Prognostic Factors for Recurrence in Pituitary Adenomas: Recent Progress and Future Directions
Abstract
1. Introduction
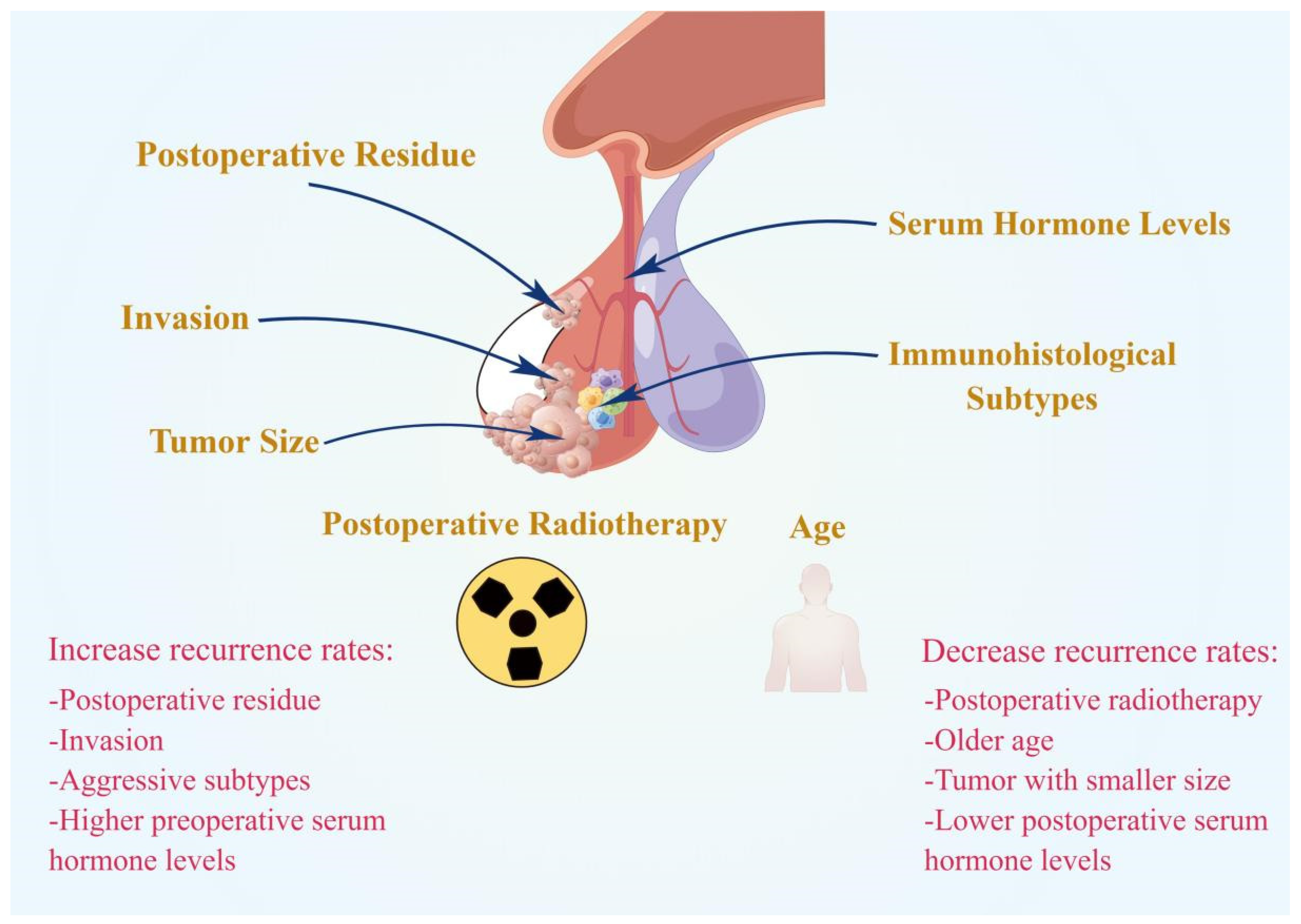
2. Clinical Factors
2.1. Postoperative Residue
2.2. Age
2.3. Immunohistological Subtypes
2.4. Invasion
2.5. Tumor Size
2.6. Hormone Levels
2.7. Postoperative Radiotherapy
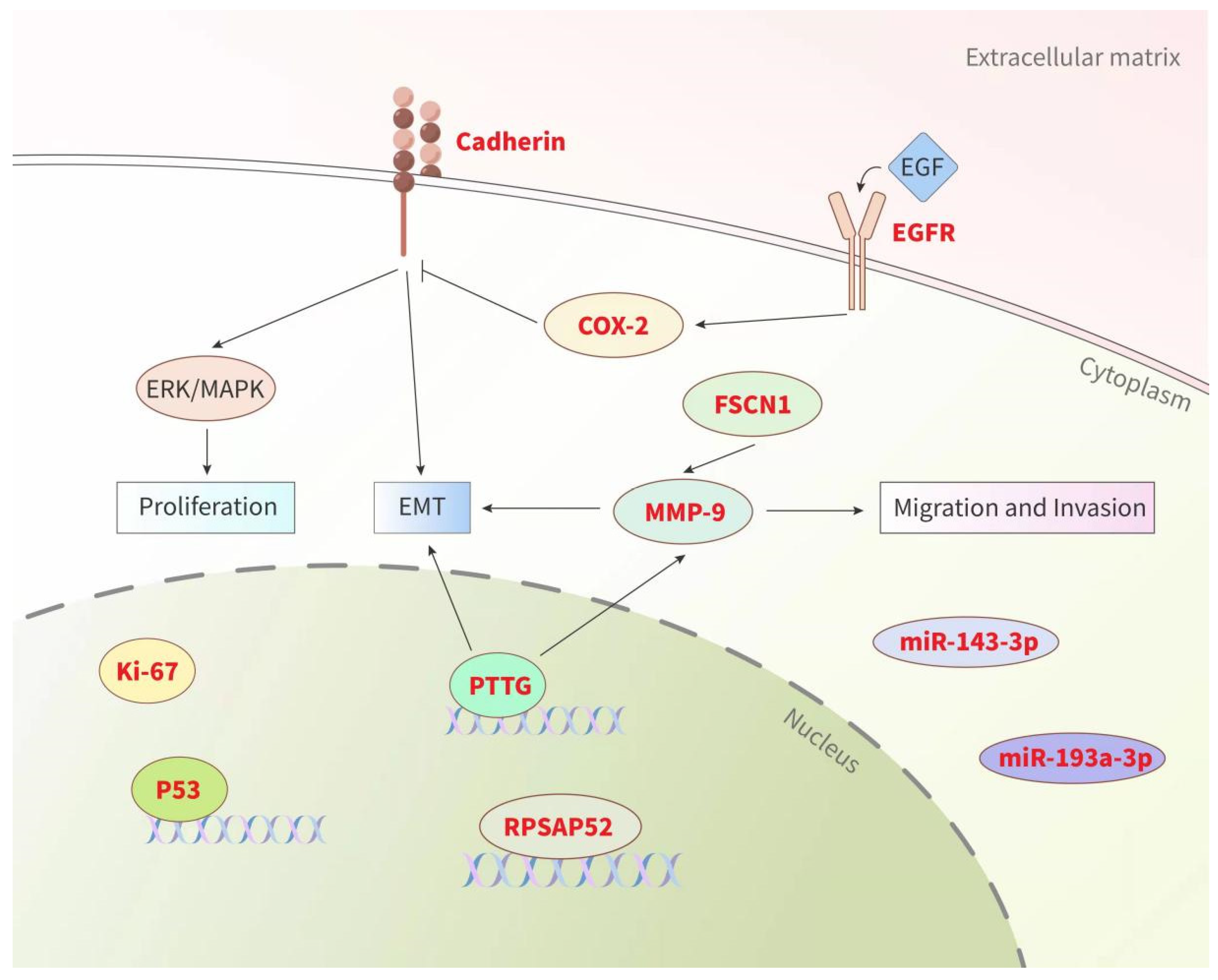
3. Biomarkers
3.1. Ki-67 and p53
3.2. Cadherin
3.3. Pituitary Tumor Transforming Gene (PTTG)
3.4. MMP-9
3.5. miRNAs and lncRNAs
3.6. Others
4. Comprehensive Models
| References | Content of the Models | Form | Sample Size | Prediction Performance |
|---|---|---|---|---|
| Pappy A. L. et al., 2019 [116] | Model 1 (CSI, diameter ≥ 2.9 cm and ki-67 > 3%) | Prognostic model | Training (n = 501) | 98.7% specificity (OR 8.6; CI 3.0–24.7) |
| Model 2 (ki-67 > 3% and CSI) | 93.1% specificity (OR 3.3; CI 1.8–6.0) | |||
| Model 3 (ki-67 > 3%, mitoses and p53, former “atypical” adenoma) | 96.0% specificity (OR 2.3; CI 1.0–5.0) | |||
| Wang X. et al., 2019 [117] | SPRY3, ZNF343, GZF1, C15orf61, SLC24A4, HOXB5, SLC9A3R2 | Prognostic model | Training (n = 57) Validation (n = 50) |
|
| Trouillas J. et al., 2013 [118] |
| Grading classification | Training (n = 410) | Invasion + proliferation (AUC = 81.4%); |
| Invasion + Ki-67 ≥ 3% (AUC = 81.4%) | ||||
| Proliferation without invasion (AUC = 0.713); | ||||
| Ki-67 ≥ 3% without invasion (AUC = 0.711) | ||||
| Wen L. et al., 2021 [124] |
| Nomogram | Training (n = 145) | AUC = 0.953 |
| A well-fitted calibration curve | ||||
| Chen Y. et al., 2021 [125] |
| Nomogram | Training (n = 172) | AUC for 1-, 2-, and 3-year survival (0.889, 0.885 and 0.832, respectively) |
| Well-fitted calibration curves |
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Wang, C.D.; Su, Z.P.; Chen, Y.X.; Cai, L.; Zhuge, Q.C.; Wu, Z.B. Natural History of Postoperative Nonfunctioning Pituitary Adenomas: A Systematic Review and Meta-Analysis. Neuroendocrinology 2012, 96, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Raappana, A.; Koivukangas, J.; Ebeling, T.; Pirilä, T. Incidence of Pituitary Adenomas in Northern Finland in 1992–2007. J. Clin. Endocrinol. Metab. 2010, 95, 4268–4275. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro-Oncology 2021, 23 (Suppl. 3), iii1–iii105. [Google Scholar] [CrossRef] [PubMed]
- Aghi, M.K.; Chen, C.C.; Fleseriu, M.; Newman, S.A.; Lucas, J.W.; Kuo, J.S.; Barkhoudarian, G.; Farrell, C.J.; Sheehan, J.; Ziu, M.; et al. Congress of neurological surgeons systematic review and evidence-based guidelines on the management of patients with nonfunctioning pituitary adenomas: Executive summary. Neurosurgery 2016, 79, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.W.; Bodach, M.E.; Tumialan, L.M.; Oyesiku, N.M.; Patil, C.G.; Litvack, Z.; Aghi, M.K.; Zada, G. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guideline on Primary Management of Patients With Nonfunctioning Pituitary Adenomas. Neurosurgery 2016, 79, E533–E535. [Google Scholar] [CrossRef]
- Karki, M.; Sun, J.; Yadav, C.P.; Zhao, B. Large and giant pituitary adenoma resection by microscopic trans-sphenoidal surgery: Surgical outcomes and complications in 123 consecutive patients. J. Clin. Neurosci. 2017, 44, 310–314. [Google Scholar] [CrossRef]
- Greenman, Y.; Cooper, O.; Yaish, I.; Robenshtok, E.; Sagiv, N.; Jonas-Kimchi, T.; Yuan, X.; Gertych, A.; Shimon, I.; Ram, Z.; et al. Treatment of clinically nonfunctioning pituitary adenomas with dopamine agonists. Eur. J. Endocrinol. 2016, 175, 63–72. [Google Scholar] [CrossRef]
- O’Sullivan, E.P.; Woods, C.; Glynn, N.; Behan, L.A.; Crowley, R.; O’Kelly, P.; Smith, D.; Thompson, C.J.; Agha, A. The natural history of surgically treated but radiotherapy-naïve nonfunctioning pituitary adenomas. Clin. Endocrinol. 2009, 71, 709–714. [Google Scholar] [CrossRef]
- Brochier, S.; Galland, F.; Kujas, M.; Parker, F.; Gaillard, S.; Raftopoulos, C.; Young, J.; Alexopoulou, O.; Maiter, D.; Chanson, P. Factors predicting relapse of nonfunctioning pituitary macroadenomas after neurosurgery: A study of 142 patients. Eur. J. Endocrinol. 2010, 163, 193–200. [Google Scholar] [CrossRef]
- Ferrante, E.; Ferraroni, M.; Castrignanò, T.; Menicatti, L.; Anagni, M.; Reimondo, G.M.; Del Monte, P.; Bernasconi, D.; Loli, P.; Faustini-Fustini, M.; et al. Non-functioning pituitary adenoma database: A useful resource to improve the clinical management of pituitary tumors. Eur. J. Endocrinol. 2006, 155, 823–829. [Google Scholar] [CrossRef]
