Abstract
Autophagy is an intracellular process that targets intracellular pathogens for lysosomal degradation. Autophagy is tightly controlled at transcriptional and post-translational levels. Nuclear receptors (NRs) are a family of transcriptional factors that regulate the expression of gene sets involved in, for example, metabolic and immune homeostasis. Several NRs show promise as host-directed anti-infectives through the modulation of autophagy activities by their natural ligands or small molecules (agonists/antagonists). Here, we review the roles and mechanisms of NRs (vitamin D receptors, estrogen receptors, estrogen-related receptors, and peroxisome proliferator-activated receptors) in linking immunity and autophagy during infection. We also discuss the potential of emerging NRs (REV-ERBs, retinoic acid receptors, retinoic acid-related orphan receptors, liver X receptors, farnesoid X receptors, and thyroid hormone receptors) as candidate antimicrobials. The identification of novel roles and mechanisms for NRs will enable the development of autophagy-adjunctive therapeutics for emerging and re-emerging infectious diseases.
1. Introduction
Autophagy, an intracellular process, is a defense against intracellular pathogens involving lysosomal degradation [,,,,,]. The signaling factors and mechanisms through which invading microbes are selectively targeted by xenophagy or LC3-associated phagocytosis (LAP) have been reported []. A variety of pathogens—including Mycobacterium tuberculosis (Mtb), Salmonella enterica serovar Typhimurium, Listeria monocytogenes, Legionella pneumophila, Anaplasma phagocytophilum, Coxiella burnetii, Francisella tularensis, and Brucella spp.—can be targeted by autophagy []. These and other pathogens, including viruses and protozoa, have evolved means of evading or circumventing autophagy, enabling their replication within host cells [,,,,,,,,].
The nuclear receptor (NR) superfamily proteins regulate genes involved in cell survival, signaling, metabolism, and reproduction [,,]. NRs promote host defense against infections by regulating innate immunity, the transcription of antimicrobial genes, and signaling pathways [,]. NRs are implicated in the regulation of autophagy at transcriptional and post-translational levels [,,,,]. Modulating NR activity by targeting the NR domains or by promoting ligand activation/suppression, modulation of the NR-DNA interaction, and/or the recruitment of coactivators may be effective against, for example, cancer, metabolic and immune diseases, inflammation, and neurodegeneration [,,,,].
The interaction of host autophagy with pathogens has been reviewed by others [,,,]. Here, we discuss the roles of autophagy-related genes (ATGs) in the immune response to pathogens and how various NR ligand-based approaches are being implemented for antimicrobial modalities based on autophagy activation.
2. Overview of Autophagy and Autophagy-Related Genes
A series of ATGs play critical roles in autophagy initiation, elongation, and maturation, and are also involved in other physiological responses, including membrane trafficking and signaling pathways [,,,]. ATG proteins in complexes with other cofactors/regulators modulate autophagy []. However, unlike in canonical autophagy, non-canonical autophagy does not necessarily require double-membraned autophagosome formation involving canonical initiation, nucleation, and an elongation step []. For example, a double-membraned autophagosome constituted from multiple isolated membranes is found in some cases of xenophagy, and even a single-membrane bound phagosome is formed in LAP []. Therefore, distinct subsets of ATGs are required for the activation of non-canonical autophagy LAP [] or selective autophagy [].
The roles of ATGs differ according to the type of autophagy. Rubicon plays opposite roles in (macro)autophagy and LAP; it is a negative regulator of autophagy, but promotes Beclin-1/VPS34 kinase activity in the phagosome [,]. Selective autophagy receptors have both a cargo recognition function and interact with the autophagosome [,]. The selective autophagy receptor p62/AQSTM1 plays several roles in numerous biological processes [,]. During nonselective autophagy, p62 degradation reflects activation of the autophagic flux, as the cargo receptor p62 is essential for selective autophagy []. Therefore, the overlapping cooperative and antagonistic roles of ATGs in autophagy need to be taken into account when developing host-directed therapeutics [,,].
Model studies using vertebrates (mice) and invertebrates (Caenorhabditis elegans and Drosophila) and involving tissue-specific ATG deletion or overexpression have provided pathogenetic insight by identifying autophagy-dependent and -independent functions of ATGs [,]. Additionally, the dysregulation or mutation of ATGs is associated with diverse human diseases []. In response to stresses, ATG upregulation at the transcriptional level induces autophagy in a manner requiring numerous transcription factors and signaling cofactors [,,]. In addition, the effect of ATGs and non-ATG proteins on autophagy is modulated by posttranslational modifications, including phosphorylation, glycosylation, ubiquitination, acetylation, and lipidation [,,,,,].
Antibacterial autophagy modulates bacterial replication and promotes innate immunity in host cells. Increasing evidence has shown that various intracellular bacteria, such as Mtb, Salmonella, Listeria, and Legionella, could be targeted by autophagy activation [,]. Autophagy not only directly causes microbial degradation, but also functions as a host defense system by participating in intracellular signaling, lysozyme secretion, the ubiquitin pathway, and antigen presentation []. However, these pathogens have also evolved strategies to evade or subvert host autophagy to survive within host cells, resulting in persistent infection and pathogenesis []. Similarly, autophagy is one of the key defense mechanisms in protecting against viral infections []. It regulates the immune response through the selective degradation of immune components associated with viral particles, followed by virus-derived antigen presentation to T lymphocytes to coordinate adaptive immunity. However, viruses manipulate and exploit autophagy for their immune evasion, replication, and release from host cells [,]. It is surprising that even if viruses target similar host defense pathways during the infection state, they differ in ways and functional outcomes, depending on each species of virus []. In order to increase the understanding on the role of autophagy dominating viral infections, not only common pathways, but also various species-specific studies, should be conducted in parallel. So far, the exact functions of ATG proteins and the detailed mechanisms that control autophagy during viral infection have not been elucidated [].
In this review, we focus on the pathophysiological roles and mechanisms underlying the NR-mediated regulation of autophagy, including the activation of antimicrobial responses in various infection models.
3. Overview of Nuclear Receptors
The NR superfamily classes are divided into four classes based on structural and functional characteristics, i.e., steroid receptors (Class I), retinoid X receptor (RXR) heterodimers (Class II), homodimeric orphan receptors (Class III), and monomeric orphan receptors (Class IV) [,]. The NR superfamily is classified as the endocrine, adopted orphan, and orphan subfamilies, depending on the existence of ligands [] (Figure 1). The differences in NR classes include their biological function, binding to a ligand or DNA, and tissue specificity. All members of the NR superfamily have a variable N-terminal domain (NTD), a DNA binding domain (DBD), a ligand-binding domain (LBD), and a variable C-terminal domain. NR DBD contains different DNA-binding recognition sequences and two zinc finger motifs for binding to chromatin [,]. NRs play a crucial role in the recruitment of co-activators within the nucleus, although there is marked variability among the binding of NTDs to co-activators [].
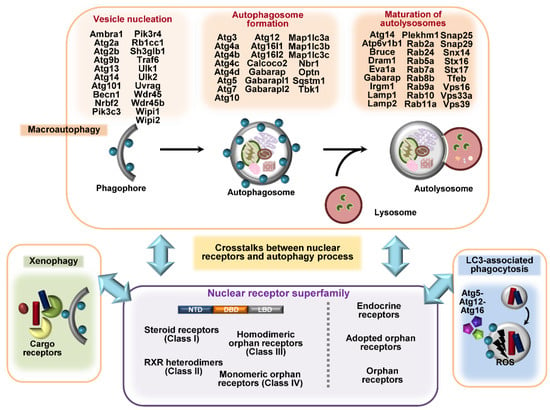
Figure 1.
A summarized figure for autophagy genes and different classes of nuclear receptors (NRs). Autophagy processes such as macroautophagy, LC3-associated phagocytosis (LAP), and xenophagy involve different autophagy-related genes (ATGs) or cargo receptors, such as p62, NDP52, and optineurin. The upper panel highlights the different sets of autophagy genes involved in vesicle nucleation, autophagosome formation, and the maturation of autolysosomes. The NR superfamily classes are divided into three or four subclasses according to their structural and functional characteristics and their ligands. NRs are implicated in the regulation of autophagy at transcriptional and post-translational levels. Understanding the mechanisms by which NRs regulate the expression and post-translational modification of ATGs will facilitate the development of novel host-directed antimicrobial agents.
A total of 48 intracellular proteins have been identified as NRs []; among them, several members are critical in the regulation of host immune responses to infection. These NRs include the vitamin D receptor (VDR), also known as nuclear receptor subfamily 1, group I, member 1 (NR1I1) [,,]; estrogen-related receptor-α (ERRα; ESRRA; NR3B1) [,]; ERRγ (ESRRG; NR3B3) []; liver X receptor-α (LXRα; LXRA; NR1H3) []; peroxisome proliferator-activated receptor-α (PPARα; PPARA; NR1C1) [,,]; PPARγ (PPARG; NR1C3) [,,]; the glucocorticoid receptor (GR; GCR; NR3C1) []; estrogen receptor-α (ERα; ESR1; NR3A); ERβ (ESR2; NR3A2) [,]; the xenobiotic pregnane X receptor (PXR; NR1I2) [,,]; rev-Erb-α (REV-ERBα; NR1D1) [,]; farnesoid X receptors (FXRs; NR1H4) [,]; nuclear receptor 4A (NR4A) family members; nuclear receptor related 1 protein (NURR1; NR4A2); and neuron-derived orphan receptor 1 (NOR1; NR4A3) []. The NRs are endogenously activated by small lipophilic ligands, such as steroid hormones, retinoids, and phospholipids; however, some of the NRs have been classified as ‘orphan’ members as their ligands have not yet been identified []. The ligands can cross the plasma membrane, directly interact with NRs inside the cells, and modulate gene transcription through different mechanisms [].
In class I NRs or steroid receptors, ligand binding at the plasma membrane is followed by a signal transduction cascade including enzymatic phosphorylation, which results in the translocation of transcription factors into the nucleus [,]. ER, a member of the class I NR superfamily, is anchored in the cytoplasm by a chaperone protein such as heat shock protein 90 (HSP90). After ligand binding, the receptor is freed from the chaperone, causing homodimerization and nuclear translocation. In the nucleus, the ligand-receptor complex associates with the transcriptional coactivator and activates the target gene []. Selective estrogen receptor modulators (SERM) such as tamoxifen and bazedoxifen have been suggested to have antimycobacterial activity [,].
The class II receptor family includes the thyroid hormone receptor (TR), VDR, retinoic acid receptor (RAR), and PPAR []. They are typically present in the nucleus and generally form heterodimers with RXR []. The heterodimers are bound to their response element, even in the absence of a ligand, where gene activation is repressed through interaction with a nuclear co-repressor (NCoR) and silencing mediator for retinoic acid and thyroid hormone receptor (SMRT) corepressor complexes. Binding of the ligand causes displacement of the NCoR/SMRT co-repressor, allowing transcriptional activation to occur [,]. For VDR, 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3), which is the active form of vitamin D3 (hereafter referred to as vitamin D), acts as a ligand and activates functional VDR, which then recognizes and binds to vitamin D response elements (VDREs) located in the promoter region of target genes to control the transcription of those genes []. VDR signaling activation during infection leads to innate immune signals for the production of antimicrobial peptides (AMPs), such as human cathelicidin AMP (CAMP) and β-defensin 2, which are important in coordinating vitamin D-induced antimicrobial responses []. PPARs include three different isotypes: PPARα, PPARβ/δ (PPARD; NR1C2), and PPARγ. Each isoform has a different distribution and ligands []. They are activated after the binding of endogenous ligands, such as fatty acids and their derivatives, or synthetic modulators, such as GW7647 and GW501516, to the ligand-binding domain. PPARs form heterodimers with RXR, which, after ligand binding, results in the transactivation or repression of target genes through PPAR responsive elements (PPREs) [,]. LXRs are the NRs which bind oxidized cholesterol derivatives such as oxysterols and intermediates of the cholesterol biosynthesis pathway []. The modulation of gene expression by LXR involves direct activation, repression, and transrepression [], and exhibits anti-inflammatory properties, as well as antimicrobial effects [].
