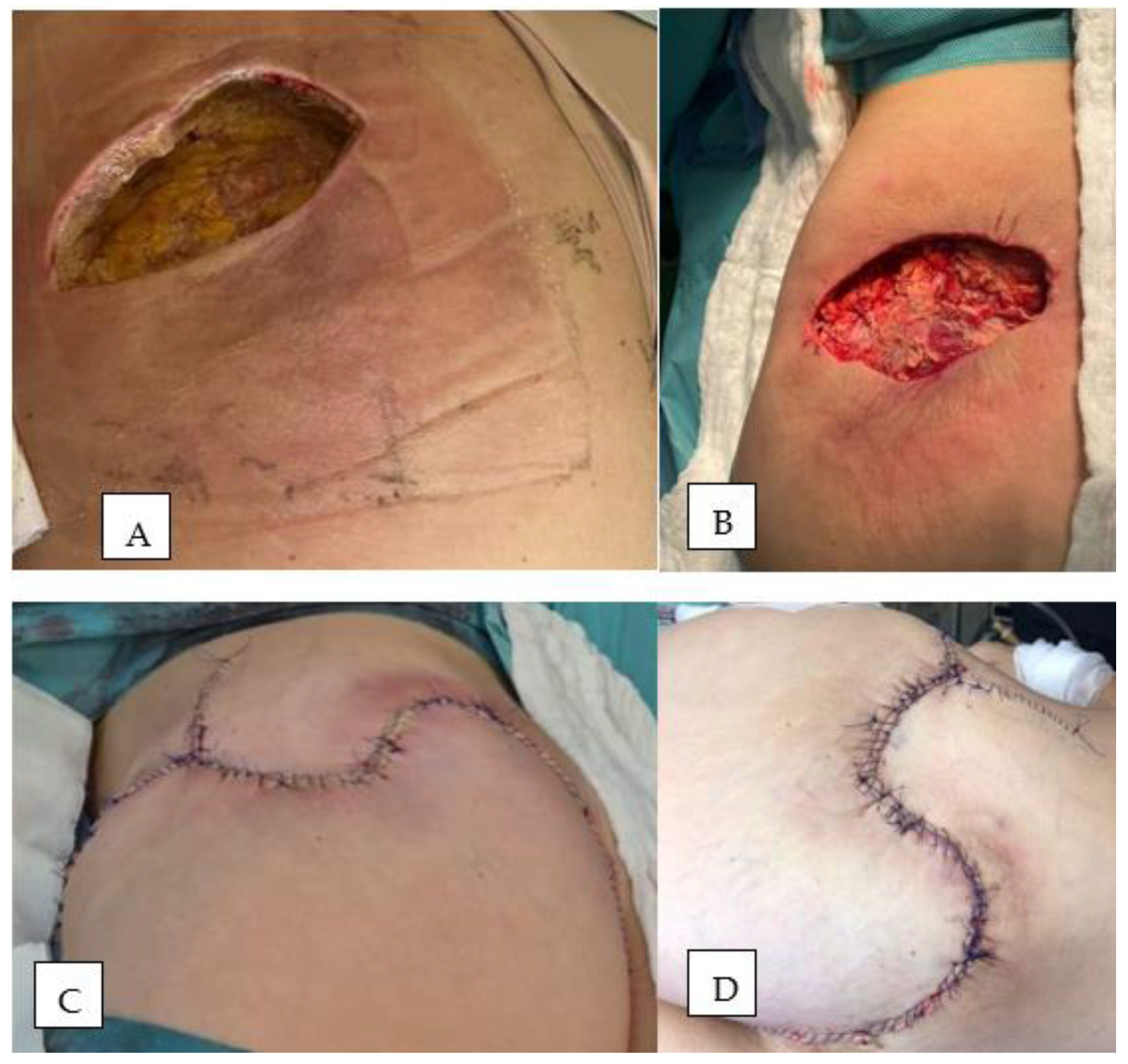
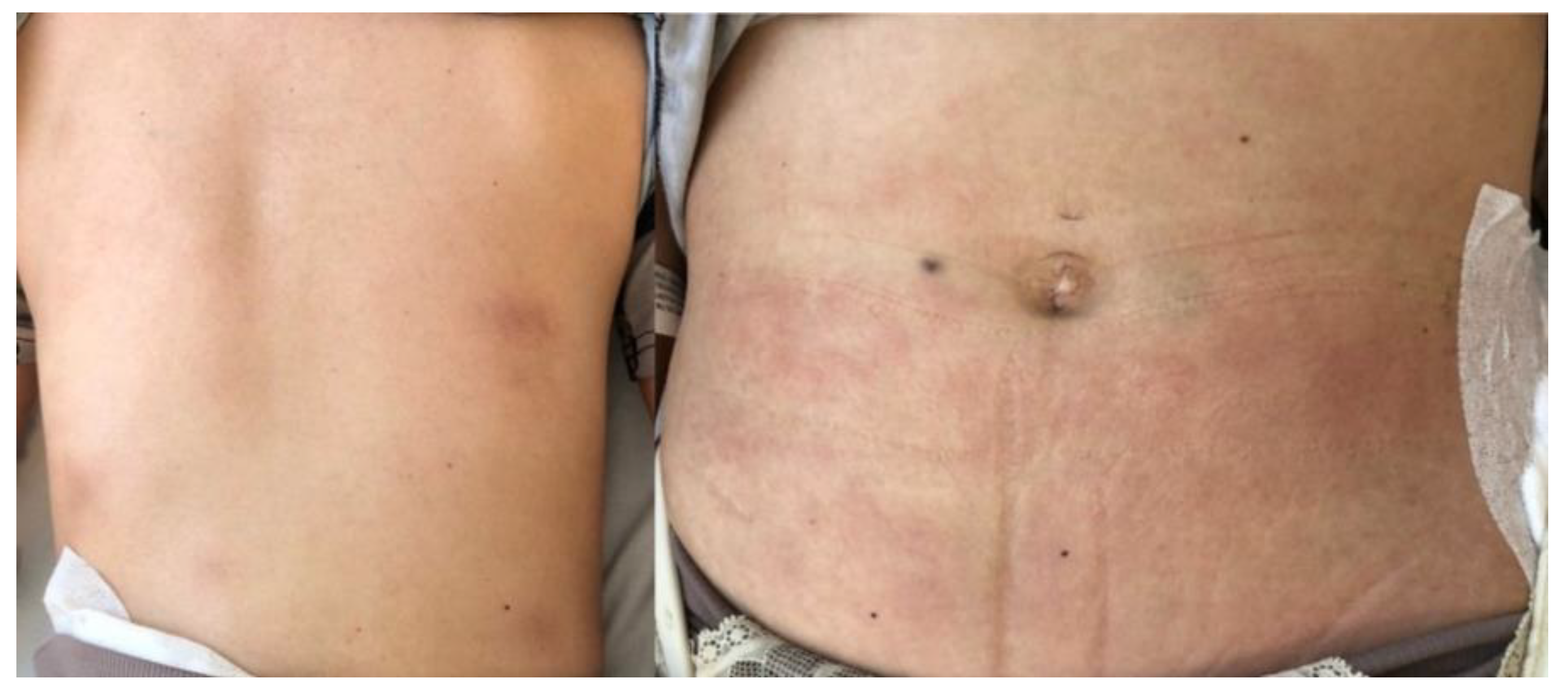
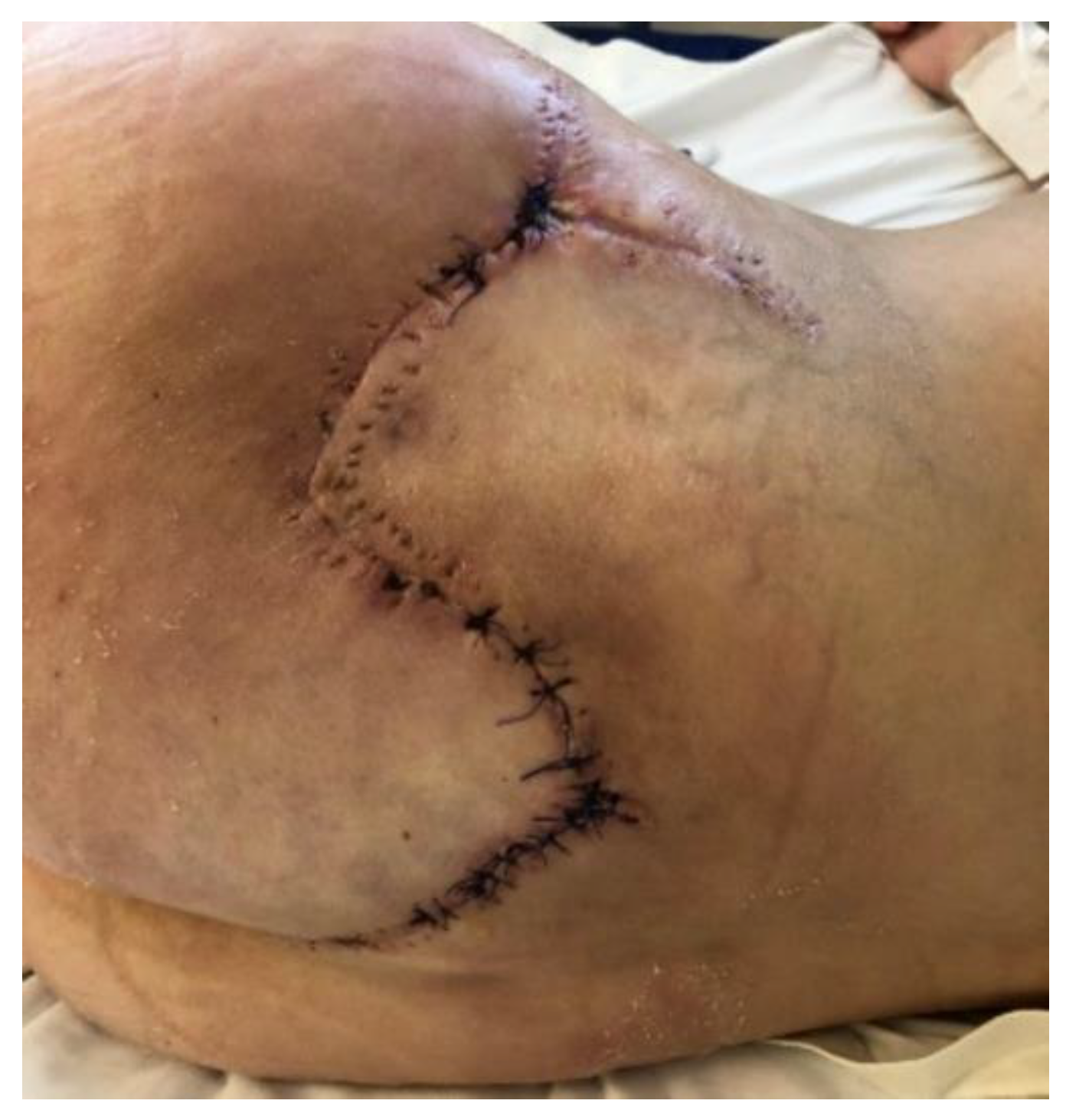