- Dekkers, O.M.; Pereira, A.M.; Roelfsema, F.; Voormolen, J.H.C.; Neelis, K.J.; Schroijen, M.A.; Smit, J.W.A.; Romijn, J.A. Observation Alone after Transsphenoidal Surgery for Nonfunctioning Pituitary Macroadenoma. J. Clin. Endocrinol. Metab. 2006, 91, 1796–1801. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergh, A.C.; van den Berg, G.; Schoorl, M.A.; Sluiter, W.J.; van der Vliet, A.M.; Hoving, E.W.; Szabó, B.G.; Langendijk, J.A.; Wolffenbuttel, B.H.; Dullaart, R.P. Immediate postoperative radiotherapy in residual nonfunctioning pituitary adenoma: Beneficial effect on local control without additional negative impact on pituitary function and life expectancy. Int. J. Radiat. Oncol. 2007, 67, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Wass, J.A.H.; Reddy, R.; Karavitaki, N. The postoperative monitoring of nonfunctioning pituitary adenomas. Nat. Rev. Endocrinol. 2011, 7, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.K.; Easwaran, A.; McNeill, P.; Wang, Y.Y.; Inder, W.J.; Caputo, C. Younger age is a risk factor for regrowth and recurrence of nonfunctioning pituitary macroadenomas: Results from a single Australian centre. Clin. Endocrinol. 2017, 87, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Sonino, N.; Zielezny, M.; Fava, G.A.; Fallo, F.; Boscaro, M. Risk factors and long-term outcome in pituitary-dependent Cushing’s disease. J. Clin. Endocrinol. Metab. 1996, 81, 2647–2652. [Google Scholar] [CrossRef]
- Braun, L.T.; Rubinstein, G.; Zopp, S.; Vogel, F.; Schmid-Tannwald, C.; Escudero, M.P.; Honegger, J.; Ladurner, R.; Reincke, M. Recurrence after pituitary surgery in adult Cushing’s disease: A systematic review on diagnosis and treatment. Endocrine 2020, 70, 218–231. [Google Scholar] [CrossRef]
- Reddy, R.; Cudlip, S.; Byrne, J.V.; Karavitaki, N.; Wass, J.A.H. Can we ever stop imaging in surgically treated and radiotherapy-naive patients with non-functioning pituitary adenoma? Eur. J. Endocrinol. 2011, 165, 739–744. [Google Scholar] [CrossRef]
- De Witte, O.; Lonneville, S.; David, P.; Devuyst, F.; Massager, N. Non-Functional Pituitary Adenoma: Is There an Interest to Treat a Residue After Surgery? World Neurosurg. 2013, 80, 668. [Google Scholar] [CrossRef]
- Losa, M.; Mortini, P.; Barzaghi, R.; Ribotto, P.; Terreni, M.R.; Marzoli, S.B.; Pieralli, S.; Giovanelli, M. Early results of surgery in patients with nonfunctioning pituitary adenoma and analysis of the risk of tumor recurrence. J. Neurosurg. 2008, 108, 525–532. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, J.H.; Seol, H.J.; Lee, J.-I.; Kim, J.H.; Kong, D.-S.; Nam, D.-H. Clinical Concerns about Recurrence of Non-Functioning Pituitary Adenoma. Brain Tumor Res. Treat. 2016, 4, 1–7. [Google Scholar] [CrossRef]
- Turner, H.E.; Stratton, I.; Byrne, J.V.; Adams, C.B.; Wass, J.A. Audit of selected patients with nonfunctioning pituitary adenomas treated without irradiation—A follow-up study. Clin. Endocrinol. 1999, 51, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Soto-Ares, G.; Cortet-Rudelli, C.; Assaker, R.; Boulinguez, A.; Dubest, C.; Dewailly, D.; Pruvo, J.P. MRI protocol technique in the optimal therapeutic strategy of non-functioning pituitary adenomas. Eur. J. Endocrinol. 2002, 146, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.F.; Zada, G.; Kim, S.; Lamborn, K.R.; Quinones-Hinojosa, A.; Tyrrell, J.B.; Wilson, C.B.; Kunwar, S. Long-term recurrence and mortality after surgery and adjuvant radiotherapy for nonfunctional pituitary adenomas. J. Neurosurg. 2008, 108, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Asa, S.L.; Mete, O.; Perry, A.; Osamura, R.Y. Overview of the 2022 WHO Classification of Pituitary Tumors. Endocr. Pathol. 2022, 33, 6–26. [Google Scholar] [CrossRef] [PubMed]
- Scheithauer, B.W.; Jaap, A.J.; Horvath, E.; Kovacs, K.; Lloyd, R.V.; Meyer, F.B.; Laws, E.R.; Young, W.F. Clinically Silent Corticotroph Tumors of the Pituitary Gland. Neurosurgery 2000, 47, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Webb, K.M.; Laurent, J.J.; Okonkwo, D.O.; Lopes, M.B.; Vance, M.L.; Laws, E.R. Clinical Characteristics of Silent Corticotrophic Adenomas and Creation of an Internet-accessible Database to Facilitate Their Multi-institutional Study. Neurosurgery 2003, 53, 1076–1084, discussion 1084–1085. [Google Scholar] [CrossRef]