Class III and IV receptors include the dimeric and monomeric orphan receptors, respectively. ERRα, one of the orphan nuclear receptors, is not regulated by the presence of natural ligands, but is regulated by post-transcriptional modifications, such as phosphorylation, sumoylation, or acetylation of the N-terminal domain [,], and is essential for antimicrobial host defense []. Other members of orphan NR include retinoic acid-related orphan receptors (ROR), whereas FXR and REV-ERB have been classified as adopted orphan receptors []. In addition, NRs are important in the regulation of autophagy [,,,], not only at the level of the transcription of ATGs, but also at the post-transcriptional level, by regulating protein–protein interactions, post-translational modification, and epigenetic mechanisms [,,,,]. The contributions of NR modulation to defense against pathogens are beginning to be deciphered. Here, we focus on the roles of VDR, ER, ERR, and PPAR in autophagic host defense against infection and discuss other NRs as links between autophagy and innate immunity during infection (Figure 2). A deeper understanding of NR signaling and its underlying mechanisms will facilitate the development of autophagy-based host-directed anti-infectives.
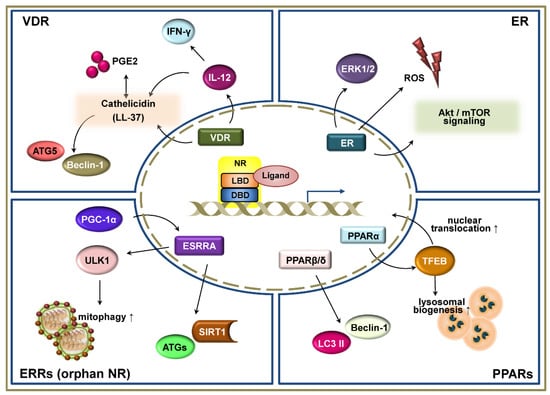
Figure 2.
Schematic representation of the signaling pathways of nuclear receptors (NRs) in autophagy-mediated host defense. NRs, including the vitamin D receptor (VDR), estrogen receptor (ER), estrogen-related receptors (ERRs), and peroxisome proliferator-activated receptors (PPARs) have been shown to play critical functions in the regulation of autophagy-mediated host defensive immune responses during infection. These NRs regulate and participate in the autophagic signaling pathways not only at the transcriptional level, but also at the post-transcriptional level. VDR is one of the best characterized NRs related to autophagic function against various infections. It is well-known that VDR signaling increases autophagy activation via the induction of cathelicidin, which is a small cationic antimicrobial peptide. In addition, VDRs functionally link adaptive and innate immune responses by regulating downstream pathways of autophagy. ER activates autophagy by increasing reactive oxygen species (ROS) generation and Akt/ mammalian target of rapamycin (mTOR) signaling. ERRs, which are one of the orphan family members of NR, also regulate a variety of cellular responses, including autophagy. The induction of PGC-1α upregulates the ERRα to promote mitophagy and an antimicrobial effect through sirtuin 1. PPARα activation leads to the expression of transcription factor EB (TFEB) and its nuclear translocation, resulting in the enhancement of lysosomal biogenesis. PPARβ/δ prevents harmful ER stress by increasing autophagy markers Beclin-1 and LC3 II.
4. Vitamin D Receptor in Autophagy-Mediated Defense against Infection
VDR signaling ameliorates infection and inflammation [,,,]. Studies on the role of vitamin D in innate immunity have revealed that autophagy enhances phagosomal maturation and lysosomal function, and ameliorates inflammation and antimicrobial protein generation [,,]. A physiological level of the active form of vitamin D (1α,25-dihydroxycholecalciferol) or functional activation of VDR signaling promotes autophagy activation in human monocytes or monocytic cells by inducing the synthesis of cathelicidin—a cationic antimicrobial peptide—which promotes phagosomal maturation in the presence of intracellular Mtb [,,] or Mycobacterium marinum []. In addition, vitamin D treatment and TLR8-mediated VDR signaling activation enhanced autophagy in human macrophages in a manner dependent on the ATG5 and Beclin-1, thereby inhibiting human immunodeficiency virus (HIV)-1 replication or the co-infection of HIV and Mtb [,,]. Vitamin D-mediated autophagy and cathelicidin expression are negatively regulated by prostaglandin (PG)E2—an arachidonic acid-derived lipid mediator—via E prostanoid (EP)2 and EP4 receptors [].
Vitamin D supplementation in mice significantly induced VDR, cathelin-related antimicrobial peptide (CRAMP), and LC3B expression, but decreased the collagenase matrix metalloproteinase-1 []. A structural equation modeling analysis suggested that vitamin D-mediated autophagy reduces necrosis []. Additionally, clinical trials of vitamin D as adjunctive therapy to standard anti-tuberculosis (TB) treatment showed a significant decline in intracellular Mtb growth and the levels of proinflammatory cytokines/chemokines [,], but no clear effect on long-term sputum-smear conversion []. These data suggest that the vitamin D-autophagy pathway is associated with clinical recovery from TB. Nevertheless, further studies are needed to determine the effects and risks of vitamin D adjunctive therapy, as discussed by others [,,]. We limit the discussion in this review to clinical trials of vitamin D therapy in TB and other infectious diseases.
IFN-γ, which is an important cytokine in the adaptive Th1 immune response, alone [] or in combination with the CD40 ligand [], enhanced VDR-mediated antimicrobial defense in human monocytes/macrophages in vitamin D-sufficient serum. Vitamin D treatment was required for the expression of IL-12 [], and the combination of IL-12 and IL-18 in human macrophages enhanced the cell-autonomous production of IFN-γ and the autophagy-cathelicidin pathway, which upregulated the antimicrobial response to Mtb []. Therefore, functional VDR signaling links the adaptive and innate immune responses by regulating autophagy, phagosome-lysosome fusion, and cytokine production in Mtb infection.
Vitamin D treatment reversed the influenza A virus-induced inhibition of autophagic flux by inducing the expression of syntaxin-17 and the V-type proton ATPase subunit (ATP6V0A2) []. In addition, probiotic lactic-acid bacteria isolated from kimchi activated VDR-autophagy responses and enhanced the expression of ATG16L1 and Beclin-1, resulting in an anti-inflammatory and anti-infective effect in the intestines []. Vitamin D treatment restored the lysosomal function impaired by Helicobacter pylori in gastric epithelial cells, by activating— protein disulfide isomerase family A member 3 (PDIA3) receptor and upregulating mucolipin-3 (MCOLN3)-mediated Ca2+ release []. In an animal study of Aspergillus fumigatus infection, vitamin D treatment led to autophagic homeostasis by reducing the number of autophagy-mediated lysosomes and regulatory T-cells, thus enhancing the antimicrobial response []. Moreover, vitamin D suppressed rotavirus infection by upregulating the autophagy gene Beclin-1 and promoting autophagic maturation and cathelicidin gene expression []. Although vitamin D-induced autophagy is critical for an effective immune response, further studies are needed to determine the ability of vitamin D to prevent and treat infectious diseases in humans. The studies on VDR-related autophagy during infection are summarized in Table 1.

Table 1.
Vitamin D receptor (VDR) in autophagy-mediated host defense against infections.
5. Estrogen Receptors
Estrogen—a female sex steroid hormone—and its receptors (ERα and ERβ) reportedly modulate autophagy, which is implicated in various human diseases and the determination of cell fate [,]. Indeed, ERs and the downstream genomic and non-genomic signaling cascades affect the outcomes of tumorigenesis and angiogenesis in breast cancer [,]. In addition, ERα activation by estrogen enhances autophagy and tumor cell survival in papillary thyroid cancer by promoting reactive oxygen species (ROS) generation and the activation of extracellular signal-regulated kinases [].
Estrogen modulators influence antimicrobial responses by influencing autophagy. The selective ER modulator bazedoxifene suppresses the intracellular growth of Mtb by activating autophagy via ROS generation and Akt/ mammalian target of rapamycin (mTOR) signaling []. Tamoxifen (TAM) is a potent inhibitor of Shiga toxin trafficking and toxicity in a manner independent on ERs []. TAM also restricted Toxoplasma replication by inducing xenophagy or autophagy []. Long-term treatment with 17β-estradiol (E2) exerted a beneficial effect on endotoxemia-associated circulatory and multiple organ dysfunction in ovariectomized rats, which was mediated, at least in part, by autophagy activation []. Clarification of the mechanisms through which ERs modulate autophagy and/or host defense, as well as the crosstalk between autophagy and immunity in the context of ER signaling, is needed for the development of novel therapeutic modalities for infection and inflammation.
6. Estrogen-Related Receptors
ERRs are orphan members of the NR family, and are involved in a variety of biological responses, including cellular metabolism and energy control [,,]. ERRα is a critical regulator of autophagy at transcriptional and post-translational levels, particularly in cooperation with sirtuin 1. These effects promoted the antimicrobial response to Mtb []. The thyroid hormone upregulated the expression of ERRα by inducing PGC-1α (PPARGC1A), thus modulating mitochondrial biogenesis and mitophagy. Mechanistically, the thyroid hormone upregulated the autophagy-regulating kinase ULK1 through ERRα, which was required for the autophagic clearance of mitochondria, i.e., mitophagy []. In contrast, the inhibition of ERRα activity by the inverse agonist XCT790 induced autophagy and promoted the clearance of toxic protein aggregates, enhancing its neuroprotective effect []. There is a role for ERRα in host defense between intracellular bacteria and viruses [,,,]. Therefore, future studies should clarify the role of ERRα in modulating autophagy and evaluate its therapeutic potential as an antimicrobial. The studies on ERs and ERRα-related autophagy during infection are summarized in Table 2.

Table 2.
Estrogen receptors (ERs)/estrogen-related receptors (ESRRs).
7. Peroxisome Proliferator-Activated Receptors
7.1. Peroxisome Proliferator-Activated Receptor-α
The nutrient-sensing NRs, PPARα and FXR, play reciprocal functions in the regulation of autophagy; PPARα enhances and FXR suppresses autophagy to enhance lipolysis [] and ciliogenesis []. PPARα ameliorates inflammatory and injurious conditions by inducing autophagy in various cells and tissues [,]. In addition, autophagy activation leads to PPARα activation by degrading nuclear receptor co-repressor 1 (NCoR1), which interacts with and suppresses the transactivation of PPARα [].
PPARα modulates antimicrobial responses to Mtb, Mycobacterium bovis bacillus Calmette-Guérin (BCG), or Mycobacterium abscessus by activating transcription factor EB (TFEB) [,,]. In addition, PPARα deficiency resulted in an exaggerated inflammatory response to mycobacterial infection. Importantly, PPARα activation significantly reduced the lipid body number and size in macrophages infected with Mtb or M. bovis BCG, suggesting that PPARα contributes to lipid catabolism and reduces the foamy refuge during mycobacterial infection []. TFEB controlled the inflammatory response of host macrophages to Mtb or BCG infection []. However, TFEB was reportedly required for the induction of inflammatory cytokines and chemokines in macrophages infected with Staphylococcus aureus [].
