The Non-Activated Thromboelastometry (NATEM) Assay’s Application among Adults and Neonatal/Pediatric Population: A Systematic Review
Abstract
1. Introduction
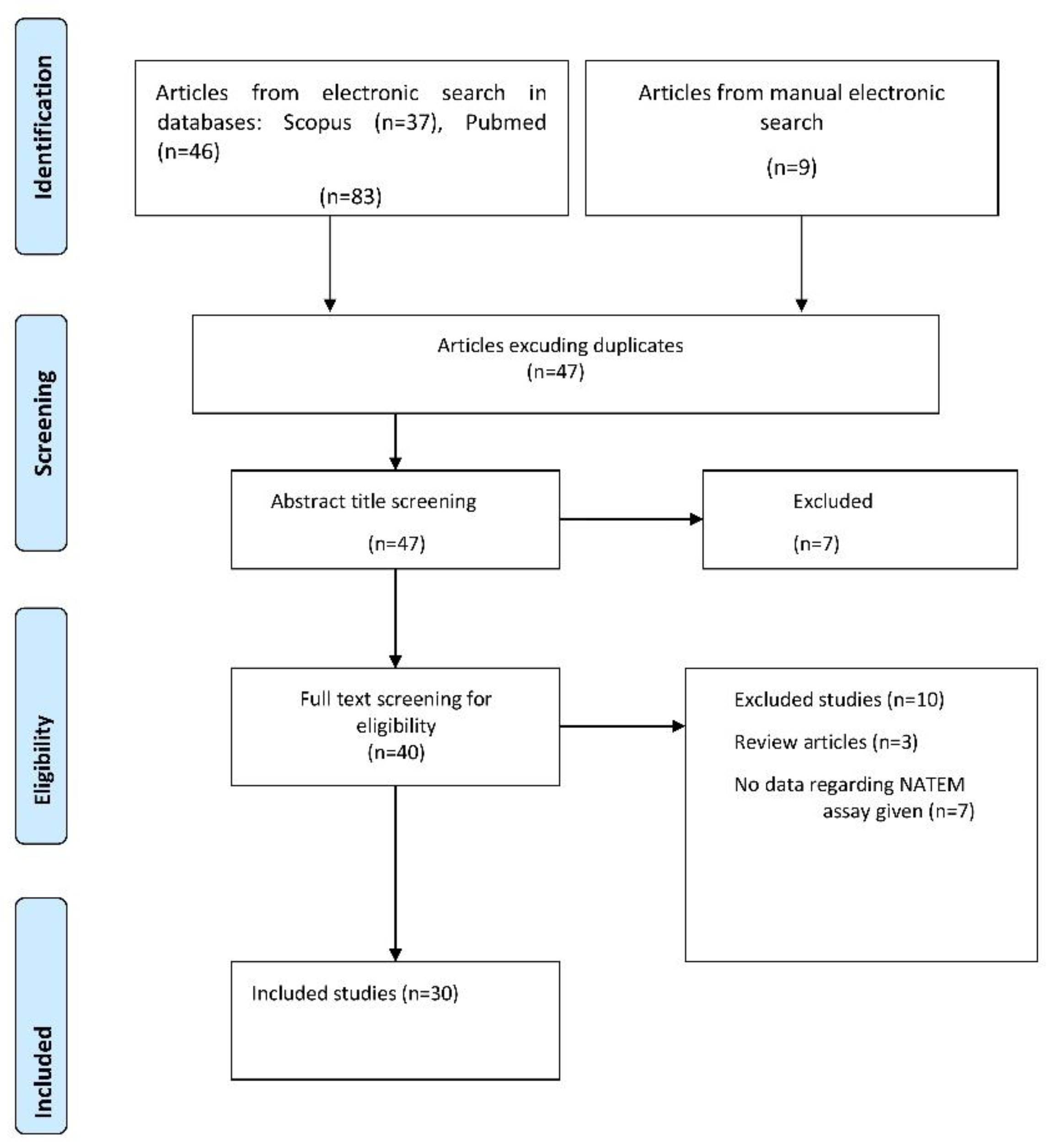
2. Methods—Material
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Collection and Analysis
3. Results
3.1. Study Characteristics
3.2. Outcomes
3.2.1. NATEM Assay Application in Experimental Studies
3.2.2. NATEM Assay Application in Healthy Human Volunteers
3.2.3. Comparison of NATEM Parameters between Healthy Adult Volunteers and Patients
3.2.4. NATEM Assay Application in Different Clinical Settings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Honickel, M.; Grottke, O. Rotational thromboelastometry for the diagnosis of coagulation disorders. Med. Klin. Intensivmed. Und Notf. 2018, 113, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Bugaev, N.; Como, J.J.; Golani, G.; Freeman, J.J.; Sawhney, J.S.; Vatsaas, C.J.; Yorkgitis, B.K.; Kreiner, L.A.; Garcia, N.M.; Aziz, H.A.; et al. Thromboelastography and rotational thromboelastometry in bleeding patients with coagulopathy: Practice management guideline from the Eastern Association for the Surgery of Trauma. J. Trauma Acute Care Surg. 2020, 89, 999–1017. [Google Scholar] [CrossRef] [PubMed]
- Da Luz, L.T.; Nascimento, B.; Shankarakutty, A.K.; Rizoli, S.; Adhikari, N.K. Effect of thromboelastography (TEG®) and rotational thromboelastometry (ROTEM®) on diagnosis of coagulopathy, transfusion guidance and mortality in trauma: Descriptive systematic review. Crit. Care 2014, 18, 518. [Google Scholar] [CrossRef] [PubMed]
- Görlinger, K.; Dirkmann, D.; Hanke, A.A. Rotational Thromboelastometry (ROTEM®). In Trauma Induced Coagulopathy; Gonzalez, E., Moore, H.B., Moore, E.E., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 267–298. [Google Scholar] [CrossRef]
- Simurda, T.; Asselta, R.; Zolkova, J.; Brunclikova, M.; Dobrotova, M.; Kolkova, Z.; Loderer, D.; Skornova, I.; Hudecek, J.; Lasabova, Z.; et al. Congenital Afibrinogenemia and Hypofibrinogenemia: Laboratory and Genetic Testing in Rare Bleeding Disorders with Life-Threatening Clinical Manifestations and Challenging Management. Diagnostics 2021, 11, 2140. [Google Scholar] [CrossRef] [PubMed]
- Sokou, R.; Piovani, D.; Konstantinidi, A.; Tsantes, A.G.; Parastatidou, S.; Lampridou, M.; Ioakeimidis, G.; Gounaris, A.; Iacovidou, N.; Kriebardis, A.G.; et al. A Risk Score for Predicting the Incidence of Hemorrhage in Critically Ill Neonates: Development and Validation Study. Thromb. Haemost. 2021, 121, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Sokou, R.; Piovani, D.; Konstantinidi, A.; Tsantes, A.G.; Parastatidou, S.; Lampridou, M.; Ioakeimidis, G.; Iacovidou, N.; Bonovas, S.; Tsantes, A.E. Prospective Temporal Validation of the Neonatal Bleeding Risk (NeoBRis) Index. Thromb. Haemost. 2021, 121, 1263–1266. [Google Scholar] [CrossRef]
- Sokou, R.; Tsantes, A.G.; Konstantinidi, A.; Ioakeimidis, G.; Lampridou, M.; Parastatidou, S.; Theodoraki, M.; Piovani, D.; Iliodromiti, Z.; Boutsikou, T.; et al. Rotational Thromboelastometry in Neonates Admitted to a Neonatal Intensive Care Unit: A Large Cross-sectional Study. Semin. Thromb. Hemost. 2021, 47, 875–884. [Google Scholar] [CrossRef]
- Durila, M.; Sevcikova, S.; Vymazal, T. Stability of Non-Activated Rotational Thromboelastometry Assay in Time of Citrated Blood (Appropriate Time Interval for Analysis). Clin. Lab. 2016, 62, 2145–2148. [Google Scholar] [CrossRef]
- Toulon, P. Developmental hemostasis: Laboratory and clinical implications. Int. J. Lab. Hematol. 2016, 38 (Suppl. S1), 66–77. [Google Scholar] [CrossRef]