- Fang, H.; Tian, R.; Wu, H.; Xu, J.; Fan, H.; Zhou, J.; Zhong, L. Cushing disease after treatment of nonfunctional pituitary adenoma a case report and literature review. Medicine 2015, 94, e2134. [Google Scholar] [CrossRef]
- Zoli, M.; Faustini-Fustini, M.; Mazzatenta, D.; Marucci, G.; De Carlo, E.; Bacci, A.; Pasquini, E.; Lanzino, G.; Frank, G. ACTH adenomas transforming their clinical expression: Report of 5 cases. Neurosurg. Focus 2015, 38, E15. [Google Scholar] [CrossRef]
- Langlois, F.; Lim, D.S.T.; Yedinak, C.G.; Cetas, I.; McCartney, S.; Cetas, J.; Dogan, A.; Fleseriu, M. Predictors of silent corticotroph adenoma recurrence; a large retrospective single center study and systematic literature review. Pituitary 2017, 21, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Saeger, W.; Koch, A. Clinical Implications of the New WHO Classification 2017 for Pituitary Tumors. Exp. Clin. Endocrinol. Diabetes 2021, 129, 146–156. [Google Scholar] [CrossRef]
- Kontogeorgos, G. Update on pituitary adenomas in the 2017 World Health Organization classification: Innovations and perspectives. Hormones 2021, 20, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.P.; Joshi, R.S.; Pereira, M.P.; Oh, T.; Haddad, A.F.; Pereira, K.M.; Osorio, R.C.; Donohue, K.C.; Peeran, Z.; Sudhir, S.; et al. Plurihormonal PIT-1–Positive Pituitary Adenomas: A Systematic Review and Single-Center Series. World Neurosurg. 2021, 151, e185–e191. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-X.; Wang, S.-Z.; Heng, L.-J.; Han, Y.; Ma, Y.-H.; Yan, L.-F.; Yu, Y.; Wang, W.; Hu, Y.-C.; Cui, G.-B. Predicting Subtype of Growth Hormone Pituitary Adenoma based on Magnetic Resonance Imaging Characteristics. J. Comput. Assist. Tomogr. 2021, 46, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Asa, S. Practical Pituitary Pathology: What Does the Pathologist Need to Know? Arch. Pathol. Lab. Med. 2008, 132, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Raverot, G.; Ilie, M.D.; Lasolle, H.; Amodru, V.; Trouillas, J.; Castinetti, F.; Brue, T. Aggressive pituitary tumours and pituitary carcinomas. Nat. Rev. Endocrinol. 2021, 17, 671–684. [Google Scholar] [CrossRef]
- Zhu, H.; Guo, J.; Shen, Y.; Dong, W.; Gao, H.; Miao, Y.; Li, C.; Zhang, Y. Functions and Mechanisms of Tumor Necrosis Factor-α and Noncoding RNAs in Bone-Invasive Pituitary Adenomas. Clin. Cancer Res. 2018, 24, 5757–5766. [Google Scholar] [CrossRef]
- Li, C.; Zhu, H.; Zong, X.; Wang, X.; Gui, S.; Zhao, P.; Bai, J.; Liu, C.; Cao, L.; Li, Z.; et al. Experience of trans-nasal endoscopic surgery for pituitary tumors in a single center in China: Surgical results in a cohort of 2032 patients, operated between 2006 and 2018. Clin. Neurol. Neurosurg. 2020, 197, 106176. [Google Scholar] [CrossRef] [PubMed]
- Cardinal, T.; Zada, G.; Carmichael, J.D. The role of reoperation after recurrence of Cushing’s disease. Best Pr. Res. Clin. Endocrinol. Metab. 2021, 35, 101489. [Google Scholar] [CrossRef]
- Lampropoulos, K.; Samonis, G.; Nomikos, P. Factors influencing the outcome of microsurgical transsphenoidal surgery for pituitary adenomas: A study on 184 patients. Hormones 2013, 12, 254–264. [Google Scholar] [CrossRef]
- Almeida, J.P.; Tabasinejad, R.; Kalyvas, A.; Takami, H.; Mohan, N.; O’Halloran, P.J.; Sanchez, M.M.; Velasquez, C.; Zadeh, G.; Gentili, F. The Importance of Long-Term Follow-up after Endoscopic Pituitary Surgery: Durability of Results and Tumor Recurrence. Neurol. India 2020, 68, S92–S100. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, G.; Ozdarski, M.; Maksymowicz, M.; Szamotulska, K.; Witek, P. Prolactinomas: Prognostic Factors of Early Remission after Transsphenoidal Surgery. Front. Endocrinol. 2020, 11, 439. [Google Scholar] [CrossRef] [PubMed]
- Pala, A.; Knoll, A.; Schneider, M.; Etzrodt-Walter, G.; Karpel-Massler, G.; Wirtz, C.R.; Hlavac, M. The Benefit of Intraoperative Magnetic Resonance Imaging in Endoscopic and Microscopic Transsphenoidal Resection of Recurrent Pituitary Adenomas. Curr. Oncol. 2022, 29, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.T.; Munoz, D.G.; Goguen, J.; Lee, J.M.; Rotondo, F.; Kovacs, K.; Cusimano, M.D. Resection of the medial wall of the cavernous sinus in functioning pituitary adenomas: Technical note and outcomes in a matched-cohort study. Clin. Neurol. Neurosurg. 2020, 200, 106306. [Google Scholar] [CrossRef]