Numerous agents have been reported to activate PPARα and enhance TFEB, thereby promoting lysosomal biogenesis in models of chronic inflammatory and degenerative diseases [,,,]. Therefore, PPARα-activating drugs have potential for various infectious diseases. HIV infection inhibited autophagy in macrophages, promoting the intracellular survival of Mtb and non-tuberculous mycobacteria (NTM) []. Trehalose, which targets TFEB and PGC-1α [], activated the xenophagic flux to eradicate intracellular Mtb and NTM []. Mechanistically, Trehalose enhanced TFEB nuclear translocation and autophagy activation in an mucolipin 1 (MCOLN1)-dependent manner []. Because trehalose promotes the functionally active conformation of the N-terminal domain of the glucocorticoid receptor [], its antimicrobial effect may be mediated by GR signaling. Therefore, trehalose-induced autophagy may be involved in controlling co-morbidities of HIV and TB infections.
7.2. PPARβ/δ and PPARγ
PPARβ/δ inhibits the ER stress induced by palmitate in AC16 cardiomyocytes by inducing expression of the autophagy markers Beclin-1 and LC3 II, thus preventing the harmful cardiac effects of ER stress []. The treatment of septic mice with the PPARβ/δ-agonist GW0742 improved long-term survival and protected against multiple organ injury and dysfunction by modulating inflammatory signaling and coagulation [,].
Amodiaquine, which is a selective anti-Plasmodium falciparum agent, suppresses autophagolysosomal degradation and PPARγ activity []. The PPARγ ligand HP24, which is a pyridinecarboxylic acid derivative, ameliorated the pathologic and inflammatory responses induced by Trypanosoma cruzi []. INT131, which is a novel non-thiazolidinedione and selective PPARγ modulator, has a beneficial anti-inflammatory effect on EcoHIV-infected glial cells and in a mouse model of EcoHIV infection []. However, whether PPARβ/δ or PPARγ activation exerts an antimicrobial effect by modulating autophagy is unclear. Further studies are needed to clarify the roles of PPARβ/δ and PPARγ in controlling the host response to infections in the context of autophagy activation. The studies on PPAR-related autophagy during infection are summarized in Table 3.

Table 3.
Peroxisome proliferator-activated receptors (PPARs).
8. Other Nuclear Receptors Potentially Linking Autophagy and Host Defenses
8.1. REV-ERBα and REV-ERBβ
The adopted orphan NR—REV-ERBα—is involved in adipogenesis, muscle differentiation, glucose/lipid metabolism, and the circadian rhythm [,,]. REV-ERBα links the circadian rhythm and autophagy, and directly regulates the rhythmic expression of ATGs in zebrafish []. The key transcriptional regulators TFEB and TFE3 are required for the expression of REV-ERBα [], suggesting another link between autophagy and the circadian cycle.
During infection, REV-ERBα activation by GSK4112 exerted an anti-mycobacterial effect in macrophages by enhancing autophagy and lysosomal biogenesis and suppressing IL-10 synthesis []. However, the pharmacological activation of REV-ERBα and REV-ERBβ (NR1D2) using agonists inhibited autophagy and lipogenesis, thereby exerting an anticancer effect []. Moreover, the lysosomotropic REV-ERBβ ligand (ARN5187) inhibited autophagy and exerted a cytotoxic effect in breast cancer cells []. The over-expression of REV-ERBα in skeletal muscle induced mitochondrial activity and respiratory capacity, but repressed autophagy []. Therefore, REV-ERBα and REV-ERBβ may play diverse roles in autophagy regulation, depending on the cell type and pathological status. Because the circadian rhythm may be linked to the immune response to infection [,,], further studies should clarify the roles of REV-ERBα and REV-ERBβ in autophagy, the circadian rhythm, and the immune response to bacterial and fungal pathogens.
8.2. Retinoic Acid Receptor-α (RARα; RARA; NR1B1), -β (RARβ; RARB; NR1B2), and -γ (RARγ; RARG; NR1B3)
All-trans retinoic acid and/or arsenic trioxide induced autophagy of the oncoprotein promyelocytic leukemia (PML)/RARA, suggesting RARα as a therapeutic target for acute PML [,]. In addition, RARα activated autophagy in human primary B cells [] and various types of cancer cells [,]. The downregulation of RARα led to the upregulation of VDR expression in acute myeloid leukemia cells []. However, its role in autophagy during the antimicrobial response is unclear. RARα is reportedly a critical regulator of the maturation of monocyte-derived dendritic cells during HIV infection [], although its relevance to autophagy has not been evaluated.
Similarly, little is known about the role of RARβ in the regulation of autophagy during infection. RARβ has a tumor suppressive function and is involved in cell differentiation and apoptosis. Interestingly, the human papillomavirus type 16 (HPV16) E7 oncoprotein upregulated the mRNA and protein levels of RARβ in cervical cancer cells and in the cervix of K14E7 transgenic mice []. During human adenovirus infection, the RARβ mRNA and protein levels were downregulated, but the overexpression of RARβ decreased human adenovirus production []. Therefore, RARβ may have therapeutic effects for adenovirus infection, although the autophagy-mediated suppression of infection is unclear in RARβ-induced antiviral and anticancer effects.
8.3. Retinoic Acid-Related Orphan-α (RORα; RORA; NR1F1), -β (RORβ; RORB; NR1F2), and -γ (RORγ; RORC; NR1F3)
RORs are important in the regulation of the circadian clock, metabolic homeostasis, and tumorigenesis [,]. Although RORs are emerging as therapeutic targets for tumors, their roles in the modulation of host defense during infection in the context of autophagy are unclear. Upon infection with highly pathogenic avian influenza viruses (HPAIV H5N1), RORα is synthesized and suppresses NF-κB signaling and the inflammatory response in monocytes, thereby contributing to the escape of H5N1 from the host inflammatory defenses []. RORα is a melatonin receptor and protects against ischemic heart injury and diabetic cardiomyopathy [,]. The effect of melatonin on autophagy regulation has been reported in various normal and cancer cells [,]. In addition, the protective effects of melatonin in various bacterial, viral, and parasitic infections have been characterized [,,,,]. Melatonin treatment of Hodgkin lymphoma cells increased the expression of RORα, RORβ, and RORγ, and enhanced autophagy activation []. Therefore, it would be interesting to investigate the involvement of RORs in melatonin-mediated autophagy activation.
8.4. Farnesoid X Receptors-α (FXR-α)
As a nutrient-sensing and autophagy-regulating NR, FXRα regulates hepatic autophagy to maintain the energy balance in the liver [,] and inhibits autophagy-mediated ciliogenesis []. The inhibitory effect of FXRα is counteracted by PPARα, which activates autophagy [,,]. Because PPARα is involved in the coordination of autophagy activation and antimicrobial defenses [], it would be interesting to investigate the role of FXRα.
In a model of cholestasis, the activation of autophagy maturation is inhibited in an FXR-dependent manner, partly as a result of the induction of Rubicon. However, ursodeoxycholic acid (UDCA), which is a non-FXR-agonistic bile acid, induced hepatic autophagy and reduced the expression of Rubicon, which is an inhibitor of autophagy []. In an autophagy-deficient liver, the expression of FXR and its downstream genes was inhibited, promoting cholestatic injury []. Therefore, the link between FXR and autophagy requires further investigation.
8.5. Liver X Receptor (LXR)-α (LXRα; NR1H3) and -β (LXRβ; NR1H2)
LXRα and LXRβ are negative regulators of cholesterol metabolism and inflammation [,]. Both LXRs are important in the antimicrobial response to viral and bacterial infections []. Three synthetic LXR agonists (T0901317, GW3965, and LXR-623) had a long-lasting inhibitory effect on hepatitis B virus replication and gene expression []. In addition, both LXRα and LXRβ were required for the suppression of gammaherpesvirus reactivation by downregulating fatty acid and cholesterol synthesis in macrophages [], and for the inhibition of herpes simplex virus type 1 (HSV-1) by 25-hydroxycholesterol []. IL-36 and LXR signaling promoted anti-mycobacterial effects by inducing the expression of cholesterol-converting enzymes and regulating the expression of antimicrobial peptides []. In addition, LXR activation inhibited Salmonella infection by inducing the expression of the multifunctional enzyme CD38 []. LXRs are involved in the regulation of autophagy in various pathological conditions, including cancers [,]. Therefore, further studies on the involvement of LXRs in modulating autophagy during infection with intracellular microbes are required.
8.6. Thyroid Hormone Receptors-α (TRα; THRA; NR1A1) and -β (TRβ; THRB; NR1A2)
The thyroid hormone is a regulator of the metabolic rate, oxidative phosphorylation (OXPHOS), and ROS production [,], and a potent inducer of autophagy/mitophagy [,]. Thyroid hormone suppresses the hepatic carcinogenesis induced by the HBV X protein by promoting mitochondrial turnover via autophagy activation. In addition, thyroid hormone/TR-induced hepatic PINK1 expression is associated with hepatic cellular carcinoma (HCC) progression and a poor prognosis []. One might expect that thyroid hormone is involved in the modulation of host antimicrobial responses through autophagy. However, it remains to be determined whether both TRα and TRβ contribute to pathogenesis or protective immunity to broader ranges of infections through autophagy modulation. The studies on several NRs with potential roles in connecting autophagy and host defense are summarized in Table 4.

Table 4.
Several nuclear receptors (NRs) with potential functions in linking autophagy and host defense.
9. Conclusions
Given the role of autophagy in controlling intracellular pathogens, there is an urgent need for autophagy-directed therapeutics and prophylactics. The targeting of autophagy in monocytes/macrophages stimulates the innate immune response, the dampening of inflammation and suppression of innate immunity, and the promotion of pathogen escape []. Therefore, a more comprehensive understanding of the molecular mechanisms of crosstalk between autophagy and the innate immunity system in acute vs. chronic infection by various pathogens in immunocompetent vs. immunocompromised hosts will facilitate the development of therapeutics and vaccines.
NR protects against a variety of infections and modulates autophagy during pathogen invasion. Early studies exploited VDR- or ERRα-targeted antimicrobial responses and were followed by several trials expanding the effects of other NRs. It is known that the ATGs involved in non-canonical autophagy are different from those involved in canonical autophagy, but only a few studies on NR-mediated non-canonical autophagy pathways have been reported to date []. We are only beginning to answer important questions on the NR regulation of autophagy and innate immune responses. It is important to understand the signaling networks connecting NRs, autophagy, and the inflammatory and immune responses according to the infection stage and pathogen. Such an enhanced understanding will facilitate the development of novel antimicrobials.
Author Contributions
E.-K.J. and P.S. conceptualized the article. E.-K.J., S.P., S.M.J., and P.S. wrote and reviewed the manuscript. The authors read and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2017R1A5A2015385 and 2019R1A2C1087686), and by the framework of the international cooperation program managed by the National Research Foundation of Korea (2015K2A2A6002008).