- Toulon, P.; Berruyer, M.; Brionne-François, M.; Grand, F.; Lasne, D.; Telion, C.; Arcizet, J.; Giacomello, R.; De Pooter, N. Age dependency for coagulation parameters in paediatric populations. Results of a multicentre study aimed at defining the age-specific reference ranges. Thromb. Haemost. 2016, 116, 9–16. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Meesters, M.I.; Koch, A.; Kuiper, G.; Zacharowski, K.; Boer, C. Instability of the non-activated rotational thromboelastometry assay (NATEM) in citrate stored blood. Thromb. Res. 2015, 136, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, V.; Spiezia, L.; Senzolo, M.; Rodriguez-Castro, K.I.; Maggiolo, S.; Simioni, P. Whole blood rotation thromboelastometry (ROTEM®) profiles in subjects with non-neoplastic portal vein thrombosis. Thromb. Res. 2013, 132, e131–e134. [Google Scholar] [CrossRef] [PubMed]
- Schöchl, H.; Solomon, C.; Laux, V.; Heitmeier, S.; Bahrami, S.; Redl, H. Similarities in thromboelastometric (ROTEM®) findings between humans and baboons. Thromb. Res. 2012, 130, e107–e112. [Google Scholar] [CrossRef]
- Zipperle, J.; Schlimp, C.J.; Holnthoner, W.; Husa, A.M.; Nürnberger, S.; Redl, H.; Schöchl, H. A novel coagulation assay incorporating adherent endothelial cells in thromboelastometry. Thromb. Haemost. 2013, 109, 869–877. [Google Scholar] [CrossRef]
- Treliński, J.; Misiewicz, M.; Robak, M.; Smolewski, P.; Chojnowski, K. Assessment of rotation thromboelastometry (ROTEM) parameters in patients with multiple myeloma at diagnosis. Thromb. Res. 2014, 133, 667–670. [Google Scholar] [CrossRef]
- Elvstam, O.; Berntorp, E.; Schött, U. ROTEM monitoring of activated and non-activated prothrombin complex concentrate correction of dilutional coagulopathy. Scand. J. Clin. Lab. Investig. 2016, 76, 202–207. [Google Scholar] [CrossRef]
- Shalaby, S.; Simioni, P.; Campello, E.; Spiezia, L.; Gavasso, S.; Bizzaro, D.; Cardin, R.; D’Amico, F.; Gringeri, E.; Cillo, U.; et al. Endothelial Damage of the Portal Vein is Associated with Heparin-Like Effect in Advanced Stages of Cirrhosis. Thromb. Haemost. 2020, 120, 1173–1181. [Google Scholar] [CrossRef]
- Spiezia, L.; Bogana, G.; Campello, E.; Maggiolo, S.; Pelizzaro, E.; Carbonare, C.D.; Gervasi, M.T.; Simioni, P. Whole blood thromboelastometry profiles in women with preeclampsia. Clin. Chem. Lab. Med. 2015, 53, 1793–1798. [Google Scholar] [CrossRef]
- Brearton, C.; Rushton, A.; Parker, J.; Martin, H.; Hodgson, J. Performance Evaluation of a New Point of Care Viscoelastic Coagulation Monitoring System in Major Abdominal, Orthopaedic and Vascular Surgery. Platelets 2020, 31, 1052–1059. [Google Scholar] [CrossRef]
- Silverberg, E.; Tornqvist, F.; Kander, T.; Bengzon, J.; Solomon, C.; Bonnevier, J.; Schött, U. Comparison of citrated and fresh whole blood for viscoelastic coagulation testing during elective neurosurgery. Thromb. Res. 2017, 156, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Bagge, A.; Schött, U.; Kander, T. Effects of naturopathic medicines on Multiplate and ROTEM: A prospective experimental pilot study in healthy volunteers. BMC Complementary Altern. Med. 2016, 16, 64. [Google Scholar] [CrossRef] [PubMed]
- Spiezia, L.; Bertini, D.; Boldrin, M.; Radu, C.; Bulato, C.; Gavasso, S.; Cozzi, E.; Simioni, P. Reference values for thromboelastometry (ROTEM®) in cynomolgus monkeys (Macaca fascicularis). Thromb. Res. 2010, 126, e294–e297. [Google Scholar] [CrossRef] [PubMed]
- Jilma-Stohlawetz, P.; Fritsche-Polanz, S.; Quehenberger, P.; Schörgenhofer, C.; Bartko, J.; Ristl, R.; Jilma, B. Evaluation of between-, within- and day-to-day variation of coagulation measured by rotational thrombelastometry (ROTEM). Scand. J. Clin. Lab. Investig. 2017, 77, 651–657. [Google Scholar] [CrossRef]