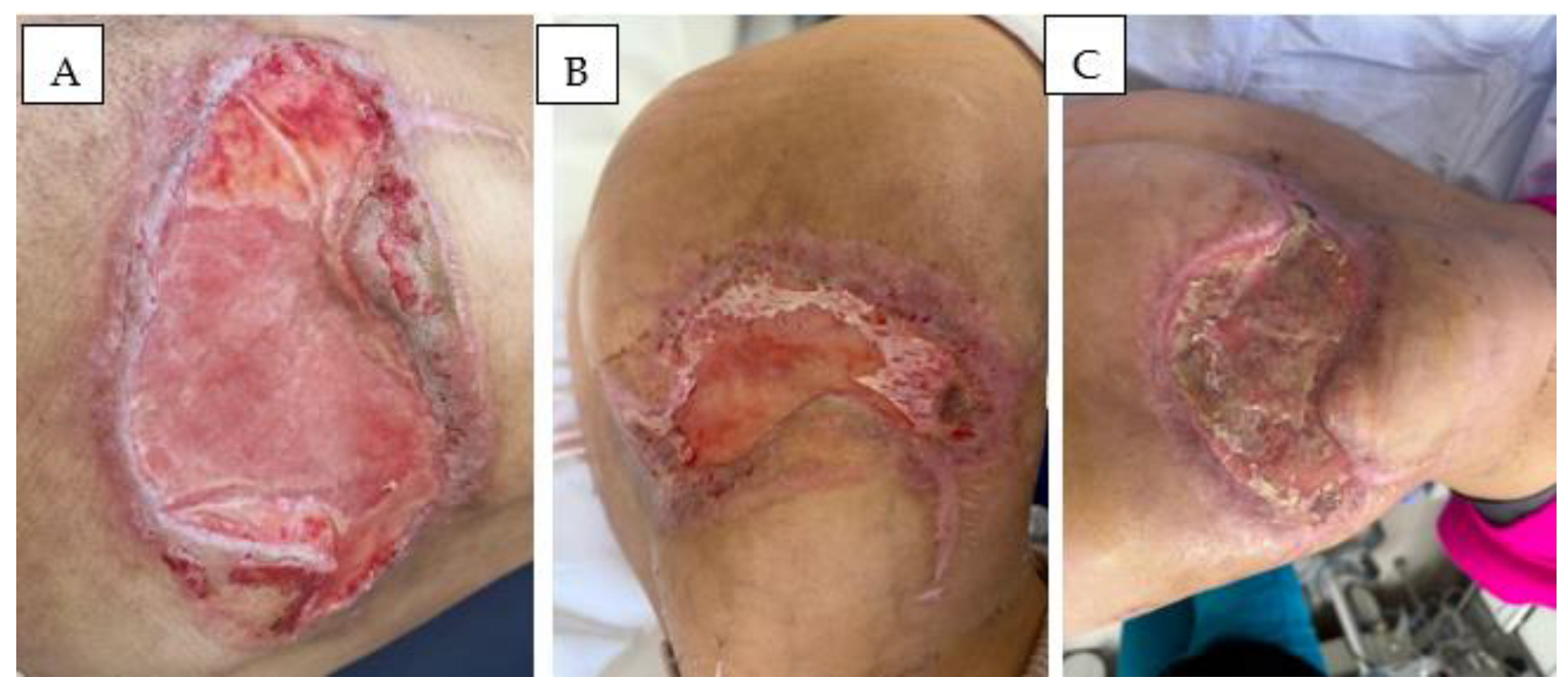
Therapeutic Management: Systemic and Surgical Wound Treatment
- Cyclophosphamide—1100 mg (Day 1);
- Doxorubicin—75 mg (Day 1);
- Vincristine—2 mg (Day 1);
- Etoposide—150 mg (Days 1, 3, 5);
- Dexamethasone—16 mg (Days 1–5).
