Mindfulness-Based Virtual Reality Intervention for Children and Young Adults with Inflammatory Bowel Disease: A Pilot Feasibility and Acceptability Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
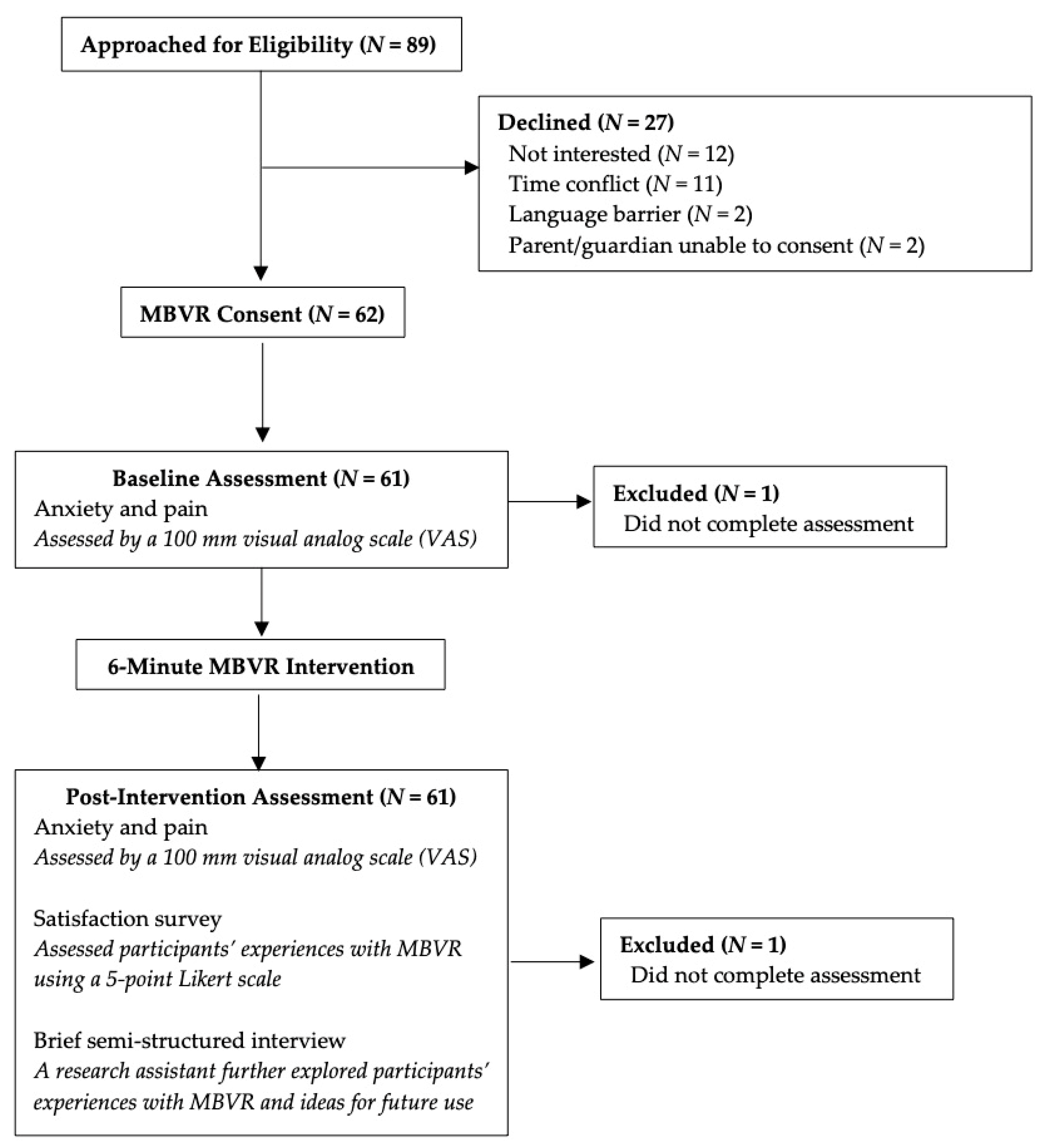
2.2. Procedures
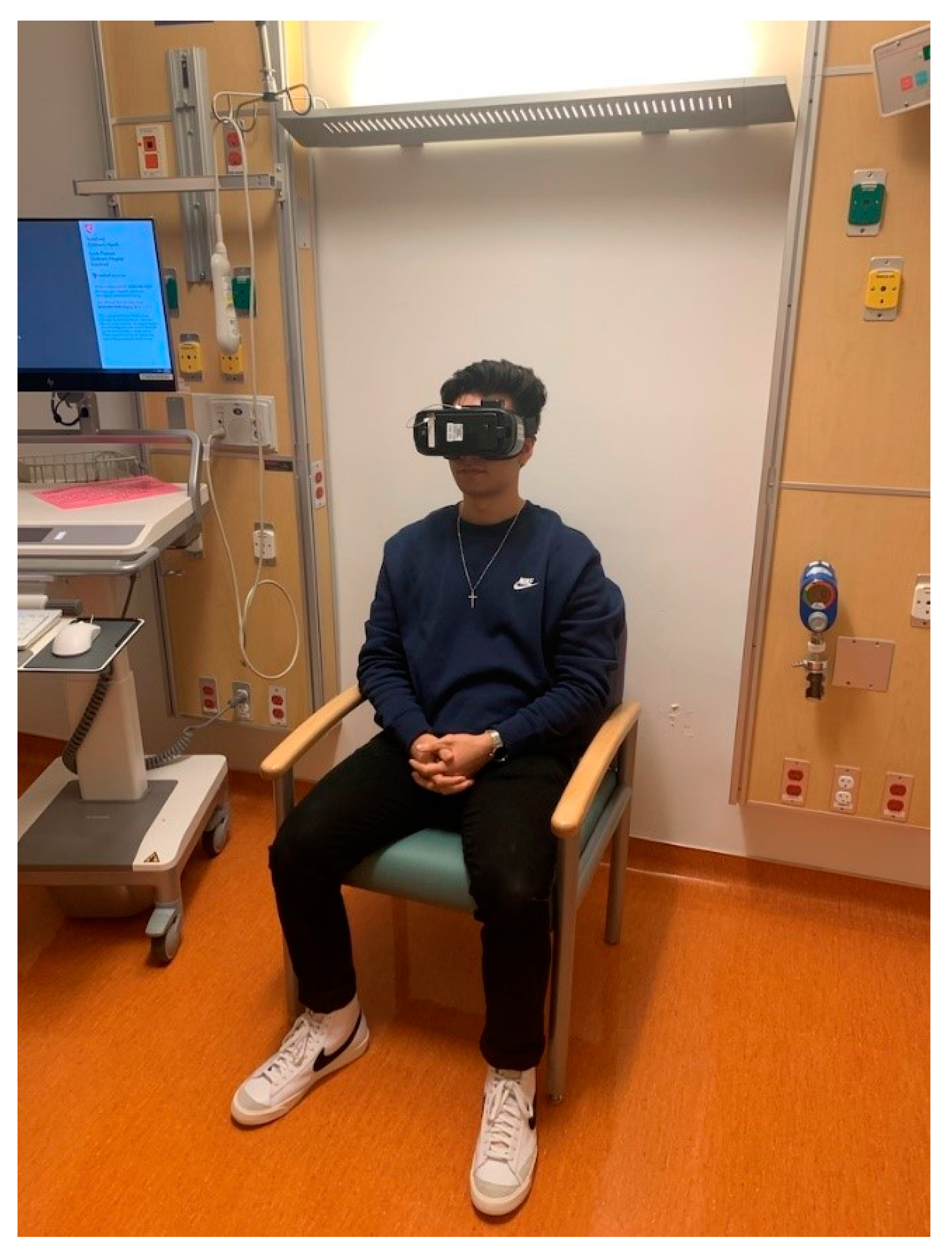
2.3. Intervention
2.4. Measures
2.5. Data Analysis
3. Results
3.1. Descriptive Statistics
3.2. Feasibility and Acceptability
3.3. Preliminary Efficacy
3.4. Exploratory Analyses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosen, M.J.; Dhawan, A.; Saeed, S.A. Inflammatory Bowel Disease in Children and Adolescents. JAMA Pediatr. 2015, 169, 1053–1060. [Google Scholar] [CrossRef]
- Baldassano, R.N.; Piccoli, D.A. Inflammatory bowel disease in pediatric and adolescent patients. Gastroenterol. Clin. N. Am. 1999, 28, 445–458. [Google Scholar] [CrossRef]
- Abramson, O.; Durant, M.; Mow, W.; Finley, A.; Kodali, P.; Wong, A.; Tavares, V.; McCroskey, E.; Liu, L.; Lewis, J.D.; et al. Incidence, prevalence, and time trends of pediatric inflammatory bowel disease in Northern California, 1996 to 2006. J. Pediatr. 2010, 157, 233–239.e231. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Manne, S.; Treem, W.R.; Bennett, D. Prevalence of Inflammatory Bowel Disease in Pediatric and Adult Populations: Recent Estimates From Large National Databases in the United States, 2007–2016. Inflamm. Bowel Dis. 2020, 26, 619–625. [Google Scholar] [CrossRef]
- Greenley, R.N.; Hommel, K.A.; Nebel, J.; Raboin, T.; Li, S.H.; Simpson, P.; Mackner, L. A meta-analytic review of the psychosocial adjustment of youth with inflammatory bowel disease. J. Pediatr. Psychol. 2010, 35, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Mackner, L.M.; Greenley, R.N.; Szigethy, E.; Herzer, M.; Deer, K.; Hommel, K.A. Psychosocial issues in pediatric inflammatory bowel disease: Report of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Stapersma, L.; van den Brink, G.; Szigethy, E.M.; Escher, J.C.; Utens, E. Systematic review with meta-analysis: Anxiety and depression in children and adolescents with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2018, 48, 496–506. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Singh, S.; Graff, L.A.; Walker, J.R.; Miller, N.; Cheang, M. A prospective population-based study of triggers of symptomatic flares in IBD. Am. J. Gastroenterol. 2010, 105, 1994–2002. [Google Scholar] [CrossRef]
- Hommel, K.A.; Greenley, R.N.; Maddux, M.H.; Gray, W.N.; Mackner, L.M. Self-management in pediatric inflammatory bowel disease: A clinical report of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 250–257. [Google Scholar] [CrossRef]