- Durila, M. Nonactivated thromboelastometry able to detect fibrinolysis in contrast to activated methods (EXTEM, INTEM) in a bleeding patient. Blood Coagul. Fibrinolysis 2016, 27, 828–830. [Google Scholar] [CrossRef]
- MacDonald, S.; Wright, A.; Beuche, F.; Downes, K.; Besser, M.; Symington, E.; Kelly, A.; Thomas, W. Characterization of a large cohort of patients with unclassified bleeding disorder; clinical features, management of haemostatic challenges and use of global haemostatic assessment with proposed recommendations for diagnosis and treatment. Int. J. Lab. Hematol. 2020, 42, 116–125. [Google Scholar] [CrossRef]
- Lancé, M.D.; Kuiper, G.J.; Sloep, M.; Spronk, H.M.; van Oerle, R.; ten Cate, H.; Marcus, M.A.; Henskens, Y.M. The effects of pneumatic tube system transport on ROTEM analysis and contact activation assessed by thrombin generation test. Thromb. Res. 2012, 130, e147–e150. [Google Scholar] [CrossRef]
- Scharbert, G.; Thaler, U.; Weilnböck, C.; Wetzel, L.; Kozek-Langenecker, S. Heparin-induced effects of prothrombin complex concentrates in thromboelastometry. Wien. Klin. Wochenschr. 2012, 124, 320–325. [Google Scholar] [CrossRef]
- Lechien, A.; Faraoni, D.; Van der Linden, P. Effective tranexamic acid concentration for 95% inhibition of tissue-type plasminogen activator-induced hyperfibrinolysis in full-term pregnant women: A prospective interventional study. Blood Coagul. Fibrinolysis 2021, 32, 186–193. [Google Scholar] [CrossRef]
- Schneider, T.; Siegemund, T.; Siegemund, R.; Petros, S. Thrombin generation and rotational thromboelastometry in the healthy adult population. Hamostaseologie 2015, 35, 181–186. [Google Scholar] [CrossRef]
- Sulaj, A.; Tsaousi, M.; Karapati, E.; Pouliakis, A.; Iliodromiti, Z.; Boutsikou, T.; Valsami, S.; Iacovidou, N.; Politou, M.; Sokou, R. Reference Values of Thromboelastometry Parameters in Healthy Term Neonates Using NATEM in Cord Blood Samples. Children 2022, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Yada, K.; Nogami, K.; Ogiwara, K.; Shida, Y.; Furukawa, S.; Yaoi, H.; Takeyama, M.; Kasai, R.; Shima, M. Global coagulation function assessed by rotational thromboelastometry predicts coagulation-steady state in individual hemophilia A patients receiving emicizumab prophylaxis. Int. J. Hematol. 2019, 110, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Oda, T.; Tamura, N.; Shen, Y.; Kohmura-Kobayashi, Y.; Furuta-Isomura, N.; Yaguchi, C.; Uchida, T.; Suzuki, K.; Itoh, H.; Kanayama, N. Amniotic fluid as a potent activator of blood coagulation and platelet aggregation: Study with rotational thromboelastometry. Thromb. Res. 2018, 172, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Nogami, K.; Ogiwara, K.; Yada, K.; Minami, H.; Shima, M. Systematic monitoring of hemostatic management in hemophilia A patients with inhibitor in the perioperative period using rotational thromboelastometry. J. Thromb. Haemost. 2015, 13, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Getrajdman, C.; Shin, D.W.; Sison, M.; Katz, D. Baseline parameters for non-activated rotational thromboelastometry tests with and without heparinase in healthy pregnant women at term gestation. J. Clin. Anesth. 2021, 75, 110484. [Google Scholar] [CrossRef]
- Bar, J.; David, A.; Khader, T.; Mulcare, M.; Tedeschi, C. Assessing Coagulation by Rotational Thromboelastometry (ROTEM) in Rivaroxaban-Anticoagulated Blood Using Hemostatic Agents. Prehospital Disaster Med. 2017, 32, 580–587. [Google Scholar] [CrossRef]