3. Discussion and Literature Review
3.1. Epidemiology
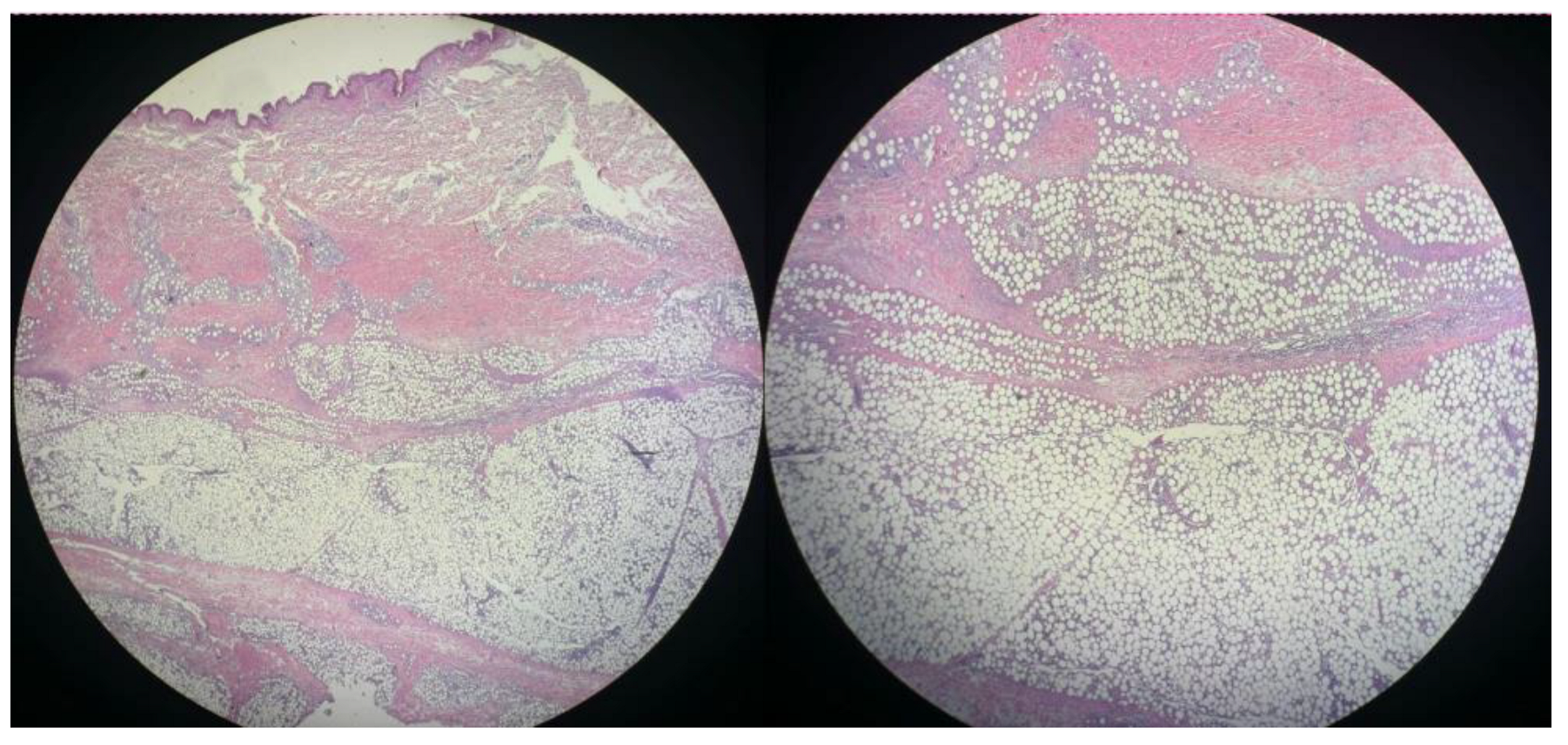
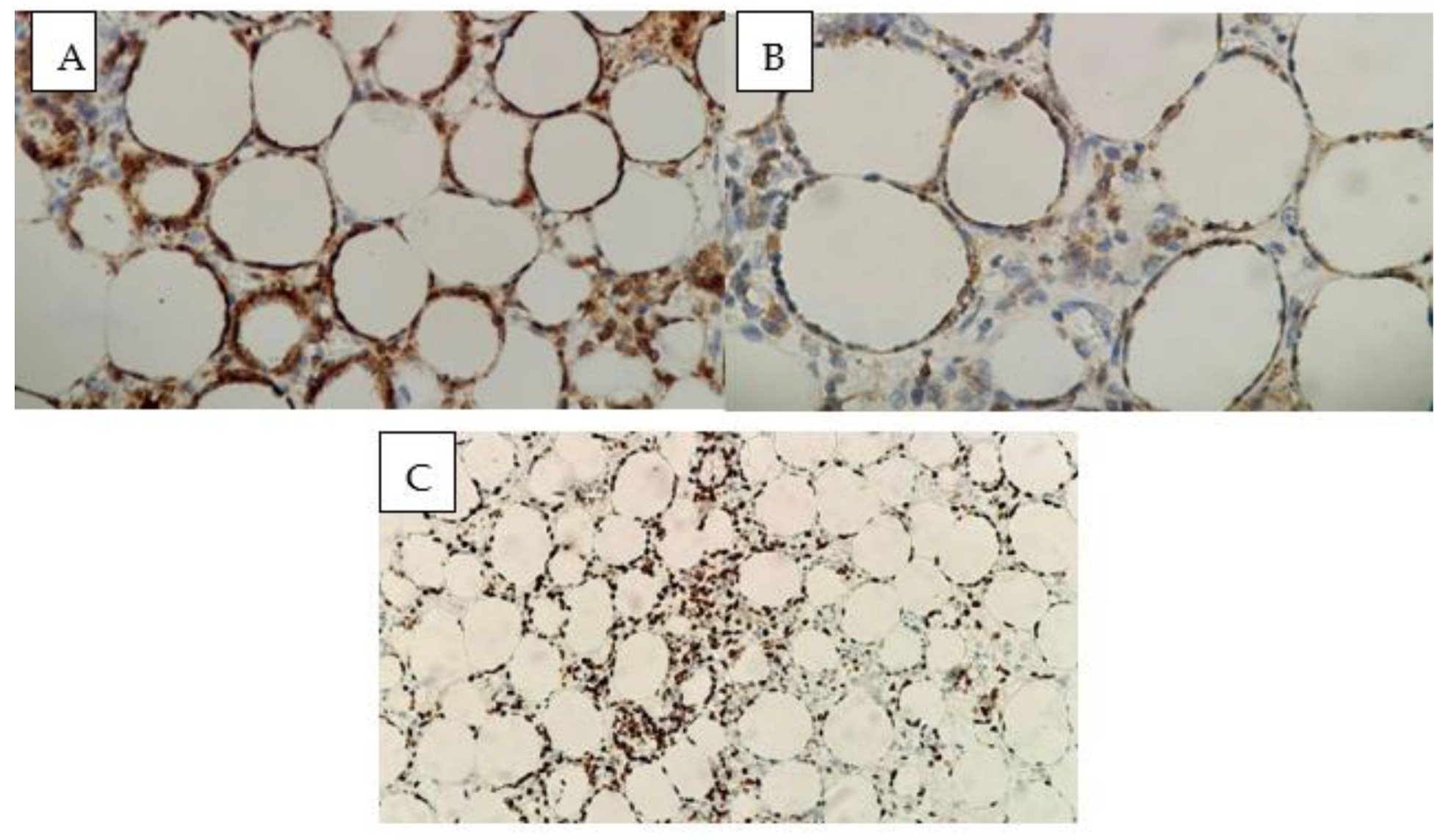
3.2. Histopathology
3.3. Clinical Manifestation and Evaluation
3.4. Treatment Options
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willemze, R.; Jansen, P.M.; Cerroni, L.; Berti, E.; Santucci, M.; Assaf, C.; Dijk, M.R.C.-V.; Carlotti, A.; Geerts, M.-L.; Hahtola, S.; et al. Subcutaneous panniculitis-like T-cell lymphoma: Definition, classification, and prognostic factors: An EORTC Cutaneous Lymphoma Group Study of 83 cases. Blood 2008, 111, 838–845. [Google Scholar] [CrossRef]
- Willemze, R.; Jaffe, E.S.; Burg, G.; Cerroni, L.; Berti, E.; Swerdlow, S.H.; Ralfkiaer, E.; Chimenti, S.; Diaz-Perez, J.L.; Duncan, L.M.; et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005, 105, 3768–3778. [Google Scholar] [CrossRef] [PubMed]
- Alsomali, D.Y.; Bakshi, N.; Kharfan-Dabaja, M.A.; El Fakih, R.; Aljurf, M. Diagnosis and treatment of subcutaneous panniculitis-like T-cell lymphoma: A systematic literature review. Hematol. Oncol. Stem Cell Ther. 2023, 16, 110–116. [Google Scholar] [CrossRef]
- Zhang, P.-S.; Wang, R.; Wu, H.-W.; Zhou, H.; Deng, H.-B.; Fan, W.-X.; Li, J.-C.; Cheng, S.-W. Non-Hodgkin’s lymphoma involving chronic difficult-to-heal wounds: A case report. World J. Clin. Oncol. 2024, 15, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Salhany, K.E.; Macon, W.R.; Choi, J.K.; Elenitsas, R.; Lessin, S.R.; Felgar, R.E.; Wilson, D.M.; Przybylski, G.K.; Lister, J.; Wasik, M.A.; et al. Subcutaneous panniculitis-like T-cell lymphoma: Clinicopathologic, immunophenotypic, and genotypic analysis of alpha/beta and gamma/delta subtypes. Am. J. Surg. Pathol. 1998, 22, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Sugaya, M. Clinical guidelines and new molecular targets for cutaneous lymphomas. Int. J. Mol. Sci. 2021, 22, 11079. [Google Scholar] [CrossRef]
- Liau, J.Y.; Chuang, S.S.; Chu, C.Y.; Ku, W.H.; Tsai, J.H.; Shih, T.F. The presence of clusters of plasmacytoid dendritic cells is a helpful feature for differentiating lupus panniculitis from subcutaneous panniculitis-like T-cell lymphoma. Histopathology 2013, 62, 1057–1066. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Takahashi, Y.; Takata, K.; Kato, S.; Sato, Y.; Asano, N.; Ogino, T.; Hashimoto, K.; Tashiro, Y.; Takeuchi, S.; Masunari, T.; et al. Clinicopathological analysis of 17 primary cutaneous T-cell lymphoma of the gamma/delta phenotype from Japan. Cancer Sci. 2014, 105, 912–923. [Google Scholar] [CrossRef]