- Mikocka-Walus, A.; Pittet, V.; Rossel, J.B.; von Känel, R. Symptoms of Depression and Anxiety Are Independently Associated With Clinical Recurrence of Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2016, 14, 829–835.e821. [Google Scholar] [CrossRef]
- Gracie, D.J.; Guthrie, E.A.; Hamlin, P.J.; Ford, A.C. Bi-directionality of Brain-Gut Interactions in Patients With Inflammatory Bowel Disease. Gastroenterology 2018, 154, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Black, D.S.; Slavich, G.M. Mindfulness meditation and the immune system: A systematic review of randomized controlled trials. Ann. N. Y. Acad. Sci. 2016, 1373, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Hilton, L.; Hempel, S.; Ewing, B.A.; Apaydin, E.; Xenakis, L.; Newberry, S.; Colaiaco, B.; Maher, A.R.; Shanman, R.M.; Sorbero, M.E.; et al. Mindfulness Meditation for Chronic Pain: Systematic Review and Meta-analysis. Ann. Behav. Med. 2017, 51, 199–213. [Google Scholar] [CrossRef]
- Ali, A.; Weiss, T.R.; Dutton, A.; McKee, D.; Jones, K.D.; Kashikar-Zuck, S.; Silverman, W.K.; Shapiro, E.D. Mindfulness-Based Stress Reduction for Adolescents with Functional Somatic Syndromes: A Pilot Cohort Study. J. Pediatr. 2017, 183, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Baer, R. Mindfulness-Based Treatment Approaches: A Clinician’s Guide; Elsevier Science: Burlington, MA, USA, 2006. [Google Scholar] [CrossRef]
- Kabat-Zinn, J. Full Catastrophe Living; Delacorte Press: New York, NY, USA, 1990; p. 453. [Google Scholar]
- Hood, M.M.; Jedel, S. Mindfulness-Based Interventions in Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 2017, 46, 859–874. [Google Scholar] [CrossRef]
- Ludwig, D.S.; Kabat-Zinn, J. Mindfulness in medicine. JAMA 2008, 300, 1350–1352. [Google Scholar] [CrossRef]
- Ewais, T.; Begun, J.; Kenny, M.; Rickett, K.; Hay, K.; Ajilchi, B.; Kisely, S. A systematic review and meta-analysis of mindfulness based interventions and yoga in inflammatory bowel disease. J. Psychosom. Res. 2019, 116, 44–53. [Google Scholar] [CrossRef]
- Ahola Kohut, S.; Stinson, J.; Jelen, A.; Ruskin, D. Feasibility and Acceptability of a Mindfulness-Based Group Intervention for Adolescents with Inflammatory Bowel Disease. J. Clin. Psychol. Med. Settings 2020, 27, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Ewais, T.; Begun, J.; Kenny, M.; Headey, A.; Tefay, M.; Kisely, S. Mindfulness-based cognitive therapy experiences in youth with inflammatory bowel disease and depression: Findings from a mixed methods qualitative study. BMJ Open 2020, 10, e041140. [Google Scholar] [CrossRef]
- Goyal, M.; Singh, S.; Sibinga, E.M.; Gould, N.F.; Rowland-Seymour, A.; Sharma, R.; Berger, Z.; Sleicher, D.; Maron, D.D.; Shihab, H.M.; et al. Meditation programs for psychological stress and well-being: A systematic review and meta-analysis. JAMA Intern. Med. 2014, 174, 357–368. [Google Scholar] [CrossRef]