- Yeom, R.S.; Wang, X.A.; Elia, E.; Yoon, U. Severe Congenital Factor VII Deficiency with Normal Perioperative Coagulation Profile Based on ROTEM Analysis in a Hepatectomy. Am. J. Case Rep. 2021, 22, e930245. [Google Scholar] [CrossRef]
- Parsi, K.; Exner, T.; Low, J.; Fung Ma, D.D.; Joseph, J.E. In vitro effects of detergent sclerosants on clot formation and fibrinolysis. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 267–277. [Google Scholar] [CrossRef][Green Version]
- Livnat, T.; Shenkman, B.; Martinowitz, U.; Zivelin, A.; Dardik, R.; Tamarin, I.; Mansharov, R.; Budnik, I.; Salomon, O. The impact of thrombin generation and rotation thromboelastometry on assessment of severity of factor XI deficiency. Thromb. Res. 2015, 136, 465–473. [Google Scholar] [CrossRef]
- Aires, R.B.; Soares, A.A.d.S.M.; Gomides, A.P.M.; Nicola, A.M.; Teixeira-Carvalho, A.; da Silva, D.L.M.; de Gois, E.T.; Xavier, F.D.; Martins, F.P.; Santos, G.P.J.; et al. Thromboelastometry demonstrates endogenous coagulation activation in nonsevere and severe COVID-19 patients and has applicability as a decision algorithm for intervention. PLoS ONE 2022, 17, e0262600. [Google Scholar] [CrossRef]
- Sidlik, R.; Strauss, T.; Morag, I.; Shenkman, B.; Tamarin, I.; Lubetsky, A.; Livnat, T.; Kenet, G. Assessment of Functional Fibrinolysis in Cord Blood Using Modified Thromboelastography. Pediatric Blood Cancer 2016, 63, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Theodoraki, M.; Sokou, R.; Valsami, S.; Iliodromiti, Z.; Pouliakis, A.; Parastatidou, S.; Karavana, G.; Ioakeimidis, G.; Georgiadou, P.; Iacovidou, N.; et al. Reference Values of Thrombolastometry Parameters in Healthy Term Neonates. Children 2020, 7, 259. [Google Scholar] [CrossRef] [PubMed]
- Sokou, R.; Foudoulaki-Paparizos, L.; Lytras, T.; Konstantinidi, A.; Theodoraki, M.; Lambadaridis, I.; Gounaris, A.; Valsami, S.; Politou, M.; Gialeraki, A.; et al. Reference ranges of thromboelastometry in healthy full-term and pre-term neonates. Clin. Chem. Lab. Med. 2017, 55, 1592–1597. [Google Scholar] [CrossRef] [PubMed]
- Van Der Weerd, N.; van Os, H.J.; Ali, M.; Schoones, J.W.; van den Maagdenberg, A.M.; Kruyt, N.D.; Siegerink, B.; Wermer, M.J. Sex Differences in Hemostatic Factors in Patients With Ischemic Stroke and the Relation With Migraine—A Systematic Review. Front. Cell. Neurosci. 2021, 15, 711604. [Google Scholar] [CrossRef]
- Tsantes, A.G.; Papadopoulos, D.V.; Trikoupis, I.G.; Tsante, K.A.; Mavrogenis, A.F.; Koulouvaris, P.; Piovani, D.; Kriebardis, A.G.; Gialeraki, A.; Nikolopoulos, G.K.; et al. Rotational Thromboelastometry Findings Are Associated with Symptomatic Venous Thromboembolic Complications after Hip Fracture Surgery. Clin. Orthop. Relat. Res. 2021, 479, 2457–2467. [Google Scholar] [CrossRef]
- Tsantes, A.G.; Trikoupis, I.G.; Papadopoulos, D.V.; Tsante, K.A.; Mavrogenis, A.F.; Koulouvaris, P.; Savvidou, O.D.; Kontogeorgakos, V.A.; Piovani, D.; Kriebardis, A.G.; et al. Higher coagulation activity in hip fracture patients: A case-control study using rotational thromboelastometry. Int. J. Lab. Hematol. 2021, 43, 477–484. [Google Scholar] [CrossRef]
- Tsantes, A.G.; Papadopoulos, D.V.; Trikoupis, I.G.; Tsante, K.A.; Mavrogenis, A.F.; Koulouvaris, P.; Vaiopoulos, A.G.; Piovani, D.; Nikolopoulos, G.K.; Kokoris, S.I.; et al. The Prognostic Performance of Rotational Thromboelastometry for Excessive Bleeding and Increased Transfusion Requirements in Hip Fracture Surgeries. Thromb. Haemost. 2021. published online ahead of print. [Google Scholar] [CrossRef]