Acknowledgments
We are indebted to current and past members of our Medical Research Center (i-MRC) for discussions and investigations that contributed to this article. We apologize to colleagues whose work and publications could not be referenced owing to space constraints.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huang, J.; Brumell, J.H. Autophagy in immunity against intracellular bacteria. Curr. Top. Microbiol. Immunol. 2009, 335, 189–215. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, M.D.S.; Ribeiro, R.M.; Travassos, L.H. Autophagy and Its Interaction With Intracellular Bacterial Pathogens. Front. Immunol. 2018, 9, 935. [Google Scholar] [CrossRef] [PubMed]
- Campoy, E.; Colombo, M.I. Autophagy in intracellular bacterial infection. Biochim. Biophys. Acta 2009, 1793, 1465–1477. [Google Scholar] [CrossRef]
- Yuk, J.M.; Yoshimori, T.; Jo, E.K. Autophagy and bacterial infectious diseases. Exp. Mol. Med. 2012, 44, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Shahnazari, S.; Brumell, J.H. Mechanisms and consequences of bacterial targeting by the autophagy pathway. Curr. Opin. Microbiol. 2011, 14, 68–75. [Google Scholar] [CrossRef]
- Huang, J.; Brumell, J.H. Bacteria-autophagy interplay: A battle for survival. Nat. Rev. Microbiol. 2014, 12, 101–114. [Google Scholar] [CrossRef]
- Winchell, C.G.; Steele, S.; Kawula, T.; Voth, D.E. Dining in: Intracellular bacterial pathogen interplay with autophagy. Curr. Opin. Microbiol. 2016, 29, 9–14. [Google Scholar] [CrossRef]
- Ogawa, M.; Nakagawa, I.; Yoshikawa, Y.; Hain, T.; Chakraborty, T.; Sasakawa, C. Streptococcus-, Shigella-, and Listeria-induced autophagy. Methods Enzymol. 2009, 452, 363–381. [Google Scholar] [CrossRef]
- Ogawa, M.; Mimuro, H.; Yoshikawa, Y.; Ashida, H.; Sasakawa, C. Manipulation of autophagy by bacteria for their own benefit. Microbiol. Immunol. 2011, 55, 459–471. [Google Scholar] [CrossRef]
- Lerena, M.C.; Vazquez, C.L.; Colombo, M.I. Bacterial pathogens and the autophagic response. Cell Microbiol. 2010, 12, 10–18. [Google Scholar] [CrossRef]
- Ogawa, M.; Sasakawa, C. Bacterial evasion of the autophagic defense system. Curr. Opin. Microbiol. 2006, 9, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Radomski, N.; Rebbig, A.; Leonhardt, R.M.; Knittler, M.R. Xenophagic pathways and their bacterial subversion in cellular self-defense—pialphanutaualpha rhoepsiloniota—everything is in flux. Int. J. Med. Microbiol. 2018, 308, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.W.; Li, F. Bacterial interaction with host autophagy. Virulence 2019, 10, 352–362. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, Y.; Chen, M. Viral strategies for triggering and manipulating mitophagy. Autophagy 2018, 14, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Porter, B.A.; Ortiz, M.A.; Bratslavsky, G.; Kotula, L. Structure and Function of the Nuclear Receptor Superfamily and Current Targeted Therapies of Prostate Cancer. Cancers (Basel) 2019, 11, 1852. [Google Scholar] [CrossRef]
- Meinsohn, M.C.; Smith, O.E.; Bertolin, K.; Murphy, B.D. The Orphan Nuclear Receptors Steroidogenic Factor-1 and Liver Receptor Homolog-1: Structure, Regulation, and Essential Roles in Mammalian Reproduction. Physiol. Rev. 2019, 99, 1249–1279. [Google Scholar] [CrossRef] [PubMed]
- Wnuk, A.; Kajta, M. Steroid and Xenobiotic Receptor Signalling in Apoptosis and Autophagy of the Nervous System. Int. J. Mol. Sci. 2017, 18, 2394. [Google Scholar] [CrossRef]
- Fiorucci, S.; Biagioli, M.; Zampella, A.; Distrutti, E. Bile Acids Activated Receptors Regulate Innate Immunity. Front. Immunol. 2018, 9, 1853. [Google Scholar] [CrossRef]
- Bakke, D.; Sun, J. Ancient Nuclear Receptor VDR With New Functions: Microbiome and Inflammation. Inflamm. Bowel Dis. 2018, 24, 1149–1154. [Google Scholar] [CrossRef]
- Kim, Y.S.; Silwal, P.; Kim, S.Y.; Yoshimori, T.; Jo, E.K. Autophagy-activating strategies to promote innate defense against mycobacteria. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef]
- Xiang, J.; Liu, X.; Ren, J.; Chen, K.; Wang, H.L.; Miao, Y.Y.; Qi, M.M. How does estrogen work on autophagy? Autophagy 2019, 15, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Wagner, M.; Xiao, R.; Kim, K.H.; Feng, D.; Lazar, M.A.; Moore, D.D. Nutrient-sensing nuclear receptors coordinate autophagy. Nature 2014, 516, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Yang, C.S.; Lee, H.M.; Kim, J.K.; Kim, Y.S.; Kim, Y.R.; Kim, J.S.; Kim, T.S.; Yuk, J.M.; Dufour, C.R.; et al. ESRRA (estrogen-related receptor alpha) is a key coordinator of transcriptional and post-translational activation of autophagy to promote innate host defense. Autophagy 2018, 14, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Veras Ribeiro Filho, H.; Tambones, I.L.; Mariano Goncalves Dias, M.; Bernardi Videira, N.; Bruder, M.; Amorim Amato, A.; Migliorini Figueira, A.C. Modulation of nuclear receptor function: Targeting the protein-DNA interface. Mol. Cell Endocrinol. 2019, 484, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Neavin, D.R.; Liu, D.; Ray, B.; Weinshilboum, R.M. The Role of the Aryl Hydrocarbon Receptor (AHR) in Immune and Inflammatory Diseases. Int. J. Mol. Sci. 2018, 19, 3851. [Google Scholar] [CrossRef]
- Scheschowitsch, K.; Leite, J.A.; Assreuy, J. New Insights in Glucocorticoid Receptor Signaling-More Than Just a Ligand-Binding Receptor. Front. Endocrinol. (Lausanne) 2017, 8, 16. [Google Scholar] [CrossRef]
- Tripathi, M.; Yen, P.M.; Singh, B.K. Estrogen-Related Receptor Alpha: An Under-Appreciated Potential Target for the Treatment of Metabolic Diseases. Int. J. Mol. Sci. 2020, 21, 1645. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef]
- Deretic, V.; Levine, B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe 2009, 5, 527–549. [Google Scholar] [CrossRef]
- Deretic, V.; Saitoh, T.; Akira, S. Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol. 2013, 13, 722–737. [Google Scholar] [CrossRef]
- Keller, M.D.; Torres, V.J.; Cadwell, K. Autophagy and microbial pathogenesis. Cell Death Differ. 2020, 27, 872–886. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T.; Ohsumi, Y. The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 2011, 27, 107–132. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N. The ATG conjugation systems in autophagy. Curr. Opin. Cell Biol. 2020, 63, 1–10. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef]
- Behrends, C.; Sowa, M.E.; Gygi, S.P.; Harper, J.W. Network organization of the human autophagy system. Nature 2010, 466, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011, 18, 571–580. [Google Scholar] [CrossRef]
- Codogno, P.; Mehrpour, M.; Proikas-Cezanne, T. Canonical and non-canonical autophagy: Variations on a common theme of self-eating? Nat. Rev. Mol. Cell Biol. 2011, 13, 7–12. [Google Scholar] [CrossRef]
- Heckmann, B.L.; Boada-Romero, E.; Cunha, L.D.; Magne, J.; Green, D.R. LC3-Associated Phagocytosis and Inflammation. J. Mol. Biol. 2017, 429, 3561–3576. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, J.; Fan, W.; Wong, K.N.; Ding, X.; Chen, S.; Zhong, Q. The RUN domain of rubicon is important for hVps34 binding, lipid kinase inhibition, and autophagy suppression. J. Biol. Chem. 2011, 286, 185–191. [Google Scholar] [CrossRef]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef]
- Sanchez-Martin, P.; Komatsu, M. Physiological Stress Response by Selective Autophagy. J. Mol. Biol. 2020, 432, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Martin, P.; Komatsu, M. p62/SQSTM1—steering the cell through health and disease. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Zhang, X.; Rodriguez-Velez, A.; Evans, T.D.; Razani, B. p62/SQSTM1 and Selective Autophagy in Cardiometabolic Diseases. Antioxid. Redox Signal. 2019, 31, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.; Cunha, L.D.; Park, S.; Yang, M.; Lu, Q.; Orchard, R.; Li, Q.Z.; Yan, M.; Janke, L.; Guy, C.; et al. Noncanonical autophagy inhibits the autoinflammatory, lupus-like response to dying cells. Nature 2016, 533, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Wesselborg, S.; Stork, B. Autophagy signal transduction by ATG proteins: From hierarchies to networks. Cell Mol. Life Sci. 2015, 72, 4721–4757. [Google Scholar] [CrossRef]
- Maruzs, T.; Simon-Vecsei, Z.; Kiss, V.; Csizmadia, T.; Juhasz, G. On the Fly: Recent Progress on Autophagy and Aging in Drosophila. Front. Cell Dev. Biol. 2019, 7, 140. [Google Scholar] [CrossRef]
- Kuo, C.J.; Hansen, M.; Troemel, E. Autophagy and innate immunity: Insights from invertebrate model organisms. Autophagy 2018, 14, 233–242. [Google Scholar] [CrossRef]
- Jin, M.; Klionsky, D.J. Regulation of autophagy: Modulation of the size and number of autophagosomes. FEBS Lett. 2014, 588, 2457–2463. [Google Scholar] [CrossRef]
- Feng, Y.; Yao, Z.; Klionsky, D.J. How to control self-digestion: Transcriptional, post-transcriptional, and post-translational regulation of autophagy. Trends Cell Biol. 2015, 25, 354–363. [Google Scholar] [CrossRef]
- Feng, Y.; He, D.; Yao, Z.; Klionsky, D.J. The machinery of macroautophagy. Cell Res. 2014, 24, 24–41. [Google Scholar] [CrossRef]
- McEwan, D.G.; Dikic, I. The Three Musketeers of Autophagy: Phosphorylation, ubiquitylation and acetylation. Trends Cell Biol. 2011, 21, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Banreti, A.; Sass, M.; Graba, Y. The emerging role of acetylation in the regulation of autophagy. Autophagy 2013, 9, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Hamai, A.; Codogno, P. New targets for acetylation in autophagy. Sci. Signal. 2012, 5, pe29. [Google Scholar] [CrossRef]