- Kral, T.R.A.; Schuyler, B.S.; Mumford, J.A.; Rosenkranz, M.A.; Lutz, A.; Davidson, R.J. Impact of short- and long-term mindfulness meditation training on amygdala reactivity to emotional stimuli. Neuroimage 2018, 181, 301–313. [Google Scholar] [CrossRef]
- McClintock, A.S.; McCarrick, S.M.; Garland, E.L.; Zeidan, F.; Zgierska, A.E. Brief Mindfulness-Based Interventions for Acute and Chronic Pain: A Systematic Review. J. Altern. Complement. Med. 2019, 25, 265–278. [Google Scholar] [CrossRef]
- Schumer, M.C.; Lindsay, E.K.; Creswell, J.D. Brief mindfulness training for negative affectivity: A systematic review and meta-analysis. J. Consult. Clin. Psychol. 2018, 86, 569–583. [Google Scholar] [CrossRef]
- Howarth, A.; Smith, J.G.; Perkins-Porras, L.; Ussher, M. Effects of Brief Mindfulness-Based Interventions on Health-Related Outcomes: A Systematic Review. Mindfulness 2019, 10, 1957–1968. [Google Scholar] [CrossRef]
- Perrier, M.F.; Gurgel-Juarez, N.; Flowers, H.L.; McCormick, A.; Short, S.J. Mindfulness-Based Interventions for children and adolescents across all settings: A scoping review protocol. Syst. Rev. 2020, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Eijlers, R.; Dierckx, B.; Staals, L.M.; Berghmans, J.M.; van der Schroeff, M.P.; Strabbing, E.M.; Wijnen, R.M.H.; Hillegers, M.H.J.; Legerstee, J.S.; Utens, E. Virtual reality exposure before elective day care surgery to reduce anxiety and pain in children: A randomised controlled trial. Eur. J. Anaesthesiol. 2019, 36, 728–737. [Google Scholar] [CrossRef]
- Ford, C.G.; Manegold, E.M.; Randall, C.L.; Aballay, A.M.; Duncan, C.L. Assessing the feasibility of implementing low-cost Virtual Reality therapy during routine burn care. Burns 2018, 44, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Griffin, A.; Wilson, L.; Feinstein, A.B.; Bortz, A.; Heirich, M.S.; Gilkerson, R.; Wagner, J.F.; Menendez, M.; Caruso, T.J.; Rodriguez, S.; et al. Virtual Reality in Pain Rehabilitation for Youth With Chronic Pain: Pilot Feasibility Study. JMIR Rehabil. Assist. Technol. 2020, 7, e22620. [Google Scholar] [CrossRef]
- Navarro-Haro, M.V.; Modrego-Alarcón, M.; Hoffman, H.G.; López-Montoyo, A.; Navarro-Gil, M.; Montero-Marin, J.; García-Palacios, A.; Borao, L.; García-Campayo, J. Evaluation of a Mindfulness-Based Intervention With and Without Virtual Reality Dialectical Behavior Therapy(®) Mindfulness Skills Training for the Treatment of Generalized Anxiety Disorder in Primary Care: A Pilot Study. Front. Psychol. 2019, 10, 55. [Google Scholar] [CrossRef]