- Lang, T.; Bauters, A.; Braun, S.L.; Pötzsch, B.; von Pape, K.W.; Kolde, H.J.; Lakner, M. Multi-centre investigation on reference ranges for ROTEM thromboelastometry. Blood Coagul. Fibrinolysis 2005, 16, 301–310. [Google Scholar] [CrossRef]
- Theusinger, O.M.; Nürnberg, J.; Asmis, L.M.; Seifert, B.; Spahn, D.R. Rotation thromboelastometry (ROTEM) stability and reproducibility over time. Eur. J. Cardio-Thorac. Surg. 2010, 37, 677–683. [Google Scholar] [CrossRef]
- Vavrecka, M.; Petrasek, R.; Komarkova, A. Citrate metabolism in human erythrocytes; preliminary report. Českoslov. Gastroenterol. Výživa 1957, 11, 383–384. [Google Scholar]
- Karpatkin, S. Studies on human platelet glycolysis. Effect of glucose, cyanide, insulin, citrate, and agglutination and contraction on platelet glycolysis. J. Clin. Investig. 1967, 46, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Davie, E.W.; Fujikawa, K. Basic mechanisms in blood coagulation. Annu. Rev. Biochem. 1975, 44, 799–829. [Google Scholar] [CrossRef] [PubMed]
- Van Der Meijden, P.E.; Van Schilfgaarde, M.; Van Oerle, R.; Renné, T.; ten Cate, H.; Spronk, H.M. Platelet- and erythrocyte-derived microparticles trigger thrombin generation via factor XIIa. J. Thromb. Haemost. 2012, 10, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Ayers, L.; Kohler, M.; Harrison, P.; Sargent, I.; Dragovic, R.; Schaap, M.; Nieuwland, R.; Brooks, S.A.; Ferry, B. Measurement of circulating cell-derived microparticles by flow cytometry: Sources of variability within the assay. Thromb. Res. 2011, 127, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Simurda, T.; Casini, A.; Stasko, J.; Hudecek, J.; Skornova, I.; Vilar, R.; Neerman-Arbez, M.; Kubisz, P. Perioperative management of a severe congenital hypofibrinogenemia with thrombotic phenotype. Thromb. Res. 2020, 188, 1–4. [Google Scholar] [CrossRef]
- Tsantes, A.G.; Trikoupis, I.G.; Papadopoulos, D.V.; Goumenos, S.; Piovani, D.; Nikolopoulos, G.K.; Gialeraki, A.; Bonovas, S.; Papagelopoulos, P.J.; Kontogeorgakos, V.A.; et al. The Safety and Efficacy of Tranexamic Acid in Oncology Patients Undergoing Endoprosthetic Reconstruction and a ROTEM-Based Evaluation of Their Hemostatic Profile: A Pilot Study. Cancers 2021, 13, 3951. [Google Scholar] [CrossRef] [PubMed]
- Mittermayr, M.; Margreiter, J.; Velik-Salchner, C.; Klingler, A.; Streif, W.; Fries, D.; Innerhofer, P. Effects of protamine and heparin can be detected and easily differentiated by modified thrombelastography (Rotem®): An in vitro study. Br. J. Anaesth. 2005, 95, 310–316. [Google Scholar] [CrossRef]
- Kuiper, G.J.A.J.M.; Kleinegris, M.-C.F.; van Oerle, R.; Spronk, H.M.H.; Lancé, M.D.; ten Cate, H.; Henskens, Y.M.C. Validation of a modified thromboelastometry approach to detect changes in fibrinolytic activity. Thromb. J. 2016, 14, 1. [Google Scholar] [CrossRef]
- Chitlur, M.; Rivard, G.E.; Lillicrap, D.; Mann, K.; Shima, M.; Young, G. Recommendations for performing thromboelastography/thromboelastometry in hemophilia: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2014, 12, 103–106. [Google Scholar] [CrossRef]
| Author, Publication Year, Country | Study Design, Centers, Time Period | Study Population | Objective | Results/Conclusions |
|---|---|---|---|---|
| Oda, 2018 Japan [34] | In vitro experimental | 21 (pregnant women with amniotic fluid infection) | NATEM in pregnant women with amniotic fluid infection | Based on the results that came from the NATEM and FIBTEM assay application, the authors came to the conclusion that amniotic fluid enhanced blood coagulation, by induced thrombin generation and activated platelet aggregation, without changes to fibrin formation and stability. |