- Nomikos, P.; Buchfelder, M.; Fahlbusch, R. The outcome of surgery in 668 patients with acromegaly using current criteria of biochemical ‘cure’. Eur. J. Endocrinol. 2005, 152, 379–387. [Google Scholar] [CrossRef]
- Dolenc, V.V. Introduction: Surgery of the Central Skull Base. Neurosurg. Focus 2008, 25, E1. [Google Scholar] [CrossRef]
- Dietemann, J.L.; Kehrli, P.; Maillot, C.; Diniz, R.; Reis, M.J.; Neugroschl, C.; Vinclair, L. Is there a dural wall between the cavernous sinus and the pituitary fossa? Anatomical and MRI findings. Neuroradiology 1998, 40, 627–630. [Google Scholar] [CrossRef]
- Ilie, M.D.; Jouanneau, E.; Raverot, G. Aggressive Pituitary Adenomas and Carcinomas. Endocrinol. Metab. Clin. N. Am. 2020, 49, 505–515. [Google Scholar] [CrossRef]
- Micko, A.; Oberndorfer, J.; Weninger, W.J.; Vila, G.; Höftberger, R.; Wolfsberger, S.; Knosp, E. Challenging Knosp high-grade pituitary adenomas. J. Neurosurg. 2020, 132, 1739–1746. [Google Scholar] [CrossRef]
- Hofstetter, C.P.; Nanaszko, M.J.; Mubita, L.L.; Tsiouris, J.; Anand, V.K.; Schwartz, T.H. Volumetric classification of pituitary macroadenomas predicts outcome and morbidity following endoscopic endonasal transsphenoidal surgery. Pituitary 2011, 15, 450–463. [Google Scholar] [CrossRef]
- Roelfsema, F.; Biermasz, N.R.; Pereira, A.M. Clinical factors involved in the recurrence of pituitary adenomas after surgical remission: A structured review and meta-analysis. Pituitary 2011, 15, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Blevins, L.S.; Christy, J.H.; Khajavi, M.; Tindall, G.T. Outcomes of Therapy for Cushing’s Disease due to Adrenocorticotropin-Secreting Pituitary Macroadenomas. J. Clin. Endocrinol. Metab. 1998, 83, 63–67. [Google Scholar] [CrossRef] [PubMed]
- De Tommasi, C.; Vance, M.L.; Okonkwo, D.O.; Diallo, A.; Laws, E.R. Surgical management of adrenocorticotropic hormone—Secreting macroadenomas: Outcome and challenges in patients with Cushing’s disease or Nelson’s syndrome. J. Neurosurg. 2005, 103, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Mampalam, T.J.; Tyrrell, J.B.; Wilson, C.B. Transsphenoidal Microsurgery for Cushing Disease. Ann. Intern. Med. 1988, 109, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.E.A.; De Mello, P.A.; De Magalhães, A.V.; Botelho, C.H.A.; Naves, L.; Nosé, V.; Schmitt, F. Non-functioning pituitary adenomas: Clinical features and immunohistochemistry. Arq. Neuropsiquiatr. 2005, 63, 1070–1078. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, F.; Catalino, M.P.; Bi, W.L.; Dunn, I.F.; Smith, T.R.; Guo, Y.; Hordejuk, D.; Kaiser, U.B.; Laws, E.R.; Min, L. Postoperative Day 1 Morning Cortisol Value as a Biomarker to Predict Long-term Remission of Cushing Disease. J. Clin. Endocrinol. Metab. 2020, 106, e94–e102. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Manzano, A.J. Detection of recurrent Cushing’s disease: Proposal for standardized patient monitoring following transsphenoidal surgery. J. Neuro-Oncol. 2014, 119, 235–242. [Google Scholar] [CrossRef]
- Estrada, J.; García-Uría, J.; Lamas, C.; Alfaro, J.; Lucas, T.; Diez, S.; Salto, L.; Barceló, B. The Complete Normalization of the Adrenocortical Function as the Criterion of Cure after Transsphenoidal Surgery for Cushing’s Disease. J. Clin. Endocrinol. Metab. 2001, 86, 5695–5699. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Hong, X.; Liu, P.; Bao, X.; Yao, Y.; Xing, B.; Li, Y.; Huang, Y.; Zhu, H.; et al. Prediction of Recurrence after Transsphenoidal Surgery for Cushing’s Disease: The Use of Machine Learning Algorithms. Neuroendocrinology 2019, 108, 201–210. [Google Scholar] [CrossRef]
- Ozgen, T.; Oruckaptan, H.H.; Ozcan, O.E.; Acıkgoz, B. Prolactin Secreting Pituitary Adenomas: Analysis of 429 Surgically Treated Patients, Effect of Adjuvant Treatment Modalities and Review of the Literature. Acta Neurochir. 1999, 141, 1287–1294. [Google Scholar] [CrossRef]
- Vladyka, V.; Liščák, R.; Novotný, J.; Marek, J.; Ježková, J. Radiation Tolerance of Functioning Pituitary Tissue in Gamma Knife Surgery for Pituitary Adenomas. Neurosurgery 2003, 52, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Gittoes, N.J. Radiotherapy for non-functioning pituitary tumors--when and under what circumstances? Pituitary 2003, 6, 103–108. [Google Scholar] [CrossRef]