- Massone, C.; Kerl, H.; Citarella, L.; Rütten, A.; Cerroni, L. The protean spectrum of non-Hodgkin lymphomas with prominent involvement of subcutaneous fat. J. Cutan. Pathol. 2006, 33, 418–425. [Google Scholar] [CrossRef]
- Toro, J.R.; Liewehr, D.J.; Pabby, N.; Sorbara, L.; Raffeld, M.; Steinberg, S.M.; Jaffe, E.S. Gamma-delta T-cell phenotype is associated with significantly decreased survival in cutaneous T-cell lymphoma. Blood 2003, 101, 3407–3412. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Xu, Y.; Li, X.; Peng, Q.; Cai, H.; Wang, J. Progressive and painful wound as a feature of subcutaneous panniculitis-like T-cell lymphoma (SPTCL): Report of a case and review of literature. Int. J. Clin. Exp. Pathol. 2015, 8, 735–742. [Google Scholar] [PubMed]
- Go, R.S.; Wester, S.M. Immunophenotypic and molecular features, clinical outcomes, treatments, and prognostic factors associated with subcutaneous panniculitis-like T-cell lymphoma: A systematic analysis of 156 patients reported in the literature. Cancer 2004, 101, 1404–1413. [Google Scholar] [CrossRef]
- Shani-Adir, A.; Zvulunov, A.; Yaniv, I.; Tamary, H.; Ben-Ami, T. Subcutaneous panniculitic T-cell lymphoma in children: Response to combination therapy with cyclosporine and chemotherapy. J. Am. Acad. Dermatol. 2004, 50 (Suppl. 2), S18–S22. [Google Scholar] [CrossRef]
- Rojnuckarin, P.; Nakorn, T.N.; Assanasen, T.; Intragumtornchai, T. Cyclosporin in subcutaneous panniculitis-like T-cell lymphoma. Leuk. Lymphoma 2007, 48, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, S.; Kuroda, J.; Shimura, Y.; Kobayashi, T.; Tsutsumi, Y.; Yamashita, M.; Yamamoto, M.; Ohshiro, M.; Sasaki, N.; Kiyota, M.; et al. Cyclosporine A for chemotherapy-resistant subcutaneous panniculitis-like T cell lymphoma with hemophagocytic syndrome. Acta Haematol. 2011, 126, 8–12. [Google Scholar] [CrossRef]
- Gibbs, J.D.; Ma, S.; Kim, A.; Seminario-Vidal, L.; Sokol, L.; Zhang, H.; Zhang, X.; Sagatys, E.; Chen, P.; Messina, J.L. Utility of flow cytometry and gene rearrangement analysis in tissue and blood of patients with suspected cutaneous t-cell lymphoma. Oncol. Rep. 2020, 45, 349–358. [Google Scholar] [CrossRef]
- Shinohara, M.; Rieger, K.; Sundram, U.; Fung, M.; Hristov, A. Assessing t-cell receptor clonality by next-generation sequencing in atypical cutaneous lymphoid infiltrates and cutaneous t-cell lymphoma: A scoping review. J. Cutan. Pathol. 2024, 51, 813–819. [Google Scholar] [CrossRef]