- Seabrook, E.; Kelly, R.; Foley, F.; Theiler, S.; Thomas, N.; Wadley, G.; Nedeljkovic, M. Understanding How Virtual Reality Can Support Mindfulness Practice: Mixed Methods Study. J. Med. Internet Res. 2020, 22, e16106. [Google Scholar] [CrossRef]
- Sliwinski, J.; Katsikitis, M.; Jones, C.M. A review of interactive technologies as support tools for the cultivation of mindfulness. Mindfulness 2017, 8, 1150–1159. [Google Scholar] [CrossRef]
- Navarro-Haro, M.V.; López-Del-Hoyo, Y.; Campos, D.; Linehan, M.M.; Hoffman, H.G.; García-Palacios, A.; Modrego-Alarcón, M.; Borao, L.; García-Campayo, J. Meditation experts try Virtual Reality Mindfulness: A pilot study evaluation of the feasibility and acceptability of Virtual Reality to facilitate mindfulness practice in people attending a Mindfulness conference. PLoS ONE 2017, 12, e0187777. [Google Scholar] [CrossRef] [PubMed]
- Chandrasiri, A.; Collett, J.; Fassbender, E.; De Foe, A. A Virtual Reality approach to mindfulness skills training. Virtual Real. 2020, 24, 143–149. [Google Scholar] [CrossRef]
- Chavez, L.J.; Kelleher, K.; Slesnick, N.; Holowacz, E.; Luthy, E.; Moore, L.; Ford, J. Virtual Reality Meditation Among Youth Experiencing Homelessness: Pilot Randomized Controlled Trial of Feasibility. JMIR Ment. Health 2020, 7, e18244. [Google Scholar] [CrossRef] [PubMed]
- Petter, M.; Chambers, C.T.; MacLaren Chorney, J. The effects of mindfulness-based attention on cold pressor pain in children. Pain Res. Manag. 2013, 18, 39–45. [Google Scholar] [CrossRef]
- Kabat-Zinn, J. Full Catastrophe Living (Revised Edition): Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness; Bantam (Random House Publishing Group): New York, NY, USA, 2013. [Google Scholar]
- Bringuier, S.; Dadure, C.; Raux, O.; Dubois, A.; Picot, M.C.; Capdevila, X. The perioperative validity of the visual analog anxiety scale in children: A discriminant and useful instrument in routine clinical practice to optimize postoperative pain management. Anesth. Analg. 2009, 109, 737–744. [Google Scholar] [CrossRef]
- Cohen, L.L.; Lemanek, K.; Blount, R.L.; Dahlquist, L.M.; Lim, C.S.; Palermo, T.M.; McKenna, K.D.; Weiss, K.E. Evidence-based Assessment of Pediatric Pain. J. Pediatr. Psychol. 2007, 33, 939–955. [Google Scholar] [CrossRef]
- Powell, C.V.; Kelly, A.M.; Williams, A. Determining the minimum clinically significant difference in visual analog pain score for children. Ann. Emerg. Med. 2001, 37, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Wren, A.A.; Bensen, R.; Sceats, L.; Dehghan, M.; Yu, H.; Wong, J.J.; MacIsaac, D.; Sellers, Z.M.; Kin, C.; Park, K.T. Starting Young: Trends in Opioid Therapy Among US Adolescents and Young Adults With Inflammatory Bowel Disease in the Truven MarketScan Database Between 2007 and 2015. Inflamm. Bowel Dis. 2018, 24, 2093–2103. [Google Scholar] [CrossRef] [PubMed]