- Xie, Y.; Kang, R.; Sun, X.; Zhong, M.; Huang, J.; Klionsky, D.J.; Tang, D. Posttranslational modification of autophagy-related proteins in macroautophagy. Autophagy 2015, 11, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Di Malta, C.; Cinque, L.; Settembre, C. Transcriptional Regulation of Autophagy: Mechanisms and Diseases. Front. Cell Dev. Biol. 2019, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.K.; Yuk, J.M.; Shin, D.M.; Sasakawa, C. Roles of autophagy in elimination of intracellular bacterial pathogens. Front. Immunol. 2013, 4, 97. [Google Scholar] [CrossRef]
- Campoy, E.; Colombo, M.I. Autophagy subversion by bacteria. Curr. Top. Microbiol. Immunol. 2009, 335, 227–250. [Google Scholar] [CrossRef]
- Paul, P.; Munz, C. Autophagy and Mammalian Viruses: Roles in Immune Response, Viral Replication, and Beyond. Adv. Virus Res. 2016, 95, 149–195. [Google Scholar] [CrossRef]
- Choi, Y.; Bowman, J.W.; Jung, J.U. Autophagy during viral infection—A double-edged sword. Nat. Rev. Microbiol. 2018, 16, 341–354. [Google Scholar] [CrossRef]
- Kudchodkar, S.B.; Levine, B. Viruses and autophagy. Rev. Med. Virol. 2009, 19, 359–378. [Google Scholar] [CrossRef]
- Mangelsdorf, D.J.; Thummel, C.; Beato, M.; Herrlich, P.; Schutz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P.; et al. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835–839. [Google Scholar] [CrossRef]
- Umesono, K.; Evans, R.M. Determinants of target gene specificity for steroid/thyroid hormone receptors. Cell 1989, 57, 1139–1146. [Google Scholar] [CrossRef]
- Huang, P.; Chandra, V.; Rastinejad, F. Structural overview of the nuclear receptor superfamily: Insights into physiology and therapeutics. Annu. Rev. Physiol. 2010, 72, 247–272. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Sousa, M.A.; Martinez, I.; Medrano, L.M.; Fernandez-Rodriguez, A.; Resino, S. Vitamin D in Human Immunodeficiency Virus Infection: Influence on Immunity and Disease. Front. Immunol. 2018, 9, 458. [Google Scholar] [CrossRef] [PubMed]
- Zdrenghea, M.T.; Makrinioti, H.; Bagacean, C.; Bush, A.; Johnston, S.L.; Stanciu, L.A. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev. Med. Virol. 2017, 27. [Google Scholar] [CrossRef]
- Wu, S.; Sun, J. Vitamin D, vitamin D receptor, and macroautophagy in inflammation and infection. Discov. Med. 2011, 11, 325–335. [Google Scholar]
- Paik, S.; Kim, J.K.; Chung, C.; Jo, E.K. Autophagy: A new strategy for host-directed therapy of tuberculosis. Virulence 2019, 10, 448–459. [Google Scholar] [CrossRef]
- Kim, D.K.; Jeong, J.H.; Lee, J.M.; Kim, K.S.; Park, S.H.; Kim, Y.D.; Koh, M.; Shin, M.; Jung, Y.S.; Kim, H.S.; et al. Inverse agonist of estrogen-related receptor gamma controls Salmonella typhimurium infection by modulating host iron homeostasis. Nat. Med. 2014, 20, 419–424. [Google Scholar] [CrossRef]
- Korf, H.; Vander Beken, S.; Romano, M.; Steffensen, K.R.; Stijlemans, B.; Gustafsson, J.A.; Grooten, J.; Huygen, K. Liver X receptors contribute to the protective immune response against Mycobacterium tuberculosis in mice. J. Clin. Investig. 2009, 119, 1626–1637. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.K.; Hanh, B.T.B.; Kim, S.Y.; Kim, H.J.; Kim, Y.J.; Jeon, S.M.; Park, C.R.; Oh, G.T.; Park, J.W.; et al. The Peroxisome Proliferator-Activated Receptor alpha-Agonist Gemfibrozil Promotes Defense Against Mycobacterium abscessus Infections. Cells 2020, 9, 648. [Google Scholar] [CrossRef]
- Kim, T.S.; Jin, Y.B.; Kim, Y.S.; Kim, S.; Kim, J.K.; Lee, H.M.; Suh, H.W.; Choe, J.H.; Kim, Y.J.; Koo, B.S.; et al. SIRT3 promotes antimycobacterial defenses by coordinating mitochondrial and autophagic functions. Autophagy 2019, 15, 1356–1375. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, H.M.; Kim, J.K.; Yang, C.S.; Kim, T.S.; Jung, M.; Jin, H.S.; Kim, S.; Jang, J.; Oh, G.T.; et al. PPAR-alpha Activation Mediates Innate Host Defense through Induction of TFEB and Lipid Catabolism. J. Immunol. 2017, 198, 3283–3295. [Google Scholar] [CrossRef] [PubMed]
- Omeragic, A.; Kara-Yacoubian, N.; Kelschenbach, J.; Sahin, C.; Cummins, C.L.; Volsky, D.J.; Bendayan, R. Peroxisome Proliferator-Activated Receptor-gamma agonists exhibit anti-inflammatory and antiviral effects in an EcoHIV mouse model. Sci. Rep. 2019, 9, 9428. [Google Scholar] [CrossRef] [PubMed]
- Leopold Wager, C.M.; Arnett, E.; Schlesinger, L.S. Mycobacterium tuberculosis and macrophage nuclear receptors: What we do and don’t know. Tuberculosis (Edinb) 2019, 116S, S98–S106. [Google Scholar] [CrossRef] [PubMed]
- Leopold Wager, C.M.; Arnett, E.; Schlesinger, L.S. Macrophage nuclear receptors: Emerging key players in infectious diseases. PLoS Pathog. 2019, 15, e1007585. [Google Scholar] [CrossRef]
- Petta, I.; Dejager, L.; Ballegeer, M.; Lievens, S.; Tavernier, J.; De Bosscher, K.; Libert, C. The Interactome of the Glucocorticoid Receptor and Its Influence on the Actions of Glucocorticoids in Combatting Inflammatory and Infectious Diseases. Microbiol. Mol. Biol. Rev. 2016, 80, 495–522. [Google Scholar] [CrossRef]
- Montoya, M.C.; Krysan, D.J. Repurposing Estrogen Receptor Antagonists for the Treatment of Infectious Disease. mBio 2018, 9. [Google Scholar] [CrossRef]
- Medina-Estrada, I.; Alva-Murillo, N.; Lopez-Meza, J.E.; Ochoa-Zarzosa, A. Immunomodulatory Effects of 17beta-Estradiol on Epithelial Cells during Bacterial Infections. J. Immunol. Res. 2018, 2018, 6098961. [Google Scholar] [CrossRef]
- Erickson, S.L.; Alston, L.; Nieves, K.; Chang, T.K.H.; Mani, S.; Flannigan, K.L.; Hirota, S.A. The xenobiotic sensing pregnane X receptor regulates tissue damage and inflammation triggered by C difficile toxins. FASEB J. 2020, 34, 2198–2212. [Google Scholar] [CrossRef]
- Qiu, Z.; Cervantes, J.L.; Cicek, B.B.; Mukherjee, S.; Venkatesh, M.; Maher, L.A.; Salazar, J.C.; Mani, S.; Khanna, K.M. Pregnane X Receptor Regulates Pathogen-Induced Inflammation and Host Defense against an Intracellular Bacterial Infection through Toll-like Receptor 4. Sci. Rep. 2016, 6, 31936. [Google Scholar] [CrossRef]
- Bhagyaraj, E.; Nanduri, R.; Saini, A.; Dkhar, H.K.; Ahuja, N.; Chandra, V.; Mahajan, S.; Kalra, R.; Tiwari, D.; Sharma, C.; et al. Human Xenobiotic Nuclear Receptor PXR Augments Mycobacterium tuberculosis Survival. J. Immunol. 2016, 197, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.M.; Bellet, M.M.; Sassone-Corsi, P.; O’Neill, L.A. Circadian clock proteins and immunity. Immunity 2014, 40, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.E.; Blaikley, J.; Beesley, S.; Matthews, L.; Simpson, K.D.; Boyce, S.H.; Farrow, S.N.; Else, K.J.; Singh, D.; Ray, D.W.; et al. The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc. Natl. Acad. Sci. USA 2012, 109, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Levy, G.; Habib, N.; Guzzardi, M.A.; Kitsberg, D.; Bomze, D.; Ezra, E.; Uygun, B.E.; Uygun, K.; Trippler, M.; Schlaak, J.F.; et al. Nuclear receptors control pro-viral and antiviral metabolic responses to hepatitis C virus infection. Nat. Chem. Biol. 2016, 12, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Prince, L.R.; Prosseda, S.D.; Higgins, K.; Carlring, J.; Prestwich, E.C.; Ogryzko, N.V.; Rahman, A.; Basran, A.; Falciani, F.; Taylor, P.; et al. NR4A orphan nuclear receptor family members, NR4A2 and NR4A3, regulate neutrophil number and survival. Blood 2017, 130, 1014–1025. [Google Scholar] [CrossRef]
- Mazaira, G.I.; Zgajnar, N.R.; Lotufo, C.M.; Daneri-Becerra, C.; Sivils, J.C.; Soto, O.B.; Cox, M.B.; Galigniana, M.D. The Nuclear Receptor Field: A Historical Overview and Future Challenges. Nucl. Receptor Res. 2018, 5. [Google Scholar] [CrossRef]
- Heldin, C.H.; Lu, B.; Evans, R.; Gutkind, J.S. Signals and Receptors. Cold Spring Harb. Perspect. Biol. 2016, 8, a005900. [Google Scholar] [CrossRef]
- Avior, Y.; Bomze, D.; Ramon, O.; Nahmias, Y. Flavonoids as dietary regulators of nuclear receptor activity. Food Funct. 2013, 4, 831–844. [Google Scholar] [CrossRef]
- Sever, R.; Glass, C.K. Signaling by nuclear receptors. Cold Spring Harb. Perspect. Biol. 2013, 5, a016709. [Google Scholar] [CrossRef]
- Ouyang, Q.; Zhang, K.; Lin, D.; Feng, C.G.; Cai, Y.; Chen, X. Bazedoxifene Suppresses Intracellular Mycobacterium tuberculosis Growth by Enhancing Autophagy. mSphere 2020, 5. [Google Scholar] [CrossRef]
- Jang, W.S.; Kim, S.; Podder, B.; Jyoti, M.A.; Nam, K.W.; Lee, B.E.; Song, H.Y. Anti-Mycobacterial Activity of Tamoxifen Against Drug-Resistant and Intra-Macrophage Mycobacterium tuberculosis. J. Microbiol. Biotechnol. 2015, 25, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Weikum, E.R.; Liu, X.; Ortlund, E.A. The nuclear receptor superfamily: A structural perspective. Protein Sci. 2018, 27, 1876–1892. [Google Scholar] [CrossRef]
- Collingwood, T.N.; Urnov, F.D.; Wolffe, A.P. Nuclear receptors: Coactivators, corepressors and chromatin remodeling in the control of transcription. J. Mol. Endocrinol. 1999, 23, 255–275. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Whitfield, G.K.; Haussler, C.A.; Hsieh, J.C.; Thompson, P.D.; Selznick, S.H.; Dominguez, C.E.; Jurutka, P.W. The nuclear vitamin D receptor: Biological and molecular regulatory properties revealed. J. Bone Miner. Res. 1998, 13, 325–349. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Silwal, P.; Kim, I.; Modlin, R.L.; Jo, E.K. Vitamin D-Cathelicidin Axis: At the Crossroads between Protective Immunity and Pathological Inflammation during Infection. Immune Netw. 2020, 20, e12. [Google Scholar] [CrossRef]
- Christofides, A.; Konstantinidou, E.; Jani, C.; Boussiotis, V.A. The role of Peroxisome Proliferator-Activated Receptors (PPAR) in immune responses. Metabolism 2020. [Google Scholar] [CrossRef]
- Wagner, K.D.; Wagner, N. Peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) acts as regulator of metabolism linked to multiple cellular functions. Pharmacol. Ther. 2010, 125, 423–435. [Google Scholar] [CrossRef]