| Bar, 2017 USA [37] | Single center, prospective, experimental pilot | 8 (adult patients) | NATEM in patients receiving Rivaroxaban | The NATEM test could detect changes in coagulation parameters induced by hemostatic agents in patients receiving Rivaroxaban/chitosan or kaolin-based hemostatic agents may be effective in improving these patients’ hemostasis profile. |
| Bagge, 2016 Sweden [23] | Single center, prospective, pilot, experimental | 35 (healthy adults) | Effects of naturopathic medicines on NATEM | Standard intake of 1260 mg Ω-3 of polyunsaturated fatty acids (fish oil) daily may decrease platelet aggregation and clot formation. |
| Elvstam, 2016 Sweden [18] | Single center, experimental | 10 (healthy adults) | NATEM and dilutional coagulopathy | The NATEM assay has been described as the most sensitive protocol for detecting endogenous tissue factors, and it may be preferred for monitoring the Prothrombin complex concentrate (PCC) effects of dilutional coagulopathy. |
| Zipperle, 2013 Austria [16] | In vitro experimental | Healthy adult volunteers | NATEM and adherent endothelial cells | Adherent endothelial cells participate in the process of hemostasis and fibrinolysis and could be incorporated into ROTEM assays via coated microbeads. In the NATEM assay, only a significant reduction in CT value was observed in the presence of unstimulated Endothelial cells, while on the other hand, CFT, a-angle, A30 and MCF values did not show any difference. |
| Scharbert, 2012 Austria [29] | Experimental | 10 (healthy adults) | NATEM and heparin-induced effects of prothrombin complex | Heparin effect was significant in thromboelastometry at clinically relevant PCC concentrations. |
| Parsi, 2011 Australia [39] | Single center, experimental | Healthy adults | NATEM and sclerosants | Detergent sclerosants indicated a trimodal reaction on clot formation, initiating strong clots at low concentrations, weak clots at midrange concentrations and preventing clot formation at higher concentrations. |
| Author, Publication Year, Country | Study Design, Centers, Time Period | Study Population | Objective | Results/Conclusions |
|---|---|---|---|---|
| Sulaj, 2021, Greece [32] | Single center, prospective, observational, March 2021–November 2021 | 189 healthy-term neonates | NATEM parameters in cord blood samples of healthy-term neonates. | Demonstrate reference ranges for healthy-term neonates in NATEM assay |
| Getrajdman, 2021 USA [36] | Single center, prospective, observational, July 2019–February 2021 | 120 (healthy pregnant women) | NATEM in healthy pregnant women | NATEM method could be useful in monitoring anticoagulant treatment in pregnant women |
| Lechien, 2021 Germany [30] | Observational | 40 (30 full term pregnant women vs. 10 healthy non pregnant women) | NATEM and tranexamic acid in Postpartum hemorrhage | Pregnant women have a higher fibrinolytic potential compared with nonpregnant women |
| Jilma-Stohlawetz, 2017 Austria [25] | Single center, observational | 42 (healthy adults) −10 healthy, BMI 23 ± 3 kg/m2 −17 healthy, BMI > 30 kg/m2 −15 healthy (12 Male/3 Female) | Circadian variation, BMI evaluation and effect of LMWH and recombinant antithrombin on coagulation and fibrinolysis | A high daily fluctuation and an influence of high BMI on clotting parameters was found. NATEM assay is sensitive to the prothrombotic phenotype in obese individuals, as well as the within one day changes of hemostatic profile of healthy adults which reflect the circadian variability of components involved in hemostasis. |