- Gittoes, N.J.; Bates, A.S.; Tse, W.; Bullivant, B.; Sheppard, M.C.; Clayton, R.N.; Stewart, P.M. Radiotherapy for non-functioning pituitary tumours. Clin. Endocrinol. 1998, 48, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Albano, L.; Losa, M.; Nadin, F.; Barzaghi, L.R.; Parisi, V.; del Vecchio, A.; Bolognesi, A.; Mortini, P. Safety and efficacy of multisession gamma knife radiosurgery for residual or recurrent pituitary adenomas. Endocrine 2019, 64, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Castinetti, F.; Brue, T.; Ragnarsson, O. Radiotherapy as a tool for the treatment of Cushing’s disease. Eur. J. Endocrinol. 2019, 180, D9–D18. [Google Scholar] [CrossRef]
- Heringer, L.C.; de Lima, M.M.; Rotta, J.M.; Botelho, R.V. Effect of Stereotactic Radiosurgery on Residual or Relapsed Pituitary Adenoma: A Systematic Review and Meta-Analysis. World Neurosurg. 2019, 136, 374–381.e4. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Z.; Tian, J.; Pan, R. Ki-67 labeling index and Knosp classification of pituitary adenomas. Br. J. Neurosurg. 2021, 27, 1–5. [Google Scholar] [CrossRef]
- Chiloiro, S.; Bianchi, A.; Doglietto, F.; De Waure, C.; Giampietro, A.; Fusco, A.; Iacovazzo, D.; Tartaglione, L.; Di Nardo, F.; Signorelli, F.; et al. Radically resected pituitary adenomas: Prognostic role of Ki 67 labeling index in a monocentric retrospective series and literature review. Pituitary 2013, 17, 267–276. [Google Scholar] [CrossRef]
- Heaney, A.P. Pituitary Carcinoma: Difficult Diagnosis and Treatment. J. Clin. Endocrinol. Metab. 2011, 96, 3649–3660. [Google Scholar] [CrossRef]
- Kovacs, K. The 2004 Who classification of pituitary tumors: Comments. Acta Neuropathol. 2005, 111, 62–63. [Google Scholar] [CrossRef]
- Raverot, G.; Wierinckx, A.; Dantony, E.; Auger, C.; Chapas, G.; Villeneuve, L.; Brue, T.; Figarella-Branger, D.; Roy, P.; Jouanneau, E.; et al. Prognostic Factors in Prolactin Pituitary Tumors: Clinical, Histological, and Molecular Data from a Series of 94 Patients with a Long Postoperative Follow-Up. J. Clin. Endocrinol. Metab. 2010, 95, 1708–1716. [Google Scholar] [CrossRef] [PubMed]
- Laws, E.R.; Penn, D.L.; Repetti, C.S. Advances and controversies in the classification and grading of pituitary tumors. J. Endocrinol. Investig. 2018, 42, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Li, S.; Zhang, W.; Zang, Z.; Hu, J.; Yang, H. Current biomarkers of invasive sporadic pituitary adenomas. Ann. Endocrinol. 2016, 77, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Marroni, C.; Pizarro, C.; Pereira-Lima, J.; Barbosa-Coutinho, L.; Ferreira, N. Expression of p53 protein in pituitary adenomas. Br. J. Med Biol. Res. 2002, 35, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Yang, L.; Li, T.; Zhang, Y. Cadherin Signaling in Cancer: Its Functions and Role as a Therapeutic Target. Front. Oncol. 2019, 9, 989. [Google Scholar] [CrossRef]
- Zhou, K.; Jin, H.; Luo, Y. Expression and Significance of E-Cadherin and β-Catenins in Pituitary Adenoma. Int. J. Surg. Pathol. 2013, 21, 363–367. [Google Scholar] [CrossRef]
- Gruppetta, M.; Formosa, R.; Falzon, S.; Scicluna, S.A.; Falzon, E.; Degeatano, J.; Vassallo, J. Expression of cell cycle regulators and biomarkers of proliferation and regrowth in human pituitary adenomas. Pituitary 2017, 20, 358–371. [Google Scholar] [CrossRef]
- Qian, Z.R.; Sano, T.; Yoshimoto, K.; Asa, S.; Yamada, S.; Mizusawa, N.; Kudo, E. Tumor-specific downregulation and methylation of the CDH13 (H-cadherin) and CDH1 (E-cadherin) genes correlate with aggressiveness of human pituitary adenomas. Mod. Pathol. 2007, 20, 1269–1277. [Google Scholar] [CrossRef]
- Qian, Z.R.; Li, C.C.; Yamasaki, H.; Mizusawa, N.; Yoshimoto, K.; Yamada, S.; Tashiro, T.; Horiguchi, H.; Wakatsuki, S.; Hirokawa, M.; et al. Role of E-Cadherin, α-, β-, and γ-Catenins, and p120 (Cell Adhesion Molecules) in Prolactinoma Behavior. Mod. Pathol. 2002, 15, 1357–1365. [Google Scholar] [CrossRef]
- Jia, W.; Zhu, J.; Martin, T.A.; Jiang, A.; Sanders, A.J.; Jiang, W.G. Epithelial-mesenchymal Transition (EMT) Markers in Human Pituitary Adenomas Indicate a Clinical Course. Anticancer. Res. 2015, 35, 2635–2643. [Google Scholar]