- Semaan, S.; Abel, M.; Raffi, J.; Murase, J. A clinician’s guide to cutaneous t-cell lymphoma presenting as recalcitrant eczematous dermatitis in adults. Int. J. Womens Dermatol. 2021, 7, 422–427. [Google Scholar] [CrossRef]
- Ivani, I.; Murasmita, A.; Mawardi, P.; Oktavriana, T. Cutaneus t-cell lymphoma in 53 years old woman: Histopathological features. J. La Medihealtico 2024, 5, 749–756. [Google Scholar] [CrossRef]
- Li, T.; Wang, G.; Zhang, C.; Wang, W.; Li, C.; Wang, Y. Case report: Cutaneous t-cell lymphoma associated with biologic therapy: Three cases and a literature review. Front. Med. 2025, 12, 1544912. [Google Scholar] [CrossRef]
- Coleman, E.; Bhawan, J. Baby wet wipes: An unusual culprit of lymphomatoid contact dermatitis mimicking mycosis fungoides. Am. J. Dermatopathol. 2022, 44, 205–206. [Google Scholar] [CrossRef] [PubMed]
- Babakoohi, S.; McCall, C. Dermatophytosis incognito mimicking cutaneous t-cell lymphoma. Cureus 2022, 14, e25809. [Google Scholar] [CrossRef] [PubMed]
- Klai, S.; Helal, I.; Jouini, R.; Hammami, H.; Kharrat, M.; Fenniche, S.; Khanchel, F.; Chadli-Debbiche, A. Diagnostic utility of molecular analysis in cutaneous t-cell lymphoma: Tunisian series on clonality of tcrg gene rearrangement. South Asian J. Cancer 2025. [Google Scholar] [CrossRef]
- Boesjes, C.M.; van der Gang, L.F.; Bakker, D.S.; Cate, T.A.T.; Spekhorst, L.S.; de Graaf, M.; van Dijk, M.R.; de Bruin-Weller, M.S. Dupilumab-associated lymphoid reactions in patients with atopic dermatitis. JAMA Dermatol. 2023, 159, 1240. [Google Scholar] [CrossRef]
- Chiba, T.; Nagai, T.; Osada, S.; Manabe, M. Diagnosis of mycosis fungoides following administration of dupilumab for misdiagnosed atopic dermatitis. Acta Derm.-Venereol. 2019, 99, 818–819. [Google Scholar] [CrossRef]
- Boh, E.; Kuraitis, D.; Jacobson, A.; Sikes, M. Healthcare provider experience in diagnosing and treating cutaneous t-cell lymphoma. Dermatol. Ther. 2023, 13, 835–842. [Google Scholar] [CrossRef]
- Pallazola, V.; Deib, G.; Soni, A.; Geha, R.; Kobayashi, K. An elusive case of mycosis fungoides: Case report and review of the literature. J. Gen. Intern. Med. 2019, 34, 2669–2674. [Google Scholar] [CrossRef]