- Greenley, R.N.; Kunz, J.H.; Schurman, J.V.; Swanson, E. Abdominal pain and health related quality of life in pediatric inflammatory bowel disease. J. Pediatr. Psychol. 2013, 38, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Watson, K.L., Jr.; Kim, S.C.; Boyle, B.M.; Saps, M. Prevalence and Impact of Functional Abdominal Pain Disorders in Children With Inflammatory Bowel Diseases (IBD-FAPD). J. Pediatr. Gastroenterol. Nutr. 2017, 65, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Rufo, P.A.; Denson, L.A.; Sylvester, F.A.; Szigethy, E.; Sathya, P.; Lu, Y.; Wahbeh, G.T.; Sena, L.M.; Faubion, W.A. Health supervision in the management of children and adolescents with IBD: NASPGHAN recommendations. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Donker, T.; Cornelisz, I.; van Klaveren, C.; van Straten, A.; Carlbring, P.; Cuijpers, P.; van Gelder, J.L. Effectiveness of Self-guided App-Based Virtual Reality Cognitive Behavior Therapy for Acrophobia: A Randomized Clinical Trial. JAMA Psychiatry 2019, 76, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Cikajlo, I.; Cizman Staba, U.; Vrhovac, S.; Larkin, F.; Roddy, M. A Cloud-Based Virtual Reality App for a Novel Telemindfulness Service: Rationale, Design and Feasibility Evaluation. JMIR Res. Protoc. 2017, 6, e108. [Google Scholar] [CrossRef] [PubMed]

| MBVR Experience | Representative Quotations |
|---|---|
| Relaxation | “Really good for soothing Crohn’s.” “Felt like a new world; Relaxing scenery; Peaceful.” “Feel calmer.” “Scenery was relaxing; Loved the aurora lights.” “It was relaxing when looking at the nature and how that was incorporated into the controlled breathing with the lights.” |
| Enjoyment | “I wish they had this technology or other anxiety/pain management offered to me when I was a younger patient.” “I really enjoyed it, I’d like a Gear VR headset for Christmas.” “I got happy symptoms, I would use it every day.” “Very effective… I experienced mindfulness before, but it was never as effective as this.” “MBVR could help in getting used to mindfulness while my mind still wanders.” “I have an HTC vive. I liked it and might try other relaxing environments now.” “I’d recommend it to friends and other patients with IBD.” |
| Future Use | |
| Clinical | “Would be helpful for people in the hospital or undergoing procedures or injections (e.g., blood draws, MRI, colonoscopy, infusion).” “Would be beneficial before and after stressful procedures.” “Would be helpful before going to surgery, MRI, CT, or other procedures and if someone was afraid of needles.” “Would be good before blood draws or anything with needles.” “Would be helpful for patients at diagnosis.” |