| Sidlik, 2016 Israel [42] | Single center, prospective, cohort | 124 (101 neonates and 23 healthy adults) | NATEM in neonatal population | Lysis parameters such as lysis index at 30 min (LI30) and time to lysis (TTL), tested with increased tPA-concentration, were found significantly lower in neonate’s cord blood fibrinolysis was more rapid in the newborns |
| Schneider, 2015 Germany [31] | Single center, observational | 132 (healthy adults) | NATEM and healthy adults | The age-related changes in calibrated automated thrombogram (CAT) and in ROTEM variables among adults are not linear |
| Schöchl, 2012 Austria/Germany [15] | Observational | 46 (25 baboons and 21 human volunteers) | NATEM between baboons and humans | Similarities in thromboelastometric measurements between humans and baboons/a higher resistance of the baboon clot to fibrinolytic breakdown measured in NATEM |
| Spiezia, 2010 Italy [24] | Observational | 93 (43 monkeys vs. 50 healthy adults) | Reference values for NATEM in monkeys | A hypercoagulable profile in monkeys as compared to humans/probably more difficult is xenotransplantation in monkeys than in humans |
| Author, Publication Year, Country | Study Design, Centers, Time Period | Study Population | Objective | Results/Conclusions |
|---|---|---|---|---|
| Rossetto, 2013 Italy [14] | Single center, observational, January 2010–September 2012 | 63 (49 cirrhotic or non-cirrhotic patients with or without PVT and 14 healthy adults) | NATEM in patients with PVT cirrhotic or non-cirrhotic | There were no significant differences in NATEM assays and in traditional coagulative parameters between the two groups. |
| Treliński,2014 Poland [17] | Single center, observational | 46 (26 patients with MM and 20 healthy adults) | NATEM and patients with multiple myeloma | A NATEM test could contribute to identify a prothrombotic situation in patients with MM. |
| Spiezia, 2015 Italy [20] | Single center, case–control, December 2013–September 2014 | 90 (30 pregnant with preeclampsia vs. 60 pregnant healthy women) | NATEM in pregnant women with preeclampsia | A prothrombotic state in pregnant women with preeclampsia was found compared to healthy pregnant women. |
| Meesters, 2015 Netherlands/Germany [13] | Multicenter, cohort | 44 (14 healthy adults and 30 patients admitted to ICU) | NATEM in citrate stored blood | A NATEM test should be carried out directly after blood sampling. |
| Livnat, 2015 Russia [40] | Cohort, January 2013–February 2014 | 41 (25 patients vs. 16 healthy adults) | NATEM and patients with FXI deficiency | A NATEM assay was incapable of predicting bleeding predisposition of the patients. |
| Furukawa, 2015 Japan [35] | Single center, cohort | 28 (8 patients vs. 20 healthy adults) | NATEM and hemophilia A | A NATEM could offer a promising strategy concerning bypassing therapy in HA patients receiving inhibitors. |
| Aires, 2022 Brazil [41] | Single center, observational, 1 August and 30 September 2020 | 61 (41 patients with COVID-19 and 20 healthy adults) | NATEM and patients with COVID-19 | The altered NATEM–CT would represent a thromboelastometric parameter useful as a predictor of disease severity. |
| Author, Publication year, Country | Study Design, Centers, Time Period | Study Population | Objective | Results/Conclusions |
|---|---|---|---|---|
| Yeom, 2021 USA [38] | Case report | 1 (patient female with severe FVII deficiency) | NATEM and patient with FVII deficiency | NATEM analysis indicated a normal coagulation condition. |
| Shalaby, 2020 Italy [19] | Single center, cohort | 45 (cirrhotic patients with PVT) | NATEM and PVT in cirrhotic patients | NATEM assay highlighted the presence of a heparin-like effect in portal blood reflecting the endothelial dysfunction in the portal vein. |