- Sano, T.; Rong, Q.; Kagawa, N.; Yamada, S. Down-Regulation of E-Cadherin and Catenins in Human Pituitary Growth Hormone-Producing Adenomas. Front. Horm. Res. 2004, 32, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Øystese, K.A.B.; Berg, J.P.; Normann, K.R.; Zucknick, M.; Casar-Borota, O.; Bollerslev, J. The role of E and N-cadherin in the postoperative course of gonadotroph pituitary tumours. Endocrine 2018, 62, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Chauvet, N.; Romanò, N.; Meunier, A.; Galibert, E.; Fontanaud, P.; Mathieu, M.; Osterstock, G.; Osterstock, P.; Baccino, E.; Rigau, V.; et al. Combining Cadherin Expression with Molecular Markers Discriminates Invasiveness in Growth Hormone and Prolactin Pituitary Adenomas. J. Neuroendocr. 2016, 28, 12352. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, Y.; Zhang, M.; Huang, G.; Zhang, Q. Wnt4 is overexpressed in human pituitary adenomas and is associated with tumor invasion. J. Clin. Neurosci. 2014, 21, 137–141. [Google Scholar] [CrossRef]
- Liu, C.; Wu, Y.; Yu, S.; Bai, J.; Li, C.; Wu, D.; Zhang, Y. Increased β-catenin and c-myc expression predict aggressive growth of non-functioning pituitary adenomas: An assessment using a tissue microarray-based approach. Mol. Med. Rep. 2017, 15, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, S.; Zheng, L.; Asa, S.L. Pituitary Tumor-Derived Fibroblast Growth Factor Receptor 4 Isoform Disrupts Neural Cell-Adhesion Molecule/N-Cadherin Signaling to Diminish Cell Adhesiveness: A Mechanism Underlying Pituitary Neoplasia. Mol. Endocrinol. 2004, 18, 2543–2552. [Google Scholar] [CrossRef]
- Chen, X.; Pang, B.; Liang, Y.; Xu, S.C.; Xin, T.; Fan, H.T.; Yu, Y.B.; Pang, Q. Overexpression of EpCAM and Trop2 in pituitary adenomas. Int. J. Clin. Exp. Pathol. 2014, 7, 7907–7914. [Google Scholar]
- Jia, W.; Lu, R.; Jia, G.; Ni, M.; Xu, Z. Expression of pituitary tumor transforming gene (PTTG) in human pituitary macroadenomas. Tumor Biol. 2013, 34, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Filippella, M.; Galland, F.; Kujas, M.; Young, J.; Faggiano, A.; Lombardi, G.; Colao, A.; Meduri, G.; Chanson, P. Pituitary tumour transforming gene (PTTG) expression correlates with the proliferative activity and recurrence status of pituitary adenomas: A clinical and immunohistochemical study. Clin. Endocrinol. 2006, 65, 536–543. [Google Scholar] [CrossRef]
- Noh, T.-W.; Jeong, H.J.; Lee, M.-K.; Kim, T.S.; Kim, S.H.; Lee, E.J. Predicting Recurrence of Nonfunctioning Pituitary Adenomas. J. Clin. Endocrinol. Metab. 2009, 94, 4406–4413. [Google Scholar] [CrossRef]
- Sánchez-Tejada, L.; Sánchez-Ortiga, R.; Moreno-Pérez, O.; Montañana, C.F.; Niveiro, M.; Tritos, N.A.; Alfonso, A.M.P. Pituitary tumor transforming gene and insulin-like growth factor 1 receptor expression and immunohistochemical measurement of Ki-67 as potential prognostic markers of pituitary tumors aggressiveness. Endocrinol. Y Nutr. 2013, 60, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Salehi, F.; Kovacs, K.; Scheithauer, B.W.; Lloyd, R.V.; Cusimano, M. Pituitary tumor-transforming gene in endocrine and other neoplasms: A review and update. Endocr. -Relat. Cancer 2008, 15, 721–743. [Google Scholar] [CrossRef] [PubMed]
- Fingleton, B. Matrix metalloproteinases: Roles in cancer and metastasis. Front. Biosci. 2006, 11, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Turner, H.E.; Nagy, Z.; Esiri, M.M.; Harris, A.L.; Wass, J.A.H. Role of Matrix Metalloproteinase 9 in Pituitary Tumor Behavior. J. Clin. Endocrinol. Metab. 2000, 85, 2931–2935. [Google Scholar] [CrossRef]
- Mao, J.; Guo, H.; Si, N.; Qiu, L.; Guo, L.; Sun, Z.; Xiang, Y.; Yang, X.; Zhao, W.; Zhang, W. Regulating effect of MMP-9 and TIMP-1 in pituitary adenoma invasion. Genet. Mol. Res. 2015, 14, 17091–17098. [Google Scholar] [CrossRef]
- Liu, X.; Feng, M.; Zhang, Y.; Dai, C.; Sun, B.; Bao, X.; Deng, K.; Yao, Y.; Wang, R. Expression of Matrix Metalloproteinase-9, Pituitary Tumor Transforming Gene, High Mobility Group A 2, and Ki-67 in Adrenocorticotropic Hormone–Secreting Pituitary Tumors and Their Association with Tumor Recurrence. World Neurosurg. 2018, 113, e213–e221. [Google Scholar] [CrossRef]
- Gültekin, G.D.; Çabuk, B.; Vural, Ç.; Ceylan, S. Matrix metalloproteinase-9 and tissue inhibitor of matrix metalloproteinase-2: Prognostic biological markers in invasive prolactinomas. J. Clin. Neurosci. 2015, 22, 1282–1287. [Google Scholar] [CrossRef]