| Non-clinical (e.g., daily life) | “Would be helpful before exams and matches.” “Would be helpful before work and to start the day and reduce stress.” “Would be helpful prior to tests in school.” “MBVR would be helpful for parents and siblings too.” “A take home version for patients and all family members.” “There should be a study including parents due to the overwhelming events that they go through with their child with IBD.” |
| User experience | “IBD kids could choose their setting (i.e., mountains, beach, forest, etc.).” “Create a more calming environment for MBVR; Turn off lights and make the room quiet to get rid of external stimuli; Use headphones.” “VR could be more interactive like involving hands to touch the waterfall or animals during mindfulness; Would be cool if you could see yourself walking in nature.” |
| Satisfaction Questions Rated on a 1 (Poor/Not at All) to 5 (Ideal/Excellent) Scale | Mean | SD |
|---|---|---|
| Participants < 18 years old | ||
| Enjoyment | 4.43 | 0.80 |
| Relaxation | 4.35 | 0.79 |
| Length of MBVR | 3.88 | 1.17 |
| Home use | 4.05 | 1.10 |
| Participants≥ 18 years old | ||
| Enjoyment | 4.26 | 1.05 |
| Relaxation | 4.37 | 0.96 |
| Length of MBVR | 4.26 | 0.99 |
| Home use | 4.21 | 1.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wren, A.A.; Neiman, N.; Caruso, T.J.; Rodriguez, S.; Taylor, K.; Madill, M.; Rives, H.; Nguyen, L. Mindfulness-Based Virtual Reality Intervention for Children and Young Adults with Inflammatory Bowel Disease: A Pilot Feasibility and Acceptability Study. Children 2021, 8, 368. https://doi.org/10.3390/children8050368
Wren AA, Neiman N, Caruso TJ, Rodriguez S, Taylor K, Madill M, Rives H, Nguyen L. Mindfulness-Based Virtual Reality Intervention for Children and Young Adults with Inflammatory Bowel Disease: A Pilot Feasibility and Acceptability Study. Children. 2021; 8(5):368. https://doi.org/10.3390/children8050368
Chicago/Turabian StyleWren, Anava A., Nicole Neiman, Thomas J. Caruso, Samuel Rodriguez, Katherine Taylor, Martine Madill, Hal Rives, and Linda Nguyen. 2021. "Mindfulness-Based Virtual Reality Intervention for Children and Young Adults with Inflammatory Bowel Disease: A Pilot Feasibility and Acceptability Study" Children 8, no. 5: 368. https://doi.org/10.3390/children8050368
APA StyleWren, A. A., Neiman, N., Caruso, T. J., Rodriguez, S., Taylor, K., Madill, M., Rives, H., & Nguyen, L. (2021). Mindfulness-Based Virtual Reality Intervention for Children and Young Adults with Inflammatory Bowel Disease: A Pilot Feasibility and Acceptability Study. Children, 8(5), 368. https://doi.org/10.3390/children8050368