- Kim, J.S.; Jeong, Y.J.; Sohn, M.H.; Jeong, H.J.; Lim, S.T.; Kim, D.W.; Kwak, J.Y.; Yim, C.Y. Usefulness of F-18 FDG PET/CT in subcutaneous panniculitis-like T cell lymphoma: Disease extent and treatment response evaluation. Radiol. Oncol. 2012, 46, 279–283. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, D.E.; Martinez-Escala, M.E.; Serrano, L.M.; Zhou, X.A.; Kaplan, J.B.; Pro, B.; Choi, J.; Guitart, J. Hemophagocytic Lymphohistiocytosis in Cutaneous T-Cell Lymphoma. JAMA Dermatol. 2018, 154, 828–831. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, Z.; Teng, J.; Zeng, M.; Lu, C. Clinical analysis of 10 cases with subcutaneous panniculitis-like T-cell lymphoma and tissue AURKA expression. Skin Res. Technol. 2024, 30, e13899. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bolognia, J.L.; Schaffer, J.V.; Cerroni, L. Dermatology, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Yared, J.; Kimball, A. The role of high dose chemotherapy and autologous stem-cell transplantation in peripheral T-cell lymphoma: A review of the literature and new perspectives. Cancer Treat. Rev. 2013, 39, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, F.; Bellosillo, B.; Serrano, S.; Pujol, R.M. Genotypic analysis in primary cutaneous lymphomas using the standardized BIOMED-2 polymerase chain reaction protocols. Actas Dermo-Sifiliográficas (Engl. Ed.) 2008, 99, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Potre, C.; Borsi, E.; Potre, O.; Ionita, I.; Samfireag, M.; Costachescu, D.; Secosan, C.; Lazar, S.; Ristescu, A.I. A Systematic Review Assessing the Impact of Vitamin D Levels on Adult Patients with Lymphoid Malignancies. Curr. Oncol. 2023, 30, 4351–4364. [Google Scholar] [CrossRef]
- Heimbach, D.; Luterman, A.; Burke, J.; Cram, A.; Herndon, D.; Hunt, J.; Jordan, M.; McMANUS, W.; Solem, L.; Warden, G.; et al. Artificial dermis for major burns: A multi-center randomized clinical trial. Ann. Surg. 1988, 208, 313–320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pit, D.; Hoinoiu, B.; Bardan, R.; Hoinoiu, T. The Impact of the Dermal Matrix in Tissue Reconstruction: A Bibliometric Perspective in Plastic Surgery. J. Funct. Biomater. 2024, 15, 189. [Google Scholar] [CrossRef]
- Yannas, I.V.; Burke, J.F. Design of an artificial skin. I. Basic design principles. J. Biomed. Mater. Res. 1980, 14, 65–81. [Google Scholar] [CrossRef]
- Moiemen, N.S.; Vlachou, E.; Staiano, J.J.; Thawy, Y.; Frame, J.D. Reconstructive surgery with Integra dermal regeneration template: Histologic study, clinical evaluation, and current practice. Plast. Reconstr. Surg. 2006, 117 (Suppl. 7), 160S–174S. [Google Scholar] [CrossRef]