- Hussaini, I.M.; Trotter, C.; Zhao, Y.; Abdel-Fattah, R.; Amos, S.; Xiao, A.; Agi, C.U.; Redpath, G.T.; Fang, Z.; Leung, G.K.; et al. Matrix Metalloproteinase-9 Is Differentially Expressed in Nonfunctioning Invasive and Noninvasive Pituitary Adenomas and Increases Invasion in Human Pituitary Adenoma Cell Line. Am. J. Pathol. 2007, 170, 356–365. [Google Scholar] [CrossRef]
- Krokker, L.; Nyírő, G.; Reiniger, L.; Darvasi, O.; Szücs, N.; Czirják, S.; Tóth, M.; Igaz, P.; Patócs, A.; Butz, H. Differentially Expressed miRNAs Influence Metabolic Processes in Pituitary Oncocytoma. Neurochem. Res. 2019, 44, 2360–2371. [Google Scholar] [CrossRef] [PubMed]
- Su, W.J.; Wang, J.S.; Ye, M.D.; Chen, W.L.; Liao, C.X. Expression and clinical significance of miR-193a-3p in invasive pituitary adenomas. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7673–7680. [Google Scholar] [CrossRef]
- D’Angelo, D.; Mussnich, P.; Sepe, R.; Raia, M.; Del Vecchio, L.; Cappabianca, P.; Pellecchia, S.; Petrosino, S.; Saggio, S.; Solari, D.; et al. RPSAP52 lncRNA is overexpressed in pituitary tumors and promotes cell proliferation by acting as miRNA sponge for HMGA proteins. Klin. Wochenschr. 2019, 97, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Guo, J.; Wang, D.; Fang, Q.; Liu, Y.; Xie, W.; Zhang, Y.; Li, C. A Novel Three-LncRNA Signature Predicting Tumor Recurrence in Nonfunctioning Pituitary Adenomas. Front. Genet. 2021, 12, 2049. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, L. The Involvement of miRNAs in Pituitary Adenomas Pathogenesis and the Clinical Implications. Eur. Neurol. 2022, 1–6. [Google Scholar] [CrossRef]
- Fosslien, E. Molecular pathology of cyclooxygenase-2 in neoplasia. Ann. Clin. Lab. Sci. 2000, 30, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Sokołowski, G.; Bałdys-Waligórska, A.; Trofimiuk, M.; Adamek, D.; Hubalewska-Dydejczyk, A.; Gołkowski, F. Expression of cyclooxygenase-2 (COX-2) in pituitary tumours. Med. Sci. Monit. 2012, 18, CR252–CR259. [Google Scholar] [CrossRef][Green Version]
- Akbari, N.; Ghorbani, M.; Salimi, V.; Alimohammadi, A.; Khamseh, M.E.; Akbari, H.; Nourbakhsh, M.; Sheikhi, A.; Taghavi, S.F.; Tavakoli-Yaraki, M. Cyclooxygenase enzyme and PGE2 expression in patients with functional and non-functional pituitary adenomas. BMC Endocr. Disord. 2020, 20, 39. [Google Scholar] [CrossRef]
- Hajjo, R.; Sweidan, K. Review on Epidermal Growth Factor Receptor (EGFR) Structure, Signaling Pathways, Interactions, and Recent Updates of EGFR Inhibitors. Curr. Top. Med. Chem. 2020, 20, 815–834. [Google Scholar] [CrossRef]
- Voellger, B.; Zhang, Z.; Benzel, J.; Wang, J.; Lei, T.; Nimsky, C.; Bartsch, J.-W. Targeting Aggressive Pituitary Adenomas at the Molecular Level—A Review. J. Clin. Med. 2021, 11, 124. [Google Scholar] [CrossRef]
- Liu, X.; Feng, M.; Dai, C.; Bao, X.; Deng, K.; Yao, Y.; Wang, R. Expression of EGFR in Pituitary Corticotroph Adenomas and Its Relationship with Tumor Behavior. Front. Endocrinol. 2019, 10, 785. [Google Scholar] [CrossRef]
- Hinojosa-Amaya, J.M.; Lam-Chung, C.E.; Cuevas-Ramos, D. Recent Understanding and Future Directions of Recurrent Corticotroph Tumors. Front. Endocrinol. 2021, 12, 392. [Google Scholar] [CrossRef]
- Chen, S.; Bangaru, M.L.Y.; Sneade, L.; Dunckley, J.A.; Ben-Jonathan, N.; Kansra, S. Epidermal growth factor receptor cross-talks with ligand-occupied estrogen receptor-α to modulate both lactotroph proliferation and prolactin gene expression. Am. J. Physiol. Metab. 2009, 297, E331–E339. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cooper, O.; Mamelak, A.; Bannykh, S.; Carmichael, J.; Bonert, V.; Lim, S.; Cook-Wiens, G.; Ben-Shlomo, A. Prolactinoma ErbB receptor expression and targeted therapy for aggressive tumors. Endocrine 2013, 46, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Y.; Li, L.; Cao, J.; Guo, Y.; Wu, Y.; Gao, W. Fascin actin-bundling protein 1 in human cancer: Promising biomarker or therapeutic target? Mol. Ther.-Oncolytics 2021, 20, 240–264. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gao, H.; Cao, L.; Gui, S.; Liu, Q.; Li, C.; Li, D.; Gong, L.; Zhang, Y. The role of FSCN1 in migration and invasion of pituitary adenomas. Mol. Cell. Endocrinol. 2016, 419, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Sav, A.; Rotondo, F.; Syro, L.V.; Altinoz, M.A.; Kovacs, K. Selective molecular biomarkers to predict biologic behavior in pituitary tumors. Expert Rev. Endocrinol. Metab. 2017, 12, 177–185. [Google Scholar] [CrossRef]