| MacDonald, 2019 UK [27] | Large cohort, Single center, 2001–October 2018 | 124 (patients with unclassified bleeding disorder) | NATEM and patients with unclassified bleeding disorder | NATEM assay was not able to contribute to the classification of hemorrhagic patients. |
| Yada, 2019 Japan [33] | Single center, cohort, May 2013–February 2016 | 7 (adult patients with HA receiving emicizumab) | NATEM in patients with hemophilia A who received emicizumab | NATEM could be useful for the evaluation of hemostasis status in patients with Hemophilia A receiving emicizumab. |
| Brearton, 2019 UK [21] | Single center, observational | 86 (patients undergoing abdominal, orthopedic or vascular surgery) | Comparison of NATEM and VCT | VCM system is not only an easy-to-use method but also capable of making measurements of patients’ coagulation system. There was reported a good correlation between the most VCM and NATEM parameters (CT, A10, A20 and MCF). |
| Silverberg, 2017 Sweden/Austria [22] | Prospective, unblinded observational cohort, September–December 2015 | 38 (patients undergoing elective brain tumor resection/biopsy) | NATEM and comparison of citrated and fresh whole blood during elective neurosurgery | The citrated blood indicated a hypercoagulative response as compared to the fresh whole blood parameters. |
| Durila, 2016 Prague [26] | Case report | 1 (patient with ards and infection) | NATEM and detection of bleeding disorder in a patient with aRSD | NATEM was capable of distinguishing fibrinolysis in a bleeding patient, in contrast to activated assays. |
| Lancé, 2012 Netherlands [28] | Observational | 44 (patients scheduled for elective cardiothoracic surgery) | NATEM and pneumatic tube system (PTS) | Among the ROTEM analysis, NATEM methods is the most sensitive tool to reflect small changes in the coagulation system. The rapid transport in pneumatic systems may influence reliability of results by platelet activation and/or contact activation as it expressed with a shortened CT revealed in NATEM assay, which reflects the initiation of clot forming. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgiadou, P.; Sokou, R.; Tsantes, A.G.; Parastatidou, S.; Konstantinidi, A.; Houhoula, D.; Kokoris, S.; Iacovidou, N.; Tsantes, A.E. The Non-Activated Thromboelastometry (NATEM) Assay’s Application among Adults and Neonatal/Pediatric Population: A Systematic Review. Diagnostics 2022, 12, 658. https://doi.org/10.3390/diagnostics12030658
Georgiadou P, Sokou R, Tsantes AG, Parastatidou S, Konstantinidi A, Houhoula D, Kokoris S, Iacovidou N, Tsantes AE. The Non-Activated Thromboelastometry (NATEM) Assay’s Application among Adults and Neonatal/Pediatric Population: A Systematic Review. Diagnostics. 2022; 12(3):658. https://doi.org/10.3390/diagnostics12030658
Chicago/Turabian StyleGeorgiadou, Petroula, Rozeta Sokou, Andreas G. Tsantes, Stavroula Parastatidou, Aikaterini Konstantinidi, Dimitra Houhoula, Styliani Kokoris, Nicoletta Iacovidou, and Argirios E. Tsantes. 2022. "The Non-Activated Thromboelastometry (NATEM) Assay’s Application among Adults and Neonatal/Pediatric Population: A Systematic Review" Diagnostics 12, no. 3: 658. https://doi.org/10.3390/diagnostics12030658
APA StyleGeorgiadou, P., Sokou, R., Tsantes, A. G., Parastatidou, S., Konstantinidi, A., Houhoula, D., Kokoris, S., Iacovidou, N., & Tsantes, A. E. (2022). The Non-Activated Thromboelastometry (NATEM) Assay’s Application among Adults and Neonatal/Pediatric Population: A Systematic Review. Diagnostics, 12(3), 658. https://doi.org/10.3390/diagnostics12030658