- Branski, L.K.; Herndon, D.N.; Pereira, C.; Mlcak, R.P.; Celis, M.M.; Lee, J.O.; Sanford, A.P.; Norbury, W.B.; Zhang, X.-J.; Jeschke, M.G. Longitudinal assessment of Integra in primary burn management: A randomized pediatric clinical trial. Crit Care Med. 2007, 35, 2615–2623. [Google Scholar] [CrossRef]
- Park, J.H.; Choe, Y.H.; Kim, Y.J.; Lee, W.S.; Yoo, J.Y. Successful reconstruction of full-thickness burn wounds in a patient with cutaneous lymphoma using dermal regeneration template. Ann. Dermatol. 2012, 24, 106–109. [Google Scholar]
- Van Kouwenberg, E.; Lee, D.C.; Dalvi, S.; Hoehn, J.; Adetayo, O.A. Primary Cutaneous Peripheral T-Cell Lymphoma of the Hand: Case Report of a Rare Diagnosis and Review of the Literature. J Hand Microsurg. 2019, 11 (Suppl. 1), S31–S35. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pit, D.; Hoinoiu, T.; Hoinoiu, B.; Cerbu, S.; Iordache, M.; Vaduva, A.; Szilagyi, D.; Bardan, C.R.; Taskov, P.; Crainiceanu, Z.P.; et al. Subcutaneous Panniculitis-like T-Cell Lymphoma: Diagnostic Challenge and Successful Multimodal Management with Integra® Dermal Matrix—Case Report and Review of the Literature. Diseases 2025, 13, 201. https://doi.org/10.3390/diseases13070201
Pit D, Hoinoiu T, Hoinoiu B, Cerbu S, Iordache M, Vaduva A, Szilagyi D, Bardan CR, Taskov P, Crainiceanu ZP, et al. Subcutaneous Panniculitis-like T-Cell Lymphoma: Diagnostic Challenge and Successful Multimodal Management with Integra® Dermal Matrix—Case Report and Review of the Literature. Diseases. 2025; 13(7):201. https://doi.org/10.3390/diseases13070201
Chicago/Turabian StylePit, Daniel, Teodora Hoinoiu, Bogdan Hoinoiu, Simona Cerbu, Maria Iordache, Adrian Vaduva, Diana Szilagyi, Claudia Ramona Bardan, Panche Taskov, Zorin Petrisor Crainiceanu, and et al. 2025. "Subcutaneous Panniculitis-like T-Cell Lymphoma: Diagnostic Challenge and Successful Multimodal Management with Integra® Dermal Matrix—Case Report and Review of the Literature" Diseases 13, no. 7: 201. https://doi.org/10.3390/diseases13070201
APA StylePit, D., Hoinoiu, T., Hoinoiu, B., Cerbu, S., Iordache, M., Vaduva, A., Szilagyi, D., Bardan, C. R., Taskov, P., Crainiceanu, Z. P., Samfireag, M., & Bardan, R. (2025). Subcutaneous Panniculitis-like T-Cell Lymphoma: Diagnostic Challenge and Successful Multimodal Management with Integra® Dermal Matrix—Case Report and Review of the Literature. Diseases, 13(7), 201. https://doi.org/10.3390/diseases13070201