- Pappy, A.L.; Savinkina, A.; Bicknese, C.; Neill, S.; Oyesiku, N.M.; Ioachimescu, A.G. Predictive modeling for pituitary adenomas: Single center experience in 501 consecutive patients. Pituitary 2019, 22, 520–531. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, D.; Ma, S.; Li, P.; Zhou, W.; Zhang, C.; Jia, W. Predicting the likelihood of early recurrence based on mRNA sequencing of pituitary adenomas. Gland Surg. 2019, 8, 648–656. [Google Scholar] [CrossRef]
- Trouillas, J.; Roy, P.; Sturm, N.; Dantony, E.; Cortet-Rudelli, C.; Viennet, G.; Bonneville, J.-F.; Assaker, R.; Auger, C.; Brue, T.; et al. A new prognostic clinicopathological classification of pituitary adenomas: A multicentric case–control study of 410 patients with 8 years post-operative follow-up. Acta Neuropathol. 2013, 126, 123–135. [Google Scholar] [CrossRef]
- Raverot, G.; Dantony, E.; Beauvy, J.; Vasiljevic, A.; Mikolasek, S.; Borson-Chazot, F.; Jouanneau, E.; Roy, P.; Trouillas, J. Risk of Recurrence in Pituitary Neuroendocrine Tumors: A Prospective Study Using a Five-Tiered Classification. J. Clin. Endocrinol. Metab. 2017, 102, 3368–3374. [Google Scholar] [CrossRef]
- Asioli, S.; Righi, A.; Iommi, M.; Baldovini, C.; Ambrosi, F.; Guaraldi, F.; Zoli, M.; Mazzatenta, D.; Faustini-Fustini, M.; Rucci, P.; et al. Validation of a clinicopathological score for the prediction of post-surgical evolution of pituitary adenoma: Retrospective analysis on 566 patients from a tertiary care centre. Eur. J. Endocrinol. 2019, 180, 127–134. [Google Scholar] [CrossRef]
- Lelotte, J.; Jouret-Mourin, A.; Fomekong, E.; Michotte, A.; Raftopoulos, C.; Maiter, D. Both invasiveness and proliferation criteria predict recurrence of non-functioning pituitary macroadenomas after surgery: A retrospective analysis of a monocentric cohort of 120 patients. Eur. J. Endocrinol. 2018, 178, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Zakir, J.C.D.O.; Casulari, L.A.; Rosa, J.W.C.; Rosa, J.W.C.; de Mello, P.A.; de Magalhães, A.V.; Naves, L.A. Prognostic Value of Invasion, Markers of Proliferation, and Classification of Giant Pituitary Tumors, in a Georeferred Cohort in Brazil of 50 Patients, with a Long-Term Postoperative Follow-Up. Int. J. Endocrinol. 2016, 2016, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Moons, K.G.M.; Altman, D.G.; Reitsma, J.B.; Ioannidis, J.P.A.; Macaskill, P.; Steyerberg, E.W.; Vickers, A.J.; Ransohoff, D.F.; Collins, G.S. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): Explanation and Elaboration. Ann. Intern. Med. 2015, 162, W1–W73. [Google Scholar] [CrossRef] [PubMed]
- Lyu, W.; Fei, X.; Chen, C.; Tang, Y. Nomogram predictive model of post-operative recurrence in non-functioning pituitary adenoma. Gland Surg. 2021, 10, 807–815. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, F.; Cao, J.; Gao, F.; Lv, Y.; Tang, Y.; Zhang, A.; Yan, W.; Wang, Y.; Hu, X.; et al. Analysis of Related Factors of Tumor Recurrence or Progression After Transnasal Sphenoidal Surgical Treatment of Large and Giant Pituitary Adenomas and Establish a Nomogram to Predict Tumor Prognosis. Front. Endocrinol. 2021, 12. [Google Scholar] [CrossRef]
- Balachandran, V.P.; Gonen, M.; Smith, J.J.; DeMatteo, R.P. Nomograms in oncology: More than meets the eye. Lancet Oncol. 2015, 16, e173–e180. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, L.; Wan, X.; Xu, Y.; Chen, J.; Shu, K.; Lei, T. Prognostic Factors for Recurrence in Pituitary Adenomas: Recent Progress and Future Directions. Diagnostics 2022, 12, 977. https://doi.org/10.3390/diagnostics12040977
Lu L, Wan X, Xu Y, Chen J, Shu K, Lei T. Prognostic Factors for Recurrence in Pituitary Adenomas: Recent Progress and Future Directions. Diagnostics. 2022; 12(4):977. https://doi.org/10.3390/diagnostics12040977
Chicago/Turabian StyleLu, Liang, Xueyan Wan, Yu Xu, Juan Chen, Kai Shu, and Ting Lei. 2022. "Prognostic Factors for Recurrence in Pituitary Adenomas: Recent Progress and Future Directions" Diagnostics 12, no. 4: 977. https://doi.org/10.3390/diagnostics12040977
APA StyleLu, L., Wan, X., Xu, Y., Chen, J., Shu, K., & Lei, T. (2022). Prognostic Factors for Recurrence in Pituitary Adenomas: Recent Progress and Future Directions. Diagnostics, 12(4), 977. https://doi.org/10.3390/diagnostics12040977