- Glaria, E.; Letelier, N.A.; Valledor, A.F. Integrating the roles of liver X receptors in inflammation and infection: Mechanisms and outcomes. Curr. Opin. Pharmacol. 2020, 53, 55–65. [Google Scholar] [CrossRef]
- Glass, C.K.; Rosenfeld, M.G. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000, 14, 121–141. [Google Scholar]
- Tremblay, A.M.; Wilson, B.J.; Yang, X.J.; Giguere, V. Phosphorylation-dependent sumoylation regulates estrogen-related receptor-alpha and -gamma transcriptional activity through a synergy control motif. Mol. Endocrinol. 2008, 22, 570–584. [Google Scholar] [CrossRef]
- Massafra, V.; van Mil, S.W.C. Farnesoid X receptor: A "homeostat" for hepatic nutrient metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Sinha, R.A.; Ohba, K.; Yen, P.M. Role of thyroid hormone in hepatic gene regulation, chromatin remodeling, and autophagy. Mol. Cell Endocrinol. 2017, 458, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Basu, J.; Shin, D.M.; Jo, E.K. Mycobacterial signaling through toll-like receptors. Front. Cell Infect. Microbiol. 2012, 2, 145. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.T.; Litonjua, A.A. Vitamin D in Host Defense: Implications for Future Research. Am. J. Respir. Cell Mol. Biol. 2017, 56, 692–693. [Google Scholar] [CrossRef] [PubMed]
- Del Pinto, R.; Ferri, C.; Cominelli, F. Vitamin D Axis in Inflammatory Bowel Diseases: Role, Current Uses and Future Perspectives. Int. J. Mol. Sci. 2017, 18, 2360. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, L.; Li, M.X.; Shen, J.; Liu, X.D.; Xiao, Z.G.; Wu, D.L.; Ho, I.H.T.; Wu, J.C.Y.; Cheung, C.K.Y.; et al. Vitamin D3 activates the autolysosomal degradation function against Helicobacter pylori through the PDIA3 receptor in gastric epithelial cells. Autophagy 2019, 15, 707–725. [Google Scholar] [CrossRef]
- Yuk, J.M.; Shin, D.M.; Lee, H.M.; Yang, C.S.; Jin, H.S.; Kim, K.K.; Lee, Z.W.; Lee, S.H.; Kim, J.M.; Jo, E.K. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe 2009, 6, 231–243. [Google Scholar] [CrossRef]
- Jo, E.K. Innate immunity to mycobacteria: Vitamin D and autophagy. Cell Microbiol. 2010, 12, 1026–1035. [Google Scholar] [CrossRef]
- Shin, D.M.; Yuk, J.M.; Lee, H.M.; Lee, S.H.; Son, J.W.; Harding, C.V.; Kim, J.M.; Modlin, R.L.; Jo, E.K. Mycobacterial lipoprotein activates autophagy via TLR2/1/CD14 and a functional vitamin D receptor signalling. Cell Microbiol. 2010, 12, 1648–1665. [Google Scholar] [CrossRef]
- Sato, E.; Imafuku, S.; Ishii, K.; Itoh, R.; Chou, B.; Soejima, T.; Nakayama, J.; Hiromatsu, K. Vitamin D-dependent cathelicidin inhibits Mycobacterium marinum infection in human monocytic cells. J. Dermatol. Sci. 2013, 70, 166–172. [Google Scholar] [CrossRef]
- Campbell, G.R.; Spector, S.A. Hormonally active vitamin D3 (1alpha,25-dihydroxycholecalciferol) triggers autophagy in human macrophages that inhibits HIV-1 infection. J. Biol. Chem. 2011, 286, 18890–18902. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.R.; Spector, S.A. Vitamin D inhibits human immunodeficiency virus type 1 and Mycobacterium tuberculosis infection in macrophages through the induction of autophagy. PLoS Pathog. 2012, 8, e1002689. [Google Scholar] [CrossRef]
- Campbell, G.R.; Spector, S.A. Toll-like receptor 8 ligands activate a vitamin D mediated autophagic response that inhibits human immunodeficiency virus type 1. PLoS Pathog. 2012, 8, e1003017. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Tang, X.; Rekha, R.S.; Muvva, S.; Brighenti, S.; Agerberth, B.; Haeggstrom, J.Z. Prostaglandin E2 suppresses hCAP18/LL-37 expression in human macrophages via EP2/EP4: Implications for treatment of Mycobacterium tuberculosis infection. FASEB J. 2018, 32, 2827–2840. [Google Scholar] [CrossRef] [PubMed]
- Wahyunitisari, M.R.; Mertaniasih, N.M.; Amin, M.; Artama, W.T.; Koendhori, E.B. Vitamin D, cell death pathways, and tuberculosis. Int. J. Mycobacteriol 2017, 6, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Rekha, R.S.; Mily, A.; Sultana, T.; Haq, A.; Ahmed, S.; Mostafa Kamal, S.M.; van Schadewijk, A.; Hiemstra, P.S.; Gudmundsson, G.H.; Agerberth, B.; et al. Immune responses in the treatment of drug-sensitive pulmonary tuberculosis with phenylbutyrate and vitamin D3 as host directed therapy. BMC Infect. Dis. 2018, 18, 303. [Google Scholar] [CrossRef] [PubMed]
- Mily, A.; Rekha, R.S.; Kamal, S.M.; Arifuzzaman, A.S.; Rahim, Z.; Khan, L.; Haq, M.A.; Zaman, K.; Bergman, P.; Brighenti, S.; et al. Significant Effects of Oral Phenylbutyrate and Vitamin D3 Adjunctive Therapy in Pulmonary Tuberculosis: A Randomized Controlled Trial. PLoS ONE 2015, 10, e0138340. [Google Scholar] [CrossRef]
- Bekele, A.; Gebreselassie, N.; Ashenafi, S.; Kassa, E.; Aseffa, G.; Amogne, W.; Getachew, M.; Aseffa, A.; Worku, A.; Raqib, R.; et al. Daily adjunctive therapy with vitamin D3 and phenylbutyrate supports clinical recovery from pulmonary tuberculosis: A randomized controlled trial in Ethiopia. J. Intern. Med. 2018, 284, 292–306. [Google Scholar] [CrossRef]
- Wallis, R.S.; Zumla, A. Vitamin D as Adjunctive Host-Directed Therapy in Tuberculosis: A Systematic Review. Open Forum Infect. Dis. 2016, 3, ofw151. [Google Scholar] [CrossRef]
- Kearns, M.D.; Alvarez, J.A.; Seidel, N.; Tangpricha, V. Impact of vitamin D on infectious disease. Am. J. Med. Sci. 2015, 349, 245–262. [Google Scholar] [CrossRef]
- Fabri, M.; Stenger, S.; Shin, D.M.; Yuk, J.M.; Liu, P.T.; Realegeno, S.; Lee, H.M.; Krutzik, S.R.; Schenk, M.; Sieling, P.A.; et al. Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages. Sci. Transl. Med. 2011, 3, 104ra102. [Google Scholar] [CrossRef] [PubMed]
- Klug-Micu, G.M.; Stenger, S.; Sommer, A.; Liu, P.T.; Krutzik, S.R.; Modlin, R.L.; Fabri, M. CD40 ligand and interferon-gamma induce an antimicrobial response against Mycobacterium tuberculosis in human monocytes. Immunology 2013, 139, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Gough, M.E.; Graviss, E.A.; May, E.E. The dynamic immunomodulatory effects of vitamin D3 during Mycobacterium infection. Innate Immun. 2017, 23, 506–523. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Yang, E.; Shen, L.; Modlin, R.L.; Shen, H.; Chen, Z.W. IL-12+IL-18 Cosignaling in Human Macrophages and Lung Epithelial Cells Activates Cathelicidin and Autophagy, Inhibiting Intracellular Mycobacterial Growth. J. Immunol. 2018, 200, 2405–2417. [Google Scholar] [CrossRef]
- Godbole, N.M.; Sinha, R.A.; Tiwari, S.; Pawar, S.D.; Dhole, T.N. Analysis of influenza virus-induced perturbation in autophagic flux and its modulation during Vitamin D3 mediated anti-apoptotic signaling. Virus Res. 2020, 282, 197936. [Google Scholar] [CrossRef]
- Lu, R.; Shang, M.; Zhang, Y.G.; Jiao, Y.; Xia, Y.; Garrett, S.; Bakke, D.; Bauerl, C.; Martinez, G.P.; Kim, C.H.; et al. Lactic Acid Bacteria Isolated From Korean Kimchi Activate the Vitamin D Receptor-autophagy Signaling Pathways. Inflamm. Bowel. Dis. 2020, 26, 1199–1211. [Google Scholar] [CrossRef]
- Dai, J.; Liang, Y.; Li, H.; Zhou, W.; Wang, B.; Gong, A.; Zhang, R. Vitamin D enhances resistance to aspergillus fumigatus in mice via inhibition of excessive autophagy. Am. J. Transl Res. 2018, 10, 381–391. [Google Scholar]
- Tian, G.; Liang, X.; Chen, D.; Mao, X.; Yu, J.; Zheng, P.; He, J.; Huang, Z.; Yu, B. Vitamin D3 supplementation alleviates rotavirus infection in pigs and IPEC-J2 cells via regulating the autophagy signaling pathway. J. Steroid Biochem. Mol. Biol 2016, 163, 157–163. [Google Scholar] [CrossRef]
- Wei, Y.; Huang, J. Role of estrogen and its receptors mediated-autophagy in cell fate and human diseases. J. Steroid Biochem. Mol. Biol. 2019, 191, 105380. [Google Scholar] [CrossRef]
- Lipovka, Y.; Konhilas, J.P. The complex nature of oestrogen signalling in breast cancer: Enemy or ally? Biosci. Rep. 2016, 36. [Google Scholar] [CrossRef]
- Fan, D.; Liu, S.Y.; van Hasselt, C.A.; Vlantis, A.C.; Ng, E.K.; Zhang, H.; Dong, Y.; Ng, S.K.; Chu, R.; Chan, A.B.; et al. Estrogen receptor alpha induces prosurvival autophagy in papillary thyroid cancer via stimulating reactive oxygen species and extracellular signal regulated kinases. J. Clin. Endocrinol. Metab. 2015, 100, E561–E571. [Google Scholar] [CrossRef] [PubMed]
- Selyunin, A.S.; Hutchens, S.; McHardy, S.F.; Mukhopadhyay, S. Tamoxifen blocks retrograde trafficking of Shiga toxin 1 and 2 and protects against lethal toxicosis. Life Sci. Alliance 2019, 2. [Google Scholar] [CrossRef] [PubMed]
- Dittmar, A.J.; Drozda, A.A.; Blader, I.J. Drug Repurposing Screening Identifies Novel Compounds That Effectively Inhibit Toxoplasma gondii Growth. mSphere 2016, 1. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.T.; Lee, Y.M.; Shen, H.H.; Cheng, P.Y.; Huang, Y.C.; Lin, Y.J.; Huang, Y.Y.; Lam, K.K. Activation of autophagy is involved in the protective effect of 17beta-oestradiol on endotoxaemia-induced multiple organ dysfunction in ovariectomized rats. J. Cell Mol. Med. 2017, 21, 3705–3717. [Google Scholar] [CrossRef] [PubMed]
- Giguere, V. Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr. Rev. 2008, 29, 677–696. [Google Scholar] [CrossRef] [PubMed]
- Huss, J.M.; Garbacz, W.G.; Xie, W. Constitutive activities of estrogen-related receptors: Transcriptional regulation of metabolism by the ERR pathways in health and disease. Biochim. Biophys. Acta 2015, 1852, 1912–1927. [Google Scholar] [CrossRef]
- Audet-Walsh, E.; Giguere, V. The multiple universes of estrogen-related receptor alpha and gamma in metabolic control and related diseases. Acta Pharmacol. Sin. 2015, 36, 51–61. [Google Scholar] [CrossRef]
- Singh, B.K.; Sinha, R.A.; Tripathi, M.; Mendoza, A.; Ohba, K.; Sy, J.A.C.; Xie, S.Y.; Zhou, J.; Ho, J.P.; Chang, C.Y.; et al. Thyroid hormone receptor and ERRalpha coordinately regulate mitochondrial fission, mitophagy, biogenesis, and function. Sci. Signal. 2018, 11. [Google Scholar] [CrossRef]
- Suresh, S.N.; Chavalmane, A.K.; Pillai, M.; Ammanathan, V.; Vidyadhara, D.J.; Yarreiphang, H.; Rai, S.; Paul, A.; Clement, J.P.; Alladi, P.A.; et al. Modulation of Autophagy by a Small Molecule Inverse Agonist of ERRalpha Is Neuroprotective. Front. Mol. Neurosci. 2018, 11, 109. [Google Scholar] [CrossRef]
- Sonoda, J.; Laganiere, J.; Mehl, I.R.; Barish, G.D.; Chong, L.W.; Li, X.; Scheffler, I.E.; Mock, D.C.; Bataille, A.R.; Robert, F.; et al. Nuclear receptor ERR alpha and coactivator PGC-1 beta are effectors of IFN-gamma-induced host defense. Genes Dev. 2007, 21, 1909–1920. [Google Scholar] [CrossRef]
- Hwang, J.; Purdy, J.G.; Wu, K.; Rabinowitz, J.D.; Shenk, T. Estrogen-related receptor alpha is required for efficient human cytomegalovirus replication. Proc. Natl. Acad. Sci. USA 2014, 111, E5706–E5715. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Ma, S.; Tian, Y.; Wei, C.; Zhu, Y.; Li, F.; Zhang, P.; Wang, P.; Zhang, Y.; Zhong, H. ERRalpha negatively regulates type I interferon induction by inhibiting TBK1-IRF3 interaction. PLoS Pathog. 2017, 13, e1006347. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Lee, J.N.; Son, M.; Lim, J.Y.; Dutta, R.K.; Maharjan, Y.; Kwak, S.; Oh, G.T.; Byun, K.; Choe, S.K.; et al. Ciliogenesis is reciprocally regulated by PPARA and NR1H4/FXR through controlling autophagy in vitro and in vivo. Autophagy 2018, 14, 1011–1027. [Google Scholar] [CrossRef] [PubMed]
- Jiao, M.; Ren, F.; Zhou, L.; Zhang, X.; Zhang, L.; Wen, T.; Wei, L.; Wang, X.; Shi, H.; Bai, L.; et al. Peroxisome proliferator-activated receptor alpha activation attenuates the inflammatory response to protect the liver from acute failure by promoting the autophagy pathway. Cell Death Dis. 2014, 5, e1397. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, X.; Qu, N.; Zhang, B.; Xia, C. Chondroprotection of PPARalpha activation by WY14643 via autophagy involving Akt and ERK in LPS-treated mouse chondrocytes and osteoarthritis model. J. Cell Mol. Med. 2019, 23, 2782–2793. [Google Scholar] [CrossRef]
- Saito, T.; Kuma, A.; Sugiura, Y.; Ichimura, Y.; Obata, M.; Kitamura, H.; Okuda, S.; Lee, H.C.; Ikeda, K.; Kanegae, Y.; et al. Autophagy regulates lipid metabolism through selective turnover of NCoR1. Nat. Commun. 2019, 10, 1567. [Google Scholar] [CrossRef]
- Visvikis, O.; Ihuegbu, N.; Labed, S.A.; Luhachack, L.G.; Alves, A.F.; Wollenberg, A.C.; Stuart, L.M.; Stormo, G.D.; Irazoqui, J.E. Innate host defense requires TFEB-mediated transcription of cytoprotective and antimicrobial genes. Immunity 2014, 40, 896–909. [Google Scholar] [CrossRef]
- Chandra, S.; Roy, A.; Patel, D.R.; Pahan, K. PPARalpha Between Aspirin and Plaque Clearance. J. Alzheimers Dis. 2019, 71, 389–397. [Google Scholar] [CrossRef]
- Chandra, S.; Roy, A.; Jana, M.; Pahan, K. Cinnamic acid activates PPARalpha to stimulate Lysosomal biogenesis and lower Amyloid plaque pathology in an Alzheimer’s disease mouse model. Neurobiol. Dis. 2019, 124, 379–395. [Google Scholar] [CrossRef]
- Chandra, S.; Jana, M.; Pahan, K. Aspirin Induces Lysosomal Biogenesis and Attenuates Amyloid Plaque Pathology in a Mouse Model of Alzheimer’s Disease via PPARalpha. J. Neurosci. 2018, 38, 6682–6699. [Google Scholar] [CrossRef]
- Ghosh, A.; Jana, M.; Modi, K.; Gonzalez, F.J.; Sims, K.B.; Berry-Kravis, E.; Pahan, K. Activation of peroxisome proliferator-activated receptor alpha induces lysosomal biogenesis in brain cells: Implications for lysosomal storage disorders. J. Biol. Chem. 2015, 290, 10309–10324. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Makhdoomi, M.; Singh, L.; Kumar, P.; Khan, N.; Singh, S.; Verma, H.N.; Luthra, K.; Sarkar, S.; Kumar, D. Trehalose limits opportunistic mycobacterial survival during HIV co-infection by reversing HIV-mediated autophagy block. Autophagy 2020. [Google Scholar] [CrossRef] [PubMed]
- Rusmini, P.; Cortese, K.; Crippa, V.; Cristofani, R.; Cicardi, M.E.; Ferrari, V.; Vezzoli, G.; Tedesco, B.; Meroni, M.; Messi, E.; et al. Trehalose induces autophagy via lysosomal-mediated TFEB activation in models of motoneuron degeneration. Autophagy 2019, 15, 631–651. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.H.; Jasuja, R.; Kumar, R. Trehalose induces functionally active conformation in the intrinsically disordered N-terminal domain of glucocorticoid receptor. J. Biomol. Struct Dyn. 2017, 35, 2248–2256. [Google Scholar] [CrossRef]
- Palomer, X.; Capdevila-Busquets, E.; Botteri, G.; Salvado, L.; Barroso, E.; Davidson, M.M.; Michalik, L.; Wahli, W.; Vazquez-Carrera, M. PPARbeta/delta attenuates palmitate-induced endoplasmic reticulum stress and induces autophagic markers in human cardiac cells. Int. J. Cardiol. 2014, 174, 110–118. [Google Scholar] [CrossRef]
- Busch, D.; Kapoor, A.; Rademann, P.; Hildebrand, F.; Bahrami, S.; Thiemermann, C.; Osuchowski, M.F. Delayed activation of PPAR-beta/delta improves long-term survival in mouse sepsis: Effects on organ inflammation and coagulation. Innate Immun. 2018, 24, 262–273. [Google Scholar] [CrossRef]
- Kapoor, A.; Shintani, Y.; Collino, M.; Osuchowski, M.F.; Busch, D.; Patel, N.S.; Sepodes, B.; Castiglia, S.; Fantozzi, R.; Bishop-Bailey, D.; et al. Protective role of peroxisome proliferator-activated receptor-beta/delta in septic shock. Am. J. Respir. Crit. Care Med. 2010, 182, 1506–1515. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, H.K.; Hwang, E.S. Novel anti-adipogenic activity of anti-malarial amodiaquine through suppression of PPARgamma activity. Arch. Pharm. Res. 2017, 40, 1336–1343. [Google Scholar] [CrossRef]
- Penas, F.N.; Carta, D.; Cevey, A.C.; Rada, M.J.; Pieralisi, A.V.; Ferlin, M.G.; Sales, M.E.; Mirkin, G.A.; Goren, N.B. Pyridinecarboxylic Acid Derivative Stimulates Pro-Angiogenic Mediators by PI3K/AKT/mTOR and Inhibits Reactive Nitrogen and Oxygen Species and NF-kappaB Activation Through a PPARgamma-Dependent Pathway in T. cruzi-Infected Macrophages. Front. Immunol. 2019, 10, 2955. [Google Scholar] [CrossRef]
- Omeragic, A.; Saikali, M.F.; Currier, S.; Volsky, D.J.; Cummins, C.L.; Bendayan, R. Selective peroxisome proliferator-activated receptor-gamma modulator, INT131 exhibits anti-inflammatory effects in an EcoHIV mouse model. FASEB J. 2020, 34, 1996–2010. [Google Scholar] [CrossRef]
- Raghuram, S.; Stayrook, K.R.; Huang, P.; Rogers, P.M.; Nosie, A.K.; McClure, D.B.; Burris, L.L.; Khorasanizadeh, S.; Burris, T.P.; Rastinejad, F. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBalpha and REV-ERBbeta. Nat. Struct. Mol. Biol. 2007, 14, 1207–1213. [Google Scholar] [CrossRef]
- Pastore, N.; Vainshtein, A.; Herz, N.J.; Huynh, T.; Brunetti, L.; Klisch, T.J.; Mutarelli, M.; Annunziata, P.; Kinouchi, K.; Brunetti-Pierri, N.; et al. Nutrient-sensitive transcription factors TFEB and TFE3 couple autophagy and metabolism to the peripheral clock. EMBO J. 2019, 38. [Google Scholar] [CrossRef] [PubMed]
- Sulli, G.; Rommel, A.; Wang, X.; Kolar, M.J.; Puca, F.; Saghatelian, A.; Plikus, M.V.; Verma, I.M.; Panda, S. Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature 2018, 553, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhang, F.; Ye, Q.; Wang, H. The circadian clock regulates autophagy directly through the nuclear hormone receptor Nr1d1/Rev-erbalpha and indirectly via Cebpb/(C/ebpbeta) in zebrafish. Autophagy 2016, 12, 1292–1309. [Google Scholar] [CrossRef] [PubMed]
- Chandra, V.; Bhagyaraj, E.; Nanduri, R.; Ahuja, N.; Gupta, P. NR1D1 ameliorates Mycobacterium tuberculosis clearance through regulation of autophagy. Autophagy 2015, 11, 1987–1997. [Google Scholar] [CrossRef] [PubMed]
- De Mei, C.; Ercolani, L.; Parodi, C.; Veronesi, M.; Lo Vecchio, C.; Bottegoni, G.; Torrente, E.; Scarpelli, R.; Marotta, R.; Ruffili, R.; et al. Dual inhibition of REV-ERBbeta and autophagy as a novel pharmacological approach to induce cytotoxicity in cancer cells. Oncogene 2015, 34, 2597–2608. [Google Scholar] [CrossRef]
- Woldt, E.; Sebti, Y.; Solt, L.A.; Duhem, C.; Lancel, S.; Eeckhoute, J.; Hesselink, M.K.; Paquet, C.; Delhaye, S.; Shin, Y.; et al. Rev-erb-alpha modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat. Med. 2013, 19, 1039–1046. [Google Scholar] [CrossRef]
- Costantini, C.; Renga, G.; Sellitto, F.; Borghi, M.; Stincardini, C.; Pariano, M.; Zelante, T.; Chiarotti, F.; Bartoli, A.; Mosci, P.; et al. Microbes in the Era of Circadian Medicine. Front. Cell Infect. Microbiol. 2020, 10, 30. [Google Scholar] [CrossRef]
- Tognini, P.; Thaiss, C.A.; Elinav, E.; Sassone-Corsi, P. Circadian Coordination of Antimicrobial Responses. Cell Host Microbe 2017, 22, 185–192. [Google Scholar] [CrossRef]
- Man, K.; Loudon, A.; Chawla, A. Immunity around the clock. Science 2016, 354, 999–1003. [Google Scholar] [CrossRef]
- Isakson, P.; Bjoras, M.; Boe, S.O.; Simonsen, A. Autophagy contributes to therapy-induced degradation of the PML/RARA oncoprotein. Blood 2010, 116, 2324–2331. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.W.; Chen, Z.H.; Zhang, X.J.; Han, B.W.; Lin, K.Y.; Li, X.J.; Wei, P.P.; Zhang, H.; Li, Y.; Chen, Y.Q. MIR125B1 represses the degradation of the PML-RARA oncoprotein by an autophagy-lysosomal pathway in acute promyelocytic leukemia. Autophagy 2014, 10, 1726–1737. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, A.B.; Torgersen, M.L.; Holm, K.L.; Abrahamsen, G.; Spurkland, A.; Moskaug, J.O.; Simonsen, A.; Blomhoff, H.K. Retinoic acid-induced IgG production in TLR-activated human primary B cells involves ULK1-mediated autophagy. Autophagy 2015, 11, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Wang, W.T.; Huang, W.; Fang, K.; Sun, Y.M.; Liu, S.R.; Luo, X.Q.; Chen, Y.Q. The lncRNA HOTAIRM1 regulates the degradation of PML-RARA oncoprotein and myeloid cell differentiation by enhancing the autophagy pathway. Cell Death Differ. 2017, 24, 212–224. [Google Scholar] [CrossRef]
- Brigger, D.; Schlafli, A.M.; Garattini, E.; Tschan, M.P. Activation of RARalpha induces autophagy in SKBR3 breast cancer cells and depletion of key autophagy genes enhances ATRA toxicity. Cell Death Dis. 2015, 6, e1861. [Google Scholar] [CrossRef]
- Marchwicka, A.; Cebrat, M.; Laszkiewicz, A.; Sniezewski, L.; Brown, G.; Marcinkowska, E. Regulation of vitamin D receptor expression by retinoic acid receptor alpha in acute myeloid leukemia cells. J. Steroid Biochem. Mol. Biol. 2016, 159, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.S.; De Veaux, N.; Rives, A.W.; Lahaye, X.; Lucas, S.Y.; Perot, B.P.; Luka, M.; Garcia-Paredes, V.; Amon, L.M.; Watters, A.; et al. A Comprehensive Map of the Monocyte-Derived Dendritic Cell Transcriptional Network Engaged upon Innate Sensing of HIV. Cell Rep. 2020, 30, 914–931 e919. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.; Garcia-Villa, E.; Ocadiz-Delgado, R.; Cortes-Malagon, E.M.; Vazquez, J.; Roman-Rosales, A.; Alvarez-Rios, E.; Celik, H.; Romano, M.C.; Uren, A.; et al. Human papillomavirus type 16 E7 oncoprotein upregulates the retinoic acid receptor-beta expression in cervical cancer cell lines and K14E7 transgenic mice. Mol. Cell Biochem. 2015, 408, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Q.; Zhou, Z.; Liu, M.; Chen, Y.; Li, J.; Xu, L.; Guo, J.; Li, Q.; Yang, J.; et al. Retinoic acid receptor beta, a potential therapeutic target in the inhibition of adenovirus replication. Antiviral Res. 2018, 152, 84–93. [Google Scholar] [CrossRef]
- Fan, J.; Lv, Z.; Yang, G.; Liao, T.T.; Xu, J.; Wu, F.; Huang, Q.; Guo, M.; Hu, G.; Zhou, M.; et al. Retinoic Acid Receptor-Related Orphan Receptors: Critical Roles in Tumorigenesis. Front. Immunol. 2018, 9, 1187. [Google Scholar] [CrossRef]
- Jetten, A.M. Retinoid-related orphan receptors (RORs): Critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl. Recept. Signal. 2009, 7, e003. [Google Scholar] [CrossRef] [PubMed]
- Friesenhagen, J.; Viemann, D.; Borgeling, Y.; Schmolke, M.; Spiekermann, C.; Kirschnek, S.; Ludwig, S.; Roth, J. Highly pathogenic influenza viruses inhibit inflammatory response in monocytes via activation of rar-related orphan receptor RORalpha. J. Innate Immun. 2013, 5, 505–518. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhao, Y.; Xu, L.; Gao, L.; Su, Y.; Lin, N.; Pu, J. The nuclear melatonin receptor RORalpha is a novel endogenous defender against myocardial ischemia/reperfusion injury. J. Pineal Res. 2016, 60, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, L.; Ding, S.; Lin, N.; Ji, Q.; Gao, L.; Su, Y.; He, B.; Pu, J. Novel protective role of the circadian nuclear receptor retinoic acid-related orphan receptor-alpha in diabetic cardiomyopathy. J. Pineal Res. 2017, 62. [Google Scholar] [CrossRef]
- Mirza-Aghazadeh-Attari, M.; Mohammadzadeh, A.; Adib, A.; Darband, S.G.; Sadighparvar, S.; Mihanfar, A.; Majidinia, M.; Yousefi, B. Melatonin-mediated regulation of autophagy: Making sense of double-edged sword in cancer. J. Cell Physiol. 2019, 234, 17011–17022. [Google Scholar] [CrossRef]
- Boga, J.A.; Caballero, B.; Potes, Y.; Perez-Martinez, Z.; Reiter, R.J.; Vega-Naredo, I.; Coto-Montes, A. Therapeutic potential of melatonin related to its role as an autophagy regulator: A review. J. Pineal Res. 2019, 66, e12534. [Google Scholar] [CrossRef]
- Brazao, V.; Santello, F.H.; Colato, R.P.; do Prado, J.C., Jr. T. cruzi infection among aged rats: Melatonin as a promising therapeutic molecule. Exp. Gerontol. 2020, 135, 110922. [Google Scholar] [CrossRef]
- Daryani, A.; Montazeri, M.; Pagheh, A.S.; Sharif, M.; Sarvi, S.; Hosseinzadeh, A.; Reiter, R.J.; Hadighi, R.; Joghataei, M.T.; Ghaznavi, H.; et al. The potential use of melatonin to treat protozoan parasitic infections: A review. Biomed. Pharmacother. 2018, 97, 948–957. [Google Scholar] [CrossRef]
- Hu, W.; Deng, C.; Ma, Z.; Wang, D.; Fan, C.; Li, T.; Di, S.; Gong, B.; Reiter, R.J.; Yang, Y. Utilizing melatonin to combat bacterial infections and septic injury. Br. J. Pharmacol. 2017, 174, 754–768. [Google Scholar] [CrossRef]
- Vielma, J.R.; Bonilla, E.; Chacin-Bonilla, L.; Mora, M.; Medina-Leendertz, S.; Bravo, Y. Effects of melatonin on oxidative stress, and resistance to bacterial, parasitic, and viral infections: A review. Acta Trop. 2014, 137, 31–38. [Google Scholar] [CrossRef]
- Anderson, G.; Reiter, R.J. Melatonin: Roles in influenza, Covid-19, and other viral infections. Rev. Med. Virol. 2020, 30, e2109. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Lei, H.; He, M.; Gong, R.; Wang, Y.; He, X.; Li, G.; Pang, P.; Li, X.; Yu, S.; et al. Melatonin triggers autophagic cell death by regulating RORC in Hodgkin lymphoma. Biomed. Pharmacother. 2020, 123, 109811. [Google Scholar] [CrossRef] [PubMed]
- Preidis, G.A.; Kim, K.H.; Moore, D.D. Nutrient-sensing nuclear receptors PPARalpha and FXR control liver energy balance. J. Clin. Investig. 2017, 127, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Panzitt, K.; Jungwirth, E.; Krones, E.; Lee, J.M.; Pollheimer, M.; Thallinger, G.G.; Kolb-Lenz, D.; Xiao, R.; Thorell, A.; Trauner, M.; et al. FXR-dependent Rubicon induction impairs autophagy in models of human cholestasis. J. Hepatol. 2020, 72, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Khambu, B.; Li, T.; Yan, S.; Yu, C.; Chen, X.; Goheen, M.; Li, Y.; Lin, J.; Cummings, O.W.; Lee, Y.A.; et al. Hepatic Autophagy Deficiency Compromises Farnesoid X Receptor Functionality and Causes Cholestatic Injury. Hepatology 2019, 69, 2196–2213. [Google Scholar] [CrossRef]
- Endo-Umeda, K.; Makishima, M. Liver X Receptors Regulate Cholesterol Metabolism and Immunity in Hepatic Nonparenchymal Cells. Int. J. Mol. Sci. 2019, 20, 5045. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, D.K.; Ma, X. Liver X receptors bridge hepatic lipid metabolism and inflammation. J. Dig. Dis. 2012, 13, 69–74. [Google Scholar] [CrossRef]
- Zeng, J.; Wu, D.; Hu, H.; Young, J.A.T.; Yan, Z.; Gao, L. Activation of the liver X receptor pathway inhibits HBV replication in primary human hepatocytes. Hepatology 2020. [Google Scholar] [CrossRef]
- Lange, P.T.; Jondle, C.N.; Darrah, E.J.; Johnson, K.E.; Tarakanova, V.L. LXR Alpha Restricts Gammaherpesvirus Reactivation from Latently Infected Peritoneal Cells. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, Z.; Zhang, Y.; Ma, X.; Chen, Y.; Yu, M.; Ma, C.; Li, X.; Cao, Y.; Liu, J.; et al. Activation of liver X receptor plays a central role in antiviral actions of 25-hydroxycholesterol. J. Lipid Res. 2018, 59, 2287–2296. [Google Scholar] [CrossRef]
- Ahsan, F.; Maertzdorf, J.; Guhlich-Bornhof, U.; Kaufmann, S.H.E.; Moura-Alves, P. IL-36/LXR axis modulates cholesterol metabolism and immune defense to Mycobacterium tuberculosis. Sci. Rep. 2018, 8, 1520. [Google Scholar] [CrossRef] [PubMed]
- Matalonga, J.; Glaria, E.; Bresque, M.; Escande, C.; Carbo, J.M.; Kiefer, K.; Vicente, R.; Leon, T.E.; Beceiro, S.; Pascual-Garcia, M.; et al. The Nuclear Receptor LXR Limits Bacterial Infection of Host Macrophages through a Mechanism that Impacts Cellular NAD Metabolism. Cell Rep. 2017, 18, 1241–1255. [Google Scholar] [CrossRef] [PubMed]
- Silvente-Poirot, S.; Segala, G.; Poirot, M.C.; Poirot, M. Ligand-dependent transcriptional induction of lethal autophagy: A new perspective for cancer treatment. Autophagy 2018, 14, 555–557. [Google Scholar] [CrossRef]
- Segala, G.; David, M.; de Medina, P.; Poirot, M.C.; Serhan, N.; Vergez, F.; Mougel, A.; Saland, E.; Carayon, K.; Leignadier, J.; et al. Dendrogenin A drives LXR to trigger lethal autophagy in cancers. Nat. Commun. 2017, 8, 1903. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.A.; Singh, B.K.; Zhou, J.; Wu, Y.; Farah, B.L.; Ohba, K.; Lesmana, R.; Gooding, J.; Bay, B.H.; Yen, P.M. Thyroid hormone induction of mitochondrial activity is coupled to mitophagy via ROS-AMPK-ULK1 signaling. Autophagy 2015, 11, 1341–1357. [Google Scholar] [CrossRef]
- Cioffi, F.; Senese, R.; Lanni, A.; Goglia, F. Thyroid hormones and mitochondria: With a brief look at derivatives and analogues. Mol. Cell Endocrinol. 2013, 379, 51–61. [Google Scholar] [CrossRef]
- Sinha, R.A.; You, S.H.; Zhou, J.; Siddique, M.M.; Bay, B.H.; Zhu, X.; Privalsky, M.L.; Cheng, S.Y.; Stevens, R.D.; Summers, S.A.; et al. Thyroid hormone stimulates hepatic lipid catabolism via activation of autophagy. J. Clin. Investig. 2012, 122, 2428–2438. [Google Scholar] [CrossRef]
- Tseng, Y.H.; Ke, P.Y.; Liao, C.J.; Wu, S.M.; Chi, H.C.; Tsai, C.Y.; Chen, C.Y.; Lin, Y.H.; Lin, K.H. Chromosome 19 open reading frame 80 is upregulated by thyroid hormone and modulates autophagy and lipid metabolism. Autophagy 2014, 10, 20–31. [Google Scholar] [CrossRef]
- Chi, H.C.; Chen, S.L.; Lin, S.L.; Tsai, C.Y.; Chuang, W.Y.; Lin, Y.H.; Huang, Y.H.; Tsai, M.M.; Yeh, C.T.; Lin, K.H. Thyroid hormone protects hepatocytes from HBx-induced carcinogenesis by enhancing mitochondrial turnover. Oncogene 2017, 36, 5274–5284. [Google Scholar] [CrossRef]
- Tao, S.; Drexler, I. Targeting Autophagy in Innate Immune Cells: Angel or Demon during Infection and Vaccination? Front. Immunol. 2020, 11, 460. [Google Scholar] [CrossRef]
- Felzen, V.; Hiebel, C.; Koziollek-Drechsler, I.; Reissig, S.; Wolfrum, U.; Kogel, D.; Brandts, C.; Behl, C.; Morawe, T. Estrogen receptor alpha regulates non-canonical autophagy that provides stress resistance to neuroblastoma and breast cancer cells and involves BAG3 function. Cell Death Dis. 2015, 6, e1812. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).